Abstract
Background
Previous observational studies have indicated that green tea (GT) consumption is associated with reduced mortality from cerebral infarction but not with mortality from cerebral hemorrhage. Therefore, we hypothesized that GT exerts a direct antiatherosclerotic effect without any effect on hypertension. To investigate this hypothesis, we focused on adiponectin that seems to be among the several key players in atherosclerosis.
Objective
The objective of this randomized controlled trial (RCT) was to assess whether the consumption of catechin-enriched GT affects serum adiponectin levels and cardiovascular disease (CVD) risk factors among apparently healthy subjects.
Design
A total of 51 individuals participated in the study. Eligible participants were randomly assigned into GT consumption groups with either high catechin (400 mg/day) or low catechin (100 mg/day). The study participants were asked to stop GT consumption for 2 weeks (washout period), following which they were to start drinking the provided GT beverages everyday for 9 weeks. The outcome measures were changes in the adiponectin levels and CVD risk factors (body weight, body mass index, waist circumference, blood pressure, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglyceride, fasting plasma glucose, as well as aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transpeptidase, uric acid, and high-sensitive C-reactive protein).
Results
After intervention for 9 weeks, we found no significant difference between the high- and low catechin group with respect to changes in the serum adiponectin level: 0.35 µg/ml (95% confidence interval (CI): −1.03, 1.74). Also, no significant difference was observed between the high- and low catechin groups with respect to changes in any of the measured CVD risk factors.
Conclusion
This RCT showed no significant difference between the high- and low catechin groups with respect to changes in the serum adiponectin level and any CVD risk factors.
Keywords: randomized controlled trial, green tea, catechin, adiponectin, cardiovascular disease risk factors
Substantial evidence from in vitro and animal studies indicates that green tea (GT) preparations inhibit cardiovascular disease (CVD) processes (1–4). In our previous observational study, we showed that GT consumption was associated with a significantly lower risk of mortality due to CVD among middle-aged adults (5). The study also indicated that GT consumption was associated with reduced mortality from cerebral infarction but not with mortality from cerebral hemorrhage. These associations were consistent with those reported in another observational study (6). Therefore, we hypothesized that GT exerts a direct antiatherosclerotic effect not mediated via any effect on hypertension. To investigate this hypothesis, we focused on adiponectin that is among the several key players that seem to play a direct role in atherosclerosis. Adiponectin inhibits proliferation of migrated smooth muscle cells (7), monocyte adhesion to endothelial cells, and oxidized low-density lipoprotein (LDL) uptake of macrophages and has been shown to have direct effects on atherosclerotic lesions (8). In addition, some human studies suggested that high plasma adiponectin concentrations are associated with a lower risk of CVD (9–12), and observational studies have indicated an antiatherosclerotic role of adiponectin (13, 14).
Several animal experiments have indicated that the intake of GT increases the adiponectin level (15–17). To date, three randomized controlled trials (RCTs) have examined the association between tea catechin consumption and adiponectin levels in humans, but none reported a significant increase in adiponectin by GT catechin consumption (18–20). Because these RCTs recruited patients with diabetes mellitus and obesity, further evidence among healthy subjects is needed to obtain some consensus on this issue. We, therefore, designed this RCT to assess whether consumption of catechin-enriched GT affects serum adiponectin levels and CVD risk factors among apparently healthy subjects.
Subjects and methods
Study participants and intervention program
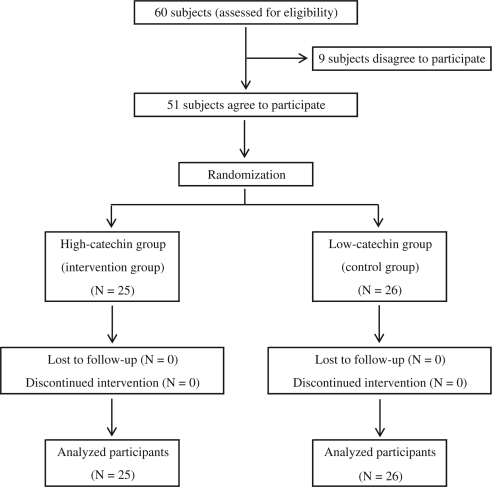
The study was conducted between June 2007 and September 2007, and between December 2007 and February 2008. The persons included in the present study were those who participated in a weight loss program at Sendai Health Promotion Center (weight loss program participants) and the staff of Sendai Health Promotion Center (weight loss program non-participants) in Japan. The inclusion criteria for the intervention program were (1) both sexes and (2) age between 20 and 70 years. The exclusion criteria were history of diabetes mellitus, cancer, ischemic heart disease, stroke, or renal disease. The weight loss program was based on exercise program (exercise guidance, stretching exercise, and strength training) and nutritional program (nutritional guidance and cooking practice). With a mean±SD value of 5±7 µg/ml in adiponectin, a minimum sample size of 50 subjects would be required to detect a difference (power=70%, two-sided α=0.05). We asked 60 subjects to participate in this study and obtained informed consent from 51 subjects. The study protocol was reviewed and approved by the Ethics Committee of Tohoku University Graduate School of Medicine.
We used commercially available catechin-containing beverages (500 ml). According to the data provided by the manufacturer, the high-concentration beverage contained 400 mg catechin and the low-concentration beverage, 100 mg (Table 1). We purchased the beverages and then delivered them to the participants’ residences. Adherence to the study protocol was confirmed by asking the subjects to return the bottle caps and by reviewing their consumption records. Eligible participants were stratified by sex (men or women) and the weight loss program (participation or non-participation), and randomization was conducted by permuted block method using a four-person block. A total of 51 participants were randomly assigned by an epidemiologist (NN) to either the high catechin group (N=25) or the low catechin group (N=26) (Fig. 1). The study participants were asked to stop GT consumption for 2 weeks (washout period), following which they were to start drinking the provided GT beverages everyday for 9 weeks. During the intervention period, the participants were asked not to drink any other catechin-containing beverage; other beverages were allowed. The participants and research assistants were blinded to the group allocation. Both the catechin-enriched beverages had similar taste and appearance. At the end of the study, the blinding of the participants was evaluated. (Register No: UMIN000000742).
Table 1.
Components of the test beverages
| Intervention beverage | Control beverage | |
|---|---|---|
| Total catechin (mg) | 400 | 100 |
| Caffeine (mg) | 105 | 80 |
| Total energy (kJ) | 0 | 0 |
| Total protein (g) | 0 | 0 |
| Total fat (g) | 0 | 0 |
| Carbohydrate (g) | 0 | 0 |
| Sodium (mg) | 46 | 53 |
Fig. 1.
Trial flow chart.
Outcome measures
The outcome measures were changes in the adiponectin levels and CVD risk factors: body weight, body mass index (BMI), waist circumference, blood pressure (BP), and levels of total cholesterol (TC), LDL cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglyceride (TG), fasting plasma glucose, as well as aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transpeptidase (γ-GTP), uric acid (UA), and high-sensitive C-reactive protein (CRP). The outcome measures were determined before and after the intervention. We also measured the nutrient intake and energy expenditure.
Venous blood was collected from the antecubital vein after the participants fasted overnight. Blood samples were collected into a tube containing ethylenediaminetetraacetic acid (EDTA)-2Na and a tube containing heparin. Serum and plasma samples were obtained by a 10-min centrifugation at 3,000 rpm within 30 min of obtaining the sample. The samples were then transported frozen to the SRL Laboratory in Hachioji, Tokyo, Japan, and stored at below −20°C until analysis. Serum adiponectin level was determined by enzyme-linked immunosorbent assay (ELISA; Otsuka Pharmaceutical, Tokyo, Japan), serum TC level, CEH-CDH-UV method (Sysmex Corporation, Hyogo, Japan), serum LDL-C level, liquid selective detergent method (Sekisui Medical, Tokyo, Japan), serum HDL-C level, accelerator selective detergent method (Sekisui Medical), TG level, enzymatic method without endogenous free glycerol (Sekisui Medical), fasting plasma glucose level, HK-G-6-PDH method (Shino-Test, Tokyo, Japan), AST, ALT, and γ-GTP levels, JSCC transferable method (Kanto Chemical, Tokyo, Japan), UA level, enzymatic method (Sekisui Medical), and the high-sensitive CRP level was assayed by nephelometric immunoassay (Siemens Healthcare Diagnostics, Tokyo, Japan).
BMI was calculated as body weight (kg) divided by squared height (m2). BP was measured using a cuff placed on the upper arm of each participant in the sitting position. Nutrition surveys were carried out using a food frequency questionnaire (Excel Eiyokun) (21). Energy expenditure was measured using Lifecorder (Suzuken).
Statistical analysis
Comparisons between the two groups were performed by Student's t-test to assess the differences in the biochemical and anthropometric parameters at baseline. Sex ratio was compared by a Chi-squared test. Effects of the intervention on serum adiponectin level and other outcome measures were tested using a paired t-test in each group before and after the intervention. Analysis of covariance was used to investigate the significance of the differences in the initial values as well as the net changes after the intervention between the two groups. We considered the following variables as potential confounders a priori: age at baseline in years (continuous variable), sex, and baseline level of each variable. All statistical analyses were performed using SAS version 9.1 (SAS Inc., Cary, NC, USA). Intention to treat analysis was adopted. Approximate variance formulas were used to calculate the 95% confidence intervals (CIs). Differences were accepted as statistically significant at p<0.05. In addition, stratified analyses according to weight loss program (participation or non-participation) were conducted.
Results
All the study participants completed the study; 95.9% of the tea bottles were consumed in the high catechin group and 97.6%, in the low catechin group. No apparent harmful effects were observed. At the end of the study, we observed that more than half of the participants were blinded.
Comparisons of baseline variables between the high- and low catechin groups are shown in Table 2. No significant difference in the baseline adiponectin level was observed between the two groups. The proportion of women was approximately 65% in both the groups. With the exception of the baseline mean γ-GTP level, no other variable showed a significant difference between the two groups.
Table 2.
Baseline characteristics of participants according to high-catechin group and low-catechin groupa
| Variables | High-catechin group (N=25) | Low-catechin group (N=26) | P-valuesb |
|---|---|---|---|
| Serum adiponectin (µg/mL) | 8.2±4.7 | 8.8±3.2 | 0.06 |
| Age (years) | 43.2±14.8 | 48.2±12.4 | 0.38 |
| Women (%) | 64.0 | 65.4 | 0.92 |
| Body weight (kg) | 66.4±13.7 | 64.8±13.7 | 0.97 |
| Body mass index (kg/m2) | 24.6±4.3 | 24.5±4.2 | 0.92 |
| Waist circumference (cm) | 85.0±12.7 | 85.7±12.0 | 0.78 |
| Systolic blood pressure (mmHg) | 123±15 | 123±16 | 0.81 |
| Diastolic blood pressure (mmHg) | 75±10 | 76±10 | 0.71 |
| Total cholesterol (mmol/L) | 4.66±0.82 | 4.96±0.67 | 0.31 |
| LDL cholesterol (mmol/L) | 2.70±0.68 | 2.98±0.75 | 0.61 |
| HDL cholesterol (mmol/L) | 1.37±0.36 | 1.46±0.37 | 0.93 |
| Triglyceride (mmol/L) | 1.20±1.15 | 1.31±1.63 | 0.09 |
| Fasting plasma glucose (mmol/L) | 5.30±0.68 | 5.48±0.53 | 0.25 |
| Aspartate aminotransferase (U/L) | 21.9±7.2 | 19.9±6.0 | 0.37 |
| Alanine aminotransferase (U/L) | 19.2±11.3 | 17.4±8.0 | 0.09 |
| Gamma-glutamyl transpeptidase (U/L) | 28.4±28.0 | 31.9±44.7 | 0.03 |
| Uric acid (µmol/L) | 308±70 | 329±76 | 0.70 |
| High-sensitive C-reactive protein (ng/mL) | 511±573 | 521±534 | 0.73 |
| Energy intake (MJ/day) | 7.82±2.12 | 8.01±1.77 | 0.39 |
| Protein intake (g/day) | 62.1±20.4 | 69.5±20.0 | 0.91 |
| Fat intake (g/day) | 55.8±21.2 | 60.9±18.6 | 0.52 |
| Carbohydrate intake (g/day) | 251±63 | 252±50 | 0.24 |
| Energy expenditure (MJ/day) | 8.75±1.33 | 8.49±1.60 | 0.56 |
Values were expressed as mean±SD.
P-values with chi-squared test for female ratio and for biochemical parameters, anthropometric parameters, nutrient intake, and energy expenditure, with Student's t-test.
Table 3 shows the changes in nutrient intake and energy expenditure. There were significant reductions in total energy intake and carbohydrate intake in any of the groups. The intake of total fat, total dietary fiber, sodium chloride, and tocopherol was significantly decreased in the low catechin group. However, the net change between the groups was not significant.
Table 3.
Change in nutrient intake and energy expenditure of participants according to high-catechin group and low-catechin group
| Variables | Baseline Mean±SD | After 9 weeks Mean±SD | P-valuesa | Net changeb (95% CI) | P-valuesb |
|---|---|---|---|---|---|
| Energy intake (MJ/day) | |||||
| High-catechin group | 7.82±2.12 | 7.00±1.17 | 0.03 | −0.14 (−0.83, 0.55) | 0.68 |
| Low-catechin group | 8.01±1.77 | 7.22±1.63 | 0.009 | ||
| Protein intake (g/day) | |||||
| High-catechin group | 62.1±20.4 | 60.8±16.5 | 0.75 | −0.91 (−8.62, 6.80) | 0.81 |
| Low-catechin group | 69.5±20.0 | 66.5±16.9 | 0.24 | ||
| Fat intake (g/day) | |||||
| High-catechin group | 55.8±21.2 | 50.8±12.4 | 0.15 | −0.01 (−6.12, 6.10) | 0.99 |
| Low-catechin group | 60.9±18.6 | 53.3±14.9 | 0.006 | ||
| Carbohydrate intake (g/day) | |||||
| High-catechin group | 251±63 | 219±34 | 0.01 | −7.82 (−30.94, 15.29) | 0.50 |
| Low-catechin group | 252±50 | 227±51 | 0.03 | ||
| Total dietary fiber intake (g/day) | |||||
| High-catechin group | 12.1±4.5 | 11.7±4.9 | 0.64 | 0.33 (−1.45, 2.10) | 0.71 |
| Low-catechin group | 13.8±4.4 | 12.7±3.6 | 0.03 | ||
| Sodium chloride intake (g/day) | |||||
| High-catechin group | 8.9±2.9 | 8.6±3.1 | 0.64 | 1.16 (−0.52, 2.83) | 0.17 |
| Low-catechin group | 10.8±4.3 | 8.7±3.9 | 0.01 | ||
| Potassium intake (g/day) | |||||
| High-catechin group | 2.16±0.78 | 2.06±0.74 | 0.49 | −0.02 (−0.30, 0.26) | 0.89 |
| Low-catechin group | 2.44±0.75 | 2.29±0.61 | 0.07 | ||
| Calcium intake (mg/day) | |||||
| High-catechin group | 575±230 | 545±213 | 0.48 | 12.29 (−67.27, 91.86) | 0.76 |
| Low-catechin group | 581±170 | 556±173 | 0.19 | ||
| Magnesium intake (mg/day) | |||||
| High-catechin group | 236±75 | 224±79 | 0.53 | −0.98 (−33.86, 31.90) | 0.95 |
| Low-catechin group | 258±78 | 244±64 | 0.14 | ||
| Iron intake (mg/day) | |||||
| High-catechin group | 6.73±2.19 | 6.90±2.32 | 0.74 | 0.44 (−0.53, 1.41) | 0.37 |
| Low-catechin group | 7.80±2.56 | 7.18±1.94 | 0.07 | ||
| Zinc intake (mg/day) | |||||
| High-catechin group | 7.62±2.23 | 7.24±1.69 | 0.40 | −0.15 (−0.93, 0.62) | 0.69 |
| Low-catechin group | 8.28±2.14 | 7.81±1.74 | 0.09 | ||
| Copper intake (mg/day) | |||||
| High-catechin group | 1.02±0.29 | 0.95±0.26 | 0.29 | −0.02 (−0.14, 0.09) | 0.68 |
| Low-catechin group | 1.11±0.32 | 1.04±0.25 | 0.10 | ||
| Tocopherol intake (mg/day) | |||||
| High-catechin group | 6.90±2.21 | 6.36±1.64 | 0.18 | −0.08 (−0.79, 0.62) | 0.81 |
| Low-catechin group | 7.93±2.53 | 7.04±1.73 | 0.006 | ||
| Vitamin K intake (µg/day) | |||||
| High-catechin group | 200±81 | 201±105 | 0.94 | 8.99 (−28.20, 46.19) | 0.63 |
| Low-catechin group | 231±89 | 218±66 | 0.19 | ||
| Vitamin C intake (mg/day) | |||||
| High-catechin group | 80±45 | 82±44 | 0.73 | 4.08 (−9.05, 17.21) | 0.54 |
| Low-catechin group | 100±44 | 95±38 | 0.22 | ||
| Energy expenditure (MJ/day) | |||||
| High-catechin group | 8.75±1.33 | 8.51±1.27 | 0.06 | −0.09 (−0.38, 0.19) | 0.51 |
| Low-catechin group | 8.49±1.50 | 8.38±1.29 | 0.28 | ||
Paired t test.
The change in high-catechin group minus the change in low-catechin group. The net differences were calculated by analysis of covariance. Adjusted for age (in years), sex, and individual baseline variables.
Table 4 shows the changes in the serum adiponectin level and CVD risk factors. After 9 weeks of catechin consumption, the mean±SD changes from baseline in the adiponectin level were 1.29±2.77 µg/ml in the high catechin group and 1.00±1.87 µg/ml in the low catechin group. We found no significant difference between the high- and low catechin group with respect to changes in the serum adiponectin level: 0.35 µg/ml (95% CI: −1.03, 1.74). There were significant decreases in the body weight, BMI, and waist circumference in both the groups, but the net change was not significant for any of these variables. Furthermore, there were no significant differences in the net change in other variables as well.
Table 4.
Change in serum adiponectin and cardiovascular risk factors of participants according to high-catechin group and low-catechin group
| Variables | Baseline Mean±SD | After 9 weeks Mean±SD | P-valuesa | Net changeb (95% CI) | P-valuesb |
|---|---|---|---|---|---|
| Serum adiponectin (µg/mL) | |||||
| High-catechin group | 8.2±4.7 | 9.5±5.7 | 0.03 | 0.35 (−1.03, 1.74) | 0.61 |
| Low-catechin group | 8.8±3.2 | 9.8±4.1 | 0.01 | ||
| Body weight (kg) | |||||
| High-catechin group | 66.4±13.7 | 64.9±13.7 | 0.002 | −0.35 (−1.44, 0.74) | 0.52 |
| Low-catechin group | 64.8±13.7 | 63.5±13.1 | 0.001 | ||
| Body mass index (kg/m2) | |||||
| High-catechin group | 24.6±4.3 | 24.0±4.1 | 0.002 | −0.18 (−0.58, 0.23) | 0.39 |
| Low-catechin group | 24.5±4.2 | 24.1±3.9 | 0.003 | ||
| Waist circumference (cm) | |||||
| High-catechin group | 85.0±12.7 | 82.7±12.2 | 0.007 | −0.73 (−2.76, 1.29) | 0.47 |
| Low-catechin group | 85.7±12.0 | 83.9±11.4 | 0.009 | ||
| Systolic blood pressure (mmHg) | |||||
| High-catechin group | 123±15 | 123±19 | 0.96 | −0.14 (−6.89, 6.61) | 0.97 |
| Low-catechin group | 123±16 | 123±13 | 0.73 | ||
| Diastolic blood pressure (mmHg) | |||||
| High-catechin group | 75±10 | 74±12 | 0.82 | −0.74 (−4.34, 2.86) | 0.68 |
| Low-catechin group | 76±10 | 76±10 | 0.92 | ||
| Total cholesterol (mmol/L) | |||||
| High-catechin group | 4.66±0.82 | 4.75±0.77 | 0.27 | 0.10 (−0.10. 0.31) | 0.32 |
| Low-catechin group | 4.96±0.67 | 4.93±0.70 | 0.59 | ||
| LDL cholesterol (mmol/L) | |||||
| High-catechin group | 2.70±0.68 | 2.76±0.73 | 0.41 | 0.07 (−0.14, 0.28) | 0.50 |
| Low-catechin group | 2.98±0.75 | 2.97±0.75 | 0.91 | ||
| HDL cholesterol (mmol/L) | |||||
| High-catechin group | 1.37±0.36 | 1.42±0.37 | 0.13 | 0.04 (−0.06, 0.15) | 0.39 |
| Low-catechin group | 1.46±0.37 | 1.47±0.37 | 0.82 | ||
| Triglyceride (mmol/L) | |||||
| High-catechin group | 1.20±1.15 | 1.14±1.06 | 0.40 | 0.25 (−0.11, 0.62) | 0.17 |
| Low-catechin group | 1.31±1.63 | 0.93±0.47 | 0.22 | ||
| Fasting plasma glucose (mmol/L) | |||||
| High-catechin group | 5.30±0.68 | 5.34±0.75 | 0.65 | 0.12 (−0.13, 0.38) | 0.34 |
| Low-catechin group | 5.48±0.53 | 5.35±0.45 | 0.20 | ||
| Aspartate aminotransferase (U/L) | |||||
| High-catechin group | 21.9±7.2 | 22.0±8.5 | 0.90 | 1.99 (−1.06, 5.05) | 0.20 |
| Low-catechin group | 19.9±6.0 | 19.2±5.7 | 0.38 | ||
| Alanine aminotransferase (U/L) | |||||
| High-catechin group | 19.2±11.3 | 20.2±9.8 | 0.52 | 1.93 (−1.78, 5.63) | 0.30 |
| Low-catechin group | 17.4±8.0 | 17.7±7.8 | 0.85 | ||
| Gamma-glutamyl transpeptidase (U/L) | |||||
| High-catechin group | 28.4±28.0 | 31.5±32.0 | 0.07 | 4.61 (−0.98, 10.19) | 0.10 |
| Low-catechin group | 31.9±44.7 | 29.4±30.7 | 0.41 | ||
| Uric acid (µmol/L) | |||||
| High-catechin group | 308±70 | 315±71 | 0.31 | 15.46 (−4.16, 35.09) | 0.12 |
| Low-catechin group | 329±76 | 318±78 | 0.16 | ||
| High-sensitive C-reactive protein (ng/mL) | |||||
| High-catechin group | 511±573 | 513±496 | 0.99 | −85.92 (−387.89, 216.06) | 0.57 |
| Low-catechin group | 521±534 | 627±692 | 0.35 | ||
Paired t test.
The change in high-catechin group minus the change in low-catechin group. The net differences were calculated by analysis of covariance. Adjusted for age (in years), sex, and individual baseline variables.
As for the net change in serum adiponectin, stratified analyses according to weight loss program (participation or non-participation) were conducted (Table 5). Among weight loss program participants and weight loss program non-participants, there were no significant differences in the net change: 0.15 µg/ml (95% CI: −1.54, 1.85) among weight loss program participants, and 1.49 µg/ml (95% CI: −0.46, 3.43) among weight loss program non-participants.
Table 5.
Change in serum adiponectin of participants according to high-catechin group and low-catechin group stratified by weight-loss program
| Variables | Baseline Mean±SD | After 9 weeks Mean±SD | P-valuesa | Net changeb (95% CI) | P-valuesb |
|---|---|---|---|---|---|
| Serum adiponectin (µg/mL) | |||||
| Weight-loss program participants | |||||
| High-catechin group (N=15) | 7.4±5.2 | 8.6±6.1 | 0.13 | 0.15 (−1.54, 1.85) | 0.86 |
| Low-catechin group (N=16) | 8.1±3.1 | 9.0±3.8 | 0.10 | ||
| Weight-loss program non-participants | |||||
| High-catechin group (N=10) | 9.5±3.6 | 10.9±5.0 | 0.15 | 1.49 (−0.46, 3.43) | 0.12 |
| Low-catechin group (N=10) | 9.9±3.1 | 11.1±4.3 | 0.06 |
Paired t test.
The change in high-catechin group minus the change in low-catechin group. The net differences were calculated by analysis of covariance. Adjusted for age (in years), sex, and baseline serum adiponectin.
Discussion
In this RCT, we tested a hypothesis that consumption of catechin-enriched GT would affect the serum adiponectin level and CVD risk factors in apparently healthy subjects. After 9 weeks of catechin consumption, the mean±SD changes from baseline in the adiponectin level were significantly increased in both groups. However, we found no significant difference between the high- and low catechin group with respect to changes in the serum adiponectin level: 0.35 µg/ml (95% CI: −1.03, 1.74). The CVD risk factors, namely, body weight, BMI, and waist circumference, were significantly decreased in both the groups, but the net change was not significant for any of these variables.
There are at least three reasons that the changes from baseline in the adiponectin level were significantly increased in the both groups. First, more than half of the study participants participated in a weight loss program. Second, lifestyle of the study participants may have been changed by this study. Third, catechin was contained not only in high-concentration beverage (high catechin group; 400 mg) but also in low-concentration beverage (low catechin group; 100 mg). Therefore, if the low concentration of the catechins might be enough to increase the adiponectin levels, an increase would be observed in the both groups.
In addition, we conducted stratified analyses according to weight loss program (participation or non-participation) because the change in adiponectin levels in both the groups may be related to the significant weight loss program. We also found that there were no significant differences in the net change among weight loss program participants and non-participants. Because the net change among weight loss program non-participants was greater than that among weight loss program participants, the change in adiponectin levels would be less affected by the weight loss program.
To date, three RCTs have examined the association between tea catechin consumption and adiponectin levels in humans (18–20). These RCTs recruited patients with diabetes mellitus and obesity. Because these patients might have had atherosclerosis before the study, the effect of tea catechin consumption on serum adiponectin level might not be well detected. Ryu et al. observed a change in the adiponectin level after the consumption of 900 ml of water containing 9 g of GT daily for 4 weeks in patients with type 2 diabetes mellitus (18). After 4 weeks, the mean±SD change in the adiponectin level from the baseline value was 6.03±3.71 µg/ml in intervention group and 6.01±3.16 µg/ml in control group, although the net change between the groups was not significant. Hsu et al. observed a significant increase in the adiponectin level in obese women who consumed one capsule containing 491 mg of total catechin daily (19). After 12 weeks, the mean±SD change in the adiponectin level from the baseline value was 2.5±4.2 µg/ml in intervention group and 2.0±5.4 µg/ml in control group, although the net change was not significantly different. Nagao et al. observed a significant increase in the adiponectin level after the consumption of 582.8 mg of catechin daily in patients with type 2 diabetes mellitus (20). After 12 weeks, the mean±SD change in the adiponectin level from the baseline value was 1.32±0.61 mg/ml in intervention group and 0.34±0.48 µg/ml in control group, although the net change was not significantly different. Thus, all the three RCTs showed that the increase in the serum adiponectin level in the intervention group was greater than that in the control group, although the net change between the groups was not significant. Although we recruited healthy participants who did not have a history of diabetes mellitus, cancer, ischemic heart disease, stroke, or renal disease, our findings were consistent with those of the above reports.
Although we found no significant difference in the net change in adiponectin level, several reasons should be considered in the interpretation of our results. First, it may be necessary to consider the difference in the catechin dose between the high- and low catechin groups and the intervention period. In the present study, the difference in the catechin dose between the high- and low catechin groups was only 300 mg/day and the intervention period was 9 weeks. Hsu et al. adopted the difference in the catechin dose of 491 mg/day and intervention period of 12 weeks, but the net change in adiponectin level was not significant (19). Also, Nagao et al. adopted the difference in the catechin dose of 486.5 mg/day and intervention period of 12 weeks, but the net change in adiponectin level was not significant (20). Therefore, the difference in the catechin dose and the intervention period could not explain our observation.
Second, because GT is consumed primarily in Japan and China (22), habitual GT consumption may have the potential to affect study results. Although we adopted the washout period for 2 weeks, we did not find any apparent association between catechin-enriched GT consumption and adiponectin. Similarly, previous studies adopted the washout period for 2 weeks (19) and 4 weeks (20), but the net change in adiponectin level was not significant. Ryu et al. also excluded subjectswho had consumed GT regularly for over a month, but the net change in adiponectin level was not significant (18). Therefore, the washout period could not explain our observation.
Third, the compounds such as caffeine found in GT may have been responsible for the association between GT consumption and CVD risk factors. A previous observational study indicated the association between consumption of caffeine-containing coffee and adiponectin. No association between consumption of caffeine-containing coffee and adiponectin was indicated in either group (quartile 1: 0–100 mg, quartile 2: 101–237 mg, quartile 3: 237–378 mg, quartile 4: 379–967 mg) among non-diabetic subjects (23). Because the difference in the caffeine dose between the high- and low catechin groups was small (25 mg/day) in our study, caffeine could not explain our observation.
Fourth, chocolate, red wine, apples, and berries are known as good source of catechin (24, 25). Although we asked participants not to drink any other catechin-containing beverage, we had no information on the intake of these food items during the 9 weeks. In addition, we had no data on the levels of the major dietary catechins (gallocatechin, epicatechin, epigallocatechin, etc.) and the total blood antioxidant levels. However, these factors may be divided equally between the high- and low catechin groups by successful randomization. Also, a previous study indicated that the half-lives of epigallocatechin-3-gallate, epigallocatechin, and epicatechin once ingested were 3.4, 1.7, and 2.0 h, respectively (26). Therefore, it is difficult to interpret the results of all-night fasting plasma levels of catechins, if measured.
Finally, our RCT design might yield a relatively small number of participants, although we made a power calculation regarding sample size. We found no significant difference between the high- and low catechin groups with respect to changes in the serum adiponectin level. Our study had a similar sample size to previous RCTs, and our results were consistent with results of previous RCTs (19, 20). Therefore, a larger sample size may be necessary to detect any effect of tea catechin consumption on serum adiponectin level.
The present study also aimed to explore the changes in CVD risk factors. We found no significant differences in CVD risk factors between the high- and low catechin groups. Many studies have assessed the relation between GT consumption and CVD risk factors. In previous studies, the GT consumption showed statistically significant reductions in body weight, BMI, and waist circumference (27–29). Our study showed that decrease in the anthropometric parameters in the high catechin group was greater than that in the low catechin group, although the net change between groups was not significant. The previous studies that suggested statistically significant changes had a larger sample size (28, 29). Therefore, a larger sample size may be necessary to detect the effect of tea catechin consumption on the anthropometric parameters.
The effect of GT consumption on BP has been investigated in meta-analysis of previous studies. These data suggested that GT consumption did not show significant effects on systolic and diastolic BP (30). Our results on BP were consistent with the previous studies.
Among previous studies that have examined the association between GT and blood cholesterol (TC, LDL-C, and HDL-C), GT consumption significantly lowered the TC and LDL-C level, but no effect on HDL-C was observed (20, 28, 30, 31). Our results on HDL-C were consistent with previous studies, but the inconsistent findings on the effect of GT consumption on TC and LDL-C were observed. There are several possible reasons for the discrepancy between our study and previous studies on TC and LDL-C. First, the subjects of the previous studies were not a healthy population (20). Therefore, one of the reasons for discrepancy might be explained by the difference in the study subjects. Second, the sample size in the previous studies was large (28). Therefore, another reason for discrepancy might be explained by sample size.
Conclusions
This RCT showed that increase in serum adiponectin level in the high catechin group was greater than that in the low catechin group, although the net change between groups was not significant. Also, no significant difference was observed between the high- and low catechin groups with respect to changes in any CVD risk factors.
Acknowledgements
We thank Yoshiko Nakata, Mika Wagatsuma, Naoko Sato, and Tomoko Muroi for their technical assistance.
Conflict of interest and funding
This study was supported by a Grant-in-Aid for Exploratory Research (Term of Project: 2007–2008, Project Number: 19659157) from the Japan Society for the Promotion of Science (JSPS), Japan. None of the authors had any conflict of interest.
References
- 1.Basu A, Lucas EA. Mechanisms and effects of green tea on cardiovascular health. Nutr Rev. 2007;65:361–75. doi: 10.1301/nr.2007.aug.361-375. [DOI] [PubMed] [Google Scholar]
- 2.Zaveri NT. Green tea and its polyphenolic catechins: medicinal uses in cancer and noncancer applications. Life Sci. 2006;78:2073–80. doi: 10.1016/j.lfs.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Cooper R, Morré DJ, Morré DM. Medicinal benefits of green tea: part I. Review of noncancer health benefits. J Altern Complement Med. 2005;11:521–8. doi: 10.1089/acm.2005.11.521. [DOI] [PubMed] [Google Scholar]
- 4.Frei B, Higdon JV. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J Nutr. 2003;133:3275S–84S. doi: 10.1093/jn/133.10.3275S. [DOI] [PubMed] [Google Scholar]
- 5.Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, et al. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA. 2006;296:1255–65. doi: 10.1001/jama.296.10.1255. [DOI] [PubMed] [Google Scholar]
- 6.Larsson SC, Männistö S, Virtanen MJ, Kontto J, Albanes D, Virtamo J. Coffee and tea consumption and risk of stroke subtypes in male smokers. Stroke. 2008;39:1681–7. doi: 10.1161/STROKEAHA.107.504183. [DOI] [PubMed] [Google Scholar]
- 7.Matsuda M, Shimomura I, Sata M, Arita Y, Nishida M, Maeda N, et al. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem. 2002;277:37487–91. doi: 10.1074/jbc.M206083200. [DOI] [PubMed] [Google Scholar]
- 8.Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24:29–33. doi: 10.1161/01.ATV.0000099786.99623.EF. [DOI] [PubMed] [Google Scholar]
- 9.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–7. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 10.Maahs DM, Ogden LG, Kinney GL, Wadwa P, Snell-Bergeon JK, Dabelea D, et al. Low plasma adiponectin levels predict progression of coronary artery calcification. Circulation. 2005;111:747–53. doi: 10.1161/01.CIR.0000155251.03724.A5. [DOI] [PubMed] [Google Scholar]
- 11.Oh DK, Ciaraldi T, Henry RR. Adiponectin in health and disease. Diabetes Obes Metab. 2007;9:282–9. doi: 10.1111/j.1463-1326.2006.00610.x. [DOI] [PubMed] [Google Scholar]
- 12.Giannessi D, Maltinti M, Del Ry S. Adiponectin circulating levels: a new emerging biomarker of cardiovascular risk. Pharmacol Res. 2007;56:459–67. doi: 10.1016/j.phrs.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Otake H, Shite J, Shinke T, Watanabe S, Tanino Y, Ogasawara D, et al. Relation between plasma adiponectin, high-sensitivity C-reactive protein, and coronary plaque components in patients with acute coronary syndrome. Am J Cardiol. 2008;101:1–7. doi: 10.1016/j.amjcard.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 14.Marso SP, Mehta SK, Frutkin A, House JA, McCrary JR, Kulkarni KR. Low adiponectin levels are associated with atherogenic dyslipidemia and lipid-rich plaque in nondiabetic coronary arteries. Diabetes Care. 2008;31:989–94. doi: 10.2337/dc07-2024. [DOI] [PubMed] [Google Scholar]
- 15.Serisier S, Leray V, Poudroux W, Magot T, Ouguerram K, Nguyen P. Effects of green tea on insulin sensitivity, lipid profile and expression of PPARalpha and PPARgamma and their target genes in obese dogs. Br J Nutr. 2008;99:1208–16. doi: 10.1017/S0007114507862386. [DOI] [PubMed] [Google Scholar]
- 16.Shimada M, Mochizuki K, Sakurai N, Goda T. Dietary supplementation with epigallocatechin gallate elevates levels of circulating adiponectin in non-obese type-2 diabetic Goto-Kakizaki rats. Biosci Biotechnol Biochem. 2007;71:2079–82. doi: 10.1271/bbb.70174. [DOI] [PubMed] [Google Scholar]
- 17.Potenza MA, Marasciulo FL, Tarquinio M, Tiravanti E, Colantuono G, Federici A, et al. EGCG, a green tea polyphenol, improves endothelial function and insulin sensitivity, reduces blood pressure, and protects against myocardial I/R injury in SHR. Am J Physiol Endocrinol Metab. 2007;292:E1378–87. doi: 10.1152/ajpendo.00698.2006. [DOI] [PubMed] [Google Scholar]
- 18.Ryu OH, Lee J, Lee KW, Kim HY, Seo JA, Kim SG, et al. Effects of green tea consumption on inflammation, insulin resistance and pulse wave velocity in type 2 diabetes patients. Diabetes Res Clin Pract. 2006;71:356–8. doi: 10.1016/j.diabres.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Hsu CH, Tsai TH, Kao YH, Hwang KC, Tseng TY, Chou P. Effect of green tea extract on obese women: a randomized, double-blind, placebo-controlled clinical trial. Clin Nutr. 2008;27:363–70. doi: 10.1016/j.clnu.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Nagao T, Meguro S, Hase T, Otsuka K, Komikado M, Tokimitsu I, et al. A catechin-rich beverage improves obesity and blood glucose control in patients with type 2 diabetes. Obesity. 2009;17:310–7. doi: 10.1038/oby.2008.505. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi K, Yoshimura Y, Kaimoto T, Kunii D, Komatsu T, Yamamoto S. Validation of a food frequency questionnaire based on food groups for estimating individual nutrient intake. Jpn J Nutr. 2001;59:221–32. (in Japanese) [Google Scholar]
- 22.Graham HN. Green tea composition, consumption, and polyphenol chemistry. Prev Med. 1992;21:334–50. doi: 10.1016/0091-7435(92)90041-f. [DOI] [PubMed] [Google Scholar]
- 23.Williams CJ, Fargnoli JL, Hwang JJ, van Dam RM, Blackburn GL, Hu FB, et al. Coffee consumption is associated with higher plasma adiponectin concentrations in women with or without type 2 diabetes: a prospective cohort study. Diabetes Care. 2008;31:504–7. doi: 10.2337/dc07-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–47. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 25.Santos-Buelga C, Scalbert A. Proanthocyanidins and tannin-like compounds: nature, occurrence, dietary intake and effects on nutrition and health. J Sci Food Agric. 2000;80:1094–117. [Google Scholar]
- 26.Lee MJ, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, et al. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol Biomarkers Prev. 2002;11:1025–32. [PubMed] [Google Scholar]
- 27.Phung OJ, Baker WL, Matthews LJ, Lanosa M, Thorne A. Coleman CI. Effect of green tea catechins with or without caffeine on anthropometric measures: a systematic review and meta-analysis. Am J Clin Nutr. 2010;91:73–81. doi: 10.3945/ajcn.2009.28157. [DOI] [PubMed] [Google Scholar]
- 28.Nagao T, Hase T, Tokimitsu I. A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity. 2007;15:1473–83. doi: 10.1038/oby.2007.176. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Wen Y, Du Y, Yan X, Guo H, Rycroft JA, et al. Effects of catechin enriched green tea on body composition. Obesity. 2010;18:773–9. doi: 10.1038/oby.2009.256. [DOI] [PubMed] [Google Scholar]
- 30.Hooper L, Kroon PA, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, et al. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2008;88:38–50. doi: 10.1093/ajcn/88.1.38. [DOI] [PubMed] [Google Scholar]
- 31.Zheng XX, Xu YL, Li SH, Liu XX, Hui R, Huang XH. Green tea intake lowers fasting serum total and LDL cholesterol in adults: a meta-analysis of 14 randomized controlled trials. Am J Clin Nutr. 2011;94:601–10. doi: 10.3945/ajcn.110.010926. [DOI] [PubMed] [Google Scholar]