Abstract
Background
Thyroidectomized patients frequently report weight gain resistant to weight loss efforts, identifying their thyroidectomy as the event precipitating subsequent weight gain. We wished to determine whether recently thyroidectomized euthyroid patients gained more weight over 1 year than matched euthyroid patients with preexisting hypothyroidism.
Methods
We performed a retrospective chart review of subjects receiving medical care at an academic medical center. One hundred twenty patients had their weight and thyroid status documented after thyroidectomy and achievement of euthyroidism on thyroid hormone replacement, and one year later. Three additional groups of 120 patients with preexisting hypothyroidism, no thyroid disease, and thyroid cancer were matched for age, gender, menopausal status, height, and weight. Anthropometric data were documented at two time points 1 year apart. We compared the weight changes and body mass index changes occurring over a 1-year period in the four groups.
Results
Patients with recent postsurgical hypothyroidism gained 3.1 kg during the year, whereas matched patients with preexisting hypothyroidism gained 2.2 kg. The patients without thyroid disease and those with iatrogenic hyperthyroidism gained 1.3 and 1.2 kg, respectively. The weight gain in the thyroidectomized group was significantly greater than that in the matched hypothyroid group (p-value 0.004), the group without thyroid disease (p-value 0.001), and the patients with iatrogenic hyperthyroidism (p-value 0.001). Within the thyroidectomized group, the weight gain in menopausal women was greater than in either premenopausal women (4.4 vs. 2.3 kg, p-value 0.007) or men (4.4 vs. 2.5 kg, p-value 0.013).
Conclusion
Patients who had undergone thyroidectomy in the previous year did, in fact, gain more weight than their matched counterparts with preexisting hypothyroidism. In addition, all patients with hypothyroidism, even though treated to achieve euthyroidism, experienced more weight gain than both subjects without hypothyroidism and subjects with iatrogenic hyperthyroidism. The greatest weight gain in the thyroidectomized group was in menopausal women. These data raise the question of an unidentified factor related to taking thyroid hormone replacement that is associated with weight gain, with an additional intriguing effect of thyroidectomy itself. Menopausal status confers additional risk. These groups should be targeted for diligent weight loss efforts.
Introduction
Agradual increase in weight over time is characteristic of developed populations. Yearly weight gains of 0.35–0.83 kg have been reported in various female populations (1–5). Yearly weight gains in men are ∼0.29–0.4 kg (1,2,4). The average age of menopause in the United States is 50–51 years (6–8). In particular, middle-age and the perimenopausal period are considered to be times when there is weight gain and a shift to visceral fat (9,10). A study of women aged 42–52 years demonstrated a weight gain of 2.1 kg (3.0%) over 3 years (11).
Both development of hypothyroidism and treatment of hyperthyroidism may be associated with weight gain. Surgical treatment of hyperthyroidism was associated with a weight gain of 6.3 kg over 1 year. Most weight gain occurred within 3 months of surgery and was not reversed with a return to euthyroidism (12). Other studies show significant weight gain after treatment of hyperthyroidism, with significantly greater weight gain when thyroidectomy was the treatment employed (13), and association between development of iatrogenic hypothyroidism and the subsequent weight gain (13–15). The weight gain averaged 3.7 kg per year in one of these studies (13) and 6 kg per year in another (15). In the third study, there was a weight gain of about 4 kg in those who were simply rendered euthyroid, compared with about 10 kg in those who had intervening hypothyroidism (14). Another study showed that treatment of hypothyroidism was only associated with loss of body water, not loss of fat mass (16). Patients who were maintained on thyroid hormone suppression therapy had some limitation of their weight gain (14,17).
However, studies of weight changes in euthyroid individuals undergoing thyroidectomy are difficult to locate in the literature. A small study employing eight patients and controls showed no weight change with thyroidectomy when euthyroidism was maintained (18). It is the authors' experience that euthyroid patients undergoing thyroidectomy, who are never permitted to develop hypothyroidism, report disturbing weight gain. This study was initiated to investigate these reports by determining whether patients with recent postsurgical hypothyroidism gained weight even if they did not sustain a period of hypothyroidism.
Methods
Study overview
This study was designed to determine whether patients who recently underwent thyroidectomy and were euthyroid on thyroid hormone replacement experienced more weight gain than a matched cohort of patients with preexisting hypothyroidism, and two other comparison groups.
Study subjects
Study participants were (i) 120 euthyroid patients who underwent thyroidectomy, had a finding of benign thyroid disease, and were subsequently treated with replacement thyroid hormone and groups of 120 age- and gender-matched subjects with (ii) preexisting Hashimoto's hypothyroidism, (iii) absence of thyroid dysfunction, and (iv) thyroid cancer.
Thyroidectomy group
Euthyroid subjects who had undergone near total or total thyroidectomy due to goiter, nodular thyroid disease, or suspected thyroid cancer were identified. Patients undergoing surgery for hyperthyroidism, or found to have thyroid cancer after their surgery, were excluded. Only those with benign thyroid pathology who were treated with levothyroxine (LT4) within 2 days of their thyroidectomy to achieve a normal serum thyroid stimulating hormone (TSH) concentration of 0.4–4.5 mIU/L were included. Patients whose TSH levels were outside this range, either at their first laboratory assessment after thyroidectomy, or 1 year later, were excluded. Patients taking any form of triiodothyronine were excluded. Data regarding anthropometric characteristics, LT4 dose, and thyroid status were collected ∼6–16 weeks after thyroidectomy and LT4 initiation and again 1 year later.
Hypothyroidism group
A group of patients with preexisting autoimmune hypothyroidism who had been taking LT4 monotherapy for at least 5 years were matched for age, weight, height, gender, and menopausal status. Initial ages were matched to within 5 years, initial weights were matched to within 2.5 kg, and heights were matched to within 5 cm. Two sets of data 1 year apart were obtained for these matched subjects. Subjects were excluded if they had any recorded serum TSH concentrations <0.4 mIU/L or >4.5 mIU/L; had any serious chronic medical conditions such as cardiac, pulmonary, or renal disease; or were taking steroids. Patients with diagnoses potentially associated with atypical weight gain such as diabetes, hypopituitarism, or intervening pregnancy were also excluded. Inclusion was limited to those who had no major medical events or interventions, other than continued routine follow-up for their hypothyroidism.
Comparison groups, small sub-groups, and nonthyroid surgery group
Identical information was obtained at the beginning and end of a 1-year period for two comparison groups, also matched for age, weight, height, gender, and menopausal status. One control group was 120 patients without thyroid disease who had a serum TSH reading of 0.4–4.5 mIU/L documented in their chart. The second comparison group was 120 patients being treated with TSH suppression therapy with LT4 for differentiated thyroid cancer, who also had no evidence of residual disease during the year of observation. To attempt to address the issue of whether thyroidectomy or LT4 initiation were events that seemed to precipitate weight gain, the charts of patients in the thyroidectomy and hypothyroid groups were examined for documentation of weight 1 year earlier. In addition, charts of healthy individuals undergoing parathyroid adenoma resection for primary hyperparathyroidism were examined. Weights were abstracted from the time of their surgery and 1 year later.
Study protocol
Following institutional review board approval, study data were abstracted from medical records completed during routine clinical visits. Information collected at both the first and last time points were age, weight, height, gender, menopausal status, LT4 dose, and serum TSH concentration. TSH values were from laboratories mandated by the patient's insurance plan; there was no central laboratory. An Ohaus DS 10 digital platform scale and Ayrton S100 stadiometer were in use in the endocrinology and internal medicine clinics during the period of the study. Waist circumference was not documented. Patients took the LT4 preparation prescribed by their managing physician. The timing of LT4 administration and adherence to LT4 therapy was not documented in most charts. The age and self-reported menopausal status used were those documented at the beginning of the year period. The TSH concentration recorded for subjects was the mean of all TSH values available during the 1-year period. The LT4 dose reported for patients was the dose that they were taking for the majority of the study. Weights and heights were recorded only at the beginning and end of the 1-year period.
Statistical analysis
Statistical services were provided by the clinical research unit biostatistics core. SAS 9.1.3 was used for all analyses. This was a retrospective study designed to answer the following questions: (i) Is the weight gain of the thyroidectomized patients significantly different from the weight gain of the hypothyroid patients? (ii) Is the weight gain of either group significantly different from the annual weight gain of developed populations of the same age of about 0.5 kg/year (1–4,11,19), or different from the weight gain of the two additional study comparison groups (euthyroid subjects and patients with thyroid cancer)? (iii) Is the weight gain significantly different for the premenopausal women, menopausal women, or men in either the thyroidectomy or hypothyroid group? The identical questions were also asked regarding the change in body mass index (BMI). The comparison used for change in BMI was an annual increase in BMI of about 0.15 units/year (1,2,4,19).
The thyroidectomized and preexisting hypothyroidism groups are subsequently referred to as the clinical groups; the premenopausal women, menopausal women, and men are referred to as gender groups; the euthyroid subjects and the patients with thyroid cancer and iatrogenic hyperthyroidism are designated as comparison groups.
For pairs of continuous variables, scatter plots were constructed to visually display the respective relations. If found to be linear with both variables normally distributed, then the Pearson correlation was computed to statistically describe the significance, strength, and nature of the relation. If the assumption of linearity was violated, then the Spearman rank correlation was used instead.
An independent two-sample t-test was used to examine the existence of a statistically significant difference between the means of two groups. When the normality assumption was violated in either or both groups, the Wilcoxon-Mann–Whitney test was used. A one-way analysis of variance (ANOVA) was used to examine the difference in means of normally distributed variables between groups of three or more. When the normality assumption was violated, then the Kruskal–Wallis test was used. The values of continuous variables whose distribution did not follow that of a normal distribution were ranked. A multifactorial ANOVA was then used to examine statistically significant differences among the ranks of the study groups. A multiple Linear Regression was used to model the relation between the dependent variable and a varying combination of predictor variables.
Results
Baseline characteristics
Study data were abstracted during November 2009–May 2010 using patient records from 2005 to 2010. The baseline characteristics of the four primary study groups are shown divided by diagnosis (thyroidectomized vs. pre-existing hypothyroidism vs. euthyroid vs. iatrogenic hyperthyroidism) in Table 1A. Each of these groups was composed of 37.5% premenopausal women, 37.5% menopausal women, and 25% men. There was no difference between the age and height of the patients in the thyroidectomy and preexisting hypothyroidism group. There was also no difference in their serum TSH concentrations (1.5 vs. 1.7 mIU/L, p-value 0.13), based on a mean of 3.1 available TSH readings. However, the mean LT4 dose was significantly higher in the thyroidectomized group (124 mcg) compared with the pre-existing hypothyroidism group (114 mcg) (p-value 0.0042). By study design, the duration of LT4 therapy was significantly shorter for the thyroidectomy group compared with the hypothyroidism group (p-value 0.0001). Table 1B shows the two primary clinical groups (thyroidectomized vs. pre-existing hypothyroidism) divided by gender (premenopausal women vs. menopausal women vs. men). The serum TSH concentrations in the three gender groups were also not significantly different. However, the menopausal group was significantly older than the other two groups, and the male group was significantly taller than the other two groups. In addition, the total daily LT4 dose successively decreased from premenopausal women to men to menopausal women.
Table 1A.
Characteristics of Primary Clinical Groups and Comparison Groups
| |
Thyroidectomy group (n=120) |
Hypothyroid group (n=120) |
Euthyroid group (n=120) |
Iatrogenic hyperthyroidism group (n=120) |
||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age, (years) | 48 | 12 | 48 | 13 | 47 | 14 | 46 | 13 |
| LT4 dose (mcg) | 124 | 30 | 114 | 24 | — | — | 150 | 45 |
| Duration of therapy for hypothyroidism (years) | 1.1 | 0.1 | 7.4 | 1.2 | — | — | 6.9 | 1.3 |
| TSH concentration (mIU/L) | 1.5 | 1.0 | 1.7 | 1.1 | 1.3 | 0.69 | 0.22 | 0.39 |
| Height (cm) | 167 | 9 | 167 | 10 | 165 | 9 | 166 | 9 |
LT4, levothyroxine; TSH, thyroid stimulating hormone.
Table 1B.
Characteristics of Gender Groups (Thyroidectomy and Hypothyroid Groups Combined)
| |
Premenopausal female group (n=90) |
Menopausal female group (n=90) |
Male group (n=60) |
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 39 | 9 | 58 | 6.5 | 46 | 12 |
| LT4 dose (mcg) | 127 | 31 | 109 | 22 | 120 | 25 |
| TSH concentration (mIU/L) | 1.6 | 1.1 | 1.4 | 1.0 | 1.7 | 1.0 |
| Height (cm) | 165 | 7 | 163 | 7 | 178 | 7 |
The characteristics of the comparison groups are shown in Table 1A. The mean TSH for the euthyroid group and the group with iatrogenic hyperthyroidism was based on 1.4 values per patient and 3.2 values per patient, respectively. The patients with differentiated thyroid cancer had the following additional characteristics: 75% had stage I and II disease, and 25% had stage III disease, as defined by using the National Thyroid Cancer Treatment Cooperative Study Group staging system (20,21). They were, on average, 6.9 years remote from their thyroidectomy, and 83% had received radioiodine therapy.
Individual weight and BMI changes for thyroidectomy and hypothyroidism groups
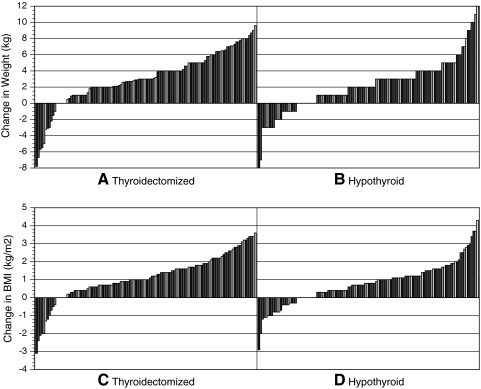
The changes in weight and BMI of the thyroidectomy and hypothyroidism group are displayed by using waterfall charts for clinical groups in Figure 1 and for gender groups in Figure 2. (The waterfalls charts show the changes in weight or BMI for each subject displayed in order of ascending magnitude from greatest decrease to greatest increase.) With regard to the pattern of weight changes in the thyroidectomy group, 11 patients (9%) lost weight, 6 patients (5%) maintained their weight, whereas the majority of patients (103 patients; 86%) gained weight (see Fig. 1A). A similar pattern was seen for the BMI changes in the same group (see Fig. 1C). Within the hypothyroid group, slightly more patients achieved weight loss. Twenty-five patients (21%) lost weight, 11 patients (9%) maintained their weight, whereas the remainder of the group (84 patients, 70%) gained weight (see Fig. 1B). Again, a similar pattern of BMI changes was seen in the hypothyroid group (see Fig. 1D).
FIG. 1.
Waterfall charts of weight changes by primary clinical groups [(A) thyroidectomy group, (B) hypothyroid group] and BMI changes by clinical groups [(C) thyroidectomy group, and (D) hypothyroid group]. BMI, body mass index.
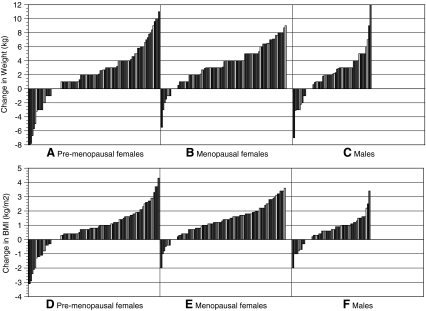
FIG. 2.
Waterfall charts of weight changes by gender groups [(A) premenopausal women, (B) postmenopausal women, (C) men] and BMI changes by gender groups [(D) premenopausal women, (E) postmenopausal women, (F) men].
Mean weight and BMI changes
The mean changes in weight and BMI for the primary clinical groups (thyroidectomy vs. hypothyroidism) are shown in Table 2A, those for the comparison groups (euthyroid without LT4 vs. iatrogenic hyperthyroidism) are shown in Table 2B, and those for the primary clinical groups combined and divided into gender groups (premenopausal vs. menopausal vs. males) are shown in Table 2C. The weight gain of the thyroidectomy group was significantly greater than the weight gain of the preexisting hypothyroidism group (3.1 vs. 2.2 kg, p-value 0.004). The patients in the thyroidectomy group also had a significantly greater increase in BMI than patients in the group with preexisting hypothyroidism (1.1 vs. 0.8 kg/m2, p-value 0.007). There was no correlation between the age of patients and their weight change in either the thyroidectomized group (Spearman Correlation: p-value=0.09) or the hypothyroid group (Spearman Correlation: p-value=0.15). Age and BMI changes were also not correlated (Spearman correlation: p-value=0.06 for the thyroidectomized group and Pearson correlation: p-value=0.38 for the hypothyroid group). Weight changes were not predicted by the achievement of different serum TSH concentrations in these groups (p-value=0.18). Although the LT4 dose was significantly higher in the thyroidectomized group than the hypothyroid group, the LT4 dose was not a statistically significant predictor (p-value=0.45) of weight change.
Table 2A.
Weight and Body Mass Index Changes by Primary Clinical Group
| |
Weight 1 (kg) |
Weight 2 (kg) |
Change in weight (kg) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinical groups | Mean | SD | Mean | SD | Mean | SD | Median | Min | Max |
| Thyroidectomy | 75.6 | 15.0 | 78.7 | 15.5 | 3.1 | 3.3 | 3.0 | −7.8 | 9.6 |
| Hypothyroid | 75.8 | 13.5 | 78.0 | 13.8 | 2.2 | 3.2 | 2.0 | −8.0 | 12 |
| p-value: 0.004 (Wilcoxon two-sample test) | |||||||||
| |
BMI 1 (kg/m2) |
BMI 2 (kg/m2) |
Change in BMI (kg/m2) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Median | Min | Max | |
| Thyroidectomy | 27.0 | 4.4 | 28.1 | 4.6 | 1.1 | 1.2 | 1.0 | −3.1 | 3.6 |
| Hypothyroid | 27.1 | 4.3 | 27.9 | 4.5 | 0.8 | 1.1 | 0.78 | −2.9 | 4.3 |
| p-value: 0.007 (Wilcoxon two-sample test) | |||||||||
BMI, body mass index.
Table 2B.
Weight Changes and Body Mass Index Changes of the Comparison Groups Studied
| |
Comparison groups |
|||
|---|---|---|---|---|
| |
Euthyroid (not on LT4) n=120 |
Iatrogenic hyperthyroidism (DTC) n=120 |
||
| Mean | SD | Mean | SD | |
| Change in Weight (kg) | 1.3 | 2.4 | 1.2 | 2.4 |
| Difference compared with thyroidectomy group | p-value 0.001 | p-value 0.001 | ||
| Difference compared with hypothyroid group | p-value 0.003 | p-value 0.003 | ||
| Change in BMI (kg/m2) | 0.5 | 0.5 | 0.4 | 0.9 |
| Difference compared with thyroidectomy group | p-value 0.001 | p-value 0.001 | ||
| Difference compared with hypothyroid group | p-value 0.007 | p-value 0.007 | ||
p-values generated using Wilcoxon two sample test.
DTC, differentiated thyroid cancer.
Table 2C.
Weight and Body Mass Index Changes by Gender Group (for Thyroidectomy and Hypothyroid Groups Combined)
| |
Weight 1 (kg) |
Weight 2 (kg) |
Change in weight (kg) |
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Premenopausal women | 71.1 | 13.2 | 73.3 | 14.4 | 2.2 | 3.7 |
| Menopausal women | 72.8 | 11.8 | 76.2 | 11.6 | 3.5 | 2.8 |
| Men | 88.1 | 12.2 | 90.1 | 12.7 | 2.0 | 3.1 |
| p-value: 0.01 (ANOVA) | ||||||
| |
BMI 1 (kg/m2) |
BMI 2 (kg/m2) |
Change in BMI (kg/m2) |
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Premenopausal women | 26.3 | 4.5 | 27 | 4.8 | 0.8 | 1.4 |
| Menopausal women | 27.4 | 4.7 | 28.8 | 4.7 | 1.3 | 1.1 |
| Men | 27.7 | 3.4 | 28.3 | 3.3 | 0.6 | 0.9 |
| p-value: 0.01 (ANOVA) | ||||||
ANOVA, analysis of variance.
The weight gain in each group was significantly greater than the weight gain of 0.5 kg/year estimated based on studies of developed populations described in the literature (1–4,11,19) (p-value <0.0001). The same was true of the increase in BMI in these groups, which was greater than the increased BMI of 0.15 kg/m2 estimated for developed populations (1,2,4,19) (p-value <0.0001). The gain in weight in the thyroidectomy group was considerably greater than in either the euthyroid group (3.1 vs. 1.3 kg, p-value 0.001) or the iatrogenic hyperthyroidism group (3.1 vs. 1.2 kg, p-value 0.001) (see Table 2B). The BMI changes were also significantly greater in the thyroidectomy group than in either of the comparison groups (see Table 2B). The same was true for the hypothyroid group, which had significantly greater weight and BMI changes than the comparison groups (p-value 0.003 for weight changes, p-value 0.007 for BMI changes) (see Table 2B).
Individual weight and BMI changes for the gender groups
The primary clinical groups (thyroidectomy and hypothyroidism groups) were combined together as one group and then instead divided by gender. Waterfall charts for the gender groups are shown in Figure 2. A different pattern of change was seen in the premenopausal and male groups compared with the menopausal group, with a greater proportion of the former groups achieving some weight loss. Sixteen premenopausal patients (13%) lost weight, 7 premenopausal patients (6%) maintained their weight, whereas 97 (81%) gained weight. Within the menopausal group, 7 patients (6%) lost weight, 5 patients (4%) maintained their weight, whereas the remaining 108 patients (90%) gained weight. Among the male group, 9 patients (15%) lost weight, 5 patients (8%) maintained their weight, and 106 patients (77%) gained weight.
Mean weight and BMI changes by gender groups
When the primary clinical groups were combined and divided by gender, there were significant differences in both the weight increases and the BMI increases according to gender (Table 2C).
Weight and BMI changes with patients divided by gender group within each of these two primary clinical groups (resulting in six different groups) are shown in Table 2D. For the thyroidectomy group alone, a statistically significant difference in mean weight change was found among the three gender groups (ANOVA: p-value: 0.0025). After multiplicity adjustments, statistically greater increases in mean weight were found in menopausal women compared with men (4.4 vs. 2.5 kg, p-value=0.013), and compared with premenopausal women (4.4 vs. 2.3 kg, p-value=0.0066). Analyses also indicated a statistically significant difference in mean BMI change between the genders within the thyroidectomized group (ANOVA: p-value: 0.0004). After multiplicity adjustments, statistically greater increases in mean BMI were found in menopausal women compared with men (1.7 vs. 0.8 kg/m2, p-value=0.0008), and compared with premenopausal women (1.7 vs. 0.8 kg/m2, p-value=0.0051).
Table 2D.
Weight Changes and Body Mass Index Changes within Primary Clinical Groups by Gender (for Thyroidectomy vs. Hypothyroid Groups)
| |
Change in weight (kg) |
Change in BMI (kg/m2) |
||||||
|---|---|---|---|---|---|---|---|---|
| |
Thyroidectomy |
Hypothyroid |
Thyroidectomy |
Hypothyroid |
||||
| Gender groups | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Premenopausal women | 2.3 | 3.7 | 2.1 | 3.7 | 0.8 | 1.4 | 0.8 | 1.4 |
| Menopausal women | 4.4 | 2.9 | 2.6 | 2.3 | 1.7 | 1.2 | 1.0 | 0.9 |
| Men | 2.5 | 2.2 | 1.6 | 3.7 | 0.8 | 0.7 | 0.5 | 1.1 |
| Significance | p-value 0.0025 (ANOVA) | p-value 0.14 (Kruskal–Wallis test) | p-value 0.0004 (ANOVA) | p-value 0.035 (ANOVA) | ||||
However, for the group with preexisting hypothyroidism alone (see Table 2D also), there was no significant difference in weight gain between the gender groups (Kruskal–Wallis Test: p-value: 0.14). When examining BMI, results indicated a statistically significant difference in mean BMI change among the 3 gender groups in the hypothyroid group (ANOVA: p-value: 0.035). After multiplicity adjustments, statistically significant differences in the mean BMI change were found between menopausal women and men (1.0 vs. 0.5 kg/m2, p-value=0.037), with the menopausal women experiencing a greater increase in BMI.
Weight changes in small sub-groups and nonthyroid surgery group
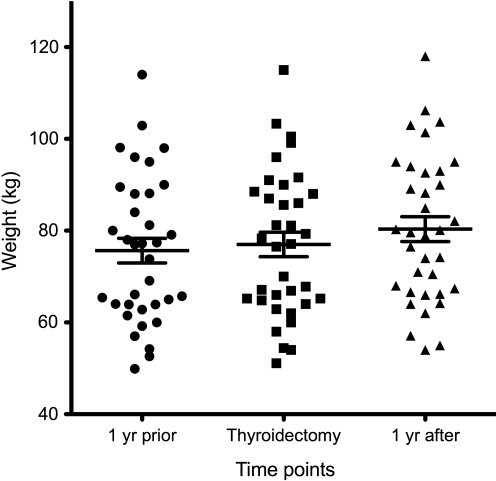
The mean weight gain of the sub-group with data available from 1 year before their thyroidectomy (n=35) was 1.3 kg (SD 2.2 kg). The mean weight gain of the same patients during the year after their thyroidectomy was 3.4 kg (SD 3.0 kg). The weights of these 35 patients are displayed in a scatter plot in Figure 3. The weight gain during the second year was significantly greater than the weight gain during the first year (p<0.001).
FIG. 3.
Weights of a subset of 35 patients who had weights available 1 year before their thyroidectomy, in addition to 1 year after their thyroidectomy. The mean weight and standard error are shown for each time point. The weight gain during the second year is significantly greater than the weight gain during the first year (p<0.001).
The mean weight gain for the sub-group with data available from 1 year before LT4 initiation (n=39) was 1.7 kg (SD 2.1). The group of 21 women who had undergone removal of a parathyroid adenoma experienced a mean weight gain of 1.1 kg (SD 1.2 kg) in the year after their surgery. These weight changes were all nonsignificant.
Discussion
This analysis confirms the patient-reported increase in weight during the year after thyroidectomy and initial treatment of postsurgical hypothyroidism. This increase is greater than that seen in subjects with treated spontaneous hypothyroidism. However, although the increase in weight is significant, it is also relatively modest. The weight gain in both the primary groups was significantly greater than that reported in a nonhypothyroid comparison group and a comparison group with iatrogenic hyperthyroidism. Thus, this study has two specific findings. First, euthyroid patients being treated for hypothyroidism of any cause experienced more weight gain than patients without hypothyroidism. Second, patients with recent postsurgical hypothyroidism gained more weight than patients with preexisting autoimmune hypothyroidism, despite immediate LT4 replacement and being rendered euthyroid within 6–16 weeks of their surgery. The greater weight gain in the two primary clinical groups was not due to inadequately normalized TSH values, as both groups had mean TSH concentrations that were similar to the median TSH reported for the healthy United States population (22).
Therefore, one could postulate that there is some factor in the physiology, lifestyle, or environment of euthyroid patients being treated for hypothyroidism which predisposes them to weight gain beyond that seen in other populations of similar ages. The comparison populations from the literature were from several developed populations from the years 1990 to 2006 (1–4,11,19); so, it is possible that this weight gain is an underestimate because of the trend for greater weight gain in the United States population in recent years. However, the primary clinical groups also had a greater weight than the two within-study comparison groups from an identical time period. The weight gain of both the clinical groups and the euthyroid control group with a documented serum TSH in their medical records may be an over-estimate, as these groups could be enriched for patients with abnormal weight gain, which lead to their negative screening for hypothyroidism, or diagnosis of thyroid disease. In addition, we cannot exclude the possibility of development of thyroid dysfunction in the euthyroid controls after their screening TSH was obtained.
Given the additional weight gain of those who had a recent thyroidectomy as the etiology of their hypothyroidism, there may be additional causative factors present in these patients. Certainly, hypothyroidism after thyroidectomy has been associated with weight gain (13–15,17). However, in our recently thyroidectomized patients, there was no intervening period of hypothyroidism. In addition, the baseline weight for the study was obtained once the patients had been documented as euthyroid. However, it is possible that either the stress of surgery or factors associated with any weight gain in the immediate postoperative period contributed to the weight gain that occurred in the subsequent year. Examination of the weight gain during the 1 year after parathyroid surgery in our small group of 21 patients and in another group reported in the literature (23) did not support this hypothesis of surgery-associated weight gain. The weight gain seen in the thyroidectomy group over 1 year was modest, but concerning. If the weight gains seen in the hypothyroid group were mirrored in the thyroidectomy group in later years, and subsequently superimposed on their initial weight gain, then this could result in clinically significant weight gain.
With regard to the thyroid cancer control group with postsurgical hypothyroidism and current iatrogenic hyperthyroidism, it would appear that their weight gain was mitigated by their hyperthyroidism or lower TSH values. This has been reported in previous studies (14,17). An association between serum TSH and weight gain has also been previously reported (24). An alternative explanation for why their weight gain was less than those with recent thyroidectomy was that an average of 6.9 years had elapsed since their thyroidectomy, and possibly this was a sufficient time period during which to reverse causative factors. Their lesser weight gain was not due to their malignancy diagnosis, as all patients had no evidence of disease during the year studied.
Many studies have suggested that the perimenopausal period is one during which women are particularly at risk for weight gain and increase in BMI (9,10). It is interesting that the menopausal women in both the study groups experienced the greatest weight gain. The magnitude was greatest in the thyroidectomy group, perhaps suggesting that sex steroid deprivation and recent thyroid surgery interact to make women particularly susceptible to weight gain.
The limitations of this study include its retrospective nature and the heterogeneity of data collection. This heterogeneity includes different laboratories employed for TSH testing, different staff obtaining anthropometric data, and the 6-year time span from which data was collected. The duration of LT4 therapy in the thyroidectomy group was ∼1 year, compared with about 7 years in the other two groups taking LT4; however, this difference is necessary for the study premise that recent onset of postsurgical hypothyroidism is a time of above-average weight gain. Despite the disadvantage of a retrospective study from a statistical standpoint, this design does ensure that the subjects' behavior with regard to weight loss efforts was unaffected by study entry, thus making the results generalizable. In other words, although we do not have information about life style and engagement in weight loss efforts, it is likely that the behavior of each of our study groups is representative of the general population. The strengths of the study include meticulous maintenance of euthyroidism and complete data collection.
The significance of these findings relates to the overall health of patients with hypothyroidism and to hypothyroid women in particular. Many health problems such as hypertension, diabetes, dyslipidemia, cardiovascular disease, and malignancy are associated with being overweight and obese (25). Decreased productivity and depression are also associated with obesity (26). Although some interventions are successful in producing weight loss, the weight loss is extremely modest and hard to sustain. For example, education interventions do not appear to impact the degree of weight gain (19), except perhaps in certain circumstances (3). Prevention of weight gain by physical activity seems to only be effective in those who are not already overweight in one study (5). Other studies show minimization of menopausal weight gain with lifestyle interventions, with a weight loss of 0.1 kg in the intervention group over 5 years compared with a weight gain of 2.4 kg in the control group (27), and increased exercise being associated with a weight loss of 0.32 kg (11).
Due to the overall limited success of weight loss programs, a propensity to weight gain is particularly concerning. From these data, the weight gain that occurs in hypothyroid patients, particularly after thyroidectomy and associated with menopause, is significant. Given the large population of individuals affected by hypothyroidism, it is important not only to target this population for intensive prevention of weight gain and weight loss programs, but also to investigate and target the cause of the weight gain. Possible causes could include an insufficient thyroid hormone dose, failure of LT4 to serve as full replacement with regard to balancing the metabolic and orexigenic actions of the thyroid hormone, or other contributing physiological or psychosocial factors in some way associated with thyroidectomy or thyroid hormone replacement. Inadequacy of thyroid hormone replacement, judged based on normalization of serum TSH, can be excluded in this particular group, as patients were maintained in the euthyroid state. It is possible that triiodothyronine deficiency could be a culprit in the weight gain. However, this would have to be subtle or present only at the tissue level (28), as serum triiodothyronine levels are, in general, adequately normalized by LT4 monotherapy (29).
In summary, physicians should counsel their hypothyroid patients and those undergoing thyroidectomy about the risk of weight gain and encourage them to engage in diligent weight loss efforts. Further, the etiology of weight gain associated with hypothyroidism, despite its treatment to achieve a normal serum TSH, deserves further investigation.
Acknowledgments
Statistical support for this project was provided through the Clinical Research Unit at Georgetown University and supported by Grant 1UL1RR031975 from the NCRR, a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Williamson DF. Kahn HS. Remington PL. Anda RF. The 10-year incidence of overweight and major weight gain in US adults. Arch Intern Med. 1990;150:665–672. [PubMed] [Google Scholar]
- 2.Rosell M. Appleby P. Spencer E. Key T. Weight gain over 5 years in 21,966 meat-eating, fish-eating, vegetarian, and vegan men and women in EPIC-Oxford. Int J Obes (Lond) 2006;30:1389–1396. doi: 10.1038/sj.ijo.0803305. [DOI] [PubMed] [Google Scholar]
- 3.Robbins AS. Chao SY. Baumgartner N. Runyan CN. Oordt MS. Fonseca VP. A low-intensity intervention to prevent annual weight gain in active duty Air Force members. Mil Med. 2006;171:556–561. doi: 10.7205/milmed.171.6.556. [DOI] [PubMed] [Google Scholar]
- 4.Ball K. Crawford D. Ireland P. Hodge A. Patterns and demographic predictors of 5-year weight change in a multi-ethnic cohort of men and women in Australia. Public Health Nutr. 2003;6:269–281. doi: 10.1079/PHN2002431. [DOI] [PubMed] [Google Scholar]
- 5.Lee IM. Djousse L. Sesso HD. Wang L. Buring JE. Physical activity and weight gain prevention. JAMA. 2010;303:1173–1179. doi: 10.1001/jama.2010.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gold EB. Bromberger J. Crawford S. Samuels S. Greendale GA. Harlow SD. Skurnick J. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153:865–874. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- 7.Weinstein M. Gorrindo T. Riley A. Mormino J. Niedfeldt J. Singer B. Rodriguez G. Simon J. Pincus S. Timing of menopause and patterns of menstrual bleeding. Am J Epidemiol. 2003;158:782–791. doi: 10.1093/aje/kwg223. [DOI] [PubMed] [Google Scholar]
- 8.McKinlay SM. Brambilla DJ. Posner JG. The normal menopause transition. Maturitas. 1992;14:103–115. doi: 10.1016/0378-5122(92)90003-m. [DOI] [PubMed] [Google Scholar]
- 9.Keller C. Larkey L. Distefano JK. Boehm-Smith E. Records K. Robillard A. Veres S. Al-Zadjali M. O'Brian AM. Perimenopausal obesity. J Womens Health (Larchmt) 2010;19:987–996. doi: 10.1089/jwh.2009.1547. [DOI] [PubMed] [Google Scholar]
- 10.Lovejoy JC. The influence of sex hormones on obesity across the female life span. J Womens Health. 1998;7:1247–1256. doi: 10.1089/jwh.1998.7.1247. [DOI] [PubMed] [Google Scholar]
- 11.Sternfeld B. Wang H. Quesenberry CP., Jr Abrams B. Everson-Rose SA. Greendale GA. Matthews KA. Torrens JI. Sowers M. Physical activity and changes in weight and waist circumference in midlife women: findings from the Study of Women's Health Across the Nation. Am J Epidemiol. 2004;160:912–922. doi: 10.1093/aje/kwh299. [DOI] [PubMed] [Google Scholar]
- 12.Pears J. Jung RT. Gunn A. Long-term weight changes in treated hyperthyroid and hypothyroid patients. Scott Med J. 1990;35:180–182. doi: 10.1177/003693309003500609. [DOI] [PubMed] [Google Scholar]
- 13.Dale J. Daykin J. Holder R. Sheppard MC. Franklyn JA. Weight gain following treatment of hyperthyroidism. Clin Endocrinol (Oxf) 2001;55:233–239. doi: 10.1046/j.1365-2265.2001.01329.x. [DOI] [PubMed] [Google Scholar]
- 14.Tigas S. Idiculla J. Beckett G. Toft A. Is excessive weight gain after ablative treatment of hyperthyroidism due to inadequate thyroid hormone therapy? Thyroid. 2000;10:1107–1111. doi: 10.1089/thy.2000.10.1107. [DOI] [PubMed] [Google Scholar]
- 15.Brunova J. Bruna J. Joubert G. Koning M. Weight gain in patients after therapy for hyperthyroidism. S Afr Med J. 2003;93:529–531. [PubMed] [Google Scholar]
- 16.Karmisholt J. Andersen S. Laurberg P. Weight loss after therapy of hypothyroidism is mainly caused by excretion of excess body water associated with myxoedema. J Clin Endocrinol Metab. 2011;96:99–103. doi: 10.1210/jc.2010-1521. [DOI] [PubMed] [Google Scholar]
- 17.Polotsky H. Brokhin M. Omry G. Tuttle R 2010 Effects of Iatrogenic Hyperthyroidism on Body Mass in Thyroid Cancer Patients. 92nd Annual Meeting of the Endocrine Society; San Diego. [Google Scholar]
- 18.Kormas N. Diamond T. O'Sullivan A. Smerdely P. Body mass and body composition after total thyroidectomy for benign goiters. Thyroid. 1998;8:773–776. doi: 10.1089/thy.1998.8.773. [DOI] [PubMed] [Google Scholar]
- 19.Jeffery RW. French SA. Preventing weight gain in adults: the pound of prevention study. Am J Public Health. 1999;89:747–751. doi: 10.2105/ajph.89.5.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jonklaas J. Sarlis NJ. Litofsky D. Ain KB. Bigos ST. Brierley JD. Cooper DS. Haugen BR. Ladenson PW. Magner J. Robbins J. Ross DS. Skarulis M. Maxon HR. Sherman SI. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid. 2006;16:1229–1242. doi: 10.1089/thy.2006.16.1229. [DOI] [PubMed] [Google Scholar]
- 21.Sherman SI. Brierley JD. Sperling M. Ain KB. Bigos ST. Cooper DS. Haugen BR. Ho M. Klein I. Ladenson PW. Robbins J. Ross DS. Specker B. Taylor T. Maxon HR., 3rd Prospective multicenter study of thyroid carcinoma treatment: initial analysis of staging and outcome. National Thyroid Cancer Treatment Cooperative Study Registry Group. Cancer. 1998;83:1012–1021. doi: 10.1002/(sici)1097-0142(19980901)83:5<1012::aid-cncr28>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 22.Hollowell JG. Staehling NW. Flanders WD. Hannon WH. Gunter EW. Spencer CA. Braverman LE. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 23.Okamoto T. Kamo T. Obara T. Outcome study of psychological distress and nonspecific symptoms in patients with mild primary hyperparathyroidism. Arch Surg. 2002;137:779–783. doi: 10.1001/archsurg.137.7.779. discussion 784. [DOI] [PubMed] [Google Scholar]
- 24.Knudsen N. Laurberg P. Rasmussen LB. Bulow I. Perrild H. Ovesen L. Jorgensen T. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab. 2005;90:4019–4024. doi: 10.1210/jc.2004-2225. [DOI] [PubMed] [Google Scholar]
- 25.Folsom AR. Kushi LH. Anderson KE. Mink PJ. Olson JE. Hong CP. Sellers TA. Lazovich D. Prineas RJ. Associations of general and abdominal obesity with multiple health outcomes in older women: the Iowa Women's Health Study. Arch Intern Med. 2000;160:2117–2128. doi: 10.1001/archinte.160.14.2117. [DOI] [PubMed] [Google Scholar]
- 26.Wolf AM. Siadaty MS. Crowther JQ. Nadler JL. Wagner DL. Cavalieri SL. Elward KS. Bovbjerg VE. Impact of lifestyle intervention on lost productivity and disability: improving control with activity and nutrition. J Occup Environ Med. 2009;51:139–145. doi: 10.1097/JOM.0b013e3181965db5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simkin-Silverman LR. Wing RR. Boraz MA. Kuller LH. Lifestyle intervention can prevent weight gain during menopause: results from a 5-year randomized clinical trial. Ann Behav Med. 2003;26:212–220. doi: 10.1207/S15324796ABM2603_06. [DOI] [PubMed] [Google Scholar]
- 28.Escobar-Morreale HF. Obregon MJ. Escobar del Rey F. Morreale de Escobar G. Replacement therapy for hypothyroidism with thyroxine alone does not ensure euthyroidism in all tissues, as studied in thyroidectomized rats. J Clin Invest. 1995;96:2828–2838. doi: 10.1172/JCI118353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jonklaas J. Davidson B. Bhagat S. Soldin SJ. Triiodothyronine levels in athyreotic individuals during levothyroxine therapy. JAMA. 2008;299:769–777. doi: 10.1001/jama.299.7.769. [DOI] [PubMed] [Google Scholar]