Abstract
Mesenchymal stem cells (MSCs) are a rare heterogeneous population of multipotent cells that can be isolated from many different adult and fetal tissues. They exhibit the capacity to give rise to cells of multiple lineages and are defined by their phenotype and functional properties, such as spindle-shaped morphology, adherence to plastic, immune response modulation capacity, and multilineage differentiation potential. Accordingly, MSCs have a wide range of promising applications in the treatment of autoimmune diseases, tissue repair, and regeneration. Recent studies have shed some light on the exact identity and native distribution of MSCs, whereas controversial results are still being reported, indicating the need for further review on their definition and origin. In this article, we summarize the important progress and describe some of our own relevant work on the developmental definition of MSCs.
Introduction
In the original definition, the term “MSC” can represent either mesenchymal stromal cells or mesenchymal stem cells. The term mesenchymal stromal cells may not be an accurate description, as stromal cells only represent connective tissue cells rather than functional cells. However, the term mesenchymal stem cells is not sufficiently descriptive either and has been questioned by Bianco et al. (2008), as cells derived from nonmesenchymal tissues have exhibited comparable properties to MSCs. Nevertheless, the term has gained such global usage that it has become the most accepted description. MSCs or MSC-like cells are widely distributed throughout tissues, including bone marrow, muscle, fat, hair follicles, tooth root, placenta, dermis, perichondrium, articular cartilage, umbilical cord, lung, and liver (Alsalameh et al., 2004; Arai et al., 2002; Forbes et al., 2002; Friedenstein et al., 1970; Fukuchi et al., 2004; Giangreco et al., 2002; Jahoda et al., 2003; Lee et al., 2004a, 2004b; Pierdomenico et al., 2005; Schultz et al., 1981; Toma et al., 2001; Williams et al., 2010; Young et al., 2001; Zuk et al., 2002). They were initially identified by their potential to differentiate upon induction, into several mesenchymal lineages such as bone and cartilage (Bianco and Gehron, 2000; Makino et al., 1999; Pittenger et al., 1999).
MSCs are comprised of a heterogeneous population of undifferentiated, committed, and lineage-primed cells, with the ability to “home” upon engraftment. As a type of adult stem cell, they show extensive proliferation, produce differentiated progeny, and functionally repair damaged tissues (Phinney et al., 1999). Since the disclosure of good immune tolerance and safety following MSC transplantation, clinical trials have been conducted to investigate the feasibility of the treatment of large bone defects, genetic bone disease such as osteogenesis imperfecta, cotransplantation with hematopoietic stem cells (HSCs) for the repair of hematopoietic stroma and graft-versus-host disease (De et al., 2003; Fang et al., 2009; Haleem et al., 2010; Joo et al., 2010; Le et al., 2005; Zhou et al., 2010). Although the previous definition of MSCs has become inadequate, due to tremendous progress achieved on the biology of MSCs, novel developmental theories have been proposed including the HSC origin of MSCs, the neural crest origin of MSCs, the pericyte origin of MSCs, and the avascular tissue origin of MSCs.
In this article we summarize the current findings on the definition of MSCs and give an overview of the recent advances in the ontogeny, characterization by surface markers, and microRNA (miRNA) profiles of MSCs. We also outline some interesting results from our latest work that may help unravel the origin of MSCs from the avascular tissue.
The Ontogeny of MSCs
MSCs were defined as mesenchymal-derived and by their functionality to generate a number of differentiated progeny and to self-renew. Alhough they were originally identified from bone marrow (BM) in the early 1970s by Friedenstein et al (1970), it is still not a well-characterized stem/progenitor cell population. It is generally accepted that MSCs may not only be isolated from the BM but also from many other tissues (Table 1). Recently, more evidences on the origin of MSCs have been brought forward in an attempt to reveal the ontogeny and physiological role of these cells.
Table 1.
Multiple Tissues Types Where MSCs Have Been Isolated
| Tissue origin | Publications |
|---|---|
| Muscle | Schultz, 1981; Med. Sci. Sports Exerc. |
| Young et al., 2001; Anat. Rec. | |
| Fat | Zuk et al., 2002; Mol. Biol. Cell |
| Hair follicels | Jahoda et al., 2003; Exp. Dermatol. |
| Tooth root | Pierdomenico et al., 2005; Transplantation |
| Placenta | Fukuchi et al., 2004; Stem Cells |
| Dermis | Toma et al., 2001; Nat. Cell Biol. |
| Perichondrium | Arai et al., 2002; J. Exp. Med. |
| articular cartiliage | Williams et al., 2010; PloS One |
| Alsalameh et al., 2004; Arthritis Rheum. | |
| Umbilical cord | Lee et al., 2004; Blood |
| Lung | Giangreco et al., 2002; Am. J. Pathol. |
| Liver | Forbes et al., 2002; J. Pathol. |
| Synovium | Sakaguchi et al., 2005; Arthritis Rheum. |
| Periodontal ligament | Seo et al., 2004; Lancet |
| Tendon | Bi et al., 2007; Nat. Med. |
| Pancreas | Sordi et al., 2010; Stem Cells |
In this regard, a few studies have suggested HSCs as an origin of MSC-like cells, as HSCs were able to differentiate into perivascular cells under an inductive condition and generated fibroblasts and their precursors (Ebihara et al., 2006; Hess et al., 2004; LaRue et al., 2006; Visconti et al., 2006). In his review, Ogawa et al. (2006) raised an hypothesis that fibroblasts and myofibroblasts in many organs and tissues are derived from HSCs, but there was no further experimental evidence to support this theory after 2006.
Recent findings have demonstrated that neural crest-derived cells exist in the adult BM and stem cells derived from BM can differentiate into neural cells (Jiang et al., 2002; Nagoshi et al., 2008). To confirm the relevance of these unexpected findings in vivo, Takashima and Morikawa demonstrated that neural crest was one of the developmental origins of MSCs in the adult BM, because the earliest wave of MSCs in the embryonic trunk was generated from Sox1+ neuroepithelium and cells derived from the neural crest possessed the similar characteristics with MSCs from other origins (Morikawa et al., 2009; Takashima et al., 2007). Similarly, Miller raised the theory that MSCs may arise from both the neural and nonneural tissues (Miller, 2007).
Probably the most interesting and so far the best accepted hypothesis is the pericyte origin of MSCs. The pericytes and smooth muscle cells have been reported to be able to transdifferentiate not only into the adult leydig cells, but also into osteocytes, adipocytes, chondrocytes, smooth muscle cells, and neural lineages (Davidoff et al., 2009; Doherty et al., 1998; Dore-Duffy et al., 2006; Farrington-Rock et al., 2004; Meyrick and Reid, 1978). The pericytes also expressed MSC markers, such as CD146, NG2, and PDGF-Rβ (Franklin et al., 1990; Ozerdem et al., 2001; Schwab and Gargett, 2007). A remarkable work conducted by Crisan and his colleagues established both the in situ and ex vivo links between human adult MSCs and pericytes (Crisan et al., 2008). The findings demonstrated that cells positive for MSC markers also expressed pericyte markers, and CD146+ pericytes (negative for CD34, CD45, and CD56) had the same multipotent capacity as MSCs (Crisan et al., 2008). Both MSCs and pericytes could migrate in response to digested extracellular matrix and other chemotactic stimuli, which could recruit MSCs to a regenerative microenvironment (Crisan et al., 2008). It is further reported that pericytes from human fetal, perinatal, and adult organ natively expressed MSC markers and possessed similar multilineage differentiation potential (Corselli et al., 2010). BMSCs expressed pericyte marker 3G5, and these cells are located in the vascular niche, where BMSCs may also provide mesenchymal elements (Kang et al., 2010; Khan et al., 2010).
However, the most recent findings are challenging the current notion, in which MSC-like cells can be derived from avascular and aneural tissue, for example, from the intervertebral disc (IVD). The IVD is a roughly cylindrical structure, comprised of a well-hydrated central nucleus pulposus (NP), an annulus fibrosus (AF) consisting of firm but flexible collagenous lamellae that surrounds the NP, and cartilaginous endplates forming an interface between disc and adjacent vertebrae (Zhou et al., 2008). Recently, a few studies have detected the existence of MSC-like cells in the degenerated human IVD and quadrupedal IVD. These cells possessed similar immunophenotype and differentiation capacity to BMSCs (Bianco et al., 2010; Henriksson et al., 2009; Risbud et al., 2007). To date, whether MSC-like cells are present in healthy human IVD has not been documented. To provide direct evidence of MSCs from the normal IVD, we have isolated cells from the IVD of healthy Rhesus monkeys, which can serve as an ideal model resembling humans in their genetic phylogeny, almost upright biomechanics, and the notochordal cell disappearance and the prevalence of disc degeneration associated with aging (Kramer et al., 2002; Pennisi, 2007; Stoeckelhuber et al., 2005; Watanabe, 1983). We found that the IVD-derived MSC-like cells express MSC markers such as CD44, CD90, CD146, and CD166, and are negative for CD34 and CD45. Furthermore, these cells demonstrate MSC-like differentiation capacities in vitro and are capable of generating IVD-like tissue in vivo. Furthermore, the cells show the self-renewal property by maintaining colony-forming ability, multidifferentiation potential, and transplantability after serials of cell divisions in vivo. In parallel, other studies have reported that pericytes are not the only cell type that acts as a source of MSCs (Feng et al., 2011). The study demonstrated that genetically marked pericytes can differentiate into odontoblasts in vivo and suggested the existence of nonpericyte-derived MSCs (Feng et al., 2011). Furthermore, the ratio of distribution was also described depending on the extent of vascularity and kinetics of growth (Feng et al., 2011).
Another crucial origin of MSCs is through the epithelial to mesenchymal transition (EMT), which is a fundamental process causing epithelial cells to lose their polarization and specialized junctional structure, undergo cytoskeleton reorganization and to acquire morphological and functional features of mesenchymal-like cells. A series of studies by Rubio et al. (2008), Limbert (2010), and Battula et al. (2010) found that EMT-derived cells shared many properties with MSCs, including the phenotype and functionality, as the EMT-derived cells expressed MSC markers and could differentiate into osteoblasts, adipocytes, and chondrocytes. However, not all epithelial cells can undergo EMT during development. Sordi and colleagues (2010) have revealed the pancreatic MSCs (PMSCs) are derived from BM by cell lineage tracing, rather than from the CD133+ duct cells through EMT.
Thus, the origin and ontogeny of adult MSCs are still complex and somewhat contradictory scenarios. Nevertheless, most findings support the overall conclusion that MSCs may originate from the perivascular sites, which is dominant in postnatal tissues. The other sources of MSCs may be related to neural crest and HSCs, and also the shielding tissues,which can maintain the protal properties to adult age, or the generation from transition.
Surface Markers of MSCs
Great efforts have been made to develop a cell-surface antigen profile for better purification and identification of MSCs. Indeed, several common mesenchymal markers have been identified for the characterization of MSCs including stro-1, CD13, CD29, CD44, CD73, CD105, and CD106, and it is generally agreed that MSC do not express CD31, CD34, CD45, and CD117 (Kucia et al., 2005; Rolf et al., 2008). Flow cytometry analysis demonstrated the basic multiple markers used to assess noncultured MSCs, such as CD105, CD166, CD90, CD44, CD29, CD73, and CD9 (Halfon et al., 2011). Changes in surface expression of MSC markers have been reported with cell growth and passage number. The expression of ITGA11, CD146, and CD106 on BMSCs decreases with increased passage number, but the expression of CD9 increases during the process, and a high level of CD9 is found in fibroblasts (Covas et al., 2008; Halfon et al., 2011). In adipose-derived MSCs (ASCs), the expression of stromal markers such as CD29, CD44, CD90, and CD166 changes during culture (Mitchell et al., 2006). Epigenetically, MSCs sequentially lose their myogenic, adipogenic, chondrogenic, osteogenic,and fibroblastic progenitor potential during growth and aging, probably indicating the hierarchical mechanism of MSC fate decisions (Sarugaser et al., 2009).
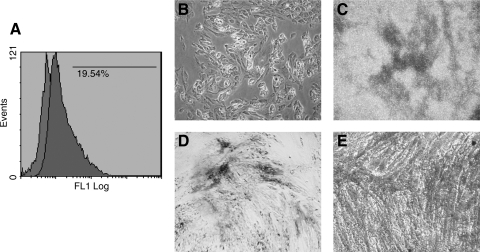
Recent studies have highlighted CD146 as a putative MSC marker for its association with the origin and aging of MSCs. This marker has been specifically suggested as one of the MSC markers in endometrium (Schwab and Gargett, 2007; Schwab et al., 2008). Other independent studies have also verified that CD146+ cells possess comparable phenotype and differentiation potential to MSCs and represent potential use in the regulation of HSCs (Covas et al., 2008; Sorrentino et al., 2008). In addition, CD146+ pericytes were reported to retain myogenicity, exhibit tripotency at the clonal level, and express MSCs markers (Crisan et al., 2009). Importantly, a subset of MSCs expressing CD146 in combination with other new markers such as NOTCH3 or ITGA11 displayed enhanced colony-forming capacities, and the CD146/NOTCH3 double-positive MSCs possessed enhanced potential to differentiate into other lineages, such as adipocytes and osteocytes (Kaltz et al., 2010). When comparing CD146 expression in MSCs and fibroblasts, most of the MSCs expressed CD146 but only around 5% of fibroblasts did, which is in line with the results from Covas reporting that 73% of BM-derived MSC and 2% of skin fibroblasts expressed CD146 (Covas et al., 2008; Kaltz et al., 2010). Interestingly, our own unpublished work has shown that the MSC-like cells isolated from the IVD tissue also expressed CD146 (Fig. 1A) and the CD146+ subsets possessed multidifferentiation capacity (Fig. 1B–E).
FIG. 1.
CD146 expression and multidifferentiation potentials of MSC-like cells isolated from the IVD (NP) tissue. (A) Flow cytometry analysis for the surface expression of CD146; adipogenic differentiation was observed using Oil Red O staining (B), chondrogenic differentiation was observed using Alican blue staining (C), and osteogenic differentiation was observed using Alkaline Phosphatase staining (D) and Alizarin Red S (E).
An additional specific surface marker for MSCs is CD271. It defines a subset of cells with high proliferative, clonogenic, and multipotential differentiation ability in adult BM (Quirici et al., 2002). Amnion mesenchymal cells (AMCs) and chorion mesenchymal cells (CMCs) have been reported to phenotypically and functionally resemble BMSCs, and the CD271+ fraction of AMCs and CMCs possess stronger stem cell potential (Soncini et al., 2007). Comparision of the CD271bright MSCs with the CD271dim MSCs from BM, only the positive subsets maintained the capacity to form colonies and coexpressed common MSC markers (Bühring et al., 2007). Other studies observed that the CD271+ fraction of MSCs have been reported to generate higher numbers of colonies and may be the most homogenous faction and better suitable for expansion (Jarocha et al., 2008). In addition, the CD271+ fraction of MSCs can improve the engraftment of CD133+ HSCs in transplantation, with immunosuppressive and lympho-hematopoietic engraftment-promoting properties (Kuçi et al., 2010). Compared with the bulk of ASCs, the CD271+ fraction of ASCs demonstrated a greater differentiation potential toward adipocytes, osteoblasts, and chondrocyte-like cells (Quirici et al., 2010). The CD271+ fraction of ASCs from aged mice possessed proliferation and differentiation potentials almost equal to the ASCs from young mice even though the differentiation potentials showed a tendency to decrease (Yamada et al., 2010). In osteoarthritic tissue, the CD45−/CD271+ MSCs abundantly existed in the trabecular bone cavity and displayed aging-related loss of proliferation (Jones et al., 2010). Osteoarthritic synovial membranes contained more CD271+ cells than healthy joints, and spontaneous cartilage repair tissue contained cells positive for CD271 antigen, suggesting the involvement of CD271 antigen in spontaneous cartilage repair (Hermida-Gómez et al., 2011).
Other cell surface molecules such as TNAP, FZD9, and Stro-4 and side population of MSCs are also focused and deemed as promising MSC markers (Battula et al., 2008; Gronthos et al., 2009; Kobayashi et al., 2008; Sobiesiak et al., 2010). Among all these potential MSC markers, we recommend that CD146 and CD271 may be the most useful markers, although their function remains to be elucidated. Cell migration, cytoskeletal response and signaling pathway stimulation assays currently used to analyze MSC membrane proteins may prove to be useful in investigating and studying the functions of those MSC markers.
Micro-RNA Profiles of MSCs
Gene expression profiling has fundamentally enhanced our understanding and approach to characterizing MSCs identifying potential biomarkers, or determining key molecules regulating biological processes. MiRNAs are likely to be invovled in gene regulation during the early stages of differentiation, which may play an important role in determining cell fate (Table 2). Although the detailed role of each miRNA in stem cell biology remains to be analyzed, understanding how miRNAs affect stem cell behavior will advance our definition of MSCs. Previous studies have demonstrated that miRNA-9 might be involved in regulating neuronal differentiation of MSC through the Notch signaling pathway (Jing et al., 2011). In adipogenic differentiation, the senescence-associated miRNAs, such as miRNA-369-5p and miRNA-371, were identified as antagonistic upstream regulators of adipogenic differentiation in MSCs. The adipogenic potential of MSCs was impaired by miRNA-369-5p but highly increased by miRNA-371. However, the adipogenic differentiation process itself required the downregulation of miRNA-369-5p while miRNA-371 was not affected (Bork et al., 2010). Another potential regulator in ASC adipogenic differentiation is miRNA-21, which mediated ASCs through the modulation of TGF-beta signaling. Overexpressing miRNA-21 decreased its target TGFBR2. The adipogenic differentiation process was accompanied with increased level of miRNA-21 and decreased level of TGFBR2. In contrast, inhibiting miRNA-21 resulted in increased TGFBR2 level in ASCs, accompanied by decreased adipogenic differentiation (Kim et al., 2009a, 2011). In osteogenic differentiation, miRNA-196a regulates osteogenic differentiation of ASCs through its target HOXC8; the overexpression of miR-196a led to increased osteogenic differentiation, whereas the inhibition of miR-196a resulted in decreased osteogenesis (Kim et al., 2009b). mi-RNA-26a, −141, and −200a were also reported to be involved in the modulation of osteogenic differentiation of MSCs (Itoh et al., 2009; Luzi et al., 2008). Furthermore, miRNA have been reported to be implicated in a chondrocytic differentiation-related expression pattern of MSCs. The reduction of miRNA-140 expression in osteoarthritic cartilage may contribute to abnormal gene expression pattern of osteoarthritis (Miyaki et al., 2009). mi-RNA-199a was found to adversely regulate early chondrocyte differentiation through the Smad pathway, as the miRNA-199a significantly inhibited Smad1/Smad4-mediated transactivation of target genes, and overexpression of Smad1 completely compensated miRNA-199a-mediated repression of early chondrogenesis (Lin et al., 2009). There are some additional potential miRNAs that have been recently reported to be involved in the chondrogenesis of MSCs, such as the hsa-miR-130b, hsa-miR-152, and hsa-miR-26b (Han et al., 2010).
Table 2.
MicroRNAs Reported to be Involved in the Regulation of MSC Fates
| MicroRNAs | Functionality | Publications |
|---|---|---|
| MiRNA-9 | Neurnal differentiation | Jing et al., 2011; Neuroreport |
| MiRNA-369-5p | Adipogenic differentiation | Bork et al., 2010; J. Cell Physiol. |
| MiRNA-371 | Adipogenic differentiation | Bork et al., 2010; J. Cell Physiol. |
| MiRNA-21 | Adipogenic differentiation | Kim et al., 2009a; Stem Cells |
| Kim et al., 2011; J. Cell Physiol. | ||
| MiRNA-335-5p | Osteogenic differentiation | Zhang et al., 2011; J. Bone Miner. Res. |
| MiRNA-196a | Osteogenic differentiation | Kim et al., 2009b; J. Bone Miner. Res. |
| MiRNA-26a | Osteogenic differentiation | Luzi et al., 2008; J. Bone Miner. Res. |
| MiRNA-141 | Osteogenic differentiation | Itoh et al., 2009; J. Biol. Chem. |
| MiRNA-200a | ||
| MiRNA-140 | Chondrogenic differentiation | Miyaki et al., 2009; Arthritis Rheum. |
| MiRNA-199a | Chondrogenic differentiation | Lin et al., 2009; J. Biol. Chem. |
| MiRNA-130b | Chondrogenic differentiation | Han et al., 2010; Int. J. Mol. Med. |
| MiRNA-152 | ||
| MiRNA-26b | ||
| MiRNA-221 | Chondrogenic differentiation | Kim et al., 2010; J. Biol. Chem. |
Summary
Up to now, the ontogeny of MSCs has been best explained by the characterization of pericytes from multiple human organs, which are myogenic, multidifferentiatial, and exhibit the features of MSCs. Recent findings suggested that the origin of MSCs is not only perivascular, but can be extended to other mesodermal lineages (such as IVD cells), neuroectodermal lineages (neurons, astrocytes, and oligodendrocytes) and endodermal lineages (hepatocytes, pancreatic cells). Such findings would question the previous definition for MSCs with mesenchymal origin, as cells derived from other tissues can bear the same properties.
As a result, based on the in vitro evidence, the MSC system may be distinct from other classic stem cell systems (such as hematopoietic or neural), in that the stem cell microenvironment varies considerably while supporting the same stem cell type with similar properties. Further work on the molecular phenotypes such as cell surface markers and critical miRNA patterns of MSCs from various origins, may be helpful in clarifying the ontogeny as well as the functional potentials of these cells.
Acknowledgments
The work was partially supported by the GRF Grant of Research Grant Concil, Hong Kong, and shenzhen Natural Science Foundation Grant (JC201005250046A).
Author Disclosure Statement
The authors declare that no conflicting financial interests exist.
References
- Alsalameh S. Amin R. Gemba T., et al. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004;50:1522–1532. doi: 10.1002/art.20269. [DOI] [PubMed] [Google Scholar]
- Arai F. Ohneda O. Miyamoto T., et al. Mesenchymal stem cells in perichondrium express activated leukocyte cell adhesion molecule and participate in bone marrow formation. J. Exp. Med. 2002;195:1549–1563. doi: 10.1084/jem.20011700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battula V.L. Treml S. Abele H., et al. Prospective isolation and characterization of mesenchymal stem cells from human placenta using a frizzled-9-specific monoclonal antibody. Differentiation. 2008;76:326–336. doi: 10.1111/j.1432-0436.2007.00225.x. [DOI] [PubMed] [Google Scholar]
- Battula V.L. Evans K.W. Hollier B.G., et al. Epithelial–mesenchymal transition-derived cells exhibit multilineage differentiation potential similar to mesenchymal stem cells. Stem Cells. 2010;28:1435–1445. doi: 10.1002/stem.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Y. Ehirchiou D. Kilts T.M., et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- Bianco J.F. Graciani I.F. Sanchez-Guijo F.M., et al. Isolation and characterization of mesenchymal stromal cells from human degenerated nucleus pulposus: comparison with bone marrow mesenchymal stromal cells from the same subjects. Spine. 2010;35:1528–1159. doi: 10.1097/BRS.0b013e3181cb8828. [DOI] [PubMed] [Google Scholar]
- Bianco P. Gehron R.P. Marrow stromal stem cells. J. Clin. Invest. 2000;105:1663–1668. doi: 10.1172/JCI10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P. Robey P.G. Simmons P.J. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork S. Horn P. Castoldi M., et al. Adipogenic differentiation of human mesenchymal stromal cells is down-regulated by microRNA-369-5p and up-regulated by microRNA-371. J. Cell. Physiol. 2010 doi: 10.1002/jcp.22557. [Epup ahead of print]. [DOI] [PubMed] [Google Scholar]
- Bühring H.J. Battula V.L. Treml S., et al. Novel markers for the prospective isolation of human MSC. Ann. N.Y. Acad. Sci. 2007;1106:262–271. doi: 10.1196/annals.1392.000. [DOI] [PubMed] [Google Scholar]
- Corselli M. Chen C.W. Crisan M., et al. Perivascular ancestors of adult multipotent stem cells. Arterioscler. Thromb. Vasc. Biol. 2010;30:1104–1109. doi: 10.1161/ATVBAHA.109.191643. [DOI] [PubMed] [Google Scholar]
- Covas D.T. Panepucci R.A. Fontes A.M., et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp. Hematol. 2008;36:642–654. doi: 10.1016/j.exphem.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Crisan M. Yap S. Casteilla L., et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Crisan M. Chen C.W. Corselli M., et al. Perivascular multipotent progenitor cells in human organs. Ann. N.Y. Acad. Sci. 2009;11:118–123. doi: 10.1111/j.1749-6632.2009.04967.x. [DOI] [PubMed] [Google Scholar]
- Davidoff M.S. Middendorff R. Müller D., et al. The neuroendocrine Leydig cells and their stem cell progenitors, the pericytes. Adv. Anat. Embryol. Cell Biol. 2009;205:1–107. [PubMed] [Google Scholar]
- De I.J. Peter S.J. Archambault M., et al. Investigation of allogeneic mesenchymal stem cell-based alveolar bone formation: preliminary findings. Clin. Oral. Implants Res. 2003;14:481–489. doi: 10.1034/j.1600-0501.2003.110770.x. [DOI] [PubMed] [Google Scholar]
- Doherty M.J. Ashton B.A. Walsh S., et al. Vascular pericytes express osteogenic potential in vitro and in vivo. J. Bone Miner. Res. 1998;13:828–838. doi: 10.1359/jbmr.1998.13.5.828. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P. Katychev A. Wang X., et al. CNS microvascular pericytes exhibit multipotential stem cell activity. J. Cereb. Blood Flow Metab. 2006;26:613–624. doi: 10.1038/sj.jcbfm.9600272. [DOI] [PubMed] [Google Scholar]
- Ebihara Y. Masuya M. Larue A.C., et al. Hematopoietic origins of fibroblasts, II: in vitro studies of fibroblasts, CFU-F, and fibrocytes. Exp. Hematol. 2006;34:219–229. doi: 10.1016/j.exphem.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Fang B. Li N. Song Y., et al. Cotransplantation of haploidentical mesenchymal stem cells to enhance engraftment of hematopoietic stem cells and to reduce the risk of graft failure in two children with severe aplastic anemia. Pediatr. Transplant. 2009;13:499–502. doi: 10.1111/j.1399-3046.2008.01002.x. [DOI] [PubMed] [Google Scholar]
- Farrington-Rock C. Crofts N.J. Doherty M.J., et al. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110:2226–2232. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- Feng J. Mantesso A. De B.C., et al. Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc. Natl. Acad. Sci. USA. 2011;108:6503–6508. doi: 10.1073/pnas.1015449108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes S. Vig P. Poulsom R., et al. Hepatic stem cells. J. Pathol. 2002;197:510–518. doi: 10.1002/path.1163. [DOI] [PubMed] [Google Scholar]
- Franklin W.A. Christison W.H. Colley M., et al. In situ distribution of the beta-subunit of platelet-derived growth factor receptor in nonneoplastic tissue and in soft tissue tumors. Cancer Res. 1990;50:6344–6348. [PubMed] [Google Scholar]
- Friedenstein A.J. Chailakhjan R.K. Lalykina K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Fukuchi Y. Nakajima H. Sugiyama D., et al. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004;22:649–658. doi: 10.1634/stemcells.22-5-649. [DOI] [PubMed] [Google Scholar]
- Giangreco A. Reynolds S.D. Stripp B.R. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am. J. Pathol. 2002;161:173–182. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S. McCarty R. Mrozik K., et al. Heat shock protein-90 beta is expressed at the surface of multipotential mesenchymal precursor cells: generation of a novel monoclonal antibody, STRO-4, with specificity for mesenchymal precursor cells from human and ovine tissues. Stem Cells Dev. 2009;18:1253–1262. doi: 10.1089/scd.2008.0400. [DOI] [PubMed] [Google Scholar]
- Halfon S. Abramov N. Grinblat B., et al. Markers distinguishing mesenchymal stem cells from fibroblasts are downregulated with passaging. Stem Cells Dev. 2011;20:53–66. doi: 10.1089/scd.2010.0040. [DOI] [PubMed] [Google Scholar]
- Haleem A.M. Singergy A.A. Sabry D., et al. The clinical use of human culture-expanded autologous bone marrow mesenchymal stem cells transplanted on platelet-rich fibrin glue in the treatment of articular cartilage defects: a pilot study and preliminary results. Cartilage. 2010;1:253–261. doi: 10.1177/1947603510366027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfon S. Abramov N. Grinblat B., et al. Markers distinguishing mesenchymal stem cells from fibroblasts are downregulated with passaging. Stem Cells Dev. 2010;43:665–674. doi: 10.1089/scd.2010.0040. [DOI] [PubMed] [Google Scholar]
- Han J. Yang T. Gao J., et al. Specific microRNA expression during chondrogenesis of human mesenchymal stem cells. Int. J. Mol. Med. 2010;25:377–384. doi: 10.3892/ijmm_00000355. [DOI] [PubMed] [Google Scholar]
- Henriksson H. Thornemo M. Karlsson C., et al. Identification of cell proliferation zones, progenitor cells and a potential stem cell niche in the intervertebral disc region: a study in four species. Spine. 2009;34:2278–2287. doi: 10.1097/BRS.0b013e3181a95ad2. [DOI] [PubMed] [Google Scholar]
- Hermida-Gómez T. Fuentes-Boquete I. Gimeno-Longas M.J., et al. Bone marrow cells immunomagnetically selected for CD271+ antigen promote in vitro the repair of articular cartilage defects. Tissue Eng. Part A. 2011;17:1169–1179. doi: 10.1089/ten.TEA.2010.0346. [DOI] [PubMed] [Google Scholar]
- Hess D.C. Abe T. Hill W.D., et al. Hematopoietic origin of microglial and perivascular cells in brain. Exp. Neurol. 2004;186:134–144. doi: 10.1016/j.expneurol.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Itoh T. Nozawa Y. Akao Y. MicroRNA-141 and −200a are involved in bone morphogenetic protein-2-induced mouse pre-osteoblast differentiation by targeting distal-less homeobox 5. J. Biol. Chem. 2009;284:19272–19279. doi: 10.1074/jbc.M109.014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahoda C.A. Whitehouse J. Reynolds A.J., et al. Hair follicle dermal cells differentiate into adipogenic and osteogenic lineages. Exp. Dermatol. 2003;12:849–859. doi: 10.1111/j.0906-6705.2003.00161.x. [DOI] [PubMed] [Google Scholar]
- Jarocha D. Lukasiewicz E. Majka M., et al. Adventage of mesenchymal stem cells (MSC) expansion directly from purified bone marrow CD105+ and CD271+ cells. Folia Histochem. Cytobiol. 2008;46:307–314. doi: 10.2478/v10042-008-0046-z. [DOI] [PubMed] [Google Scholar]
- Jiang Y. Jahagirdar B.N. Reinhardt R.L., et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Jing L. Jia Y. Lu J., et al. MicroRNA-9 promotes differentiation of mouse bone mesenchymal stem cells into neurons by Notch signaling. Neuroreport. 2001;22:206–211. doi: 10.1097/WNR.0b013e328344a666. [DOI] [PubMed] [Google Scholar]
- Jing L. Jia Y. Lu J., et al. MicroRNA-9 promotes differentiation of mouse bone mesenchymal stem cells into neurons by Notch signaling. Neurorcport. 2011;22:206–211. doi: 10.1097/WNR.0b013e328344a666. [DOI] [PubMed] [Google Scholar]
- Jones E. English A. Churchman S.M., et al. Large-scale extraction and characterization of CD271+ multipotential stromal cells from trabecular bone in health and osteoarthritis: implications for bone regeneration strategies based on uncultured or minimally cultured multipotential stromal cells. Arthritis Rheum. 2010;62:1944–1954. doi: 10.1002/art.27451. [DOI] [PubMed] [Google Scholar]
- Joo S.Y. Cho K.A. Jung Y.J., et al. Mesenchymal stromal cells inhibit graft-versus-host disease of mice in a dose-dependent manner. Cytotherapy. 2010;12:361–370. doi: 10.3109/14653240903502712. [DOI] [PubMed] [Google Scholar]
- Kaltz N. Ringe J. Holzwarth C., et al. Novel markers of mesenchymal stem cells defined by genome-wide gene expression analysis of stromal cells from different sources. Exp. Cell Res. 2010;316:2609–2617. doi: 10.1016/j.yexcr.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Kang S.G. Shinojima N. Hossain A., et al. Isolation and perivascular localization of mesenchymal stem cells from mouse brain. Neurosurgery. 2010;67:711–720. doi: 10.1227/01.NEU.0000377859.06219.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan W.S. Adesida A.B. Tew S.R., et al. Bone marrow-derived mesenchymal stem cells express the pericyte marker 3G5 in culture and show enhanced chondrogenesis in hypoxic conditions. J. Orthop. Res. 2010;28:834–840. doi: 10.1002/jor.21043. [DOI] [PubMed] [Google Scholar]
- Kim D. Song J. Jin E.J., et al. MicroRNA-221 regulates chondrogenic differentiation through promoting proteosomal degradation of slug by targeting Mdm2. J. Biol. Chem. 2010;285:26900–26907. doi: 10.1074/jbc.M110.115105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kim Y.J. Hwang S.J. Bae Y.C., et al. MiR-21 regulates adipogenic differentiation through the modulation of TGF-beta signaling in mesenchymal stem cells derived from human adipose tissue. Stem Cells. 2009a;27:3093–3102. doi: 10.1002/stem.235. [DOI] [PubMed] [Google Scholar]
- Kim Y.J. Bae S.W. Yu S.S., et al. miR-196a regulates proliferation and osteogenic differentiation in mesenchymal stem cells derived from human adipose tissue. J. Bone Miner. Res. 2009b;24:816–825. doi: 10.1359/jbmr.081230. [DOI] [PubMed] [Google Scholar]
- Kim Y.J. Hwang S.H. Cho H.H., et al. MicroRNA 21 regulates the proliferation of human adipose tissue-derived mesenchymal stem cells and high-fat diet-induced obesity alters microRNA 21. J. Cell. Physiol. 2011 Mar 4; doi: 10.1002/jcp.22716. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Kobayashi M. Yakuwa T. Sasaki K., et al. Multilineage potential of side population cells from human amnion mesenchymal layer. Cell Transplant. 2008;17:291–301. doi: 10.3727/096368908784153904. [DOI] [PubMed] [Google Scholar]
- Kramer P.A. Newell-Morris L.L. Simkin P.A. Spinal degenerative disk disease (DDD) in female macaque monkeys: epidemiology and comparison with women. J. Orthop. Res. 2002;20:399–408. doi: 10.1016/S0736-0266(01)00122-X. [DOI] [PubMed] [Google Scholar]
- Kuçi S. Kuçi Z. Kreyenberg H., et al. CD271 antigen defines a subset of multipotent stromal cells with immunosuppressive and lymphohematopoietic engraftment-promoting properties. Haematologica. 2010;95:651–659. doi: 10.3324/haematol.2009.015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucia M. Reca R. Jala V.R., et al. Bone marrow as a home of heterogenous populations of nonhematopoietic stem cells. Leukemia. 2005;19:1118–1127. doi: 10.1038/sj.leu.2403796. [DOI] [PubMed] [Google Scholar]
- LaRue A.C. Masuya M. Ebihara Y., et al. Hematopoietic origins of fibroblasts: I. In vivo studies of fibroblasts associated with solid tumors. Exp. Hematol. 2006;34:208–218. doi: 10.1016/j.exphem.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Le B.K. Götherström C. Ringdén O., et al. Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation. 2005;79:1607–1614. doi: 10.1097/01.tp.0000159029.48678.93. [DOI] [PubMed] [Google Scholar]
- Lee O.K. Kuo T.K. Chen W.M., et al. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–1675. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- Lee R.H. Kim B. Choi I., et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol. Biochem. 2004;14:311–324. doi: 10.1159/000080341. [DOI] [PubMed] [Google Scholar]
- Limbert C. Ebert R. Schilling T., et al. Functional signature of human islet-derived precursor cells compared to bone marrow-derived mesenchymal stem cells. Stem Cells Dev. 2010;19:679–691. doi: 10.1089/scd.2009.0241. [DOI] [PubMed] [Google Scholar]
- Lin E.A. Kong L. Bai X.H., et al. miR-199a, a bone morphogenic protein 2-responsive MicroRNA, regulates chondrogenesis via direct targeting to Smad1. J. Biol. Chem. 2009;284:11326–11335. doi: 10.1074/jbc.M807709200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzi E. Marini F. Sala S.C., et al. Osteogenic differentiation of human adipose tissue-derived stem cells is modulated by the miR-26a targeting of the SMAD1 transcription factor. J. Bone Miner. Res. 2008;23:287–295. doi: 10.1359/jbmr.071011. [DOI] [PubMed] [Google Scholar]
- Makino S. Fukuda K. Miyoshi S., et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J. Clin. Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyrick B. Reid L. The effect of continued hypoxia on rat pulmonary arterial circulation an ultrastructural study. Lab. Invest. 1978;38:188–200. [PubMed] [Google Scholar]
- Miller F.D. Riding the waves: neural and nonneural origins for mesenchymal stem cells. Cell Stem Cell. 2007;1:129–130. doi: 10.1016/j.stem.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Mitchell J.B. McIntosh K. Zvonic S., et al. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- Miyaki S. Nakasa T. Otsuki S., et al. MicroRNA-140 is expressed in differentiated human articular chondrocytes and modulates interleukin-1 responses. Arthritis Rheum. 2009;60:2723–2730. doi: 10.1002/art.24745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa S. Mabuchi Y. Niibe K., et al. Development of mesenchymal stem cells partially originate from the neural crest. Biochem. Biophys. Res. Commun. 2009;379:1114–1119. doi: 10.1016/j.bbrc.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Nagoshi N. Shibata S. Kubota Y., et al. ontogeny and multipotent of neural crest-derived stem cells in mouse bone marrow, dorsal root ganglia, and whisker pad. Cell Stem Cell. 2008;2:392–403. doi: 10.1016/j.stem.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Ogawa M. LaRue A.M. Drake C.J. Hematopoietic origin of fibroblasts/myofibroblasts: Its pathophysiologic implications. Blood. 2006;9:2893–2896. doi: 10.1182/blood-2006-04-016600. [DOI] [PubMed] [Google Scholar]
- Ozerdem U. Grako K.A. Dahlin-Huppe K., et al. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev. Dyn. 2001;222:218–227. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- Pennisi E. Boom time for monkey research. Science. 2007;316:216–218. doi: 10.1126/science.316.5822.216. [DOI] [PubMed] [Google Scholar]
- Phinney D.G. Kopen G. Isaacson R.L., et al. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, and differentiation. J. Cell Biochem. 1999;72:570–585. [PubMed] [Google Scholar]
- Pierdomenico L. Bonsi L. Calvitti M., et al. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation. 2005;80:836–842. doi: 10.1097/01.tp.0000173794.72151.88. [DOI] [PubMed] [Google Scholar]
- Pittenger M.F. Mackay A.M. Beck S.C., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Quirici N. Soligo D. Bossolasco P., et al. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp. Hematol. 2002;30:783–791. doi: 10.1016/s0301-472x(02)00812-3. [DOI] [PubMed] [Google Scholar]
- Quirici N. Scavullo C. De G.L., et al. Anti-L-NGFR and -CD34 monoclonal antibodies identify multipotent mesenchymal stem cells in human adipose tissue. Stem Cells. 2010;19:915–925. doi: 10.1089/scd.2009.0408. [DOI] [PubMed] [Google Scholar]
- Risbud M.V. Guttapalli A. Tsai T.T. Evidence for skeletal progenitor cells in the degenerate human intervertebral disc. Spine. 2007;32:2537–2544. doi: 10.1097/BRS.0b013e318158dea6. [DOI] [PubMed] [Google Scholar]
- Rolf H.J. Kierdorf U. Kierdorf H., et al. Localization and characterization of STRO-1 cells in the deer pedicle and regenerating antler. PLoS One. 2008;3:e2064. doi: 10.1371/journal.pone.0002064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio D. Garcia S. De C.T., et al. Human mesenchymal stem cell transformation is associated with a mesenchymal-epithelial transition. Exp. Cell Res. 2008;314:691–698. doi: 10.1016/j.yexcr.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Sakaguchi Y. Sekiya I. Yagishita K., et al. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- Sarugaser R. Hanoun L. Keating A., et al. Human mesenchymal stem cells self-renew and differentiate according to a deterministic hierarchy. Plos One. 2009;4:e6498. doi: 10.1371/journal.pone.0006498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz E. Satellite cell behaviour during skeletal muscle growth and regeneration. Med. Sci. Sports Exerc. 1981;21:S181–S186. [PubMed] [Google Scholar]
- Schwab K.E. Gargett C.E. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum. Reprod. 2007;22:2903–2911. doi: 10.1093/humrep/dem265. [DOI] [PubMed] [Google Scholar]
- Schwab K.E. Hutchinson P. Gargett C.E. Identification of surface markers for prospective isolation of human endometrial stromal colony-forming cells. Hum. Reprod. 2008;23:934–943. doi: 10.1093/humrep/den051. [DOI] [PubMed] [Google Scholar]
- Seo B.M. Miura M. Gronthos S., et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- Sobiesiak M. Sivasubramaniyan K. Hermann C., et al. The mesenchymal stem cell antigen MSCA-1 is identical to tissue non-specific alkaline phosphatase. Stem Cells Dev. 2010;19:669–677. doi: 10.1089/scd.2009.0290. [DOI] [PubMed] [Google Scholar]
- Soncini M. Vertua E. Gibelli L., et al. Isolation and characterization of mesenchymal cells from human fetal membranes. J. Tissue Eng. Regen. Med. 2007;1:296–305. doi: 10.1002/term.40. [DOI] [PubMed] [Google Scholar]
- Sordi V. Melzi R. Mercalli A., et al. Mesenchymal cells appearing in pancreatic tissue culture are bone marrow-derived stem cells with the capacity to improve transplanted islet function. Stem Cells. 2010;28:140–151. doi: 10.1002/stem.259. [DOI] [PubMed] [Google Scholar]
- Sorrentino A. Ferracin M. Castelli G. Isolation and characterization of CD146+ multipotent mesenchymal stromal cells. Exp. Hematol. 2008;36:1035–1046. doi: 10.1016/j.exphem.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Stoeckelhuber M. Brueckner S. Spohr G., et al. Proteoglycans and collagen in the intervertebral disc of the rhesus monkey (Macaca mulatta) Ann. Anat. 2005;187:35–42. doi: 10.1016/j.aanat.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Takashima Y. Era T. Nakao K., et al. Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell. 2007;7:1377–1388. doi: 10.1016/j.cell.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Toma J.G. Akhavan M. Fernandes K.J.L., et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat. Cell Biol. 2001;3:778–784. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- Visconti R.P. Ebihara Y. LaRue A.C., et al. An in vivo analysis of hematopoietic stem cell potential: hematopoietic origin of cardiac valve interstitial cells. Circ. Res. 2006;98:690–696. doi: 10.1161/01.RES.0000207384.81818.d4. [DOI] [PubMed] [Google Scholar]
- Watanabe T. The mechanism of transition from notochordal nucleus to fibrocartilagenous nucleus of the disc in the young monkey—an electron microscopic study. Nippon Seikeigeka Gakkai Zasshi. 1983;57:519–532. [PubMed] [Google Scholar]
- Williams R. Khan I.M. Richardson K., et al. Identification and clonal characterisation of a progenitor cell sub-population in normal human articular cartilage. PloS One. 2010;14:e13246. doi: 10.1371/journal.pone.0013246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T. Akamatsu H. Hasegawa S., et al. Age-related changes of p75 neurotrophin receptor-positive adipose-derived stem cells. J. Dermatol. Sci. 2010;58:36–42. doi: 10.1016/j.jdermsci.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Young H.E. Steele T.A. Bray R.A. Human reserve pluripotent mesenchymal stem cells are present in the connective tissues of skeletal muscle and dermis derived from fetal, adult, and geriatric donors. Anat. Rec. 2001;264:51–62. doi: 10.1002/ar.1128. [DOI] [PubMed] [Google Scholar]
- Zhang J. Tu Q. Bonewald L.F., et al. Effects of miR-335-5p in modulating osteogenic differentiation by specifically down-regulating Wnt antagonist DKK1. J. Bone Miner. Res. 2011;26:1953–1963. doi: 10.1002/jbmr.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G.Q. Yang F. Leung V.L., et al. Molecular and cellular biology of the intervertebral disc and the use of animal models. Curr. Orthop. 2008;22:267–273. [Google Scholar]
- Zhou H. Guo M. Bian C., et al. Efficacy of bone marrow-derived mesenchymal stem cells in the treatment of sclerodermatous chronic graft-versus-host disease: clinical report. Biol. Blood Marrow Transplant. 2010;16:403–412. doi: 10.1016/j.bbmt.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Zuk P.A. Zhu M. Ashjian P., et al. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]