Abstract
This paper presents the latest progress on quantitative, in vivo, transcutaneous glucose sensing using surface enhanced spatially offset Raman spectroscopy (SESORS). Silver film over nanosphere (AgFON) surfaces were functionalized with a mixed self-assembled monolayer (SAM) and implanted subcutaneously in Sprague-Dawley rats. The glucose concentration was monitored in the interstitial fluid of six separate rats. The results demonstrated excellent accuracy and consistency. Remarkably, the root mean square error of calibration (RMSEC) (3.6 mg/dL) and the root mean square error of prediction (RMSEP) (13.7 mg/dL) for low glucose concentration (< 80 mg/dL) is lower than the current International Organization Standard (ISO/DIS 15197) requirements. None of the commercially available glucose sensing techniques can achieve enough accuracy during hypoglycemic episodes. Additionally, our sensor demonstrated functionality up 17 days after implantation, including 12 days under the laser safety level for human skin exposure with only one time calibration. Therefore, our SERS based sensor shows promise for the challenge of reliable continuous glucose sensing systems for optimal glycemic control.
Keywords: surface-enhanced Raman spectroscopy, SERS, in vivo glucose sensing, transcutaneous, hypoglycemic, accuracy, stability, reliability, calibration
The high sensitivity and selectivity of surface enhanced Raman spectroscopy (SERS) make it an ideal method for the detection and characterization of low concentration analytes in a complex biological environment.1–5 The advancement in fabrication of reproducible, large area, high enhancement substrates has paved the way for biologically relevant small molecules sensing via SERS.4, 6–13 The recent emergence of spatially offset Raman spectroscopy (SORS) has provided significant increases in depth penetration and high depth resolution Raman signals.14, 15 Medically relevant applications of SORS range from bone disease diagnosis to cancer detection.16, 17 Currently, the approach of combining SERS and SORS (SESORS) has opened new pathways for in vivo, continuous sensing of metabolic analytes.18, 19
The use of SERS for in vivo sensing mostly relies on injection of SERS nanoprobes because the background signals from the complex biological environment can mask direct signals from analytes of interest.5 An early report of in vivo SERS detection was given by the Nie group where PEGylated gold nanoparticles functionalized with a tumor-targeting ligand were used to identify cancerous areas.20 Later, the Gambhir group showed a step forward with in vivo multiplexed SERS imaging in a nude mouse.21 The problem associated with these studies is that SERS signals can be acquired only from the nanoparticles accumulating at the surface of the animal.19 Recently, the Matousek group has pursued the application of SESORS as a potential in vivo SERS detection tool for low concentration small molecule targets that are deeply buried within tissues.18 The capability of multiplexed SESORS imaging of SERS nanoparticles in porcine tissue has also been demonstrated.19 However, the majority of the in vivo SERS detection work performed to date are qualitative analyses providing only molecular identification,4, 5 and all of these rely on injection of SERS nanoprobes.20, 21 Currently, the consequence of introducing nanoparticles into the body remains an active area of research.22–24
One important area for in vivo small molecule sensing is glucose detection due to its intimate connection with diabetes, which affects 11.3% of Americans over the age of 20 and 26.9% of those over the age of 65.25 Prevention of hypoglycemia is a critical component of diabetes management since it is the leading limiting factor in glycemic management.26 Given sufficient insulin doses, patients with diabetes can hold plasma glucose concentrations at non-diabetic levels yet they are likely to suffer iatrogenic hypoglycemia at other times. Continuous glucose monitoring (CGM) is particular useful in those with hypoglycemia unawareness and/or frequent episodes of hypoglycemia.27 However, all of the glucose sensing techniques available today including commercially available electrochemical methods of CGM suffer from inaccuracy in the low concentration range (<80 mg/dL).28–31 The fear of hypoglycemia hinders strict glycemic control in both diabetic and critically ill patients.28
The CGM devices available on the market today can function for up to 7 days.28, 30 For these CGM sensors, multiple calibrations with optimal timing are needed to ensure the reliability and accuracy of the glucose measurements.28, 32 Much effort has been applied to develop long-term implantable sensors. Armour et al. has demonstrated a sensor placed in the vena cava functioning up to 10 months with weekly basis calibration.33 However, this sensor associates with increased invasiveness. Another long-term sensor implanted in the subcutaneous tissue has functioned up to 6 months and operated over 100 days without the need of calibration. However, the sensor did not function well during the first 3–4 weeks after implantation.34 Clearly, there is a need to develop a glucose monitoring device that can give an improved assessment of glycemic variations, especially the detection of hypoglycemia, over a longer period.
In order to maintain euglycemia while avoiding debilitating hypoglycemia and intermittent hyperglycemia, optical glucose detection has been explored as a useful technique.28, 29 Among various optical techniques, Raman spectroscopy stands out because it relies on the unique vibrational signatures of each molecule, which allows direct and selective identification of glucose. Although Raman spectroscopy has been promising for non-invasive glucose sensing,35–37 the inherently weak Raman signal of glucose makes it difficult to apply this technique in vivo. SERS has the advantage of greatly enhancing the signal strength. The Van Duyne lab has made great strides in developing a continuous, direct, and quantitative in vivo glucose sensor based on SERS.3, 6, 13, 38, 39 The ability of the decanethiol (DT)/6-mercapto-1-hexanol (MH) functionalized silver film over nanosphere (AgFON) substrates to detect glucose has been studied extensively both in vitro and in vivo.3, 13, 39 The DT/MH mixed self-assembled monolayer (SAM) partitions glucose and localizes it within the SERS-active field on the AgFON surface. The exact mechanism of mixed SAM formation and the partitioning of glucose has not yet been investigated as well as we would like. We do, however, have a working hypothesis- the longer chain length of DT combined with the shorter chain length of MH creates a dynamic “pocket” that is approximately the size of glucose as can be seen in space-filling computer models.13 Further, this pocket enables glucose to reside closer to the SERS-active surface.13
Our previous work reached a key milestone by demonstrating the capability of in vivo transcutaneous glucose sensing via SESORS.40 In this report, we significantly extend the SESORS approach by demonstrating its reliability, accuracy, and long-term stability for in vivo glucose sensing in multiple animals. Further, our data show a RMSEC of 3.6 mg/dL and a RMSEP 13.7 mg/dL in the hypoglycemic range, which is lower than the present International Organization Standard (ISO/DIS 15197), and meets the requirements for ICU patients.30 Additionally, our results demonstrate that our SERS sensor is capable of monitoring glucose in vivo transcutaneously for up to 17 days without multiple calibrations and under the laser power safety level for human skin exposure.
EXPERIMENTAL SECTION
Materials
All the chemicals were reagent grade or better and used as purchased. Silver pellets (99.99%) were purchased from the Kurt J. Lesker Co. (Clairton, PA). Titanium was obtained from McMaster-Carr (Chicago, IL) and cut into 0.5 mm thick, 8 mm diameter disks. NH4OH (28–30% in H20), H2O2 (30% in H20), and ethanol were purchased from Fisher Scientific (Fairlawn, VA) for cleaning substrates. Silica nanosphere solution (600 nm ± 10–15% diameter, 10.2% solid) was purchased from Bangs Laboratories, Inc. (Fishers, IN). Only ultrapure water (18.2 MΩ cm−1) from a Millipore system (Marlborough, MA) was used for substrate preparation. Glucose, albumin from bovine serum (BSA), decanethiol (DT), and 6-mercapto-1-hexanol (MH) were purchased from Sigma-Aldrich (St. Louis, MO). Insulin (100 U/mL) was acquired from Eli Lilly (Indianapolis, IN).
AgFON Fabrication and Incubation Procedure
The titanium substrates were cleaned by sonicating in a 5:1:1 H2O/H2O2/NH4OH solution. In previous studies, the nanosphere solution was directly drop-coated onto the titanium substrate.3, 13, 39, 40 Here, an improved fabrication technique was employed where silica nanospheres were first isolated from solution by centrifugation and removal of the supernatant. The nanospheres were then dispersed in ultrapure water and sonicated to disperse particle aggregates. This procedure ensures that a more uniform close-packed array of nanospheres will form on the titanium substrate surface. Approximately 20 µL of nanosphere solution was drop-coated onto each clean titanium substrate and allowed to dry under ambient conditions. An Ag film (200 nm thick) was deposited over the nanosphere mask using a home-built thermal deposition system to form silver film over nanosphere (AgFON) substrates. The substrates were incubated in 1 mM DT in ethanol for 45 min and transferred to 1 mM MH in ethanol for at least 12 h to form a mixed DT/MH SAM. The AgFONs were kept in the 1 mM MH solution until used.
Instrumentation
The SESORS system previously described was used in the present study.40 The only change was a new 785 nm diode laser (Renishaw, RL785, 300 mW, < 1 cm−1).
Surgical Implantation
All surgical procedures followed protocols filed with the Northwestern University IACUC. Male Sprague-Dawley rats (300–500g, N=5) were anesthetized with isoflurane (1.5–3%) throughout the surgical procedure and the duration of the experiment. The animal was checked for pain reactions by toe-tug and blink tests. None were observed. After the anesthetic had taken effect, the surgical areas were prepared by removal of hair (shaving and chemical depilatory) and cleaning. The femoral vein and artery were cannulated using PE 50 tubing for drug/glucose injections and blood glucose measurements, respectively. An incision was made in the skin and a pocket was blunt dissected into the subcutaneous space. A single DT/MH AgFON was placed in the pocket. All incisions were closed with surgical clips. The rats were thermally stabilized by an electric heating pad throughout the course of the surgery and experiment. Following the experiment, the animals were sacrificed with an overdose of sodium pentabarbitol (150 mg/kg) and bilateral thorachotomy.
Experimental Procedure and Spectroscopic Measurement
The rats were placed in the SESORS apparatus. The glucose concentration in the rats was increased through intermittent intravenous infusion (1 g/mL in sterile saline) and decreased by IV insulin injection (0.2 mL of 2 U/mL) over the course of the experiment. A droplet of blood was drawn from the rats, the glucose level was measured with the OneTouch® Ultra® 2 home blood glucometer, and corresponding SESORS measurements were taken (λex = 785 nm, Pex = 50 mW, tacq = 2 min). To keep the osmotic pressure of the rats at normal physiological levels, a volume of BSA (0.8% in sterile saline) equal to the blood removed was injected following each blood glucose measurement via femoral cannula. The data were collected and analyzed by the partial least-squares leave-one-out (PLS-LOO) method described in our previous papers.3, 13, 38–40 The calculations were performed with MATLAB (MathWorks, Inc., Natick, MA) and PLS_Toolbox (Eignevector Research, Inc., Manson, WA).
RESULTS AND DISCUSSION
Reliability and Hypoglycemic Accuracy of SESORS In Vivo Glucose Sensing
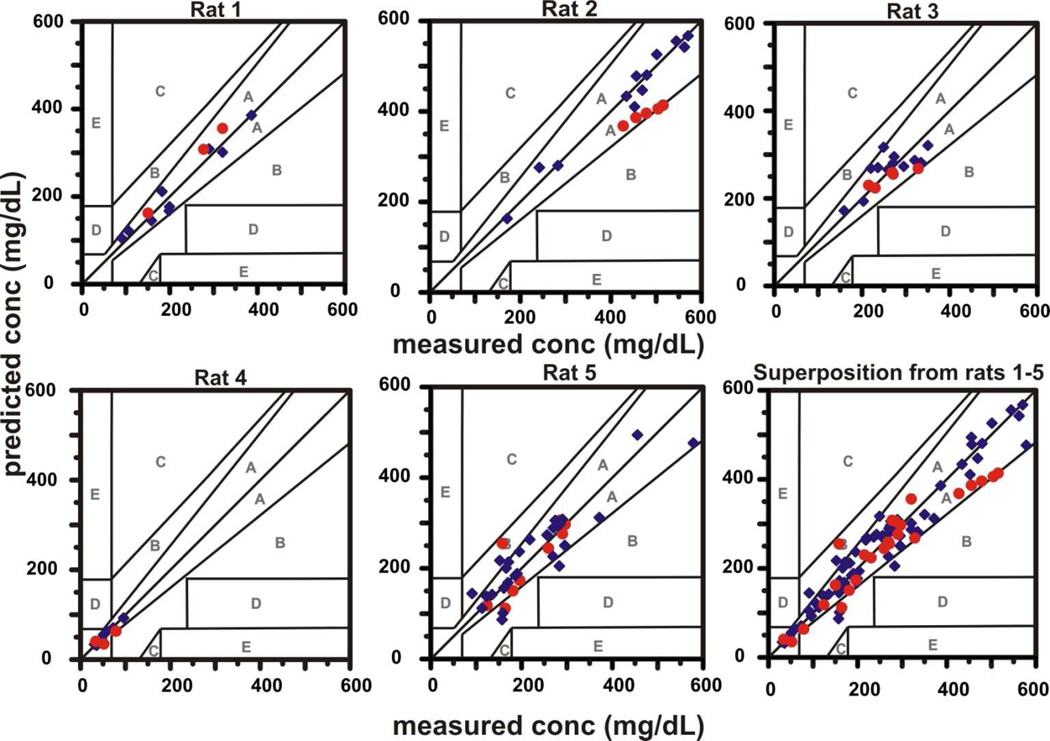
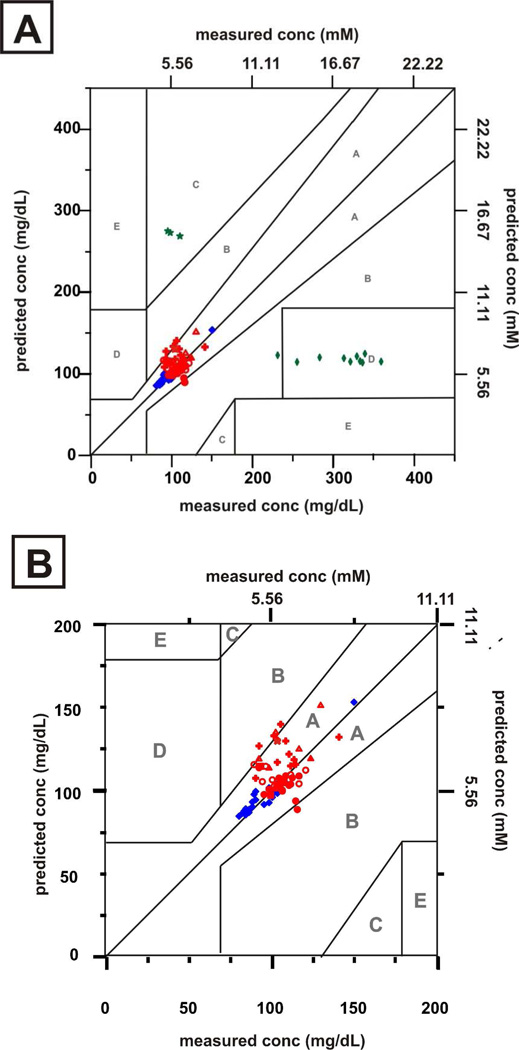
Over the past 20 years, the Clarke error grid has became the most common standard for evaluating the accuracy and performance of glucose sensors in clinically relevant concentration ranges.41, 42 The grid is divided into five zones with measured concentrations on the x-axis and predicted concentrations on the y-axis. Predictions that fall in these zones lead to the following: (A) clinically accurate measurements and treatment, (B) benign errors or no action, (C) unnecessary action, (D) a lack of action, and (E) actions that are opposite to those that are clinically necessary. Accurate measurements only result in data points within the A and B zone of the grid.43 Five separate in vivo transcutaneous SESORS glucose experiments are presented on Clarke error grids in Figure 1. Measurements were taken from multiple spots of the implanted sensor due to movement of body of the rat as it breathed. The relative motion between the sensor and the SORS probe did not cause consistent problems due to the spatially averaged collection of the annular fiber bundle. The results of the five in vivo experiments are summarized in Table I. For each experiment, all points of the calibration and validation fall in zone A and B range, indicating high sensor accuracy. Note that all points for rats 1–4 fall only within zone A. Both the mean absolute relative difference values for calibration and validation (MARDC and MARDV) and root mean square error for calibration and prediction (RMSEC and RMSEP) are lower than the previous in vivo transcutaneous results and comparable to the previous windowed chamber results.3, 40 These improved results may be due to a refined fabrication technique for preparing the SERS substrates that has been developed in the Van Duyne laboratory.44 The new fabrication procedure for preparing AgFONs yields a more uniform, robust, and reproducibly close-packed array of nanospheres. This, in turn, yields a spatially more uniform surface plasmon resonance, higher SERS enhancement factors, and improved S/N for the SERS glucose sensor. For our previous in vivo studies, the AgFONs used provided enhancement factors of 106.3, 39, 40 The refined process developed in the Van Duyne lab has increased the enhancement factors to mid-107 level, and possibly to 109 under optimal conditions (unpublished results).
Figure 1.
Calibration ( ) and validation (
) and validation ( ) data sets for in vivo transcutaneous SESORS glucose measurements on 5 rats. All data points were acquired with λex = 785 nm, Pex = 50 mW, tacq = 2 min.
) data sets for in vivo transcutaneous SESORS glucose measurements on 5 rats. All data points were acquired with λex = 785 nm, Pex = 50 mW, tacq = 2 min.
Table I.
Quantitative in vivo transcutaneous glucose detection using PLS calibration for five rats
| Rat number | Points used for | RMSEC | RMSEP | MARDC | MARDV | |
|---|---|---|---|---|---|---|
| Calibration | Validation | |||||
| 1 | 9 | 3 | 20.4 mg/dL (1.1 mM) | 28.4 mg/dL (1.6 mM) | 10.9% | 10.3% |
| 2 | 12 | 5 | 20.6 mg/dL (1.1 mM) | 83.2 mg/dL (4.6 mM) | 4.13% | 17.0% |
| 3 | 14 | 5 | 35.3 mg/dL (2.0 mM) | 28.8 mg/dL (1.6 mM) | 10.8% | 7.40% |
| 4 | 9 | 3 | 3.6 mg/dL (0.2 mM) | 13.7 mg/dL (0.8 mM) | 6.84% | 24.0% |
| 5 | 27 | 9 | 43.1 mg/dL (2.4 mM) | 40.0 mg/dL (2.2 mM) | 16.2% | 16.0% |
The error can be further improved by increasing the number of data points in the calibration.40 Ideally, the calibration model would be created with a large number of data points spanning the whole range of physically relevant glucose concentration (0–450 mg/dL). However, since only a limited number of data points can be collected from each rat due to the lifetime of the rat during an experiment, we use a specific ratio of calibration points to validation points to create a robust model. The ratio of calibration to validation points that is used in our studies is between 2:1 and 3:1. Based on our previous experiments,40 a ratio in this range builds a relatively accurate calibration model for validation. In the five in vivo transcutaneous SESORS glucose experiments, the data from rat 5 has a greater number of calibration points than the other four rats, but has higher error. This is because glucose concentrations span around two times the range in rat 5 than in other rats. A wider range of concentrations results in more variation in the spectra which leads to great error in the PLS-LOO calibration model. This error can be reduced by including even more data points in the calibration model Nevertheless, the results from the five experiments show that our SESORS glucose sensor can make accurate and consistent in vivo transcutaneous glucose measurements.
Strict glycemic control benefits both diabetic and ICU patients.28 Reliable CGM plays a key role in optimal glycemic control.28, 30 To date, most commercially available CGM devices have 14–20% error range, and none of them can achieve 100% accuracy in terms of Clarke error grid analysis.28 In comparison, our SERS based glucose sensor shows great promise for an accurate GCM sensor. 100% of measurements from all the rats are in the clinically acceptable range (zone A and B range). Moreover, the experiment with rat 4 demonstrated high accuracy for low glucose concentrations (31–79 mg/dL). At the center of diabetes management is prevention of hypoglycemia.26 However, all of the sensors available today have lower accuracy at low glucose levels than they do at higher levels, causing unreliable detection of hypoglycemia.28–30 The ISO/DIS 15197 requires that the sensor should detect a result within 15 mg/dL (0.83 mmol/L) for reference glucose values ≤ 75 mg/dL (4.2 mmol/L) and, the sensor should be within 20% for reference glucose values ≥ 75 mg/dL. Clearly, our SERS based sensor show the potential to meet and possibly exceed the requirements of the standard.
Long Term Stability of SESORS In Vivo Glucose Sensing
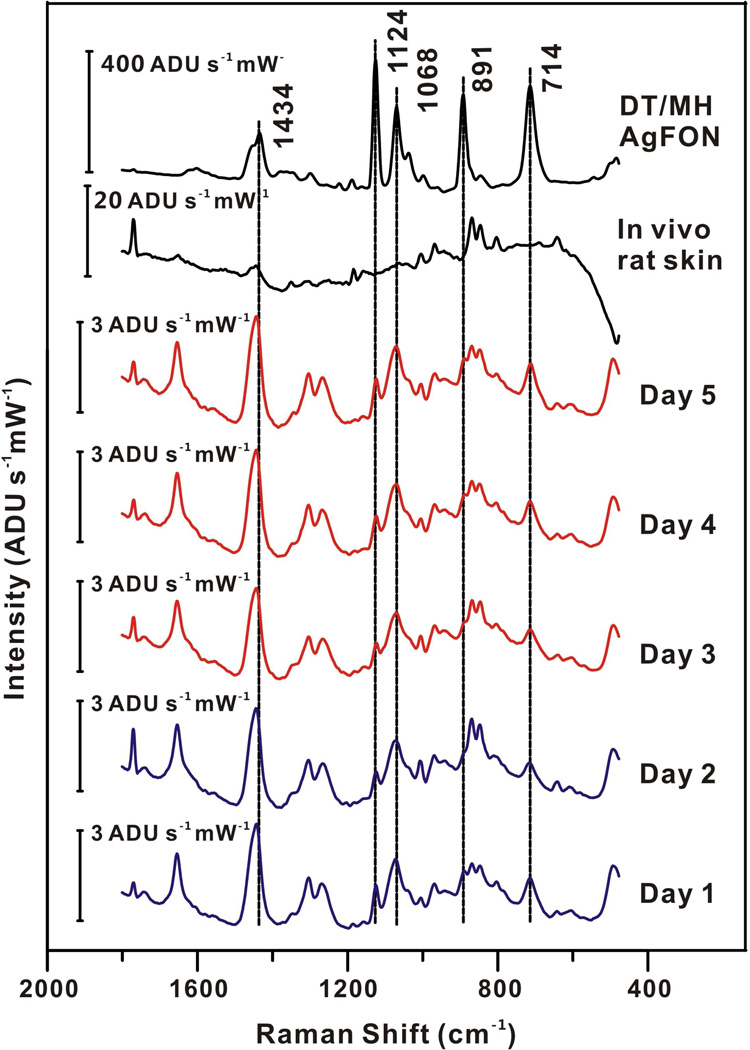
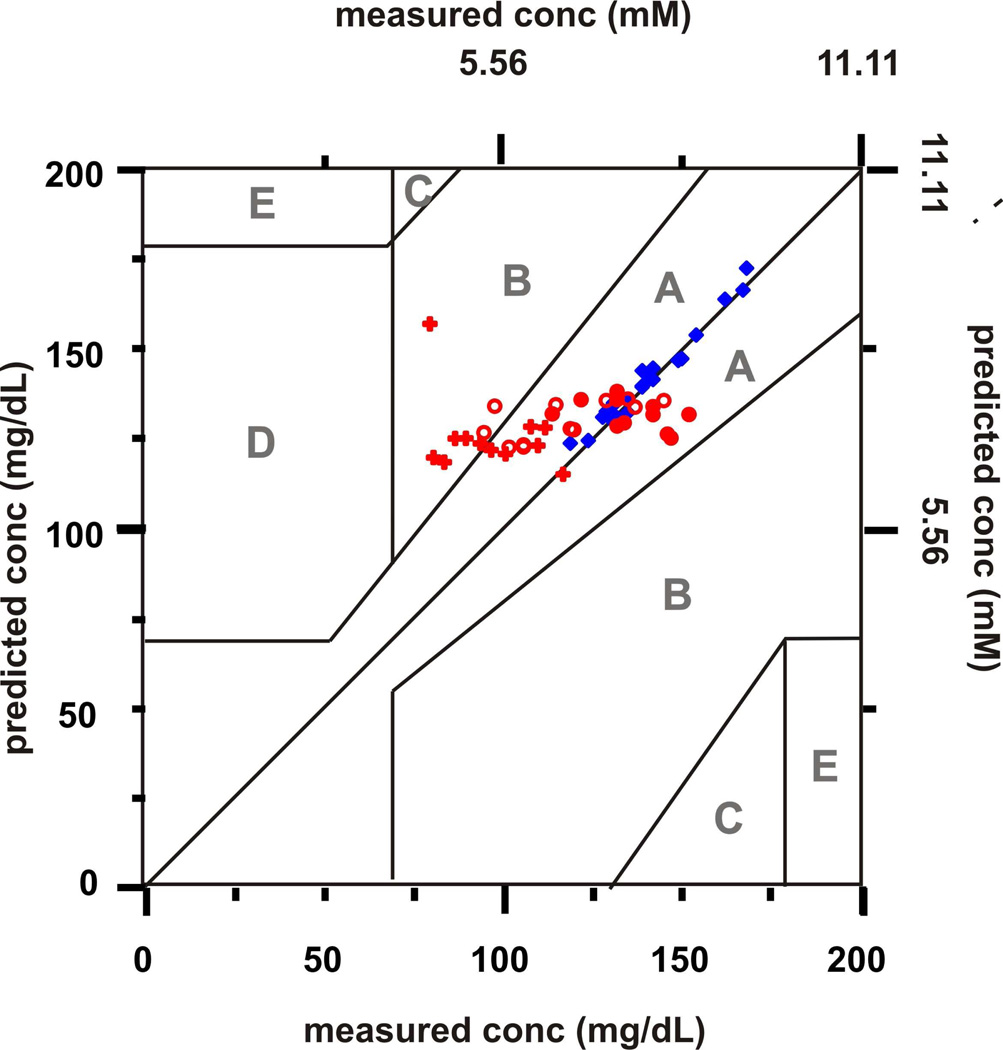
An implantable glucose sensor must be stable for at least 3 days for continuously in vivo glucose sensing.45 Herein, the stability of the DT/MH functionalized AgFON for transcutaneously monitoring glucose was studied over a period of 20 days in a randomly chosen rat. For days 1–5, SESOR spectra were captured every hour for 12 hours a day from the same implanted sensor in the same rat with a laser beam at 785 nm yielding 50 mW at the sample (tacq = 2 min). One of the acquired SESOR spectra from each day is shown in Figure 2. The DT/MH peaks are clearly present among the peaks of the rat skin in each day’s spectrum as compared to the spectrum of in vivo rat skin and DT/MH AgFON. Representative peaks can be seen at 1434, 1124, 1068, 891, and714 cm−1. Peaks in the region between 1050 and 700 cm−1 correspond to the skin and hair of the rat. Their positions and intensities varied across different days due to the regrowth of hair. Otherwise, the spectral band positions and intensities of each day’s spectrum in other region did not vary significantly over the course of 5 days. To evaluate the accuracy and performance of our sensor over the first 5 days, the in vivo transcutaneous SESORS glucose measurements were analyzed by Clarke error grid as shown in Figure 3. The measurements from the first 2 days were used as a calibration sets and those from the rest of the 3 days were used as a validation set. All calibration and validation points fall in zones A and B, showing excellent accuracy. The MARD and RMSE for calibration are 2.3 mg/dL (0.1 mM) and 1.42%, respectively. The results of the validation measurements for the remaining 3 days are summarized in Table II. Both MARD and RMSE for validation did not show a significant increase over the 3 days period, indicating good sensor stability.
Figure 2.
Comparison of SESOR spectra from day 1–5, in vivo rat skin, and DT/MH-functionalized AgFON. λex = 785 nm, Pex = 50 mW, tacq = 2 min.
Figure 3.
Calibration and validation data sets for a period of 5-day in vivo transcutaneous SESORS glucose measurements on one rat. Measurements from day 1 and 2 ( ) were used for calibration sets. Measurements from day 3 (
) were used for calibration sets. Measurements from day 3 ( ), 4 (
), 4 ( ) and 5(
) and 5( ) were used for validation sets. All data points were acquired in vivo transcutaneously with λex = 785 nm, Pex = 50 mW, tacq = 2 min.
) were used for validation sets. All data points were acquired in vivo transcutaneously with λex = 785 nm, Pex = 50 mW, tacq = 2 min.
Table II.
Quantitative in vivo transcutaneous glucose detection using PLS calibration for 5-day monitoring
| Day | Measurement Number |
Daily RMSEP |
Daily MARDV |
Overall RMSEP |
Overall MARDV |
|---|---|---|---|---|---|
| 3 | 12 | 13.5 mg/dL (0.8 mM) | 8.32% | 13.5 mg/dL (0.8 mM) | 8.32% |
| 4 | 12 | 17.4 mg/dL (1.0 mM) | 13.3% | 15.6 mg/dL (0.9 mM) | 10.8% |
| 5 | 12 | 33.3 mg/dL (1.9 mM) | 31.6% | 23.0 mg/dL (1.3 mM) | 17.7% |
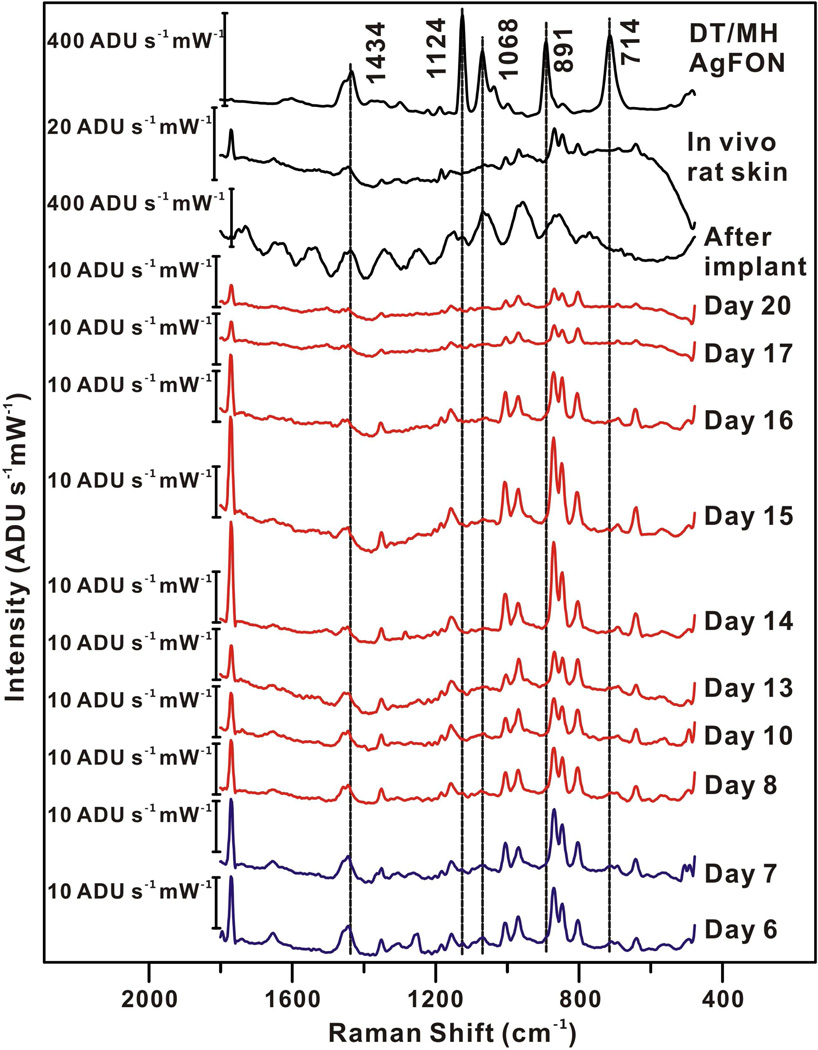
For this SESORS technique to ever be used as a practical approach in glucose sensing, the incident laser power on the skin must be below the safety level for skin exposure. To demonstrate that meaningful data can be collected at low laser power, starting on day 6 the incident power on the sample was attenuated to 2 mW, which is approximately an order of magnitude below the safe level for skin illumination in the NIR spectral region. For day 6–13, SESOR spectra were captured every hour for 12 hours each day from the same implanted sensor in the same rat (λex = 785 nm, tacq = 2 min). After day 13, four spectra were acquired each day (except day 16). Figure 4 presents the representative SESOR spectra acquired from each day. As time progressed, the daily spectrum showed diminished DT/MH features and increased skin features. One DT/MH peak (891 cm−1) does disappear after lowering the laser power due to obfuscation by hair and skin peaks, but this is somewhat expected with the lower signal intensity. The data collection was stopped at day 20 due to the significant change of spectral band positions and intensities. Glucose measurements from days 6 and 7 were used a calibration set and the measurements from the rest of days were used as a validation set (see Figure 5). Measurements from days 6 to 17 fall in the zones A and B. Data from day 20 fell in zone C, indicating that our SERS glucose sensor functioned properly up to at least 17 days. In order to prove that the SERS sensor read glucose signals rather than random noises, ten SESOR spectra were taken from an area not over the implanted sensor. These ten measurements are also presented in Figure 5. Nine of ten measurements fall in zone D, demonstrating our glucose sensor detects indeed glucose signal. The MARD and RMSE for calibration of 2 mW measurements are 4.2 mg/dL (0.2 mM) and 3.69%, respectively. The results of the validation measurements of the 2 mW illumination power for the rest of the days are summarized in Table III. Again, both MARD and RMSE for validation did not show significant increase over the 12 days period.
Figure 4.
Comparison of SESOR spectra from day 6–20, after implant, in vivo rat skin, and DT/MH-functionalized AgFON. λex = 785 nm, Pex = 2 mW, tacq = 2 min.
Figure 5.
Calibration and validation data sets for a period of 12-day in vivo transcutaneous SESORS glucose measurements on one rat. Measurements from day 6 and 7 ( ) were used for calibration sets. Measurements from day 8 (
) were used for calibration sets. Measurements from day 8 ( ), 10 (
), 10 ( ), 13(
), 13( ), 14 (
), 14 ( ), 15 (
), 15 ( ), 16 (
), 16 ( ), 17 (
), 17 ( ), 20 (
), 20 ( ), and in vivo rat skin (
), and in vivo rat skin ( ) were used for validation sets. All data points were acquired in vivo transcutaneously with λex = 785 nm, Pex = 2 mW, tacq = 2 min.
) were used for validation sets. All data points were acquired in vivo transcutaneously with λex = 785 nm, Pex = 2 mW, tacq = 2 min.
Table III.
Quantitative in vivo transcutaneous glucose detection using PLS calibration for 12-day monitoring
| Day | Measurement Number |
Daily RMSEP |
Daily MARDV |
Overall RMSEP |
Overall MARDV |
|---|---|---|---|---|---|
| 8 | 12 | 11.2 mg/dL (0.6 mM) | 6.66% | 11.2 mg/dL (0.6 mM) | 6.66% |
| 10 | 12 | 12.5 mg/dL (0.7 mM) | 10.3% | 11.9 mg/dL (0.7 mM) | 8.49% |
| 13 | 12 | 20.1 mg/dL (1.1 mM) | 16.0% | 15.1 mg/dL (0.8 mM) | 11.0% |
| 14 | 4 | 7.7 mg/dL (0.4 mM) | 5.33% | 14.6 mg/dL (0.8 mM) | 10.4% |
| 15 | 4 | 23.4 mg/dL (1.3 mM) | 20.4% | 15.6 mg/dL (0.9 mM) | 11.4% |
| 16 | 1 | N/A | 24.2% | 15.9 mg/dL (0.9 mM) | 11.6% |
| 17 | 4 | 10.4 mg/dL (0.6 mM) | 7.13% | 15.5 mg/dL (0.9 mM) | 11.3% |
Overall, our SERS glucose sensor showed excellent accuracy and reliability over a period of 17 days. The longest life span of GCM sensors currently available on the market is 7 days.28, 30 Although some long-term implanted sensors showed longer functional time, they do not function well during the first 3–4 weeks after implantation due to the foreign body response.34 In contrast, our SERS sensor functions immediately after implantation, indicating that the foreign body response does not affect the glucose sensing ability of the sensor. Furthermore, our SERS sensor was calibrated just once during both the 5-day and 12-day measurements. One of the main disadvantages of current GCM devices is that repeated calibration is needed for obtaining reliable glycemic profiles.28 Most of the devices need calibration at least four times a day.28 The accuracy of the sensor is greatly affected by the number and timing of the calibrations.32 Our SERS sensor showed consistent accuracy during the multiple days measurement period with only one calibration at the initial stage of sensor utilization.
CONCLUSION
These experiments show a significant step forward in our quantitative in vivo transcutaneous glucose sensing work.40 Most notably, the sensor system is now able to perform more accurately over a low range of glucose concentrations, over a long period of time, and over multiple rats. The high accuracy, consistent, and stable glucose measurements provide promise for an implantable, real time, continuous glucose sensor based on SERS. Our SESORS approach allows us to detect glucose directly with high accuracy especially in the low glucose concentration range as well as over a long period of time with only one time calibration. We have shown it is also possible to achieve this under the laser safety level for human skin exposure. To date, none of the commercially available devices can achieve enough accuracy in the hypoglycemic range and can function for more than 7 days. Currently, we are actively developing the next generation of SERS substrates with higher enhancement.10 This will further increase the accuracy of our SERS based glucose sensor. With high enough accuracy, our sensor could serve as a research tool for investigating the role of glycemic control in ICU patients.28, 30 Furthermore, new partition layers are being developed in our lab to expand the biological targets accessible to in vivo SERS from glucose to those included in the Chem 7 panel and others. Looking to the future, we believe that our SESORS sensor approach will have important applications in both opening up new areas of fundamental research and treatment and care of diabetic and ICU patients.
ACKNOWLEDGMENT
This work was supported by NIH Grant 5R56DK078691-02, NSF Grant CHE-0911145, and AFOSR/DARPA Grant FA9550-08-1-0221.
REFERENCES
- 1.Kneipp K, Kneipp H, Kartha VB, Manoharan R, Deinum G, Itzkan I, Dasari RR, Feld MS. Physical Review E. 1998;57:R6281–R6284. [Google Scholar]
- 2.Das G, Mecarini F, Gentile F, De Angelis F, Kumar HGM, Candeloro P, Liberale C, Cuda G, Di Fabrizio E. Biosensors & Bioelectronics. 2009;24:1693–1699. doi: 10.1016/j.bios.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 3.Stuart DA, Yuen JM, Lyandres NSO, Yonzon CR, Glucksberg MR, Walsh JT, Van Duyne RP. Analytical Chemistry. 2006;78:7211–7215. doi: 10.1021/ac061238u. [DOI] [PubMed] [Google Scholar]
- 4.Xie W, Qiu PH, Mao CB. Journal of Materials Chemistry. 2011;21:5190–5202. doi: 10.1039/C0JM03301D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bantz KC, Meyer AF, Wittenberg NJ, Im H, Kurtulus O, Lee SH, Lindquist NC, Oh SH, Haynes CL. Physical Chemistry Chemical Physics. 2011;13:11551–11567. doi: 10.1039/c0cp01841d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stuart DA, Yonzon CR, Zhang XY, Lyandres O, Shah NC, Glucksberg MR, Walsh JT, Van Duyne RP. Analytical Chemistry. 2005;77:4013–4019. doi: 10.1021/ac0501238. [DOI] [PubMed] [Google Scholar]
- 7.Zhang XY, Zhao J, Whitney AV, Elam JW, Van Duyne RP. Journal of the American Chemical Society. 2006;128:10304–10309. doi: 10.1021/ja0638760. [DOI] [PubMed] [Google Scholar]
- 8.Stiles PL, Dieringer JA, Shah NC, Van Duyne RR. Annual Review of Analytical Chemistry. Vol. 1. Palo Alto: Annual Reviews; 2008. pp. 601–626. [DOI] [PubMed] [Google Scholar]
- 9.Wustholz KL, Henry AI, McMahon JM, Freeman RG, Valley N, Piotti ME, Natan MJ, Schatz GC, Van Duyne RP. Journal of the American Chemical Society. 2010;132:10903–10910. doi: 10.1021/ja104174m. [DOI] [PubMed] [Google Scholar]
- 10.Henry AI, Bingham JM, Ringe E, Marks LD, Schatz GC, Van Duyne RP. Journal of Physical Chemistry C. 2011;115:9291–9305. [Google Scholar]
- 11.Huang GG, Han XX, Hossain MK, Ozaki Y. Analytical Chemistry. 2009;81:5881–5888. doi: 10.1021/ac900392s. [DOI] [PubMed] [Google Scholar]
- 12.Singhal R, Bhattacharyya S, Orynbayeva Z, Vitol E, Friedman G, Gogotsi Y. Nanotechnology. 2010:21. doi: 10.1088/0957-4484/21/1/015304. [DOI] [PubMed] [Google Scholar]
- 13.Lyandres O, Shah NC, Yonzon CR, Walsh JT, Glucksberg MR, Van Duyne RP. Analytical Chemistry. 2005;77:6134–6139. doi: 10.1021/ac051357u. [DOI] [PubMed] [Google Scholar]
- 14.Matousek P, Clark IP, Draper ERC, Morris MD, Goodship AE, Everall N, Towrie M, Finney WF, Parker AW. Applied Spectroscopy. 2005;59:393–400. doi: 10.1366/0003702053641450. [DOI] [PubMed] [Google Scholar]
- 15.Matousek P, Morris MD, Everall N, Clark IP, Towrie M, Draper E, Goodship A, Parker AW. Applied Spectroscopy. 2005;59:1485–1492. doi: 10.1366/000370205775142548. [DOI] [PubMed] [Google Scholar]
- 16.Matousek P, Draper ERC, Goodship AE, Clark IP, Ronayne KL, Parker AW. Applied Spectroscopy. 2006;60:758–763. doi: 10.1366/000370206777886955. [DOI] [PubMed] [Google Scholar]
- 17.Stone N, Baker R, Rogers K, Parker AW, Matousek P. Analyst. 2007;132:899–905. doi: 10.1039/b705029a. [DOI] [PubMed] [Google Scholar]
- 18.Stone N, Faulds K, Graham D, Matousek P. Analytical Chemistry. 2010;82:3969–3973. doi: 10.1021/ac100039c. [DOI] [PubMed] [Google Scholar]
- 19.Stone NSN, Kerssens M, Lloyd GR, Faulds K, Graham D, Matousek P. Chemical Science. 2011;2:776–780. [Google Scholar]
- 20.Qian XM, Peng XH, Ansari DO, Yin-Goen Q, Chen GZ, Shin DM, Yang L, Young AN, Wang MD, Nie SM. Nature Biotechnology. 2008;26:83–90. doi: 10.1038/nbt1377. [DOI] [PubMed] [Google Scholar]
- 21.Zavaleta CL, Smith BR, Walton I, Doering W, Davis G, Shojaei B, Natan MJ, Gambhir SS. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13511–13516. doi: 10.1073/pnas.0813327106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewinski N, Colvin V, Drezek R. Small. 2008;4:26–49. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- 23.Oberdorster G, Stone V, Donaldson K. Nanotoxicology. 2007;1:2–25. [Google Scholar]
- 24.Murphy CJ, Gole AM, Stone JW, Sisco PN, Alkilany AM, Goldsmith EC, Baxter SC. Accounts of Chemical Research. 2008;41:1721–1730. doi: 10.1021/ar800035u. [DOI] [PubMed] [Google Scholar]
- 25.Atlanta, GA: 2011. [Google Scholar]
- 26.Cryer PE. Diabetologia. 2002;45:937–948. doi: 10.1007/s00125-002-0822-9. [DOI] [PubMed] [Google Scholar]
- 27.Diabetes Care. 2011;34 Suppl 1:S11–S61. [Google Scholar]
- 28.Kondepati VR, Heise HM. Analytical and Bioanalytical Chemistry. 2007;388:545–563. doi: 10.1007/s00216-007-1229-8. [DOI] [PubMed] [Google Scholar]
- 29.Oliver NS, Toumazou C, Cass AEG, Johnston DG. Diabetic Medicine. 2009;26:197–210. doi: 10.1111/j.1464-5491.2008.02642.x. [DOI] [PubMed] [Google Scholar]
- 30.Brauker J. Diabetes Technology & Therapeutics. 2009;11:S25–S36. doi: 10.1089/dia.2008.0137. [DOI] [PubMed] [Google Scholar]
- 31.Aye T, Block J, Buckingham B. Endocrinology and Metabolism Clinics of North America. 2010;39:609–624. doi: 10.1016/j.ecl.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buckingham BA, Kollman C, Beck RW, Kalajian A, Fiallo-Scharer R, Tansey MJ, Fox LA, Wilson DM, Weinzimer SA, Ruedy KJ, Tamborlane WV DirecNet Study, G. Diabetes Technology & Therapeutics. 2006;8:318–325. doi: 10.1089/dia.2006.8.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Renard E. Current Opinion in Pharmacology. 2002;2:708–716. doi: 10.1016/s1471-4892(02)00216-3. [DOI] [PubMed] [Google Scholar]
- 34.Gilligan BC, Shults M, Rhodes RK, Jacobs PG, Brauker JH, Pintar TJ, Updike SJ. Diabetes Technology & Therapeutics. 2004;6:378–386. doi: 10.1089/152091504774198089. [DOI] [PubMed] [Google Scholar]
- 35.Dingari NC, Barman I, Singh GP, Kang JW, Dasari RR, Feld MS. Analytical and Bioanalytical Chemistry. 2011;400:2871–2880. doi: 10.1007/s00216-011-5004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipson J, Bernhardt J, Block U, Freeman WR, Hofmeister R, Hristakeva M, Lenosky T, McNamara R, Petrasek D, Veltkamp D, Waydo S. J Diabetes Sci Technol. 2009;3:233–241. doi: 10.1177/193229680900300203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Enejder AMK, Scecina TG, Oh J, Hunter M, Shih WC, Sasic S, Horowitz GL, Feld MS. Journal of Biomedical Optics. 2005;10 doi: 10.1117/1.1920212. [DOI] [PubMed] [Google Scholar]
- 38.Lyandres O, Van Duyne RP, Walsh JT, Glucksberg MR, Mehrotra S. Analyst. 2010;135:2111–2118. doi: 10.1039/c0an00134a. [DOI] [PubMed] [Google Scholar]
- 39.Lyandres O, Yuen JM, Shah NC, VanDuyne RP, Walsh JT, Glucksberg MR. Diabetes Technology & Therapeutics. 2008;10:257–265. doi: 10.1089/dia.2007.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuen JM, Shah NC, Walsh JT, Glucksberg MR, Van Duyne RP. Analytical Chemistry. 2010;82:8382–8385. doi: 10.1021/ac101951j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clarke WL, Cox D, Gonderfrederick LA, Carter W, Pohl SL. Diabetes Care. 1987;10:622–628. doi: 10.2337/diacare.10.5.622. [DOI] [PubMed] [Google Scholar]
- 42.Clarke WL. Diabetes Technology & Therapeutics. 2005;7:776–779. doi: 10.1089/dia.2005.7.776. [DOI] [PubMed] [Google Scholar]
- 43.Sheffield CA, Kane MP, Bakst G, Busch RS, Abelseth JM, Hamilton RA. Diabetes Technology & Therapeutics. 2009;11:587–592. doi: 10.1089/dia.2008.0143. [DOI] [PubMed] [Google Scholar]
- 44.Greeneltch N, Dieringer JA, Van Duyne RP. in preparation. Evanston: Northwestern University; 2011. [Google Scholar]
- 45.Kaufman FR, Gibson LC, Halvorson M, Carpenter S, Fisher LK, Pitukcheewanont P. Diabetes Care. 2001;24:2030–2034. doi: 10.2337/diacare.24.12.2030. [DOI] [PubMed] [Google Scholar]