Abstract
Extensive developmental research has linked peer rejection during adolescence with a host of psychopathological outcomes, including depression. Moreover, recent neuroimaging research has suggested that increased activity in the subgenual region of the anterior cingulate cortex (subACC), which has been consistently linked with depression, is related to heightened sensitivity to peer rejection among adolescents. The goal of the current study was to directly test the hypothesis that adolescents’ subACC responses are predictive of their risk for future depression, by examining the relationship between subACC activity during peer rejection and increases in depressive symptoms during the following year. During a functional magnetic resonance imaging scan, 20 13-year-olds were ostensibly excluded by peers during an online social interaction. Participants’ depressive symptoms were assessed via parental reports at the time of the scan and 1 year later. Region of interest and whole-brain analyses indicated that greater subACC activity during exclusion was associated with increases in parent-reported depressive symptoms during the following year. These findings suggest that subACC responsivity to social exclusion may serve as a neural marker of adolescents’ risk for future depression and have implications for understanding the relationship between sensitivity to peer rejection and the increased risk of depression that occurs during adolescence.
As children transition into adolescence, they face a unique challenge: peer relationships become more important (Brown, 1990) at the same time as peer rejection becomes more prevalent (Coie, Dodge, & Kupersmidt, 1990; Juvonen, Graham, & Shuster, 2003). At this age there is a well-documented shift from relying on parents for social support to relying on peer relationships (Rubin, Bukowski, & Parker, 2006). Upon entering adolescence, youth spend increased time with peers (Csikszentmihalyi & Larson, 1984), seek out peers’ opinions and place increased value on gaining their approval (Brown, 1990), and are generally more concerned with maintaining peer acceptance (Parkhurst & Hopmeyer, 1998). However, along with this heightened emphasis on social relationships with peers comes increased risk for peer rejection, which is a particularly prevalent form of negative treatment at this age (Coie et al., 1990). Given adolescents’ reliance on peer relationships and the degree to which they value peer acceptance, it is not surprising that this increase in peer rejection has significant negative consequences for adolescents’ emotional well-being and mental health.
During adolescence, instances of interpersonal stress become increasingly predictive of depression (Hankin, Mermelstein, & Roesch, 2007; Larson & Ham, 1993; Leadbeater, Kuperminc, Blatt, & Hertzog, 1999; Nolan, Flynn, & Garber, 2003; Rudolph, 2002; Rudolph et al., 2000; Rudolph & Hammen, 1999; Rudolph, Hammen, & Burge, 1994), and overall there is a significant spike in the onset of depression (Pine, Cohen, Gurley, Brook, & Ma, 1998; Pine, Cohen, Johnson, & Brook, 2002; Klerman & Weissman, 1989). Specifically, peer rejection and conflict have been linked with increased rates of depression (French, Conrad, & Turner, 1995; Larson, Moneta, Richards, & Wilson, 2002; Nolan et al., 2003; Panak & Garber, 1992; Prinstein & Aikins, 2004; Rigby, 2003), increased internalizing and externalizing symptoms over time (Carter, Garber, Ciesla, & Cole, 2006), increased social withdrawal (Abecassis, Hartup, Haselager, Scholte, & Lieshout, 2002), and other adverse mental health outcomes that persist across development (Lev-Wiesel, Nuttman-Shwartz, & Sternberg, 2006; Prinstein & Aikins, 2004; Prinstein, Sheah, & Guyer, 2005).
Furthermore, several researchers have specifically shown that incidences of peer rejection and interpersonal stress lead to increases in depression, rather than the converse possibility that depressed individuals elicit more interpersonal stressors (Hammen & Goodman-Brown, 1990; Rudolph & Clark, 2001; Panak & Garber, 1992; Hankin et al., 2007; Nolan et al., 2003). Thus, adolescents’ responses to social stressors in peer contexts may precipitate increases in internalizing symptoms and depression over time. Finally, some research has suggested that adolescents not only experience an increase in peer-related stressors that likely contributes to these symptom increases but also are more sensitive to these stressors (Hankin & Abramson, 2001; Nelson, Leibenluft, McClure, & Pine, 2005; Rudolph, 2002). One study actually demonstrated that sensitivity to rejection predicted psychopathological outcomes, even after controlling for the experience of being rejected (Sandstrom, Cillessen, & Eisenhower, 2003). In other words, adolescents likely experience more peer-related stress in adolescence because of both an increased number of stressful events as well as heightened sensitivity to these events, and individuals’ responses to negative events like peer rejection are likely an important contributor to adolescents’ heightened risk for depression.
Building on this literature, researchers have suggested that many of the changes that occur during adolescence, including a reorientation toward peers and away from parents, heightened stress responses to peer rejection, and the increasing onset of mood disorders, may partially reflect underlying changes in neural responses to social events (Nelson et al., 2005; Steinberg, 2008). The degree of neural activity that adolescents display in brain regions responsible for affective processing, particularly in response to social rejection may directly relate to their emotional sensitivity to these events and predict their likelihood of developing psychopathology (Nelson et al., 2005). This theory is consistent with the robust developmental literature indicating that heightened sensitivity to social stressors during adolescence contributes to depression onset and suggests a parallel contribution of neural sensitivity to adolescents’ risk for depression.
Despite the growing body of evidence that responses to peer rejection contribute to adolescents’ risk for depression through both behavioral and neural pathways, specific neurobiological markers that might predict future outcomes remain unexplored in adolescents. Fortunately, however, recent neuroimaging studies of adult populations have begun to elucidate the brain systems involved in depression, and they provide a framework for examining these neural processes in adolescence prior to the typical age of depression onset. These studies have focused largely on the subgenual anterior cingulate cortex (subACC) and its role in depressive symptomatology. For example, research examining depressed populations has indicated that the subACC is more responsive to negative emotional stimuli among depressed patients (Chen et al., 2007; Davidson, Irwin, Anderle, & Kalin, 2003). In addition, heightened subACC activity is indicative of the severity of depressive symptoms (Saxena et al., 2003), and responsiveness to clinical treatment (Brody et al., 1999; Mayberg et al., 1997). Given the robustness of these findings among adults, examination of the role of subACC activity in predicting depressive symptoms during adolescence prior to disorder onset is clearly warranted. Specifically, examining subACC responses to peer rejection, a major adolescent stressor, may be useful in predicting adolescents’ risk for depression.
It is interesting that the subACC and several of its surrounding subcortical structures have already been implicated in adolescents’ experiences of peer rejection as well as other affective experiences. A recent neuroimaging study examining 13-year-olds’ neural responses to peer rejection found heightened sub-ACC activity during adolescents’ experiences of peer exclusion compared to peer inclusion, and this activity was positively related to adolescents’ reported distress resulting from the exclusion (Masten et al., 2009). This finding suggests that overlapping neural systems are involved in both sensitivity to peer rejection among adolescents and neural dysregulation among depressed adults, and supports the possibility that heightened subACC activity might be predictive of both sensitivity to peer rejection and heightened risk for depression during adolescence. Additional studies examining social processing among adolescents have implicated other subcortical regions in affective processing that are highly interconnected with the subACC, including the ventral striatum, hypothalamus, amygdala, orbitofrontal cortex, and anterior cingulate (Guyer et al., 2008; Guyer, McClure-Tone, Shiffrin, Pine, & Nelson, 2009; Monk et al., 2003). These findings further support the possibility that subACC activity among adolescents might be an important index of sensitivity to social stressors like peer rejection, and that this subcortical activity might act as a marker of adolescents’ risk for depression.
The goal of the current study was to directly test this hypothesis. One route via which peer-related stressors likely contribute to adolescents’ risk for depression is through altered neural sensitivities (see Nelson et al., 2005), and the subACC has been shown to index responses to one of the most pervasive and stressful types of peer-related stressors, that is, peer rejection (Masten et al., 2009), as well as emotional processing among depressed adults (Chen et al., 2007; Davidson, Irwin, Anderle, & Kalin, 2003). Thus, our goal was to examine whether heightened subACC activity in response to peer rejection among adolescents was associated with increases in depressive symptoms over time. To examine this, healthy adolescents were ostensibly excluded during a functional magnetic resonance imaging (fMRI) scan in order to measure subACC responses to peer rejection. These subACC responses were then correlated with concurrent, and increases in, depressive symptoms over the following year. We expected that adolescents displaying greater subACC activity would be more likely to develop depressive symptoms over time.
In this study we aimed to expand on previous research in several ways. First, although research has examined neural correlates of depression among adults, this is the first neuro-imaging study to examine antecedents of risk for depression during adolescence when disorder onset has not yet occurred. Our sample consisted of young adolescents who had recently begun middle school—the period of development during which peers relationships are highly salient and peer rejection is most prevalent (Brown, 1990; Coie et al., 1990; Juvonen et al., 2003; Rubin et al., 2006). In addition, these young adolescents were all typically developing and within the normal range of depressive symptomatology. Thus, we were able to examine predictors of adolescents’ risk for depression prior to any potential disorder onset, as well as developmental processes relevant for understanding changing depressive symptoms across this period of development. Second, we used an ecologically valid task to examine emotional responses to a salient, real-life, social stressor. Previous neuroimaging studies examining depression in adults have typically relied on resting state responses or simple emotion-processing tasks, whereas previous behavioral studies examining adolescents have relied largely on reports of past experiences or imagined vignettes. Thus, using an ecologically valid, experimental approach to simulate a real, highly relevant, social experience is much needed (Nelson et al., 2005). Third, to our knowledge, no prior neuro-imaging studies have examined predictive links between social or emotional experiences and mental health-related outcomes across time. Thus, we employed a longitudinal design in order to better examine the neural antecedents of adolescents’ risk for depression and to complement the many well-designed, longitudinal, behavioral studies examining this topic.
Fourth, in the current study we also explored potential sex differences, given that these differences have been well established in both clinical and affective neuroimaging research on social/emotional processing and depression. Specifically, research has shown that the onset of depression is earlier and more prevalent among females (Weissman & Klerman, 1977; Wolk & Weissman, 1995), and that these differences in depression first reliably emerge in adolescence (Nolen-Hoeksema & Girgus, 1994; Peterson et al., 1993). In addition, adolescent girls are more likely to develop depression as a result of certain depression precursors, including heightened social evaluative concerns (Rudolph & Conley, 2005), and both increased frequency of stressful events and greater sensitivity to these events (Hankin & Abramson, 1999; Wagner & Compas, 1990). Furthermore, neuroimaging studies have also shown sex differences in affective and emotional processing in adolescents (e.g., Guyer et al., 2009). Thus, although our sample size did not permit definitive tests of sex differences, we explored potential differential patterns in the links between subACC activity and development of depressive symptoms among boys and girls.
Method
Participants
A socioeconomically diverse sample of 20 adolescents (13 females), representing a range of ethnic backgrounds (45% Caucasian, 30% Latino, 10% African American, 10% Asian, and 5% Native American), were recruited from the greater Los Angeles area through mass mailings, summer camps, and fliers distributed in the community. Adolescents and their parents underwent extensive screening and participants showed no self- or parent-reported evidence of any psychiatric disorder, and were not taking any psychiatric medications at any point during the study. At the first time point (age range = 12.4–13.6 years, M = 12.94 years), participants completed an fMRI scan during which they experienced a simulated experience of peer rejection and subsequently self-reported their distress, and their parents completed a measure assessing their child’s depressive symptoms (see below). At the second time point (12–14 months later), participants’ parents reported their child’s depressive symptoms again. The age range in this study is particularly relevant given prior research characterizing the middle school transition as a time of heightened salience of peer relationships resulting from both concern about peer acceptance as well as increased prevalence of peer rejection (Brown, 1990). All participants and their parents provided assent/consent in accordance with UCLA’s institutional review board.
fMRI-simulated peer exclusion task
In order to simulate peer rejection during the fMRI scan, adolescents played two rounds of a computerized game called “Cyberball” (Williams, Cheung, & Choi, 2000; Williams et al., 2002), in which participants experienced simulated peer exclusion. This simulation of exclusion was used as a proxy for peer rejection based on research indicating that during early adolescence, isolating peers from social groups is one of the dominant methods used to reject peers (Coie et al., 1990). Moreover, Cyberball has been used successfully to elicit feelings of rejection in previous neuroimaging studies with adults (Eisenberger, Lieberman, & Williams, 2003) and adolescents (Masten et al., 2009).
During the instructions for the Cyberball game, participants were told that they would be playing a ball-tossing game via the Internet with two other adolescents in other scanners, in order to examine coordinated neural activity. To increase ecological validity, participants were given the first names, ages (which matched that of the participant) and genders (one boy, one girl) of these other players. Once in the scanner, the Cyberball game was displayed on a computer screen through MR-compatible goggles (Resonance Technology, Inc.). Participants saw cartoon images representing the other players, as well as a cartoon image of their own “hand” that they controlled using a button box. Throughout the game the ball was thrown back and forth among the three players, with the participant choosing the recipient of his or her own throws, and the throws of the other two “players” determined by the preset program. Participants played two rounds of Cyberball during two sequential fMRI scans: one round in which they were “included” throughout the game, and one round in which they were “excluded” by the other participants. Throughout the inclusion round the computerized players were equally likely to throw the ball to the participant or the other player. However, during the exclusion round, the two computerized players stopped throwing the ball to the participant after the participant had received a total of 10 throws and threw the ball only to each other for the remainder of the game. Upon leaving the scanner, participants self-reported their distress resulting from the exclusion condition (see below) and were then debriefed regarding the deception used in the study.
Measure of distress resulting from peer exclusion
Immediately following completion of the Cyberball task, adolescents completed the Need–Threat Scale (NTS; Williams et al., 2000; Williams et al., 2002) in order to measure distress associated with the exclusion condition. The NTS assesses 12 subjectively experienced consequences of being excluded during the game, including ratings of self-esteem (“I felt liked”), belongingness (“I felt rejected”), meaningfulness (“I felt invisible”), and control (“I felt powerful”), on a scale ranging from 1 (not at all) to 5 (very much).
Measures of depressive symptoms
Depressive symptoms were assessed at both time points through parental reports on the withdrawn/depressed subscale of the Childhood Behavior Checklist (CBCL; Achenbach & Rescorla, 2001), which assesses an array of internalizing symptoms and negative affect typical of depression and other mood disorders. Participants were specifically recruited so as not to meet clinical or subclinical criteria for any psychiatric condition including depression (Ts > 65). However, a range of CBCL scores was reported on this subscale at both time points (see behavioral results). Participants’ scores at Time 1 reflect their concurrent depressive symptoms at the time of the fMRI scan. Scores at Time 2, after controlling for Time 1, reflect increases (or decreases) in participants’ depressive symptoms during the year following the scan. To control for scores at Time 1, residualized scores for Time 2 were calculated, whereby the group-level variance in Time 2 scores that was explained by Time 1 scores was removed. There were no sex differences in depressive symptoms at either time point, and there were no sex differences in the amount of increase in depressive symptoms from Time 1 to Time 2.
fMRI data acquisition
Images were collected using a Siemens Allegra 3-Tesla MRI scanner. Extensive instructions and reminders were given to decrease motion, and head motion was restrained with foam padding. For each participant, an initial two-dimensional spin–echo image (repetition time [TR] = 4000 ms, echo time [TE] = 40 ms, matrix size 256 × 256, 4-mm thickness, 1-mm gap) in the sagittal plane was acquired in order to enable prescription of slices obtained in structural and functional scans. In addition, a high-resolution structural scan (echo planar spin–spin relaxation time [T2] weighted spin–echo, TR = 4000 ms, TE = 54 ms, matrix size 128 × 128, field of view = 20 cm, 36 slices, 1.56-mm in-plane resolution, 3-mm thickness) coplanar with the functional scans was obtained for functional image registration during fMRI analysis preprocessing. Each of the two rounds of Cyberball was completed during a functional scan lasting 2 min, 48 s (echo planar combined magnetic field inhomogeneities and spin–spin relaxation time [T2*] weighted gradient echo, TR =2000 ms, TE =25 ms, flip angle =90 degrees, matrix size 64 × 64, 36 axial slices, field of view = 20 cm, 3-mm thickness, 1-mm skip).
fMRI data analysis
Neuroimaging data were preprocessed and analyzed using statistical parametric mapping (SPM5; Wellcome Department of Cognitive Neurology, Institute of Neurology, Lon-don), and region of interest (ROI) extraction was performed using the MARsBaR toolbox within SPM (Marseille boîte à région d’intérêt; Brett, Anton, Valabregue & Poline, 2002). Preprocessing included image realignment to correct for head motion, normalization into a standard stereotactic space defined by the Montreal Neurological Institute and the International Consortium for Brain Mapping, and spatial smoothing using an 8-mm Gaussian kernel at full width at half-maximum to increase the signal/noise ratio.
Modeling of contrasts
The Cyberball task was modeled as a block design. Each round of Cyberball was modeled as a run with each period of inclusion and exclusion modeled as blocks within the run for a total of two inclusion blocks (one during the first run and one during the short period of inclusion in the second run prior to exclusion) and one exclusion block. After modeling the Cyberball paradigm, linear contrasts were calculated for each planned condition comparison for each participant. These individual contrast images were then used in ROI and whole-brain, group-level, random-effects analyses across all participants.
ROI analyses
Given our specific interest in the relationship between subACC activity and adolescents’ risk for depression, we first performed ROI analyses to examine whether subACC activity in response to peer rejection was associated with either concurrent depressive symptoms or increases in depressive symptoms during the following year. The ROI was functionally defined (using the MARsBaR toolbox) as the cluster in the subACC that was previously found to show greater activation to peer exclusion compared to inclusion, among a larger group of adolescents that included those in the current study (see Masten et al., 2009; peak voxel [x y z in millimeters (8 22 −4)], t = 4.06, p = .0005, k = 151 voxels). Mean parameter estimates for each participant (which model the amplitude of the blood oxygen level-dependent response during exclusion vs. inclusion) were then extracted and averaged across all voxels in the ROI. Standard statistical software (SPSS 16.0, Chicago) was used to conduct correlations to determine whether these parameter estimates were correlated with concurrent (scores at Time 1) and longitudinal increases in (scores at Time 2, controlling for Time 1) depressive symptom scores. To examine whether activity in this same region of the subACC correlated with participants’ self-reported distress following the exclusion round of Cyber-ball, we examined whether these parameter estimates were correlated with NTS scores. Because we predicted that greater subACC activity would be associated specifically with greater increases in depressive symptoms over time, as well as greater self-reported distress, all tests were one tailed.
Whole-brain analyses
In order to supplement the ROI analyses and examine the relationships between brain activity during peer rejection and depressive symptoms, as well as self-reported distress following exclusion, the following group-level tests were run at each voxel across the entire brain volume: (a) examination of differences between exclusion and inclusion that were associated with individuals’ concurrent depressive symptoms (parent-reported scores at Time 1), (b) examination of differences between exclusion and inclusion that were associated with longitudinal increases in individuals’ depressive symptoms (parent-reported scores at Time 2, controlling for Time 1 scores), and (c) examination of differences between exclusion and inclusion that were associated with NTS scores. Reported correlational findings reflect regions of the brain identified using these whole-brain regressions, in which depressive symptoms or NTS scores were significantly associated with the difference in activity between exclusion and inclusion. All whole-brain, group-level regression analyses were thresholded at p < .001 for magnitude, with a minimum cluster size threshold of 10 voxels. All coordinates are reported in Montreal Neurological Institute format.
Analyses of sex differences
Finally, given the established sex differences in depression onset during adolescence (Nolen-Hoeksema & Girgus, 1994; Peterson et al., 1993; Weissman & Klerman, 1977; Wolk & Weissman, 1995), we also performed exploratory ROI and whole-brain regressions to examine sex differences in the relationship between subACC activity and longitudinal increases in depressive symptoms.
Results
Behavioral analyses
For subjective distress reported immediately following the Cyberball game, participants’ mean score was 2.90 (SD = 0.73) and ranged from 1.58 to 4.50 out of a possible 5; these scores did not differ by sex. For parent-reported depression symptoms, CBCL subscale scores ranged from T = 50 to 57 at both time points. Scores were similar on average at Time 1 (M = 51.35, SD = 2.35) and Time 2 (M = 51.85, SD = 2.43), suggesting that across the whole sample there was no overall increase in depressive symptoms. These sub-scale scores did not differ by sex at either time point, and there was no sex difference in the amount of increase in depression symptoms from Time 1 to Time 2. Finally, there were no significant correlations between self-reported distress following the experience of peer exclusion during the fMRI scan and either increases in depression symptoms (r =−.13, ns), or depression scores at Time 1 (r = −.02, ns) or Time 2 (r = −.12, ns), perhaps because of our relatively small sample size, which is typical of current neuroimaging studies.
ROI analyses
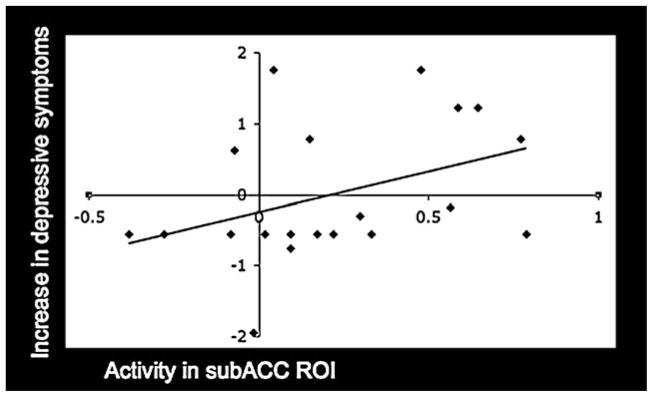
ROI analyses revealed that activity during peer rejection in the subACC was not associated with concurrent depressive symptoms (r = .01, ns), but was significantly correlated with subsequent increases in depressive symptoms (r = .39, p < .05; see Figure 1). There was no sex difference in this effect (Z = 0.24, ns; girls: r = .34, p = .13; boys: r = .46, p = .15). In addition, activity in this ROI was marginally correlated with self-reported social distress following the exclusion episode (r = .32, p = .08). Thus, greater subACC activity in response to peer rejection was associated with greater subsequent increases in depressive symptoms among adolescents as well as greater self-reported distress in response to rejection.
Figure 1.
A scatterplot depicting the relationship between increases in depressive symptoms scores and mean parameter estimates extracted for each individual from the subgenual anterior cingulate cortex (subACC) region of interest (ROI; r = .39; ROI is functionally defined as the region that showed greater activity among adolescents experiencing peer exclusion compared to inclusion in a previous study; see Masten et al., 2009).
Whole-brain analyses
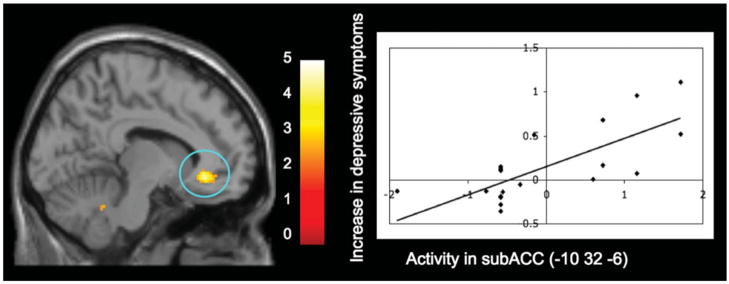
Consistent with the ROI analyses, whole-brain analyses indicated that greater subACC activity during peer rejection was not related to concurrent depressive symptoms (see Table 1). However, activity in two regions of the subACC was significantly associated with increases in depressive symptoms during the year following the fMRI scan ([12 36 −10], t =5.31, r =.78, p <.0001, k =11; [−10 32 −6], t =4.65, r =.74, p <.0001, k =21, see Figure 2). Again, there were no sex differences in these effects (Zs < 0.15, ns).1 In addition, as reported previously (see Masten et al., 2009), whole-brain analyses also revealed that activity in a similar region of the subACC correlated significantly with self-reported social distress following rejection (r = .70, p <.001 for the 20 participants included in the current sample). Moreover, greater activity during peer rejection in two additional regions—the dorsomedial prefrontal cortex (DMPFC; [14 44 44], t = 5.14, r = .80, p < .0001, k = 60) and the middle temporal gyrus ([56 4 −26], t =4.47, r =.72, p <.0005, k =10)—was associated with a longitudinal increase in depressive symptoms as well. There were no negative correlations between brain activity and increases in depressive symptom scores.
Table 1.
Anatomical regions activated during the exclusion condition versus the inclusion condition that correlated significantly with concurrent depressive symptoms
| Anat. Region | BA | x | y | z | t | r | k | p | |
|---|---|---|---|---|---|---|---|---|---|
| Positive Associations With Concurrent Depressive Symptoms
| |||||||||
| VLPFC | 46 | L | −44 | 28 | 10 | 4.35 | .71 | 24 | <.0005 |
| DMPFC | 8 | L | −8 | 56 | 40 | 4.38 | .70 | 14 | <.0005 |
| Precuneus | 7 | L | −20 | −56 | 34 | 4.92 | .72 | 28 | <.0001 |
| PCC | 29 | R | 14 | −46 | 8 | 6.49 | .79 | 47 | <.0001 |
|
| |||||||||
| Negative Associations With Concurrent Depressive Symptoms
| |||||||||
| Cuneus | 18 | R | 6 | −84 | 22 | 7.26 | −.81 | 42 | <.0001 |
| IPL | 40 | R | 44 | −36 | 24 | 5.47 | −.77 | 151 | <.0001 |
| Precuneus/IPL | 19 | R | 34 | −64 | 40 | 5.19 | −.74 | 79 | <.0001 |
| DLPFC | 9 | R | 28 | 8 | 42 | 5.06 | −.74 | 19 | <.0001 |
| 6 | R | 44 | 2 | 44 | 4.16 | −.73 | 17 | <.0005 | |
| 9/46 | R | 36 | 30 | 30 | 3.77 | −.68 | 10 | <.001 | |
| SMA | 6 | R | 24 | −8 | 74 | 4.55 | −.74 | 21 | <.0005 |
| rACC | 24 | L | −14 | 36 | 10 | 4.48 | −.75 | 19 | <.0005 |
| Precuneus | 7 | L | −16 | −56 | 76 | 4.33 | −.71 | 19 | <.0005 |
| STG | 39 | L | −42 | −50 | 26 | 4.19 | −.71 | 12 | <.0005 |
| dACC | 24 | R | 14 | 0 | 44 | 4.06 | −.70 | 21 | <.0005 |
Note: BA, putative Brodmann area; L, R, respective left and right hemispheres; x, y, and z, Montreal Neurological Institute coordinates in respective left–right, anterior–posterior, and interior–superior dimensions; t, t score at those coordinates (local maxima); r, correlation coefficient representing the strength of the association between concurrent depression symptom scores and the difference between activity during exclusion and activity during inclusion in the specified clusters; VLPFC, ventrolateral prefrontal cortex; DMPFC, dorsomedial prefrontal cortex; PCC, posterior cingulate cortex; IPL, inferior parietal lobe; DLPFC, dorsolateral PFC; SMA, supplementary motor area; rACC, rostral anterior cingulate cortex; STG, superior temporal gyrus; dACC, dorsal ACC.
Figure 2.
The whole-brain regression analysis displaying activity in the subgenual anterior cingulate cortex (subACC) during peer exclusion compared to inclusion that was associated with increases in depressive symptoms over the following year. The scatterplot is provided to illustrate the relationship between increases in depressive symptoms and the mean parameter estimates extracted for each individual from the significant subACC cluster. For display purposes only, activation shown here is thresholded at p = .01 to better depict the location and nature of this activation. [A color version of this figure can be viewed online at journals.cambridge.org/dpp]
Discussion
Findings from this study indicate that healthy adolescents displaying greater subACC activation in response to peer rejection are more likely to exhibit an increase in depressive symptoms during the following year. To our knowledge, this is the first study to establish a neurobiological link between a social stress or and depressive symptoms during adolescence, as well as the first longitudinal, neuroimaging study to examine these types of predictive links. Our findings provide promising support for the hypothesis that subACC responsiveness may be predictive of healthy adolescents’ risk for future depression, and extends behavioral research that has consistently linked experiences of peer rejection with depressive symptoms during adolescence (French et al., 1995; Larson et al., 2002; Nolan et al., 2003; Panak & Garber, 1992; Prinstein & Aikins, 2004; Rigby, 2003).
These findings also build on previous work with adults linking subACC activity with functioning among depressed patients (Brody et al., 1999; Chen et al., 2007; Davidson et al., 2003; Mayberg et al., 1997; Saxena et al., 2003) in two ways. First, these findings indicated that subACC activity is a potentially important neural marker of depressive symptoms that can be assessed prior to any diagnosis of depression. In other words, subACC responses may be predictive of individuals’ risk for developing depression during late adolescence or adulthood, before symptoms potentially reach a clinical level. Second, these previous studies of depression in adults relied on resting state activity (Brody et al., 1999; Mayberg et al., 1997; Saxena et al., 2003) or responses to simple emotional stimuli (Chen et al., 2007; Davidson et al., 2003). The current findings demonstrate a link between neural sensitivity during a real, social experience with peers and depressive symptom ratings, which is an extension of previous work that has been needed for a long time (Nelson et al., 2005). Thus, our findings extend previous work by demonstrating that subACC activity in response to a socially relevant task, rather than just baseline levels of subACC activity, may be indicative of future depression.
Given that subACC activity during peer rejection did not relate to concurrent depressive symptoms, but rather was associated with longitudinal increases in depressive symptoms, heightened subACC activity during peer rejection may specifically indicate an increased risk for developing depressive symptoms over time. Thus, individuals who show greater subACC responses to peer rejection early in adolescence may be more likely to subsequently experience increases in depressive symptoms, and may face a greater likelihood of eventually developing a clinical disorder. The absence of a link between subACC activity and concurrent depressive symptoms could indicate that this heightened activity in response to peer rejection represents a vulnerability among certain individuals that is cumulative over time. It is possible that the downstream effects of this neural sensitivity include increases in internalizing symptoms that could eventually reach a clinical level. Given behavioral research indicating that sensitivity to peer-related negative events may be predictive of depression over time, above and beyond the frequency of these events (Sandstrom et al., 2003), this subACC responsivity could represent an early indication of which individuals will be at greater risk for psychiatric problems over time as a result of their sensitivity to peer rejection. Of course, causality cannot be determined from the correlational methods used in this study; however, future research with adolescent participants should continue to probe the relationships between subACC activity, depressive symptoms, and eventual disorder onset.
Although understanding the mechanism responsible for the link between adolescents’ peer rejection and risk for depression goes beyond the current data, there are several possibilities suggested by these results. First, greater subACC sensitivity to peer rejection might actually alter adolescents’ subjective emotional experiences and result in more acute emotional responses and more negative interpretations of both current and future instances of peer rejection. As a result, the peers of these adolescents might respond to them more negatively in these situations and potentially reject them more frequently in the future. Thus, over time, sensitivity at the neural level might actually elicit more negative peer rejection experiences, from both the victim’s perspective and in terms of frequency, that put adolescents at greater risk for psychopathology.
Second, in the current study we found some indication that activity during peer rejection in regions other than the subACC, including the DMPFC, posterior cingulate cortex, and precuneus, also related to depressive symptoms both concurrently and over time. Based on prior research linking these areas with “mentalizing,” or thinking about the thoughts and perspectives of others (Frith & Frith, 1999, 2003, 2006; Mitchell et al., 2005), one possibility is that adolescents displaying greater activity in these regions are thinking more about the negative social interaction or worrying more about why they were rejected. Over time, frequent mentalizing associated with negative peer interactions could lead to chronic rumination and other depressive symptomatology.
Third, another possibility is that greater responsivity in the subACC during peer rejection reflects an inability to properly regulate emotions resulting from such negative events.2 One previously proposed mechanism for depression is corticolimbic dysregulation (Mayberg, 2007; Mayberg et al., 1997), and dysregulation of the subACC in particular has been implicated in susceptibility for depression (Pezawas et al., 2005). Moreover, the positive relationship found in the current findings and in previous findings (Masten et al., 2009) between subACC activity and adolescents’ distress following peer rejection further suggests that activity in this region is greater among individuals who are less able to regulate negative emotion. Examining the link between subcortical regions and emotion regulation in the context of adolescents’ risk for depression will be a fruitful avenue for future research.
The findings of the current study should be considered in light of several limitations, which might also help direct future studies. First, the adolescent participants did not meet clinical criteria for depression; thus, the links found between subACC responses during peer rejection and depressive symptom ratings do not necessarily reflect patterns representative of a depressed population. Given that depression onset is most common later in adolescence, we believe the findings reported here contribute to the current literature on adolescents’ risk for depression, prior to actual disorder onset. However, it will be crucial for future studies to examine depressed adolescent populations with longitudinal data that taps brain function spanning the period during which onset occurs. Data of this kind would permit examination of neural markers important for the onset of clinically significant depression. Second, the measure of depressive symptoms employed was not ideal. Although the withdrawn/depressed subscale of the CBCL is useful for measuring an array of internalizing symptoms typical of depressive disorders, future studies should use more comprehensive diagnostic tests with multiple reporters (e.g., self-reports in addition to parental reports) to measure both depressive symptomatology among typically developing populations and to confirm diagnoses in depressed populations. Third, although the goal of the present investigation was to specifically examine subACC responsivity, future studies would benefit from using other tasks that are known to engage activity in additional subcortical regions, such as those identified in previous studies to be relevant to adolescents’ social and emotional processing (e.g., amygdala, ventral striatum, orbitofrontal cortex, hypothalamus; Guyer et al., 2009; Monk et al., 2003; Nelson et al., 2005), as well as areas implicated in the current study that have been previously linked with cognitive control and mentalizing processes (i.e., DMPFC, posterior cingulate cortex, precuneus; Frith & Frith, 1999, 2003, 2006; Mitchell et al., 2005). Understanding this larger network of neural regions will be invaluable for understanding causal links between adolescents’ social experiences and the development of psychopathology.
Fourth, future research should further explore potential sex differences in neural systems underlying the development of depressive symptoms. Although the current findings provide no evidence of sex differences in the relationship between subACC activity and increases in depressive symptoms over time, the sample size for girls and particularly for boys was too small in this investigation to permit conclusive results. Given the well-established sex differences in frequency of social stressors, sensitivity to social stressors, and depression during adolescence (Hankin & Abramson, 1999; Nolen-Hoeksema, Girgus, 1994; Peterson, et al., 1993; Wagner & Compas, 1990), larger studies could focus specifically on differences between boys and girls, and take into account other maturational factors such as pubertal status and pubertal timing that might play a key role in producing sex differences in the development of depression.
Conclusion
These findings are the first to demonstrate a neural link between peer rejection and depressive symptoms during adolescence and suggest that heightened subACC responsivity may be a marker of adolescents’ risk for later depression. In addition, these findings contribute to the growing body of neuro-psychiatric research implicating the subACC as a region that may be central to our understanding of the neural substrates of depression, as well as its developmental course. Finally, this work as a whole links the fields of adolescent peer relations and clinical neuroscience, and contributes to our knowledge about how risk for depression may develop in the context of heightened peer salience during adolescence.
Acknowledgments
This work was supported by the Santa Fe Institute Consortium. Support was also provided by an Elizabeth Munsterberg Koppitz Award and a Ruth L. Kirschstein National Research Service Award (to C.M.). The authors are grateful for the generous support from the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson–Lovelace Foundation, Ahmanson Foundation, Tamkin Foundation, Jennifer Jones–Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, and Northstar Fund. This project was also partially supported by grants (RR12169, RR13642 and RR00865) from the National Center for Research Resources, a component of the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors thank Elliot Berkman for statistical assistance.
Footnotes
In addition, when whole-brain regression analyses were run separately for boys and girls (examined at a lowered threshold given the small number of participants in each group; p = .05, minimum cluster = 10 voxels), there was little indication of sex differences in the relationship between subACC activity and increases in depressive symptom scores. For both girls and boys, the subACC was related to increases in depressive symptoms: girls, [12 36 −10], t = 6.39, r = .89, p <.0001, k = 589; boys, [6 30 −10], t = 5.37, r = .92, p < .005, k = 332. Although our sample size was not large enough to permit a definitive investigation of sex differences, these analyses provide little evidence that the relationship between subACC activity and increases in depressive symptoms varies in any meaningful way across sexes.
We thank an anonymous reviewer for suggesting this possibility and contributing to the subsequent discussion of this topic.
References
- Abecassis M, Hartup WW, Haselager GJT, Scholte RHJ, Lieshout CFM. Mutual antipathies and their developmental significance. Child Development. 2002;73:1543–1556. doi: 10.1111/1467-8624.00489. [DOI] [PubMed] [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth & Families; 2001. [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. NeuroImage. 2002;16:1140–1141. [Google Scholar]
- Brody AL, Saxena S, Silverman DHS, Alborzian S, Fairbanks LA, Phelps ME, et al. Brain metabolic changes in major depressive disorder from pre- to post-treatment with paroxetine. Psychiatry Research. 1999;91:127–139. doi: 10.1016/s0925-4927(99)00034-7. [DOI] [PubMed] [Google Scholar]
- Brown BB. Peer groups and peer cultures. In: Feldman SS, Elliot GR, editors. At the threshold: The developing adolescent. Cambridge, MA: Harvard University Press; 1990. pp. 171–196. [Google Scholar]
- Carter JS, Garber J, Ciesla JA, Cole DA. Modeling relations between hassles and internalizing and externalizing symptoms in adolescents: A four-year prospective study. Journal of Abnormal Psychology. 2006;115:428–442. doi: 10.1037/0021-843X.115.3.428. [DOI] [PubMed] [Google Scholar]
- Chen CH, Ridler K, Suckling J, Williams S, Fu CH, Merlo-Pich E, Bullmore E. Brain imaging correlates of depressive symptom severity and predictors of symptom improvement after antidepressant treatment. Biological Psychiatry. 2007;62:407–414. doi: 10.1016/j.biopsych.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Coie JD, Dodge KA, Kupersmidt JB. Peer group behavior and social status. In: Asher SR, Coie JD, editors. Peer rejection in childhood. Cambridge studies in social and emotional development. New York: Cambridge University Press; 1990. pp. 17–59. [Google Scholar]
- Csikszentmihalyi M, Larson R. Being adolescent: Conflict and growth in the teenage years. New York: Basic Books; 1984. [Google Scholar]
- Davidson RJ, Irwin W, Anderle MJ, Kalin NH. The neural substrates of affective processing in depressed patients treated with venlafaxine. American Journal of Psychiatry. 2003;160:64–75. doi: 10.1176/appi.ajp.160.1.64. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- French DC, Conrad J, Turner TM. Adjustment of antisocial and nonantisocial rejected adolescents. Development and Psychopathology. 1995;7:857–874. [Google Scholar]
- Frith CD, Frith U. Interacting minds—a biological basis. Science. 1999;286:1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. How we predict what other people are going to do? Brain Research. 2006;1079:36–46. doi: 10.1016/j.brainres.2005.12.126. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society of London Series B. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Lau JY, McClure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, et al. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Archives of General Psychiatry. 2008;65:1303–1312. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, McClure-Tone EB, Shiffrin ND, Pine DS, Nelson EE. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Development. 2009;80:1000–1015. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C, Goodman-Brown T. Self-schemas and vulnerability to specific life stress in children at risk for depression. Cognitive Therapy and Research. 1990;14:215–227. [Google Scholar]
- Hankin BL, Abramson LY. Development of gender differences in depression: Description and possible explanations. Annals of Medicine. 1999;31:372–379. doi: 10.3109/07853899908998794. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY. Development of gender differences in depression: An elaborated cognitive vulnerability–transactional stress theory. Psychological Bulletin. 2001;127:773–796. doi: 10.1037/0033-2909.127.6.773. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Mermelstein R, Roesch L. Sex differences in adolescent depression: Stress exposure and reactivity models. Child Development. 2007;78:279–295. doi: 10.1111/j.1467-8624.2007.00997.x. [DOI] [PubMed] [Google Scholar]
- Juvonen J, Graham S, Schuster MA. Bullying among young adolescents: The strong, the weak, and the troubled. Pediatrics. 2003;112:1231–1237. doi: 10.1542/peds.112.6.1231. [DOI] [PubMed] [Google Scholar]
- Klerman GL, Weissman MM. Increasing rates of depression. Journal of the American Medical Association. 1989;261:2229–2235. [PubMed] [Google Scholar]
- Larson R, Ham M. Stress and “storm and stress” in early adolescence: The relationship of negative events with dysphoric affect. Developmental Psychology. 1993;29:130–140. [Google Scholar]
- Larson R, Moneta G, Richards M, Wilson S. Continuity, stability, and change in daily emotional experience across adolescence. Child Development. 2002;73:1151–1165. doi: 10.1111/1467-8624.00464. [DOI] [PubMed] [Google Scholar]
- Leadbeater BJ, Kuperminc GP, Blatt SJ, Hertzog C. A multivariate model of gender differences in adolescents’ internalizing and externalizing problems. Developmental Psychology. 1999;35:1268–1282. doi: 10.1037//0012-1649.35.5.1268. [DOI] [PubMed] [Google Scholar]
- Lev-Wiesel R, Nuttman-Shwartz O, Sternberg R. Peer rejection during adolescence: Psychological long-term effects—A brief report. Journal of Loss and Trauma. 2006;11:131–142. [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, et al. Neural correlates of social exclusion during adolescence: Understanding the distress of peer rejection. Social Cognitive Affective Neuroscience. 2009;4:143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS. Defining the neural circuitry of depression: Toward a new nosology with therapeutic implications. Biological Psychiatry. 2007;61:729–730. doi: 10.1016/j.biopsych.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, et al. Cingulate function in depression: A potential predictor of treatment response. NeuroReport. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. Journal of Cognitive Neuroscience. 2005;17:1306–1315. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, et al. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. NeuroImage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Nolan SA, Flynn C, Garber J. Prospective relations between rejection and depression in young adolescents. Journal of Personality and Social Psychology. 2003;85:745–755. doi: 10.1037/0022-3514.85.4.745. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Girgus JS. The emergence of gender differences in depression during adolescence. Psychological Bulletin. 1994;115:424–443. doi: 10.1037/0033-2909.115.3.424. [DOI] [PubMed] [Google Scholar]
- Panak WF, Garber J. Role of aggression, rejection, and attributions in the prediction of depression in children. Development and Psychopathology. 1992;4:145–165. [Google Scholar]
- Parkhurst JT, Hopmeyer A. Sociometric popularity and peer-perceived popularity: Two distinct dimensions of peer status. Journal of Early Adolescence. 1998;18:125–144. [Google Scholar]
- Peterson AC, Compas BE, Brooks-Gunn J, Stemmler M, Ey S, Grant KE. Depression in adolescence. American Psychologist. 1993;45:513–520. doi: 10.1037//0003-066x.48.2.155. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Archives of General Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Johnson JG, Brook JS. Adolescent life events as predictors of adult depression. Journal of Affective Disorders. 2002;68:49–57. doi: 10.1016/s0165-0327(00)00331-1. [DOI] [PubMed] [Google Scholar]
- Prinstein MJ, Aikins JW. Cognitive moderators of the longitudinal association between peer rejection and adolescent depressive symptoms. Journal of Abnormal Child Psychology. 2004;32:147–158. doi: 10.1023/b:jacp.0000019767.55592.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinstein MJ, Sheah CS, Guyer AE. Peer victimization, cue interpretation, and internalizing symptoms: Preliminary concurrent and longitudinal findings for children and adolescents. Journal of Clinical Child and Adolescent Psychology. 2005;34:11–24. doi: 10.1207/s15374424jccp3401_2. [DOI] [PubMed] [Google Scholar]
- Rigby K. Consequences of bullying in schools. Canadian Journal of Psychiatry. 2003;48:583–590. doi: 10.1177/070674370304800904. [DOI] [PubMed] [Google Scholar]
- Rubin KH, Bukowski WM, Parker JG. Peer interactions, relationships, and groups. In: Eisenberg N, Damon W, Lerner RM, editors. Handbook of child psychology: Vol. 3. Emotional and personality development. 6. Hoboken, NJ: Wiley; 2006. pp. 571–652. [Google Scholar]
- Rudolph KD. Gender differences in emotional responses to interpersonal stress during adolescence. Journal of Adolescent Health. 2002;30:3–13. doi: 10.1016/s1054-139x(01)00383-4. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Clark AG. Conceptions of relationships in children with depressive and aggressive symptoms: Social–cognitive distortion or reality? Journal of Abnormal Child Psychology. 2001;29:41–56. doi: 10.1023/a:1005299429060. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Conley CS. The socioemotional costs and benefits of social–evaluative concerns: Do girls care too much? Journal of Personality. 2005;73:115–138. doi: 10.1111/j.1467-6494.2004.00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Hammen C. Age and gender as determinants of stress exposure, generation, and reactions in youngsters: A transactional perspective. Child Development. 1999;70:660–677. doi: 10.1111/1467-8624.00048. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Hammen C, Burge D. Interpersonal functioning and depressive symptoms in childhood: Addressing the issues of specificity and comorbidity. Journal of Abnormal Child Psychology. 1994;22:355–371. doi: 10.1007/BF02168079. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Hammen C, Burge D, Lindberg N, Herzberg D, Daley SE. Toward an interpersonal life-stress model of depression: The developmental context of stress generation. Development and Psychopathology. 2000;12:215–234. doi: 10.1017/s0954579400002066. [DOI] [PubMed] [Google Scholar]
- Sandstrom MJ, Cillessen AHN, Eisenhower A. Children’s appraisal of peer rejection experiences: Impact on social and emotional adjustment. Social Development. 2003;12:530–550. [Google Scholar]
- Saxena S, Brody AL, Ho ML, Zohrabi N, Maidment KM, Baxter LR. Differential brain metabolic predictors of response to paroxetine in obsessive–compulsive disorder versus major depression. American Journal of Psychiatry. 2003;160:522–532. doi: 10.1176/appi.ajp.160.3.522. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A neurobehavioral perspective on adolescent risk-taking. Developmental Review. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner BM, Compas BE. Gender, instrumentality, and expressivity: Moderates of the relation between stress and psychological symptoms during adolescence. American Journal of Community Psychology. 1990;18:383–406. doi: 10.1007/BF00938114. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Klerman GL. Sex differences and the epidemiology of depression. Archives of General Psychiatry. 1977;34:98–111. doi: 10.1001/archpsyc.1977.01770130100011. [DOI] [PubMed] [Google Scholar]
- Williams KD, Cheung CK, Choi W. CyberOstracism: Effects of being ignored over the internet. Journal of Personality and Social Psychology. 2000;79:748–762. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Williams KD, Goven CL, Croker V, Tynan D, Cruickshank M, Lam A. Investigations into differences between social- and cyberostracism. Group Dynamics. 2002;6:65–77. [Google Scholar]
- Wolk SI, Weissman MM. Women and depression: An update. Review of Psychiatry. 1995;14:227–259. [Google Scholar]