Abstract
Background
Language delay is a hallmark feature of autism spectrum disorders (ASD). The identification of word boundaries in continuous speech is a critical first step in language acquisition that can be accomplished via statistical learning and reliance on speech cues. Importantly, early word segmentation skills have been shown to predict later language development in typically developing (TD) children.
Methods
Here we investigated the neural correlates of online word segmentation in children with and without ASD with a well-established behavioral paradigm previously validated for functional magnetic resonance imaging. Eighteen high-functioning boys with ASD and 18 age- and IQ-matched TD boys underwent functional magnetic resonance imaging while listening to two artificial languages (containing statistical or statistical + prosodic cues to word boundaries) and a random speech stream.
Results
Consistent with prior findings, in TD control subjects, activity in fronto-temporal-parietal networks decreased as the number of cues to word boundaries increased. The ASD children, however, did not show this facilitatory effect. Furthermore, statistical contrasts modeling changes in activity over time identified significant learning-related signal increases for both artificial languages in basal ganglia and left temporo-parietal cortex only in TD children. Finally, the level of communicative impairment in ASD children was inversely correlated with signal increases in these same regions during exposure to the artificial languages.
Conclusions
This is the first study to demonstrate significant abnormalities in the neural architecture subserving language-related learning in ASD children and to link the communicative impairments observed in this population to decreased sensitivity to the statistical and speech cues available in the language input.
Keywords: Autism, implicit learning, language, neuroimaging, speech perception
A hallmark feature of autism is a delay in language development (1–3). The absence of functional speech before age 5 years indicates a poor prognosis (3), and up to one-half of individuals with autism never acquire useful language (1). Even those individuals that acquire language typically display abnormalities in syntax (4–6), semantics (6,7), and pragmatics (8–10) (see Groen et al. [11] for review). Moreover, even high-functioning individuals with autism with verbal IQ scores well within the normal range often show a developmental history of delayed or abnormal language development. Previous investigations into the neural substrates of language processing in autism have identified abnormal structure (12–16), function (17–22), and connectivity (23,24) within language networks. However, to date, the neural substrate of online language-related learning in autism has not been investigated.
One of the first steps in language learning is the identification of word boundaries within a continuous speech stream (i.e., word segmentation). Behavioral studies in infants suggest that the developing brain computes statistical probabilities of syllable co-occurrence within speech streams to guide the identification of word boundaries (25–27). Importantly, the ability to identify words in fluent speech in infancy has been linked to higher vocabulary scores at 2 years of age and better overall language skills in preschool (28). In fact, it was found that children with Williams Syndrome, a rare genetic disorder characterized by language delay, also show severely delayed word segmentation abilities (29). Additionally, a recent study found that the learning of new object labels in 17-month-old infants was facilitated by prior exposure to a continuous speech stream containing those novel object names (30), providing further evidence for a direct connection between early word segmentation and language learning. In addition, acquisition of other language skills, such as grammar, might also rely on calculations of statistical dependencies across linguistic units (31).
We previously examined the neural correlates of word segmentation in both healthy adults and typically developing (TD) children and showed the involvement of unique frontal, temporal, and striatal networks during implicit word segmentation (32,33). The aim of the present study was to identify potential system-level differences in the neural correlates of word segmentation in children with autism spectrum disorders (ASD), and to determine whether children with ASD are able to capitalize on statistical and speech cues available in the input to identify word boundaries.
Methods and Materials
Participants
Participants were recruited through flyers posted around the University of California at Los Angeles (UCLA) campus and the greater Los Angeles area as well as through referrals from the UCLA Autism Evaluation Clinic. Twenty-four high-functioning boys with ASD (12.62 ± 2.50 years) with normal full-scale IQ (FSIQ; 102.17 + 19.82) as assessed by the Wechsler Abbreviated Scales of Intelligence—Revised (WASI-R) (34) or Wechsler Intelligence Scale for Children—3rd edition (WISC-III) (35) and 24 age- and IQ-matched TD boys (11.64 ± 1.58 years; FSIQ = 104.00 ± 12.36) were scanned in this study. Of the ASD participants, five subjects were excluded due to excess motion and one subject was excluded for falling asleep during the scan, resulting in a final group of 18 participants. Within the TD subjects, five participants were excluded due to excessive motion, resulting in a final group of 18 subjects. The final groups did not significantly differ in age, FSIQ, performance IQ or verbal IQ (TD: verbal IQ = 102.83 ± 12.92; ASD: verbal IQ = 96.11 ± 17.93). For the ASD group, prior clinical autism diagnosis was confirmed by the Autism Diagnostic Observation Scale-General (ADOS-G) (36) and/or Autism Diagnostic Interview—Revised (ADI-R) (37) (Table 1). Sixteen participants met full criteria for autism, and 2 participants met criteria for autism spectrum. Additionally, participants with ASD were given the Peabody Picture Vocabulary Test—3rd ed. (PPVT-III) (Pearson Assessments; Minneapolis, Minnesota) to measure receptive language abilities. The average PPVT standard score was within the normal range (103.47 ± 21.33). Table 1 includes a full description of the demographic information for each of the ASD participants. By report, none of the participants had any known loss of consciousness longer than 5 min or any neurological (e.g., epilepsy), genetic (e.g., Fragile X), or major psychiatric (e.g., schizophrenia) disorder other than autism. Additional exclusion criteria for the TD children included any first-degree relative with an ASD. Written informed assent and parental consent was obtained from participants and their parents, respectively, according to the specifications of the UCLA Institutional Review Board.
Table 1.
Detailed Demographic Information on Participants with an Autism Spectrum Disorder
| Subject | Age | Verbal IQ | Full Scale IQ | PPVT |
|---|---|---|---|---|
| 1 | 9.01 | 104 | 130 | — |
| 2 | 9.26 | 105 | 125 | 116 |
| 3 | 9.56 | 111 | 122 | 133 |
| 4 | 9.66 | 79 | 89 | 112 |
| 5 | 9.78 | 107 | 120 | 94 |
| 6 | 11.14 | 127 | 129 | 124 |
| 7 | 11.35 | 74 | 80 | 87 |
| 8 | 12.43 | 104 | 108 | 132 |
| 9 | 12.54 | 91 | 89 | 96 |
| 10 | 13.02 | 89 | 83 | 74 |
| 11 | 13.27 | 74 | 88 | 83 |
| 12 | 14.18 | 81 | 87 | 81 |
| 13 | 14.28 | 86 | 80 | 85 |
| 14 | 14.54 | 98 | 99 | 128 |
| 15 | 14.99 | 102 | 106 | 109 |
| 16 | 15.62 | 94 | 94 | 95 |
| 17 | 15.7 | 69 | 76 | 76 |
| 18 | 16.91 | 135 | 134 | 134 |
All patients were diagnosed with autism, except for Subjects 16 and 18, who had a diagnosis of autism spectrum disorder.
PPVT, Peabody Picture Vocabulary Test.
Experimental Stimuli and Activation Paradigm
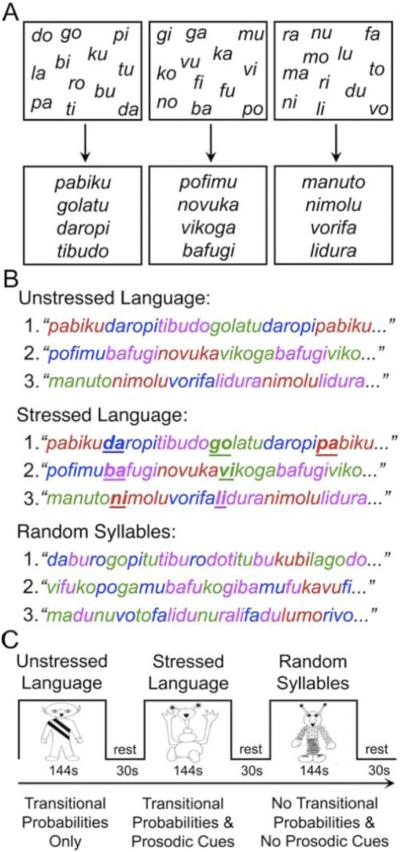
We used the exact same speech stream exposure procedure as described in McNealy et al. (32,33). Children listened to three counterbalanced streams of nonsense speech, which lasted for 144 sec each and were separated by 30 sec of rest, within one continuous block. Children were not explicitly instructed to perform a task except to listen, in light of a recent study that demonstrated that implicit learning can be attenuated by explicit memory processes during sequence learning (38). The paradigm construction is depicted in Figure 1. The artificial speech streams were created from three different sets of 12 syllables following the same procedure used in previous infant and adult behavioral studies (25–27,32,33,39). Within the speech stream, first-order transitional probabilities (i.e., the odds that one syllable will follow another in a given language) for syllables within a word and across word boundaries were 1 and .33, respectively. Thus, as the words were repeated, transitional probabilities could be computed and used to segment the speech stream. In the Unstressed Language condition (U), the stream contained only transitional probabilities as cues to word boundaries. In the Stressed Language condition (S), the speech stream contained transitional probabilities as well as prosodic cues introduced by adding stress (i.e., increasing amplitude, duration, and pitch) to the initial syllable of each word, one-third of the time it occurred. A Random Syllables condition (R) was also created to control for activity related to merely listening to a series of concatenated syllables. In this condition, the 12 syllables were arranged pseudorandomly such that no three-syllable string occurred more than twice in the stream (the frequency with which two-syllable strings occurred was also minimized). Therefore, in this condition, the statistical likelihood of any one syllable following another was very low (with an average transitional probability between syllables in the stream of .1; range .02–.22), thus affording minimal cues to word boundaries. Please note that participants heard a different set of syllables associated with each of the speech streams, ensuring that any difference between conditions could not be attributed to different degrees of familiarity with a given set of syllables.
Figure 1.
Activation paradigm. In the speech stream exposure task, three different sets of 12 syllables were used to create the three sets of four words (A). The Unstressed and Stressed languages were formed by concatenating these words to form two artificial languages, whereas the random syllables stream was formed by pseudorandomly concatenating individual syllables (B). Participants listened to each of three counterbalanced speech streams (C) containing statistical regularities (unstressed language), statistical regularities and prosodic cues (stressed language), and minimal cues to guide word segmentation (random syllables). Reprinted from McNealy et al. (32), with permission.
Behavioral Task
A post-test was given outside the scanner to investigate whether children were able to explicitly discriminate between words and part words (i.e., trisyllabic sets of syllables spanning word boundaries) from the speech streams, and behavioral measures (response times and accuracy scores) were collected. Children listened to the trisyllabic sets of syllables and responded yes or no as to whether they thought each combination could be a word in the artificial languages they had previously heard. Responses from six ASD and three TD children were not recorded due to a computer malfunction.
Functional Magnetic Resonance Imaging Data Acquisition
Functional images were collected with a Siemens Allegra 3 Tesla head-only magnetic resonance imaging scanner. For each child, a high-resolution structural T2-weighted echo-planar imaging volume (spin-echo, repetition time = 5,000 msec, echo time = 33 msec, matrix size = 128 × 128, field-of-view = 20 cm, 36 slices, 1.56-mm in-plane resolution, 3-mm thick) was acquired coplanar with the functional scans to allow for spatial registration of each child's data into a standard coordinate system. One functional scan lasting 8 min and 48 sec was acquired covering the whole cerebral volume (174 images, echo planar imaging gradient-echo, repetition time = 3,000 msec, echo time = 25 msec, flip angle = 90°, matrix size = 64 × 64, field-of-view = 20 cm, 36 slices, 3.125-mm in-plane resolution, 3-mm thick, 1-mm gap).
Children listened to the auditory stimuli through a set of magnet-compatible stereo headphones (Resonance Technology, Northridge, California). Stimuli were presented with MacStim 3.2 psychological experimentation software (Darby, WhiteAnt Occasional Publishing and CogState, 2000, Melbourne, Australia).
fMRI Data Analysis
Preprocessing of the functional magnetic resonance imaging (fMRI) data were conducted in exactly the same manner as described in McNealy et al. (32,33). Statistical analyses were implemented in SPM99 (http://www.fil.ion.ucl.ac.uk/spm/;Wellcome Department of Cognitive Neurology, London, United Kingdom). For each child, contrasts of interest were estimated according to the general linear model with a canonical hemodynamic response function. We investigated changes in neural activity as a function of exposure to the speech stream within each activation block—similar to our previous studies with neurotypical populations (32,33). Each condition (R, U, S) was modeled with the exponential decay function in SPM99 (which closely approximates a linear function) with respect to the baseline condition. Contrast images from these fixed effects analyses were then entered into second-level analyses with random effects models to allow for inferences to be made at the population level (40). Corrected cluster volumes were determined by applying Random Field Theory (full-width-at-half-maximum = 8.8, 8.5, 8.1 mm) to estimate significant cluster volumes at a corrected α= .05.
Separate one-sample t tests were implemented for each condition (U, S, and R vs. resting baseline) to identify blood-oxygenation level dependent signal increases associated with listening to each speech stream within each group. Direct contrasts between conditions were masked by the combined liberal (p < .05 uncorrected) within-group contrasts for all conditions compared with rest. To compare neural activity between children with and without ASD, two-sample t tests were run on primary contrasts of interest, including the combined U+S contrast (mean and signal increases) versus rest. There were no between-group differences in the mean amount of head motion, calculated as the average displacement across all voxels in all functional images relative to their mean position in millimeters (41) [ASD mean = .63 mm; TD mean = .54 mm; t(34) = .66, p = .50]. Correlation analysis within the ASD group to identify regions related to the Qualitative Impairments in Communication subscale of the ADI-R was conducted. This analysis was masked by the combined (ASD + TD) mask of liberal (p < .05 uncorrected) within-group contrasts for the U+S conditions vs. rest and including the basal ganglia, an a priori region of interest. Small volume correction for a 9-mm sphere was applied to clusters falling within basal ganglia nuclei. Parameter estimates for significant clusters were extracted from each participant and plotted for graphical representation. For all comparisons reported activity survived correction for multiple comparisons at the cluster level (voxel: t > 2.54, p < .01, cluster: p < .05, corrected; reported peaks are t > 3.33).
Results
Behavioral Results
Response times and accuracy on the postscan behavioral test are reported in Table S1 in Supplement 1. Children were not expected, on the basis of evidence from prior behavioral studies, to be able to explicitly identify whether these trisyllabic combinations were words in the artificial languages after such a short exposure to the streams (26,42). Participants were unable to explicitly recognize trisyllabic word combinations from the artificial languages they heard during the exposure task, consistent with previous studies with this paradigm in adults (32) and children (33). Accuracy for both groups was at chance, and there were no significant differences in reaction time between words compared with part words. There were no significant between-group differences on any of the behavioral measures.
fMRI Results
Main Effect of Language Cues
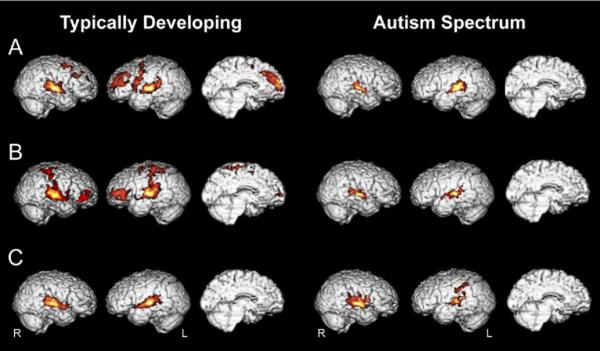
Contrasts examining main effects of each artificial language condition compared with rest revealed a pattern of increasingly more focal activity as the number of available language cues to word boundaries increased (i.e., R > U > S activity) in TD children. That is, as the number of cues increased, activity in several regions decreased. Children with ASD, however, did not show a clear decrease in cortical recruitment among the R, U, and S language conditions (Figure 2; Table S2 in Supplement 1) but rather activated a more circumscribed network including primarily bilateral superior temporal cortices for all conditions. Direct contrasts between conditions in the TD group support significantly greater activity in the R condition relative to U in left dorsolateral frontal cortices and in U relative to S in bilateral middle frontal gyrus (Table S3 in Supplement 1). Contrasts between the R and U conditions revealed significantly increased frontal activity in the ASD group such that R > U. However, no regions were significantly more active during the U condition relative to S. Between-group comparisons further support these findings and are reported in Table S4 in Supplement 1.
Figure 2.
Mean activity for each condition compared with resting baseline within groups; t > 2.57, p < .05, cluster corrected. (A) Random Syllable. (B) Unstressed language. (C) Stressed language.
Learning-Related Changes in Neural Activity as a Function of Exposure to Speech Streams
Because transitional probabilities and the identification of word boundaries are calculated online, we examined where activity might be increasing as a function of exposure to the speech streams. To do so, we modeled signal increases over the course of the block for the two language conditions (↑U and ↑S) within each group with respect to rest. The same patterns of activation were observed for both the U and S conditions separately within each group, and as such we report results collapsing across these two conditions. Similar to previous findings in typical children and adults (32,33), this contrast identified significant increases in left supramarginal gyrus (SMG), left inferior parietal lobule (IPL), and bilateral striatum in the TD group (Figure 3A, Table 2). In contrast, there were no significant signal increases observed in the ASD group for either the U or S language conditions. This difference was reliable in a between-group comparison, such that TD children showed greater signal increases than ASD children in left superior temporal gyrus, SMG, and IPL as well as in the right superior parietal lobule and right caudate (Figure 3B, Table 3). Importantly, there were no significant signal increases observed during the R condition in either group, nor were there any regions that showed greater signal increases in ASD than TD children. These results held when the increases in the two language (↑U and ↑S) conditions were compared with increases in the R condition.
Figure 3.
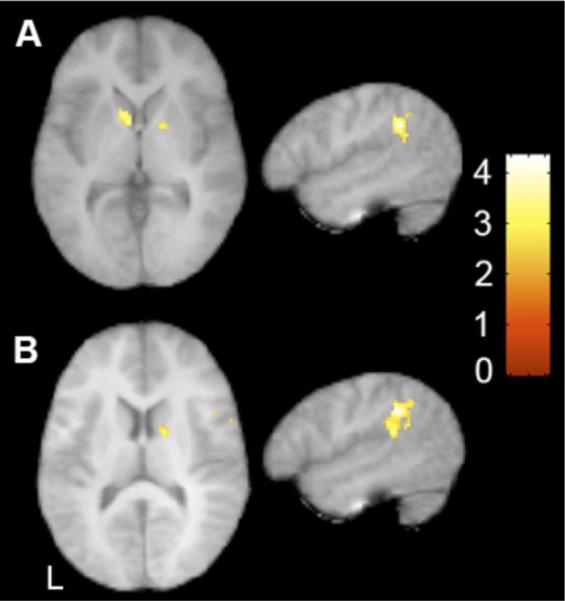
Signal increases during both artificial language conditions (↑U+↑S). (A) Significant increases in typically developing children in bilateral basal ganglia and left supramarginal gyrus (x = −52, z = 4). (B) Significant between group differences in signal increases in right caudate and left supramarginal gyrus. x = −52, z = 2, t > 2.57, p < .05 cluster corrected. L, left; U, Unstressed language; S, Stressed language.
Table 2.
Signal Increases During Language Conditions in TD Children
| TD | Anatomical Location | BA | X | y | z | t |
|---|---|---|---|---|---|---|
| ↑U+S | ||||||
| L | Supramarginal gyrus | 40 | −52 | −44 | 30 | 4.34 |
| L | Inferior parietal lobule | 40 | −54 | −50 | 38 | 3.72 |
| L | Caudate | −8 | 4 | 4 | 3.67 | |
| R | Putamen | 14 | 2 | 6 | 3.32 |
Significant signal increases as a function of language exposure in parietal and temporal cortices and striatum in typically developing (TD) children during the stressed (S) and unstressed (U) language conditions. There were no significant signal increases during the random syllables condition. Results corrected for multiple comparisons at voxel: t > 2.54, p < .01, cluster: k > 63, p < .05. Reported peaks are t > 3.33. BA refers to putative Brodmann's area; L and R refer to left and right hemispheres, respectively; x, y, and z refer to the left-right, anterior-posterior, and inferior-superior dimensions, respectively; t refers to the t score at those coordinates (local maxima or submaxima). Reported peaks are t > 3.33, results cluster corrected for multiple comparisons at t > 2.57 and p < .05.
Table 3.
Differences in Signal Increases During Language Conditions Between TD and ASD Children
| TD > ASD | Anatomical Location | BA | X | y | z | t |
|---|---|---|---|---|---|---|
| ↑U+S | ||||||
| L | Superior temporal gyrus | 22 | −50 | −38 | 18 | 3.69 |
| L | Supramarginal gyrus | 40 | −52 | −42 | 32 | 4.32 |
| L | Inferior parietal lobule | 40 | −54 | −50 | 38 | 3.86 |
| R | Superior parietal lobule | 40 | 24 | −52 | 54 | 3.66 |
| R | Caudate | 12 | 0 | 12 | 3.73 |
Significantly greater signal increases in parietal and temporal cortices and caudate in TD children than children with autism spectrum disorders (ASD). There were no significant effects in the opposite direction (>TD). Results corrected for multiple comparisons at voxel: t > 2.54, p < .01, cluster: k > 88, p < .05. Reported peaks are t > 3.33. Caudate cluster small-volume corrected (9 mm). Other abbreviations as in Table 2.
Correlation with Symptom Severity in ASD
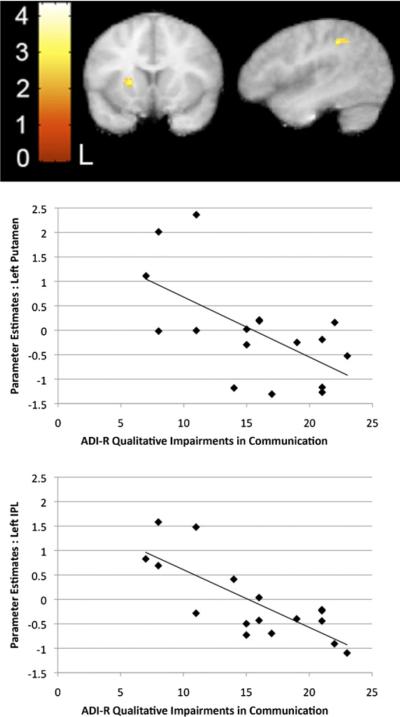
We next examined the relationship between changes as a function of exposure to the artificial languages (↑U+↑S) and impairments in communication as measured by the ADI-R subscale algorithm in all regions active during exposure to the two languages compared with rest. We found reliable negative correlations between the score on the ADI-R communication subscale and signal increases during the language conditions (↑U+↑S) in left IPL and putamen (Figure 4, Table 4), such that children with more severe communicative deficits showed smaller signal increases in these regions. Importantly, these are the same regions where activity was shown to increase significantly during exposure to the artificial languages (↑U+↑S) in TD children.
Figure 4.
Correlation with communicative impairment. Significant negative correlation in left putamen and left inferior parietal lobule (IPL) between signal increases during the two languages and total score on the qualitative impairment in communication subscale of the Autism Diagnostic Interview-Revised (ADI-R). Scatterplot displays mean parameter estimates extracted from each significant cluster as a function of ADI-R subscale score. x = −48, y = 12. Putamen cluster: t = 3.59 pSVC(9 mm) < .05. IPL cluster t = 3.71, p < .05 cluster corrected. L, left.
Table 4.
Correlation with ADI-R in ASD
| Anatomical Location | BA | X | y | z | t | |
|---|---|---|---|---|---|---|
| ↑U+↑S | ||||||
| L | Inferior parietal lobule | 40 | −36 | −32 | 42 | 4.75 |
| L | Inferior parietal lobule | 40 | −48 | −38 | 40 | 3.71 |
| L | Putamen | −22 | 12 | 8 | 3.59 |
Negative correlation between severity rating on a measure of impairments in communication and signal increases during the two language conditions in children with ASD in inferior parietal lobule and putamen. Results corrected for multiple comparisons at voxel: t > 2.54, p < .01, cluster: k > 22, p < .05, and reported peaks are t > 3.33.
ADI-R, Autism Diagnostic Interview—Revised; other abbreviations as in Table 2.
Discussion
We found evidence of disrupted language network activity in children with ASD, consistent with previous investigations of language processing in autism (17,18,20,21,23,24). Previous fMRI findings of word segmentation in healthy adults (32) revealed a consistent pattern of decreasing cortical activity within fronto-temporal-parietal networks as the number of cues to word boundaries increased from the R (minimal statistical cues) to the U (strong statistical cues) and S (strong statistical cues + prosodic cues) conditions. The TD children in this study demonstrated the same pattern of more focal activity as speech parsing cues increased, a pattern supported by direct comparisons between conditions. Specifically, we found that recruitment of dorsolateral frontal cortices, including middle frontal gyrus, decreased with increasing cues to word boundaries. These findings further support a role for the middle frontal gyrus, previously shown to be important for phonological and sequential processing (43), in language learning. In contrast, the ASD children showed more similar activation profiles across conditions, involving primarily bilateral temporal cortices, without evidence for frontal involvement. Direct comparisons between conditions in this group revealed some greater activity for the R than the U condition in right and left dorsolateral prefrontal cortices but no regions of greater activity for the U compared with the S conditions. These results suggest that ASD children might be less sensitive to the implicit cues that guide word segmentation during language acquisition, particularly when both statistical and prosodic cues (e.g., stress) are present. This is noteworthy because infants as young as 8 months of age have been shown to weight prosodic cues more heavily than statistical regularities to identify word boundaries (39,44).
As in our prior studies with neurotypical populations (32,33), we further characterized the neural correlates of online word segmentation by examining changes in activity that occurred as a function of exposure to the speech streams (i.e., as transitional probabilities are computed as stimuli repeat over time). The TD children showed increased recruitment of regions subserving statistical learning (i.e., basal ganglia) (33) and language processing (i.e., left SMG) (see Gitelman et al. [45] for review) while listening to the speech streams containing high transitional probabilities and prosodic cues, but no significant signal increases while listening to the speech stream containing minimal cues to word boundaries. In contrast, children with ASD did not demonstrate significant signal increases over time for any speech stream. A between-group comparison indicated that the difference for the two artificial language conditions was reliable between groups, further suggesting that the ASD brain might fail to capitalize on two important cues to word boundaries (i.e., transitional probabilities and prosodic cues).
Next, we investigated whether this failure to rely on statistical and speech cues to guide word segmentation might be related to the linguistic and communicative impairments that are hallmark features of autism. To address this question we examined the relationship between communicative impairments in our sample of children with ASD (as indexed by the ADI-R communication subscale) and signal increases during exposure to the two artificial languages (↑U+↑S). We found a significant negative correlation between a participant's communicative deficits and signal increases in the left putamen and IPL during exposure to the artificial language streams, such that children with less impairment showed greater signal increases in these areas. Importantly, the regions where activity was found to correlate with the degree of communicative impairments are the same as those showing significant learning-related increases during the artificial language conditions in TD children. The ADI-R communication subscale taps into atypical development of communicative skills and captures delayed language abilities. The negative correlation observed between this reliable index of atypical development and signal increases in regions shown to be involved in word segmentation supports the hypothesis that involvement of these structures during word segmentation is related to the development of language and communicative skills. Behavioral research has shown that the ability to correctly segment words from continuous speech is predictive of future language development (28). Our results further indicate a critical role of word segmentation abilities for normative language development while also implicating a corticostriatal network as the neural substrate of this fundamental process.
We hypothesized, on the basis of the persistent impairments in higher-order syntactic abilities in individuals with ASD (2) and the demonstrated link between statistical learning and grammar acquisition (46), that differences in the neural substrate of statistical learning might underlie the abnormal development of language in children with ASD, a population characterized by language impairment. Our findings indicate significant abnormalities in the neural architecture subserving language-related learning in ASD children and relate communicative impairments observed in this population to decreased sensitivity to the statistical and speech cues available in the language input. To elucidate the underlying causes of these abnormalities, further studies are warranted to determine whether longer exposures to similar speech streams would result in significant behavioral learning and to characterize the functioning of this corticostriatal network in ASD. An additional limitation of the current study is that our ASD and TD participants were matched on verbal IQ. Although this controls a potential confound in the interpretation of our findings, future studies should include individuals with autism who continue to demonstrate language impairments. Furthermore, greater characterization of the linguistic abilities of individuals with ASD (i.e., central auditory processing) and their relation to word segmentation abilities would be valuable in future studies. Interestingly, genetic investigations of language ability in ASD have identified a variant that is associated with the disorder (47,48) and is expressed in fronto-temporal and striatal regions of the developing brain (49). Thus, it might be particularly informative to examine the role that this genetic variant plays in establishing aberrant patterns of corticostriatal connectivity in ASD as well as in other developmental disorders characterized by marked linguistic impairments, such as Williams syndrome and specific language impairment.
Supplementary Material
Acknowledgments
This work was supported in part by grants from the National Institute of Child Health and Human Development (PO1 HD035470), the National Alliance for Autism Research, Autism Speaks, a National Science Foundation predoctoral fellowship to KM; the Foundation for Psychocultural Research Center for Culture, Brain and Development; as well as the Training Program in Neurobehavioral Genetics (T32 MH073526) and an National Research Service Award predoctoral fellowship to AS (F31 MH079645). The project described was also supported in part by grants (RR12169, RR13642, and RR00865) from the National Center for Research Resources, a component of the National Institutes of Health (NIH); its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Research or NIH.
For generous support, we also wish to thank the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, Ahmanson Foundation, William M. And Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family, and North-star Fund.
Footnotes
All authors reported no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online.
References
- 1.Bailey A, Phillips W, Rutter M. Autism: Towards an integration of clinical, genetic, neuropsychological, and neurobiological perspectives. J Child Psychol Psychiatry. 1996;37:89–126. doi: 10.1111/j.1469-7610.1996.tb01381.x. [DOI] [PubMed] [Google Scholar]
- 2.Tager-Flusberg H, Calkins S, Nolin T, Baumberger T, Anderson M, Chadwick-Dias A. A longitudinal study of language acquisition in autistic and Down syndrome children. J Autism Dev Disord. 1990;20:1–21. doi: 10.1007/BF02206853. [DOI] [PubMed] [Google Scholar]
- 3.Venter A, Lord C, Schopler E. A follow-up study of high-functioning autistic children. J Child Psychol Psychiatry. 1992;33:489–507. doi: 10.1111/j.1469-7610.1992.tb00887.x. [DOI] [PubMed] [Google Scholar]
- 4.Bartolucci G, Albers RJ. Deictic categories in the language of autistic children. J Autism Child Schizophr. 1974;4:131–141. doi: 10.1007/BF02105366. [DOI] [PubMed] [Google Scholar]
- 5.Bartolucci G, Pierce SJ, Streiner D. Cross-sectional studies of grammatical morphemes in autistic and mentally retarded children. J Autism Dev Disord. 1980;10:39–50. doi: 10.1007/BF02408431. [DOI] [PubMed] [Google Scholar]
- 6.Kjelgaard MM, Tager-Flusberg H. An investigation of language impairment in autism: Implications for genetic Subgroups. Lang Cogn Process. 2001;16:287–308. doi: 10.1080/01690960042000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howlin P. Outcome in high-functioning adults with autism with and without early language delays: Implications for the differentiation between autism and Asperger syndrome. J Autism Dev Disord. 2003;33:3–13. doi: 10.1023/a:1022270118899. [DOI] [PubMed] [Google Scholar]
- 8.Happe FG. Communicative competence and theory of mind in autism: A test of relevance theory. Cognition. 1993;48:101–119. doi: 10.1016/0010-0277(93)90026-r. [DOI] [PubMed] [Google Scholar]
- 9.Martin I, McDonald S. An exploration of causes of non-literal language problems in individuals with Asperger Syndrome. J Autism Dev Disord. 2004;34:311–328. doi: 10.1023/b:jadd.0000029553.52889.15. [DOI] [PubMed] [Google Scholar]
- 10.Rutherford MD, Baron-Cohen S, Wheelwright S. Reading the mindin the voice: A study with normal adults and adults with Asperger syndrome and high functioning autism. J Autism Dev Disord. 2002;32:189–194. doi: 10.1023/a:1015497629971. [DOI] [PubMed] [Google Scholar]
- 11.Groen WB, Zwiers MP, van der Gaag RJ, Buitelaar JK. The pheno-type and neural correlates of language in autism: An integrative review. Neurosci Biobehav Rev. 2008;32:1416–1425. doi: 10.1016/j.neubiorev.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Bigler ED, Mortensen S, Neeley ES, Ozonoff S, Krasny L, Johnson M, et al. Superior temporal gyrus, language function, and autism. Dev Neuropsychol. 2007;31:217–238. doi: 10.1080/87565640701190841. [DOI] [PubMed] [Google Scholar]
- 13.De Fossé L, Hodge SM, Makris N, Kennedy DN, Caviness VS, Jr, McGrath L, et al. Language-association cortex asymmetry in autism and specific language impairment. Ann Neurol. 2004;56:757–766. doi: 10.1002/ana.20275. [DOI] [PubMed] [Google Scholar]
- 14.Herbert MR, Harris GJ, Adrien KT, Ziegler DA, Makris N, Kennedy DN, et al. Abnormal asymmetry in language association cortex in autism. Ann Neurol. 2002;52:588–596. doi: 10.1002/ana.10349. [DOI] [PubMed] [Google Scholar]
- 15.Herbert MR, Ziegler DA, Deutsch CK, O'Brien LM, Kennedy DN, Filipek PA, et al. Brain asymmetries in autism and developmental language disorder: A nested whole-brain analysis. Brain. 2005;128:213–226. doi: 10.1093/brain/awh330. [DOI] [PubMed] [Google Scholar]
- 16.Rojas DC, Bawn SD, Benkers TL, Reite ML, Rogers SJ. Smaller left hemisphere Planum temporale in adults with autistic disorder. Neurosci Lett. 2002;328:237–240. doi: 10.1016/s0304-3940(02)00521-9. [DOI] [PubMed] [Google Scholar]
- 17.Gaffrey MS, Kleinhans NM, Haist F, Akshoomoff N, Campbell A, Courchesne E, Müller RA. Atypical [corrected] participation of visual cortex during word processing in autism: An fMRI study of semantic decision. Neuropsychologia. 2007;45:1672–1684. doi: 10.1016/j.neuropsychologia.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris GJ, Chabris CF, Clark J, Urban T, Aharon I, Steele S, et al. Brain activation during semantic processing in autism spectrum disorders via functional magnetic resonance imaging. Brain Cogn. 2006;61:54–68. doi: 10.1016/j.bandc.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 19.Redcay E, Courchesne E. Deviant functional magnetic resonance imaging patterns of brain activity to speech in 2–3-year-old children with autism spectrum disorder. Biol Psychiatry. 2008;64:589–598. doi: 10.1016/j.biopsych.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang AT, Lee SS, Sigman M, Dapretto M. Neural basis of irony comprehension in children with autism: The role of prosody and context. Brain. 2006;129:932–943. doi: 10.1093/brain/awl032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang AT, Lee SS, Sigman M, Dapretto M. Reading affect in the face and voice: Neural correlates of interpreting communicative intent in children and adolescents with autism spectrum disorders. Arch Gen Psychiatry. 2007;64:698–708. doi: 10.1001/archpsyc.64.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tesink CM, Buitelaar JK, Petersson KM, van der Gaag RJ, Kan CC, Tendolkar I, et al. Neural correlates of pragmatic language comprehension in autism spectrum disorders. Brain. 2009;132:1941–1952. doi: 10.1093/brain/awp103. [DOI] [PubMed] [Google Scholar]
- 23.Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: Evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- 24.Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: Thinking in pictures with decreased functional connectivity. Brain. 2006;129:2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aslin RN, Saffran JR, Newport EL. Computation of conditional probability Statistics by 8-month-old infants. Psych Sci. 1998;9:321–324. [Google Scholar]
- 26.Saffran JR, Aslin RN, Newport EL. Statistical learning by 8-month-old infants. Science. 1996;274:1926–1928. doi: 10.1126/science.274.5294.1926. [DOI] [PubMed] [Google Scholar]
- 27.Thiessen ED, Saffran JR. When cues collide: Use of stress and statistical cues to word boundaries by 7 to 9-month-old infants. Dev Psychol. 2003;39:706–716. doi: 10.1037/0012-1649.39.4.706. [DOI] [PubMed] [Google Scholar]
- 28.Newman R, Ratner NB, Jusczyk AM, Jusczyk PW, Dow KA. Infants' early ability to segment the conversational speech signal predicts later language development: A retrospective analysis. Dev Psychol. 2006;42:643–655. doi: 10.1037/0012-1649.42.4.643. [DOI] [PubMed] [Google Scholar]
- 29.Nazzi T, Paterson S, Karmiloff-Smith A. Early word Segmentation by infants and toddlers with Williams Syndrome. Infancy. 2003;4:251–271. [Google Scholar]
- 30.Graf Estes K, Evans JL, Alibali MW, Saffran JR. Can infants map meaning to newly segmented words? Statistical segmentation and word learning. Psychol Sci. 2007;18:254–260. doi: 10.1111/j.1467-9280.2007.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomez RL, Gerken L. Artificial grammar learning by 1-year-olds leads to specific and abstract knowledge. Cognition. 1999;70:109. doi: 10.1016/s0010-0277(99)00003-7. [DOI] [PubMed] [Google Scholar]
- 32.McNealy K, Mazziotta JC, Dapretto M. Cracking the language code: Neural mechanisms underlying speech parsing. J Neurosci. 2006;26:7629–7639. doi: 10.1523/JNEUROSCI.5501-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNealy K, Mazziotta JC, Dapretto M. The neural basis of speech parsing in children and adults. Dev Sci. 2010;13:385–406. doi: 10.1111/j.1467-7687.2009.00895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wechsler D. Wechsler Abbreviated Scale of Intelligence. Psychological Corporation; San Antonio, Texas: 1999. [Google Scholar]
- 35.Wechsler D. Wechsler Intelligence Scale for Children. 3rd ed. Psychological Corporation; San Antonio, Texas: 1991. [Google Scholar]
- 36.Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- 37.Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 38.Fletcher PC, Zafiris O, Frith CD, Honey RA, Corlett PR, Zilles K, Fink GR. On the benefits of not trying: Brain activity and connectivity reflecting the interactions of explicit and implicit sequence learning. Cereb Cortex. 2005;15:1002–1015. doi: 10.1093/cercor/bhh201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson EK, Jusczyk PW. Word segmentation by 8-month-olds: When speech cues count more than statistics. J Mem Lang. 2001;44:548. [Google Scholar]
- 40.Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ. Multisubject fMRI studies and conjunction analyses. NeuroImage. 1999;10:385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- 41.Woods RP. Characterizing volume and surface deformations in an atlas framework: Theory, applications, and implementation. NeuroImage. 2003;18:769–788. doi: 10.1016/s1053-8119(03)00019-3. [DOI] [PubMed] [Google Scholar]
- 42.Sanders LD, Newport EL, Neville HJ. Segmenting nonsense: An event-related potential index of perceived onsets in continuous speech. Nat Neurosci. 2002;5:700–703. doi: 10.1038/nn873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gelfand JR, Bookheimer SY. Dissociating neural mechanisms of temporal sequencing and processing phonemes. Neuron. 2003;38:831–842. doi: 10.1016/s0896-6273(03)00285-x. [DOI] [PubMed] [Google Scholar]
- 44.Johnson EK, Seidl AH. At 11 months, prosody still outranks statistics. Dev Sci. 2009;12:131–141. doi: 10.1111/j.1467-7687.2008.00740.x. [DOI] [PubMed] [Google Scholar]
- 45.Gitelman DR, Nobre AC, Sonty S, Parrish TB, Mesulam MM. Language network specializations: An analysis with parallel task designs and functional magnetic resonance imaging. NeuroImage. 2005;26:975–985. doi: 10.1016/j.neuroimage.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 46.Gomez RL, Gerken L. Infant artificial language learning and language acquisition. Trends Cogn Sci. 2000;4:178–186. doi: 10.1016/s1364-6613(00)01467-4. [DOI] [PubMed] [Google Scholar]
- 47.Alarcón M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, et al. Linkage, Association and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am J Hum Genet. 2008;82:150–159. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alarcon M, Cantor RM, Liu J, Gilliam TC, Geschwind DH. Evidence for a language quantitative trait locus on chromosome 7q in multiplex autism families. Mol Psychiatry. 2002;70:60–71. doi: 10.1086/338241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abrahams BS, Tentler D, Perederiy JV, Oldham MC, Coppola G, Geschwind DH. Genome-wide analyses of human perisylvian cerebral cortical patterning. Proc Natl Acad Sci U S A. 2007;104:17849–17854. doi: 10.1073/pnas.0706128104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.