Abstract
FDA-approved treatment for metastatic melanoma, including interferon alpha and interleukin-2, offer a modest benefit. Immunotherapy, although it has not enjoyed high overall response rates, is capable of providing durable responses in a subset of patients. In recent years, new molecular targeted therapies have become available and offer promise of clinical benefit, though low durability of response. It is not yet clear how best to integrate these two novel modalities that target the immune response to melanoma (immune therapy) or that target molecular signaling pathways in the melanoma cells (targeted therapy). Many signal transduction pathways are important in both tumor cell and T cell proliferation and survival, which generates risk in combining targeted therapy and immunotherapy. This review will focus on the role of targeted therapy and immunotherapy in melanoma, and discusses how to combine the two modalities rationally for increased duration and response.
Keywords: melanoma, immunotherapy, signal transduction
Metastatic melanoma typically has a poor prognosis, with a median survival of 7.5 months after diagnosis of distant metastatic disease, and it has a poor response to traditional chemotherapy(1). There are two FDA-approved immunotherapy options for advanced melanoma: Interferon alpha (IFN-α2β) and interleukin-2 (IL-2). IFN- α2β is approved for use in the adjuvant setting for resected stage IIB-III disease and reduces recurrence rates by about 10%(2). It may also effect clinical regression in 10–20% of patients and has been associated with clinical regressions in about half of patients when treating in a neoadjuvant setting(2–3). For treatment of advanced melanoma, high dose IL-2 has a response rate of 10–20%, and has the ability to provide durable responses in 5–7% of patients(4). In recent years, new molecular targeted therapies have become available and offer promise of clinical benefit. Tumor survival mechanisms, angiogenesis, growth, and proliferation are popular targets of directed therapies. Early experience with a specific inhibitor of mutant B-Raf was associated with clinical responses in over half of patients, albeit transiently. Key roles for targeted therapies include induction of tumor apoptosis, suppression of lymphocyte apoptosis, and reversal of tumor microenvironment immunosuppression. It is not yet clear how best to integrate these two novel modalities that target the immune response to melanoma (immune therapy) or that target molecular signaling pathways in the melanoma cells (targeted therapy). This review will focus on the role of targeted therapy and immunotherapy in melanoma, and how to combine the two modalities rationally for increased duration and response. Particular attention will be paid to immune effects of small-molecule targeted therapy and how these may be incorporated into successful therapy. Combining immunotherapy, with its durable response, and targeted therapies, with their potential high response rates, may produce a highly successful and durable approach to treating cancers such as melanoma. Often-cited issues impeding success in immunotherapy include T cell death, immune evasion by tumor cells, and immunosuppression driven by the tumor microenvironment(5). Some of these are potentially addressed with the addition of targeted therapies. The use of targeted agents for tumor immunosensitization, in combination with immunotherapy for tumor recognition and killing, is a promising strategy already employed in a number of cancer types, both in vitro and in clinical studies. Many of these pathways are important in T cell proliferation and survival, which creates a risk in combining targeted therapies with immunotherapies. The ideal agent will specifically target a key oncogenic signaling protein, and affect tumor cells without impacting normal tissue. These targeted therapies should avoid or minimize toxicity to immune cells, and allow T cells and NK cells to interact with tumor for optimal tumor surveillance and killing.
Immunotherapy includes stimulation of the immune system using vaccines, cytokines, antibodies, or T cells. The desired effect is stimulation of an anti-tumor response, decreasing tumor suppressor mechanisms, or alteration of the tumor itself to make it susceptible to the immune system. Immunotherapy, particularly active immunotherapy with vaccines, has the capability of creating endogenous responses with low toxicity and the potential for durable memory. Immunotherapy, although it has not enjoyed high overall response rates, is capable of providing durable responses in a subset of patients(6). Potentially higher response rates have been reported with adoptive T cell transfer therapy and systemic lymphodepletion, which has produced an objective response in 49–72%, albeit of carefully selected patients(7). Overall anti-tumor response rates for vaccine therapies are 2.6–3.3%, while overall immune response rates range from 50–100%(8–10). Other immunotherapies discussed in this review, Anti-CTLA4 antibody therapy and IL-2, have response rates of 13% and 10–20%, respectively(4, 11).
Targeted therapy aims to inhibit tumor growth and metastasis by blocking commonly overexpressed signal transduction molecules. These molecules comprise signaling pathways that cause proliferation and migration of all cells, but are often overexpressed in cancer cells. One of the most important of these pathways is RAF/RAS/MEK, involved in cell proliferation and resistance to apoptosis(12). Other key signaling pathways overexpressed in melanoma include PTEN, PI3K/Akt/mTOR and STAT3. Therapies targeting each of these pathways have been developed with varying degrees of success. An example is PLX-4032, with a 71% response rate in properly selected patients (56). However, an issue for PLX-4032 as well as other targeted therapies is a low duration of response and a high relapse rate.
Immunologic Therapies
Anti-CTLA-4 Antibody
Many tumors secrete cytokines and other factors that suppress T cells, allowing immune escape of tumors. Cytotoxic T lymphocyte associated protein 4 (CTLA-4) is a molecule that limits proliferation of T cells and is active in most melanomas. Strategies to increase T cell activation through blockade of this molecule with a monoclonal antibody have shown anticancer immune response in preclinical studies and in patients(13). Often, the responses to this therapy are durable, even lasting years(14). Treatment with anti-CTLA4 antibody ipilimumab had an overall response rate of 5.8%, and tremelimumab had an overall response rate of 6.6–10% in recent phase II trials(15–17).
Therapy blocking CTLA-4 increases tumor immunity in previously vaccinated patients(18), which created interest in combining CTLA-4 blockade with vaccine therapy. Combining a dendritic cell vaccine with anti-CTLA-4 antibody produced a 25% objective response rate, which was better than the success of either agent alone(19). A study combining anti CTLA-4 antibody and multipeptide vaccine demonstrated tumor infiltrating lymphocytes (TIL) on some biopsies, and development of antigen-specific immune response in a subset of patients(20). Interestingly, anti-CTLA-4 treatment increases T cell immune responses to NYESO1 protein(21), and this correlates with presence of durable clinical response(22). It follows that use of anti-CTLA-4 therapy in combination with vaccination using NYESO1 peptides might augment specific immune responses and increase the number of durable responders to this therapy. A study of anti-CTLA-4 plus autologous NYESO1 expressing CD8 cells is underway in melanoma (NCT00871481).
CTLA-4 molecules are frequently expressed on regulatory T cells (Tregs)(23), and an anticipated effect of anti-CTLA-4 antibody therapy was to decrease Treg-driven immunosuppression. However, current data suggests that this is not a significant effect of CTLA-4 antibody therapy. Evaluation of tumor post CTLA-4 antibody treatment reveals an increase in CD8+ CTL infiltrate, but no definite change in Treg populations near tumor(19).
Toll-like receptor (TLR) agonists
TLR agonists are commonly used cancer vaccine adjuvants to augment local immune responses(24). They activate and direct dendritic cells (DC) to the tumor microenvironment, stimulate production of Th1 cytokines such as IL-12 and IFN-γ, and also augment tumor-specific cytotoxic lymphocyte responses(25, 26). This has led to decreased tumor progression and prolonged survival in preclinical studies(25). TLR7 agonist imiquimod is the only FDA-approved TLR agonist, used topically for treatment of actinic keratoses, basal cell carcinomas, and warts. Imiquimod has also shown promise in topical treatment of melanoma of the skin(27). TLR9 agonists have demonstrated anti-melanoma activity in preclinical studies and are being evaluated in clinical trials(28).
The local immunostimulatory effect of TLR therapy is being investigated in combination with other immunotherapies. Topical TLR7 agonist imiquimod in combination with intralesional IL-2 increased CD25+ cells in the CD4+ population in tumors, and shifted cytokine secretion to a Th1 bias, accompanied by decreased in transit metastasis of melanoma(29). TLR agonists are also being used in combination with several types of vaccine in clinical trials (NCT00118313, NCT00304057, NCT00142454, NCT00960752, NCT00112229, and NCT00304057).
Recent studies have shown that some TLR agonists, such as TLR9 agonist CpG, upregulate STAT3 and thus decrease immunostimulatory effects(30). Other studies have discovered that TLR agonists increase Tregs and IL-10 secretion, signaled through the p38MAPK pathway. However, TLR8 activation has been linked to reduction of T regs(31). In vitro, blockade of the p38MAPK pathway decreased Treg induction and IL-10 production by DCs(32). The combination of MAPK inhibitors and TLR agonists may be able to decrease the local immunosuppression in the tumor microenvironment.
Signal transduction therapeutics with immune effects
Other tumor functions that can be exploited with targeted therapy include apoptosis, angiogenesis, growth, and proliferation. Although many types of immunotherapy produce a systemic response, measurable in blood and serum, local immunosuppressive factors stimulated by the tumor itself can result in local failure to mount an immune response. Key components of this local immunosuppression include tolerogenic DCs, CD4+CD25(hi)FoxP3+ Tregs, and myeloid-derived suppressor cells (MDSC).
Sunitinib
Several targeted therapies have effects on the tumor microenvironment and Tregs. Sunitinib is a multikinase inhibitor with targets including platelet-derived growth factor (PDGF), c-Kit, RET, VEGFR2, and Flt3. It is currently approved for use in renal cell cancer (RCC) and imatinib-resistant GIST. In RCC, sunitinib has had success as a single agent, with some studies reporting a disease control rate of 50%(33). Along with its blockade of multiple tyrosine kinases, sunitinib has relevant immune effects. In renal cell cancer (RCC) patients, sunitinib reverses type I immune suppression(34). The Th2 bias common in RCC patients was reversible with sunitinib therapy, with a majority of patients experiencing an increase in IFN-γ producing cells and decrease in IL-4 producing T cells(34). This was not correlative with progression-free survival however. Increased numbers of Tregs near the tumor has been associated with poor prognosis(35–37). Patients with RCC have increased levels of CD3+CD4+CD25(hi)FoxP3+ Tregs in the tumor itself, and decreased Tregs in the peripheral blood(34). Sunitinib treatment decreases the percentage of CD4+CD25(hi)FoxP3+ Treg cells in peripheral blood, and may also reduce the function of the remaining Tregs(34). Sunitinib treated mice have improved infiltration of tumor by CD8+ T cells(38).
MDSCs, which infiltrate the tumor and interfere with anti-tumor responses by T cells, are another method for tumors to evade the innate immune system(39). It is hypothesized that sunitinib may modulate antitumor immunity by reversing MDSC-mediated tumor immunosuppression(40). VEGFR signaling has been implicated in MDSC generation(41), and sunitinib’s anti-VEGFR2 activity may be the reason that MDSC populations decrease in RCC patients treated with sunitinib(40).The depletion of MDSC after sunitinib correlated with improved T cell function. C-Kit has also been implicated in MDSC and Treg accumulation(38), and sunitinib’s ability to block c-Kit activation may contribute to its effects on both cell populations.
Sunitinib’s ability to affect T cell function and to counteract tumor-induced immunosuppression provides evidence that targeted tyrosine kinase inhibitors (TKI) have dual signaling and immune effects. Thus, sunitinib and other TKIs may enhance efficacy of immunotherapies. Sunitinib is being used alone and in combination with chemotherapy in multiple clinical trials in melanoma and other solid tumors. Combinations of sunitinib and more traditional immunotherapy are underway in RCC as well as urothelial carcinoma (NCT00890110, NCT00678119, and NCT00794950).
Signal transducer and activator of transcription 3 (STAT3)
STAT3 is a key mediator of melanoma-induced immunosuppression, and is overexpressed in most melanomas(35, 42). STAT3 activation inhibits DC maturation and impairs T cell response to tumor(43, 44). Ablating STAT3 increases DC maturation, T cell activation, generation of tumor antigen specific T cells, and long-lasting anti-tumor immunity(30). STAT3 is a key component for generation of Treg populations, and STAT3 ablation reduces the number of tumor-infiltrating Tregs(30). STAT3 inhibitor WP1066 has been used in vitro to inhibit Treg induction in melanoma(42). Interferon effects are mediated through the JAK/STAT pathway, and IFN treatment is associated with decreased phosphorylated STAT3 and increased phosphorylated STAT1 in atypical nevi of patients undergoing IFN(45). However, STAT3 phosphorylation is increased in 17% of melanoma patients treated with IFN, and its expression correlates negatively with survival (46). Although this only assumes an association between STAT3 activation and interferon, it suggests that combining IFN therapy with STAT3 inhibition may be beneficial in a subset of patients.
Some TLRs, such as TLR9, upregulate STAT3 signaling upon stimulation with an agonist. TLRs have beneficial roles as vaccine adjuvants to augment local immune response(24). Their immunostimulatory properties activate and direct DC to the tumor microenvironment and stimulate production of immunostimulatory cytokines(25). To combat the increase in Treg infiltration seen after TLR agonist therapy, blockade of STAT3 in combination with TLR agonists may improve local immunostimulation. In mice, combining STAT3 inhibition with activation of TLR4 increased tumor-infiltrating lymphocytes (TIL) and decreased tumor burden when compared to either treatment alone(35). Thus, priming the tumor microenvironment by blocking STAT3 might augment immunotherapy. There are two clinical trials evaluating blockade of STAT3 in solid tumors(NCT00696176, NCT00955812), but none evaluating it in combination with immunotherapy.
Traditional signal transduction pathways
MAPK pathways: RAS/RAF/MEK and p38MAPK
Overactivation in the Mitogen activated protein kinase (MAPK) pathway is common in many tumor types including melanoma(47). NRAS and BRAF mutations occur in 20% and 60% of melanomas, respectively(48), and melanomas have been accurately subtyped using mutation status at these two genes(49,50). Sorafenib is a multikinase inhibitor that targets B-Raf, C-raf, VEGF, and PDGF. No trials have demonstrated single agent effectiveness in melanoma, and sorafenib has had promising anti-tumor effects when combined with chemotherapy that did not persist in randomized trials(51–53). Its disappointing effects thus far may be due to its nonselectivity for B-Raf mutants in preclinical studies(52). Also of concern for sorafenib is its ability to diminish activation of both T cells and DC in vitro and ex vivo(54,55). A much more selective B-Raf inhibitor, PLX-4032, has greater single agent activity with tumor regression rates of 71% in patients containing the B-RafV600E mutation(56). Because B-RafV600E is the most common oncogenic mutation in melanoma, the success of PLX-4032 has garnered a great deal of interest. However, the failure of PLX-4032 to produce complete responses, coupled with the high relapse rate, indicates that combinations with chemotherapy or immunotherapy are appealing options to improve its efficacy. PLX-4032 is being studied as a single agent and in combination with chemotherapy in clinical trials (NCT 01006980, NCT00949702, and NCT00405587).
MAPK pathway overactivation has been linked to immunosuppressive effects, such as induction of IL-10 production in DC(57). Blockade of MEK signaling is able to suppress IL-10 production and enhance IL-12 production(58,59). This Th1 shift allows DC to activate T cells properly, and this activation persists even in the presence of immunosuppressive melanoma cell lysate(57,58). Activation of p38MAPK has also been associated with a Th2 phenotype and suppression of DC activation(32,57). Blockade of p38MAPK suppresses IL-10, increases IL-12 production by DC and increases IFN-γ secretion by T cells(32). p38MAPK overexpression is also linked to increased Foxp3+ Treg infiltration in tumors, which is reversible by p38MAPK inhibition(32). Thus, blockade of MAPK in combination with immunotherapy may allow DC to become resistant to the immunosuppressive functions of tumors in vivo and thus gain more effective anti-tumor activity. Also, the increase in Tregs seen after TLR agonist therapy can be counteracted by addition of MAPK inhibition(32). Although there are no clinical trials evaluating MAPK inhibition in combination with vaccine therapy, there is preclinical evidence to suggest that combining these modalities will be beneficial.
Loss of tumor-associated antigens is a common evasion mechanism for melanoma. Activating mutations in the MAPK pathway, most notably B-RafV600E, reduce antigen levels in melanomas in vitro. Inhibition of the MAPK pathway is able to counteract this decrease in antigen expression of melanoma cells(60, 61). MEK inhibitor U0126 is able to induce increased antigen expression and cause apoptosis of melanoma cells. The melanoma cells not killed by this inhibitor are those with high antigen expression, and thus, the combination of MAPK inhibitors and immunotherapy may be useful to augment tumor antigen expression while targeting the critical survival MAPK pathway.
PI3K/Akt/mTOR
The phosphoinositide 3 kinase (PI3K) pathway is a critical survival pathway, and is activated in many cancer types including melanoma. This pathway is activated in 30% of melanoma cell lines and 5–10% of melanoma tissue specimens, typically through a loss of phosphatase and tensin (PTEN)(62, 63). Inhibitors of Akt and PI3K are being investigated in phase I studies, and Inhibitors of mammalian target of rapamycin (mTOR) have been increasingly used as chemoimmunotherapy in phase II and III trials in melanoma and other cancers. Several mTOR inhibitors are under clinical investigation in cancer treatment, including rapamycin, sirolimus, temsirolimus/CCI-779, and everolimus/RAD001.
Inhibition of the PI3K/Akt/mTOR pathway has a considerable effect on immune regulation. Both DC and Tregs are sensitive to Akt-mTOR signaling. Preclinical studies have demonstrated that Akt-mTOR signaling is essential for DC survival and maturation(64). In a mouse model, presence of Akt allowed sufficient DC maturation for eradication of lymphoma. Although mTOR is required for DC activation and maturation during acute responses, continued mTOR stimulation enhances IL-10 production and can limit T cell activity(23). Conversely, mTOR inhibition causes IL-12 production and inhibits IL-10 production(65, 66). From these studies, it appears that blocking mTOR selectively while avoiding Akt blockade would be most advantageous to DC function.
Rapamycin has well-known immunosuppressive effects, and may selectively deplete or increase Tregs in vitro(67, 68). Treating T cells with rapamycin during stimulation and expansion in the presence of IL-2 creates a population of lymphocytes with a high percentage of Tregs(69). A contrasting study found apoptosis of Tregs after treatment with sirolimus. This effect was reversed in the presence of IL-2, perhaps accounting for the contrasting results seen in other studies(70). Additionally, activated Akt impedes development of Tregs in vitro(71). For optimal downregulation of Tregs, Akt inhibition appears appropriate. The use of mTOR inhibitors in suppressing Tregs has been mixed, and further evaluation of mTOR inhibitors is forthcoming. Importantly, this finding emphasizes the fact that combination therapies must be rationally designed to optimize both tumor killing and anti-cancer immunity. Rapamycin is able to stimulate and enhance survival of memory CD8+ T cells in mice, through upregulation of phenotypes associated with protective immunity (CD127high, CD62Lhigh, Bcl2high)(72). Thus mTOR inhibition to increase function of memory T cells may be used to enhance vaccine therapy in melanoma.
Treatment with mTOR inhibitors may sensitize cancers to subsequent immunotherapy, and thus may be an important way to combine signal transduction therapy and immunotherapy(73). It is important to evaluate immune function as part of clinical trials of small molecule inhibitors, both to avoid excessive immune toxicity and to determine the appropriateness of combining immune therapy with small molecule targeted therapy. mTOR inhibitors have been investigated in combination with tyrosine kinase inhibitors(67,74,75). The vascular endothelial growth factor (VEGF) antibody bevacizumab in combination with rapamycin analog CCI-779 is being investigated in multiple tumor types including melanoma(NCT00397982)(76).
VEGF
Angiogenesis is critical for the growth of tumors. Tumor cells upregulate VEGF expression by both tumor cells and stromal cells. Melanoma tumors tend to be vascular, and thus anti-angiogenic agents are promising(77). Bevacizumab is a monoclonal antibody to VEGF that is FDA approved for use in RCC, lung and colorectal cancer, and has been effective in clinical trials in melanoma(78,79). A number of other agents targeting VEGF receptors, sunitinib, vendetanib, and AG013736, are currently in use in clinical trials. Some of these, such as sunitinib, have immune effects discussed above as well as signaling effects.
Additionally, VEGF is an inhibitor of immune function in the tumor microenvironment. In vitro, a Th2 bias in melanoma cell lines has been linked to the presence of VEGF(80). Melanoma cells have upregulated VEGF transcription, and tumor bearing patients have higher levels of circulating VEGF as well as an increase in Th2 cytokines(80). Additionally, overexpression of VEGF correlates with a high number of Tregs in the tumor environment(81). VEGF impairs DC activation, and anti-VEGF therapy increases peripheral DC in vivo(82). One way to counteract the inability to generate DC in the setting of cancer is using anti-VEGF and anti-VEGFR-2 therapy in combination with traditional immunotherapies. Both the ligand VEGF and its common receptor VEGFR-2 have been targets of anti-melanoma therapy. Immunotherapy has been used in combination with the ligand VEGF in preclinical studies. Mouse studies combining VEGF blockade (with a recombinant adeno-associated virus vector expressing VEGF) with GM-CSF secreting tumor immunotherapy had prolonged overall survival, with the added benefit of increasing TILs and decreasing Tregs in the tumor microenvironment as compared to untreated mice(81). Using anti-VEGF antibodies with antibodies to melanoma antigen TYRP-1/gp75 decreased local tumor growth and distant metastasis of murine melanoma tumors more than each therapy alone(83).
Targeting the VEGF receptor has had success in mouse models as well. Use of a DC vaccine modified with VEGFR-2 in mice led to induction of CTL activity against VEGFR-2 positive melanomas(84). Combining a DNA vaccine with pDNAs encoding angiostatin or endostatin increased tumor free survival in mice(85). Another vaccine targeting VEGFR-2 and other anti-angiogenic targets was able to suppress angiogenesis in the tumor microenvironment as well as decrease in tumor growth and angiogenesis(86). Immunization with DC transfected with mRNA for VEGFR-2 led to partial inhibition of angiogenesis and decreased tumor growth in mice(87).
There are two clinical investigations of VEGF therapy coupled with traditional immunotherapy, both in RCC. One trial is evaluating anti-VEGF antibody bevacizumab in combination with an autologous DC vaccine and IL-2(NCT00913913). The second is evaluating bevacizumab in combination with IL-2(NCT00301990).
Apoptosis pathways
Tumor cells have upregulated pathways to induce anti-apoptotic effects of tumor cells within the tumor microenvironment. Targeting these pathways to shift the balance toward a pro-apoptotic bias may assist in effective tumor killing by immune cells(88). Anti-apoptotic proteins Bcl-2, Bcl-XL, and XIAP are overexpressed in melanoma(89). TNF related apoptosis inducing ligand (TRAIL) signaling is often impaired in melanoma.
Bcl-2 is the most commonly targeted apoptotic protein, and several therapies are under preclinical and early clinical evaluation. Curcumin, a molecule that has shown anti-cancer activity in vitro, induced apoptosis in melanoma cells in vitro via reduction of Bcl-2 phosphorylation. Curcumin can also affect responsiveness of immune effector cells to antitumor cytokines, which may increase tumor microenvironment immunosuppression by melanoma cells(90). Thus for curcumin to be a useful targeted therapy in a proapoptotic sense, it may need to be combined with IL-12 or interferon gamma to increase local immune effector cells. Oblimersen sodium and AT-101 are additional Bcl-2 targeted therapies that have shown promise preclinically and are in clinical studies in melanoma (NCT00518895, NCT00988169).
TRAIL signaling is the predominant mechanism for cancer cell killing via apoptosis(12), but is impaired in melanomas. Use of resveratrol to upregulate TRAIL expression can result in enhanced melanoma cell apoptosis(91). Alternatively, interferon beta can induce TRAIL expression and lead to apoptosis in vitro(92). Interferon alpha also induces apoptosis in melanoma. While the use of pro-apoptotic therapy both alone and in combination with chemotherapy has shown some success, in vitro studies have demonstrated a potential role for pro-apoptotic therapy in combination with immune therapies such as vaccine. Mouse studies combining IFN-alpha with tumor cell vaccines led to a synergistic effect on tumor growth and in fact led to an 80% rejection of established tumors. The use of IFN-alpha at the vaccine site allowed priming of the vaccine and optimized a pro-apoptotic environment that led to efficient immune-initiated tumor killing(93). Also in mice, a DC vaccine in combination with Bcl-2 inhibitor ABT-737 enhances antitumor activity in colon cancer, but not in melanoma(94). A single mouse melanoma line, B16, was evaluated, and study of other melanoma cell lines including human xenografts should be evaluated as well. Interferon gamma also has antiapoptotic effects, and when combined with an antibody against anti-apoptotic molecule Fas, creates synergistic anti-proliferative effects against melanoma cells in vitro and in a mouse model(95). This combination also had increased tumor apoptosis in a mouse model as compared to tumors treated with either agent alone(95).
While it is important to activate apoptotic pathways in melanoma cells, it is equally important to avoid apoptosis in T cells that are pivotal in development of immune responses to tumor, both innate and in response to therapy. Transfection of tumor reactive T cells with Bcl-2 provides a survival benefit and improves T-cell resistance to cell death. In mice, this has led to enhanced rates of successful adoptive immunotherapy(96). The decision whether to block Bcl-2 for anti-tumor benefit or upregulate Bcl-2 for lymphocyte benefit is a difficult one. Directing Bcl-2 blockade specifically to tumor cells while avoiding its effect on lymphocytes is ideal.
Conclusion
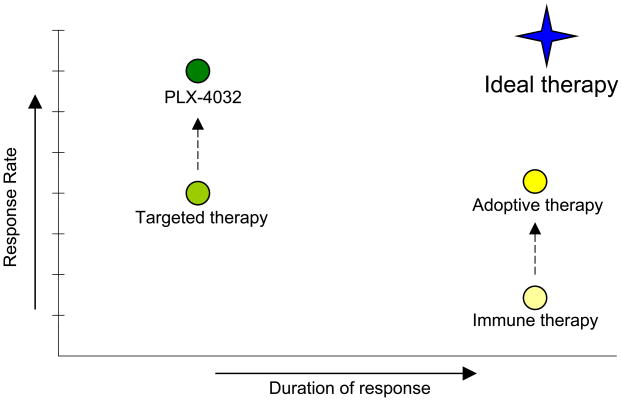
Melanoma is a difficult disease to treat effectively, as it uses multiple mechanisms for survival and signals through many pathways. Targeted therapies have shown clinical promise, but are limited by high relapse rates. Immunotherapy techniques have had much lower clinical response rates, but the success they do have tends to be more durable. Both targeted therapies and immunotherapy continue to be studied extensively individually and in combination with chemotherapy. However, preclinical and clinical evidence exists that combining targeted therapy with immunotherapy will be more successful than either type of therapy alone in halting the growth and metastasis of melanoma (Figure 1). It has been well established that the immune system plays a significant role in the development and progression of cancers such as melanoma. The ability of immunotherapy such as anti-CTLA4 antibody to generate long lasting responses is significant. The ability of targeted therapy such as PLX-4032 to generate a clinical response in a majority of patients is also significant. Combining the two therapy types in a rational way may be able to garner the most important characteristics of the two types. Additionally, targeted therapies with immune effects, like sunitinib, have immune effects and are being studied in combination with other immunotherapies. Because targeted small molecule inhibitors affect molecules and pathways that are common to both tumor cells and immune cells, knowledge of the immune effects of signal transduction pathways is important to develop rational combinations of targeted therapy and immune therapy.
Figure 1.
Schematic of average duration and response for typical immunotherapy and signal transduction targeted therapy. Also pictured are recent improvements in each field (PLX-4032 as a targeted B-Raf inhibitor and adoptive immunotherapy). The ideal treatment combination would capitalize off of the individual strengths of each treatment type to produce a durable anti-tumor therapy with a high response rate.
Acknowledgments
Grant Support: NCI grant R01 CA57653 to Dr. Craig L. Slingluff, Jr., University of Virginia Cancer Center Support Grant (NIH/NCI P30 CA44579), the Commonwealth Foundation for Cancer Research, and the James and Rebecca Craig Foundation. Kerrington R. Molhoek, Ph.D. was supported by the American Cancer Society, California Division Campaign for Research 2007 Postdoctoral Fellowship. Amber L. Shada, M.D. was supported by the UVa Cancer Center through the Farrow Fellowship Fund and the NCI Cancer Center Support Grant, P30CA44579.
Footnotes
Conflict of Interest: The authors declare no conflicts.
References
- 1.Lachiewicz AM, Berwick M, Wiggins CL, et al. Survival differences between patients with scalp or neck melanoma and those with melanoma of other sites in the Surveillance, Epidemiology, and End Results (SEER) program. Arch Dermatol. 2008;144:515–521. doi: 10.1001/archderm.144.4.515. [DOI] [PubMed] [Google Scholar]
- 2.Tarhini AA, Agarwala SS. Cutaneous melanoma: available therapy for metastatic disease. Dermatol Ther. 2006;19:19–25. doi: 10.1111/j.1529-8019.2005.00052.x. [DOI] [PubMed] [Google Scholar]
- 3.Moschos SJ, Edington HD, Land SR, et al. Neoadjuvant treatment of regional stage IIIB melanoma with high-dose interferon alfa-2b induces objective tumor regression in association with modulation of tumor infiltrating host cellular immune responses. J Clin Oncol. 2006;24:3164–3171. doi: 10.1200/JCO.2005.05.2498. [DOI] [PubMed] [Google Scholar]
- 4.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 5.Jandus C, Speiser D, Romero P. Recent advances and hurdles in melanoma immunotherapy. Pigment Cell Melanoma Res. 2009;22:711–723. doi: 10.1111/j.1755-148X.2009.00634.x. [DOI] [PubMed] [Google Scholar]
- 6.Slingluff CLJ, Speiser DE. Progress and controversies in developing cancer vaccines. J Transl Med. 2005;3:18. doi: 10.1186/1479-5876-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slingluff CLJ, Chianese-Bullock KA, Bullock TN, et al. Immunity to melanoma antigens: from self-tolerance to immunotherapy. Adv Immunol. 2006;90:243–295. doi: 10.1016/S0065-2776(06)90007-8. [DOI] [PubMed] [Google Scholar]
- 10.Riker AI, Jove R, Daud AI. Immunotherapy as part of a multidisciplinary approach to melanoma treatment. Front Biosci. 2006;11:1–14. doi: 10.2741/1775. [DOI] [PubMed] [Google Scholar]
- 11.Ku GY, Yuan J, Page DB, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116:1767–1775. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hersey P, Zhang XD. Treatment combinations targeting apoptosis to improve immunotherapy of melanoma. Cancer Immunol Immunother. 2009;58:1749–1759. doi: 10.1007/s00262-009-0732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber JS. Tumor evasion may occur via expression of regulatory molecules: a case for CTLA-4 in melanoma. J Invest Dermatol. 2008;128:2750–2752. doi: 10.1038/jid.2008.341. [DOI] [PubMed] [Google Scholar]
- 14.Ribas A. Anti-CTLA4 Antibody Clinical Trials in Melanoma. Update Cancer Ther. 2007;2:133–139. doi: 10.1016/j.uct.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Day SJ, Maio M, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010 doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 16.Camacho LH, Antonia S, Sosman J, et al. Phase I/II trial of tremelimumab in patients with metastatic melanoma. J Clin Oncol. 2009;27:1075–1081. doi: 10.1200/JCO.2008.19.2435. [DOI] [PubMed] [Google Scholar]
- 17.Kirkwood JM, Lorigan P, Hersey P, et al. Phase II trial of tremelimumab (CP-675,206) in patients with advanced refractory or relapsed melanoma. Clin Cancer Res. 2010;16:1042–1048. doi: 10.1158/1078-0432.CCR-09-2033. [DOI] [PubMed] [Google Scholar]
- 18.Hodi FS, Mihm MC, Soiffer RJ, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribas A, Comin-Anduix B, Chmielowski B, et al. Dendritic cell vaccination combined with CTLA4 blockade in patients with metastatic melanoma. Clin Cancer Res. 2009;15:6267–6276. doi: 10.1158/1078-0432.CCR-09-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanderson K, Scotland R, Lee P, et al. Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma. J Clin Oncol. 2005;23:741–750. doi: 10.1200/JCO.2005.01.128. [DOI] [PubMed] [Google Scholar]
- 21.Fong L, Kwek SS, O'Brien S, et al. Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res. 2009;69:609–615. doi: 10.1158/0008-5472.CAN-08-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan J, Gnjatic S, Li H, et al. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc Natl Acad Sci U S A. 2008;105:20410–20415. doi: 10.1073/pnas.0810114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delgoffe GM, Powell JD. mTOR: taking cues from the immune microenvironment. Immunology. 2009;127:459–465. doi: 10.1111/j.1365-2567.2009.03125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams S, O'Neill DW, Nonaka D, et al. Immunization of malignant melanoma patients with full-length NY-ESO-1 protein using TLR7 agonist imiquimod as vaccine adjuvant. J Immunol. 2008;181:776–784. doi: 10.4049/jimmunol.181.1.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garay RP, Viens P, Bauer J, et al. Cancer relapse under chemotherapy: why TLR2/4 receptor agonists can help. Eur J Pharmacol. 2007;563:1–17. doi: 10.1016/j.ejphar.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 26.Ito T, Amakawa R, Kaisho T, et al. Interferon-alpha and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. J Exp Med. 2002;195:1507–1512. doi: 10.1084/jem.20020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turza K, Dengel LT, Harris RC, et al. Effectiveness of imiquimod limited to dermal melanoma metastases, with simultaneous resistance of subcutaneous metastasis. J Cutan Pathol. 2009 doi: 10.1111/j.1600-0560.2009.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lonsdorf AS, Kuekrek H, Stern BV, et al. Intratumor CpG-oligodeoxynucleotide injection induces protective antitumor T cell immunity. J Immunol. 2003;171:3941–3946. doi: 10.4049/jimmunol.171.8.3941. [DOI] [PubMed] [Google Scholar]
- 29.Green DS, Dalgleish AG, Belonwu N, et al. Topical imiquimod and intralesional interleukin-2 increase activated lymphocytes and restore the Th1/Th2 balance in patients with metastatic melanoma. Br J Dermatol. 2008;159:606–614. doi: 10.1111/j.1365-2133.2008.08709.x. [DOI] [PubMed] [Google Scholar]
- 30.Kortylewski M, Kujawski M, Herrmann A, et al. Toll-like receptor 9 activation of signal transducer and activator of transcription 3 constrains its agonist-based immunotherapy. Cancer Res. 2009;69:2497–2505. doi: 10.1158/0008-5472.CAN-08-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang RF, Miyahara Y, Wang HY. Toll-like receptors and immune regulation: implications for cancer therapy. Oncogene. 2008;27:181–189. doi: 10.1038/sj.onc.1210906. [DOI] [PubMed] [Google Scholar]
- 32.Jarnicki AG, Conroy H, Brereton C, et al. Attenuating regulatory T cell induction by TLR agonists through inhibition of p38 MAPK signaling in dendritic cells enhances their efficacy as vaccine adjuvants and cancer immunotherapeutics. J Immunol. 2008;180:3797–3806. doi: 10.4049/jimmunol.180.6.3797. [DOI] [PubMed] [Google Scholar]
- 33.Machiels JP, Henry S, Zanetta S, et al. Phase II study of sunitinib in recurrent or metastatic squamous cell carcinoma of the head and neck: GORTEC 2006–01. J Clin Oncol. 2010;28:21–28. doi: 10.1200/JCO.2009.23.8584. [DOI] [PubMed] [Google Scholar]
- 34.Finke JH, Rini B, Ireland J, et al. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res. 2008;14:6674–6682. doi: 10.1158/1078-0432.CCR-07-5212. [DOI] [PubMed] [Google Scholar]
- 35.Molavi O, Ma Z, Hamdy S, et al. Immunomodulatory and Anticancer Effects of Intra-Tumoral Co-Delivery of Synthetic Lipid A Adjuvant and STAT3 Inhibitor, JSI-124. Immunopharmacol Immunotoxicol. 2008:1–14. doi: 10.1080/08923970802380452. [DOI] [PubMed] [Google Scholar]
- 36.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 37.Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 38.Ozao-Choy J, Ma G, Kao J, et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69:2514–2522. doi: 10.1158/0008-5472.CAN-08-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagaraj S, Schrum AG, Cho HI, et al. Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J Immunol. 2010;184:3106–3116. doi: 10.4049/jimmunol.0902661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ko JS, Zea AH, Rini BI, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 41.Gabrilovich D, Ishida T, Oyama T, et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150–4166. [PubMed] [Google Scholar]
- 42.Kong LY, Wei J, Sharma AK, et al. A novel phosphorylated STAT3 inhibitor enhances T cell cytotoxicity against melanoma through inhibition of regulatory T cells. Cancer Immunol Immunother. 2009;58:1023–1032. doi: 10.1007/s00262-008-0618-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 44.Wang T, Niu G, Kortylewski M, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 45.Wang W, Edington HD, Rao UN, et al. STAT3 as a biomarker of progression in atypical nevi of patients with melanoma: dose-response effects of systemic IFNalpha therapy. J Invest Dermatol. 2008;128:1997–2002. doi: 10.1038/jid.2008.26. [DOI] [PubMed] [Google Scholar]
- 46.Humpolikova-Adamkova L, Kovarik J, Dusek L, et al. Interferon-alpha treatment may negatively influence disease progression in melanoma patients by hyperactivation of STAT3 protein. Eur J Cancer. 2009;45:1315–1323. doi: 10.1016/j.ejca.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 47.Fecher LA, Amaravadi RK, Flaherty KT. The MAPK pathway in melanoma. Curr Opin Oncol. 2008;20:183–189. doi: 10.1097/CCO.0b013e3282f5271c. [DOI] [PubMed] [Google Scholar]
- 48.Haluska F, Pemberton T, Ibrahim N, et al. The RTK/RAS/BRAF/PI3K pathways in melanoma: biology, small molecule inhibitors, and potential applications. Semin Oncol. 2007;34:546–554. doi: 10.1053/j.seminoncol.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 49.Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 50.Viros A, Fridlyand J, Bauer J, et al. Improving melanoma classification by integrating genetic and morphologic features. PLoS Med. 2008;5:e120. doi: 10.1371/journal.pmed.0050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flaherty K. Phase I study of PLX4032: Proof of concept for V600E BRAF mutation as a therapeutic target in human cancer. ASCO. 2009 [Google Scholar]
- 52.Eisen T, Ahmad T, Flaherty KT, et al. Sorafenib in advanced melanoma: a Phase II randomised discontinuation trial analysis. Br J Cancer. 2006;95:581–586. doi: 10.1038/sj.bjc.6603291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amaravadi RK, Schuchter LM, McDermott DF, et al. Phase II Trial of Temozolomide and Sorafenib in Advanced Melanoma Patients with or without Brain Metastases. Clin Cancer Res. 2009;15:7711–7718. doi: 10.1158/1078-0432.CCR-09-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Houben R, Voigt H, Noelke C, et al. MAPK-independent impairment of T-cell responses by the multikinase inhibitor sorafenib. Mol Cancer Ther. 2009;8:433–440. doi: 10.1158/1535-7163.MCT-08-1051. [DOI] [PubMed] [Google Scholar]
- 55.Hipp MM, Hilf N, Walter S, et al. Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood. 2008;111:5610–5620. doi: 10.1182/blood-2007-02-075945. [DOI] [PubMed] [Google Scholar]
- 56.Flaherty K. BRAF Validation in Melanoma. Clin Adv Hematol Oncol. 2010;8:31–34. [PubMed] [Google Scholar]
- 57.Zhao F, Falk C, Osen W, et al. Activation of p38 mitogen-activated protein kinase drives dendritic cells to become tolerogenic in ret transgenic mice spontaneously developing melanoma. Clin Cancer Res. 2009;15:4382–4390. doi: 10.1158/1078-0432.CCR-09-0399. [DOI] [PubMed] [Google Scholar]
- 58.Jackson AM, Mulcahy LA, Zhu XW, et al. Tumour-mediated disruption of dendritic cell function: inhibiting the MEK1/2-p44/42 axis restores IL-12 production and Th1-generation. Int J Cancer. 2008;123:623–632. doi: 10.1002/ijc.23530. [DOI] [PubMed] [Google Scholar]
- 59.Sumimoto H, Imabayashi F, Iwata T, et al. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med. 2006;203:1651–1656. doi: 10.1084/jem.20051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dunn IS, Haggerty TJ, Kono M, et al. Enhancement of human melanoma antigen expression by IFN-beta. J Immunol. 2007;179:2134–2142. doi: 10.4049/jimmunol.179.4.2134. [DOI] [PubMed] [Google Scholar]
- 61.Kono M, Dunn IS, Durda PJ, et al. Role of the mitogen-activated protein kinase signaling pathway in the regulation of human melanocytic antigen expression. Mol Cancer Res. 2006;4:779–792. doi: 10.1158/1541-7786.MCR-06-0077. [DOI] [PubMed] [Google Scholar]
- 62.Tsao H, Zhang X, Fowlkes K, et al. Relative reciprocity of NRAS and PTEN/MMAC1 alterations in cutaneous melanoma cell lines. Cancer Res. 2000;60:1800–1804. [PubMed] [Google Scholar]
- 63.Hersey P, Bastholt L, Chiarion-Sileni V, et al. Small molecules and targeted therapies in distant metastatic disease. Ann Oncol. 2009;20(Suppl 6):vi35–40. doi: 10.1093/annonc/mdp254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park D, Lapteva N, Seethammagari M, et al. An essential role for Akt1 in dendritic cell function and tumor immunotherapy. Nat Biotechnol. 2006;24:1581–1590. doi: 10.1038/nbt1262. [DOI] [PubMed] [Google Scholar]
- 65.Ohtani M, Nagai S, Kondo S, et al. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood. 2008;112:635–643. doi: 10.1182/blood-2008-02-137430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weichhart T, Costantino G, Poglitsch M, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 67.Molhoek KR, Brautigan DL, Slingluff CLJ. Synergistic inhibition of human melanoma proliferation by combination treatment with B-Raf inhibitor BAY43-9006 and mTOR inhibitor Rapamycin. J Transl Med. 2005;3:39. doi: 10.1186/1479-5876-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Strauss L, Czystowska M, Szajnik M, et al. Differential responses of human regulatory T cells (Treg) and effector T cells to rapamycin. PLoS One. 2009;4:e5994. doi: 10.1371/journal.pone.0005994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Battaglia M, Stabilini A, Migliavacca B, et al. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177:8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 70.Molhoek KR, McSkimming CC, Olson WC, et al. Apoptosis of CD4(+)CD25(high) T cells in response to Sirolimus requires activation of T cell receptor and is modulated by IL- 2. Cancer Immunol Immunother. 2009;58:867–876. doi: 10.1007/s00262-008-0602-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu G, Burns S, Huang G, et al. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat Immunol. 2009;10:769–777. doi: 10.1038/ni.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Araki K, Turner AP, Shaffer VO, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hahnel PS, Thaler S, Antunes E, et al. Targeting AKT signaling sensitizes cancer to cellular immunotherapy. Cancer Res. 2008;68:3899–3906. doi: 10.1158/0008-5472.CAN-07-6286. [DOI] [PubMed] [Google Scholar]
- 74.Lasithiotakis KG, Sinnberg TW, Schittek B, et al. Combined inhibition of MAPK and mTOR signaling inhibits growth, induces cell death, and abrogates invasive growth of melanoma cells. J Invest Dermatol. 2008;128:2013–2023. doi: 10.1038/jid.2008.44. [DOI] [PubMed] [Google Scholar]
- 75.Molhoek KR, Griesemann H, Shu J, et al. Human melanoma cytolysis by combined inhibition of mammalian target of rapamycin and vascular endothelial growth factor/vascular endothelial growth factor receptor-2. Cancer Res. 2008;68:4392–4397. doi: 10.1158/0008-5472.CAN-07-5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Merchan JR, Liu G, Fitch T, et al. Phase I/II trial of CCI-779 and bevacizumab in stage IV renal cell carcinoma: Phase I safety and activity results. J Clin Oncol, 2007 ASCO Annual Meeting Proceedings. 2007;25:5034. [Google Scholar]
- 77.Velazquez OC, Herlyn M. The vascular phenotype of melanoma metastasis. Clin Exp Metastasis. 2003;20:229–235. doi: 10.1023/a:1022987201264. [DOI] [PubMed] [Google Scholar]
- 78.Varker KA, Biber JE, Kefauver C, et al. A randomized phase 2 trial of bevacizumab with or without daily low-dose interferon alfa-2b in metastatic malignant melanoma. Ann Surg Oncol. 2007;14:2367–2376. doi: 10.1245/s10434-007-9389-5. [DOI] [PubMed] [Google Scholar]
- 79.Flaherty KT, Puzanov I. Building on a foundation of VEGF and mTOR targeted agents in renal cell carcinoma. Biochem Pharmacol. 2010 doi: 10.1016/j.bcp.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 80.Nevala WK, Vachon CM, Leontovich AA, et al. Evidence of systemic Th2-driven chronic inflammation in patients with metastatic melanoma. Clin Cancer Res. 2009;15:1931–1939. doi: 10.1158/1078-0432.CCR-08-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li B, Lalani AS, Harding TC, et al. Vascular endothelial growth factor blockade reduces intratumoral regulatory T cells and enhances the efficacy of a GM-CSF-secreting cancer immunotherapy. Clin Cancer Res. 2006;12:6808–6816. doi: 10.1158/1078-0432.CCR-06-1558. [DOI] [PubMed] [Google Scholar]
- 82.Osada T, Chong G, Tansik R, et al. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol Immunother. 2008;57:1115–1124. doi: 10.1007/s00262-007-0441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patel D, Bassi R, Hooper AT, et al. Enhanced suppression of melanoma tumor growth and metastasis by combined therapy with anti-VEGF receptor and anti-TYRP-1/gp75 monoclonal antibodies. Anticancer Res. 2008;28:2679–2686. [PubMed] [Google Scholar]
- 84.Pan JP, Weng YS, Wu QQ. Anti-metastatic effect of vascular endothelial growth factor receptor 2 extracellular domain gene-modified dendritic cell vaccination in murine model with experimental pulmonary metastasis. Zhonghua Zhong Liu Za Zhi. 2006;28:646–649. [PubMed] [Google Scholar]
- 85.Chan RC, Gutierrez B, Ichim TE, et al. Enhancement of DNA cancer vaccine efficacy by combination with anti-angiogenesis in regression of established subcutaneous B16 melanoma. Oncol Rep. 2009;22:1197–1203. doi: 10.3892/or_00000555. [DOI] [PubMed] [Google Scholar]
- 86.Xiang R, Luo Y, Niethammer AG, et al. Oral DNA vaccines target the tumor vasculature and microenvironment and suppress tumor growth and metastasis. Immunol Rev. 2008;222:117–128. doi: 10.1111/j.1600-065X.2008.00613.x. [DOI] [PubMed] [Google Scholar]
- 87.Nair S, Boczkowski D, Moeller B, et al. Synergy between tumor immunotherapy and antiangiogenic therapy. Blood. 2003;102:964–971. doi: 10.1182/blood-2002-12-3738. [DOI] [PubMed] [Google Scholar]
- 88.Hersey P. Apoptosis and melanoma: how new insights are effecting the development of new therapies for melanoma. Curr Opin Oncol. 2006;18:189–196. doi: 10.1097/01.cco.0000208794.24228.9f. [DOI] [PubMed] [Google Scholar]
- 89.Bush JA, Li G. The role of Bcl-2 family members in the progression of cutaneous melanoma. Clin Exp Metastasis. 2003;20:531–539. doi: 10.1023/a:1025874502181. [DOI] [PubMed] [Google Scholar]
- 90.Bill MA, Bakan C, Benson DMJ, et al. Curcumin induces proapoptotic effects against human melanoma cells and modulates the cellular response to immunotherapeutic cytokines. Mol Cancer Ther. 2009;8:2726–2735. doi: 10.1158/1535-7163.MCT-09-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Johnson GE, Ivanov VN, Hei TK. Radiosensitization of melanoma cells through combined inhibition of protein regulators of cell survival. Apoptosis. 2008;13:790–802. doi: 10.1007/s10495-008-0212-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chawla-Sarkar M, Leaman DW, Jacobs BS, et al. IFN-beta pretreatment sensitizes human melanoma cells to TRAIL/Apo2 ligand-induced apoptosis. J Immunol. 2002;169:847–855. doi: 10.4049/jimmunol.169.2.847. [DOI] [PubMed] [Google Scholar]
- 93.Prell RA, Li B, Lin JM, et al. Administration of IFN-alpha enhances the efficacy of a granulocyte macrophage colony stimulating factor-secreting tumor cell vaccine. Cancer Res. 2005;65:2449–2456. doi: 10.1158/0008-5472.CAN-04-1975. [DOI] [PubMed] [Google Scholar]
- 94.Begley J, Vo DD, Morris LF, et al. Immunosensitization with a Bcl-2 small molecule inhibitor. Cancer Immunol Immunother. 2009;58:699–708. doi: 10.1007/s00262-008-0592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kamei T, Inui M, Nakase M, et al. Experimental therapy using interferon-gamma and anti-Fas antibody against oral malignant melanoma cells. Melanoma Res. 2005;15:393–400. doi: 10.1097/00008390-200510000-00007. [DOI] [PubMed] [Google Scholar]
- 96.Charo J, Finkelstein SE, Grewal N, et al. Bcl-2 overexpression enhances tumor-specific T-cell survival. Cancer Res. 2005;65:2001–2008. doi: 10.1158/0008-5472.CAN-04-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]