Abstract
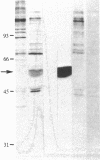
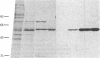
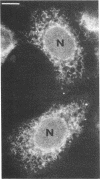
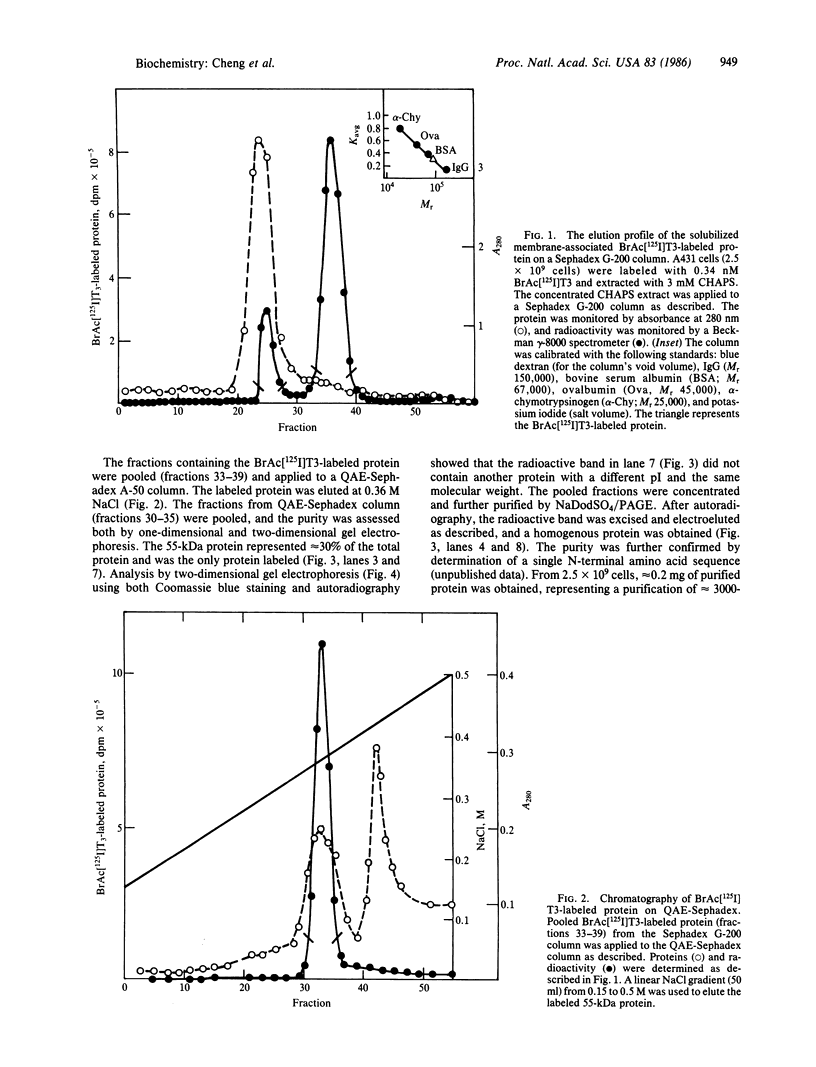
A membrane-associated binding protein for 3,3',5-triiodo-L-thyronine (T3) was purified to apparent homogeneity from A431 human epidermoid carcinoma cells. A431 cells were specifically labeled with the N-bromoacetyl derivative of T3 labeled with 125I at the 3' position (BrAc[125I]T3) and were extracted with 3-[3-(cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), a zwitterionic detergent. The solubilized BrAc[125I]T3-labeled protein was successively purified by chromatography on Sephadex G-200 and QAE-Sephadex followed by NaDod-SO4/PAGE. Approximately 0.2 mg of purified protein was obtained from 2.5 X 10(9) cells, which represents a 3000-fold purification. The membrane-associated T3 binding protein is an acidic protein with a pI of 5.1 and an apparent molecular mass of 55,000 daltons determined by NaDodSO4/PAGE. Polyclonal antibodies against the 55-kDa protein were prepared and used in indirect immunofluorescence to show that the 55-kDa protein was mainly found in the nuclear envelope and endoplasmic reticulum.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderson R., Pastan I., Cheng S. Characterization of the 3,3',5-triiodo-L-thyronine-binding site on plasma membranes from human placenta. Endocrinology. 1985 Jun;116(6):2621–2630. doi: 10.1210/endo-116-6-2621. [DOI] [PubMed] [Google Scholar]
- Botta J. A., de Mendoza D., Morero R. D., Farías R. N. High affinity L-triiodothyronine binding sites on washed rat erythrocyte membranes. J Biol Chem. 1983 Jun 10;258(11):6690–6692. [PubMed] [Google Scholar]
- Cheng S. Y. Characterization of binding and uptake of 3,3',5-triido-L-thyronine in cultured mouse fibroblasts. Endocrinology. 1983 May;112(5):1754–1762. doi: 10.1210/endo-112-5-1754. [DOI] [PubMed] [Google Scholar]
- Cheng S. Y. Structural similarities in the plasma membrane 3,3',5-triiodo-L-thyronine receptors from human, rat and mouse cultured cells. Analysis by affinity labeling. Endocrinology. 1983 Sep;113(3):1155–1157. doi: 10.1210/endo-113-3-1155. [DOI] [PubMed] [Google Scholar]
- Hasumura S., Rossi B., Alderson R., Pastan I., Cheng S. Antibodies against the plasma membrane 3,3',5-triiodo-L-thyronine binding protein of rat pituitary GH3 cells: partial characterization and cross-species immunoreactivity. Biochem Biophys Res Commun. 1984 Nov 14;124(3):956–962. doi: 10.1016/0006-291x(84)91051-9. [DOI] [PubMed] [Google Scholar]
- Horiuchi R., Cheng S. Y., Willingham M., Pastan I. Inhibition of the nuclear entry of 3,3',5'-triiodo-L-thyronine by monodansylcadaverine in GH3 cells. J Biol Chem. 1982 Mar 25;257(6):3139–3144. [PubMed] [Google Scholar]
- Horiuchi R., Johnson M. L., Willingham M. C., Pastan I., Cheng S. Affinity labeling of the plasma membrane 3,3',5-triiodo-L-thyronine receptor in GH3 cells. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5527–5531. doi: 10.1073/pnas.79.18.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenning E. P., Docter R., Bernard H. F., Visser T. J., Hennemann G. The essential role of albumin in the active transport of thyroid hormones into primary cultured rat hepatocytes. FEBS Lett. 1979 Nov 1;107(1):227–230. doi: 10.1016/0014-5793(79)80501-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Latham K. R., Ring J. C., Baxter J. D. Solubilized nuclear "receptors" for thyroid hormones. Physical characteristics and binding properties, evidence for multiple forms. J Biol Chem. 1976 Dec 10;251(23):7388–7397. [PubMed] [Google Scholar]
- Lefebvre Y. A., Venkatraman J. T. Characterization of a thyroid-hormone-binding site on nuclear envelopes and nuclear matrices of the male-rat liver. Biochem J. 1984 May 1;219(3):1001–1007. doi: 10.1042/bj2191001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H. Thyroid hormone action at the cellular level. Science. 1979 Mar 9;203(4384):971–979. doi: 10.1126/science.218285. [DOI] [PubMed] [Google Scholar]
- Pliam N. B., Goldfine I. D. High affinity thyroid hormone binding sites on purified rat liver plasma membranes. Biochem Biophys Res Commun. 1977 Nov 7;79(1):166–172. doi: 10.1016/0006-291x(77)90075-4. [DOI] [PubMed] [Google Scholar]
- Robbins J., Cheng S. Y., Gershengorn M. C., Glinoer D., Cahnmann H. J., Edelnoch H. Thyroxine transport proteins of plasma. Molecular properties and biosynthesis. Recent Prog Horm Res. 1978;34:477–519. doi: 10.1016/b978-0-12-571134-0.50017-x. [DOI] [PubMed] [Google Scholar]
- SOKOLOFF L., FRANCIS C. M., CAMPBELL P. L. THYROXINE STIMULATION OF AMINO ACID INCORPORATION INTO PROTEIN INDEPENDENT OF ANY ACTION ON MESSENGER RNA SYNTHESIS. Proc Natl Acad Sci U S A. 1964 Sep;52:728–736. doi: 10.1073/pnas.52.3.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal J., Ingbar S. H. Specific binding sites for the triiodothyronine in the plasma membrane of rat thymocytes. Correlation with biochemical responses. J Clin Invest. 1982 Nov;70(5):919–926. doi: 10.1172/JCI110703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff L., Roberts P. A., Januska M. M., Kline J. E. Mechanisms of stimulation of protein synthesis by thyroid hormones in vivo. Proc Natl Acad Sci U S A. 1968 Jun;60(2):652–659. doi: 10.1073/pnas.60.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling K., Campbell G. A., Brenner M. A. Purification of the mitochondrial triiodothyronine (T3) receptor from rat liver. Acta Endocrinol (Copenh) 1984 Mar;105(3):391–397. doi: 10.1530/acta.0.1050391. [DOI] [PubMed] [Google Scholar]
- Surks M. I., Koerner D., Dillman W., Oppenheimer J. H. Limited capacity binding sites for L-triiodothyronine in rat liver nuclei. Localization to the chromatin and partial characterization of the L-triiodothyronine-chromatin complex. J Biol Chem. 1973 Oct 25;248(20):7066–7072. [PubMed] [Google Scholar]