Abstract
Objective
To find the best lead exposure assessment marker for children.
Methods
We recruited 11 children, calculated a cumulative blood lead index (CBLI) for the children, measured their concurrent BLL, assessed their development, and measured their bone lead level.
Results
Nine of 11 children had clinically significant neurodevelopment problems. CBLI and current blood lead level, but not the peak lead level, were significantly or marginally negatively associated with the full-scale IQ score.
Conclusion
Lead exposure at younger age significantly impacts a child’s later neurodevelopment. CBLI may be a better predictor of neurodevelopment than are current or peak blood lead levels.
Keywords: Heavy metal toxicity, neurological disease, environmental pollution/ecotoxicology
Introduction
Lead exposure has declined in the United States over the last two decades. Nonetheless, lead toxicity remains a significant pediatric public health issue due to the extensive use of leaded-paint in housing, the normal hand to mouth activities of toddlers, and the sensitivity of a child’s developing brain. Blood lead concentration has been used as the primary biomarker to assess lead exposure in children. However, it mainly reflects current- or short-term lead exposure due to lead’s relatively short half-life in blood (Rabinowitz, 1991). Bone lead, on the other hand, has a half-life of years to decades (at least in adults), which reflects long term or cumulative lead exposure (Hu et al., 1995). KXRF technology has been used to measure lead in bone for over three decades and many studies in adults have demonstrated that bone lead is a better biomarker than blood lead in addressing long-term health effects related to cumulative lead exposure (Shih et al., 2007; Navas-Acien et al., 2007; Navas-Acien et al., 2008; Weisskopf et al., 2009).
Whether children’s bone lead stores persist into adolescence or adulthood is unknown. Previous kinetic models have predicted that the combination of growth and bone turnover would wash out lead in bone during adolescence (O’Flaherty, 1998), or that there is not much accumulation of lead in bone for toddlers even at high lead exposure levels because of the high bone turnover rate in childhood (Leggett, 1993). Whether lead accumulates in bone for toddlers and whether lead in bone would persist is important because (1) if so bone lead would be a useful biomarker of cumulative exposure in children if it could be measured and (2) lead present in bone during adolescence could be remobilized causing toxicity as it would represent a “second dose” of lead, endogenous in origin. Because neurodevelopment is a long-term process, bone lead levels might be a better biomarker than blood for characterizing the association between lead exposure and neurodevelopment. The sensitivity of previous KXRF bone lead measurement systems has limited its use as a biomarker to assess lead exposure in children. Recently, a highly sensitive KXRF bone lead measurement system has been developed and validated (Nie et al., 2006), making bone lead assessment for children potentially more informative.
The first objective of this study was to determine whether it would be possible to measure the lead concentrations in bone in 6–16 year old children with a history of lead poisoning as toddlers. The second objective was to examine the children’s current neurodevelopment functioning in relation to their lead exposure histories.
Patients and methods
Subjects and recruitment
The base population for the present study was 1442 children age of 6–16 years old who were followed in the Children’s Hospital Pediatric Environmental Health Specialty Units (PEHSU) in Boston for elevated lead exposure at the age of 1–4 years.
We mailed the recruitment letters to 110 families of children who met the following eligibility criteria: ages 8–13 years with a past peak blood lead >30 µg/dl. Some of these families were unavailable for such follow-up 6–11 years from when they had been originally treated in the PEHSU. Of the 110 invitations mailed, 28 were undeliverable; 5 families declined to participate; 15 families were interested in the study, and 62 did not respond. Of the 15 families who were interested, 11 completed the study, 2 canceled appointments and 2 refused consent at a later contact. Thus 11 children and their parents completed the bone lead test, blood lead measurement, and neurodevelopmental testing.
The study protocol was approved by the institutional review board of the Children’s Hospital Boston. Informed consent was obtained from the parents of all subjects, and assent was obtained from all the subjects as well. Parents were later informed of study results on their child.
KXRF technology and bone lead measurement
The in vivo bone lead measurements were performed with an advanced KXRF system. The physical principles, technical advances, and in vivo investigations of this instrument have been described elsewhere (Nie et al., 2006). In summary, the system consists of four 16-mm diameter Canberra HpGe detectors (model GL0210R/S) of 10 mm thickness, four resistive feedback pre-amplifiers with resistors of about 50 GΩ, 4 Canberra digital signal analyzers (DSA-1000) and a computer.
We measured bone lead in the tibia and calcaneus of each subject for 30 min. We took the measurements at the mid-shaft of the left tibia and at the right calcaneus after each region had been washed with a 50% solution of isopropyl alcohol. Interaction signals, which include characteristic lead X-rays, were collected by the four HpGe detectors and processed by 4 sets of digital signal processing electronics. We then analyzed the spectra with an in-house peak fitting program based on Marquardt least-square algorithm and calculated the final lead concentrations.
Blood lead measurement
Whole blood samples were collected in lead-free tubes and analyzed by inductively coupled plasma-mass spectrometry (ICP-MS) in the Chemistry Laboratory at Harvard School of Public Health (Farias et al., 2005). Results are given as the average of 5 replicate measurements.
Cumulative blood lead index (CBLI)
CBLI reflects cumulative lead exposure and it can be estimated from a series of blood lead levels recorded over a period of time. The CBLI (CBLI) for these children when they were 1.5–5 years old is estimated from the child’s medical record. The children enrolled in the study had been followed in the PEHSU for an average of 1.5 years. The CBLI is calculated as (Somervaille et al., 1988):
Where PbBi and PbBi+1 are the blood lead concentrations at two consecutive times ti and ti+1. and t1 and tN are the beginning and ending time for the blood lead measurements. Among these subjects, 7 had their first blood lead test between 1.5 and 2 years of age, and 4 had their first blood lead test between 2 and 4 years of age. Among the 4 who had first blood lead measurement after 2 years of age, 1 had only three blood tests in total. This subject was removed from the analysis involving CBLI due to the lack of blood lead data. The average number of visits for the 10 subjects who are included in the analysis was 12 (range 6–17). Because the children only had blood lead records for a certain time period between 1.5–5 years, the blood lead levels at 1.5 years was arbitrarily set at 10 µg/dl and at 5 years was set at the level for the last visit. This is a valid estimation because: (a) the blood lead levels were recorded during the period when the children had the highest lead exposures; (b) the blood lead level between 1.5 years and the first visit (when the peak blood lead level was recorded) is partially weighted by the peak blood lead level; (c) the blood lead level between the last visit and 5 years old is fully weighted by the blood lead level for the last visit.
To assess the impact of setting the blood lead level at 1.5 years of age at 10 µg/dl, we also set the blood lead level at 1.5 years to be the same as the blood lead level at the first visit for the children who had their first visit between 1.5 and 2 years. We performed analyses with the CBLI calculated using this slightly different assumption.
Neurodevelopmental assessments
An experienced neuropsychologist (D.B.) administered the neurodevelopmental tests. The neurodevelopmental assessment consisted of one individually-administered test of intelligence, the Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV), and three parent-completed questionnaires. The three questionnaires were the Behavior Assessment System for Children–2 (BASC-2), which provides a broad-based assessment of behavioral problems, the Behavior Rating Inventory of Executive Function (BRIEF), which assesses executive functions, and the Conners ADHD/DSM-IV Scale (CADS-IV), which assesses attention and hyperactivity.
The WISC-IV has five scores, which are verbal comprehension, perceptual reasoning, working memory, processing speed, and full-scale IQ. The BASC-2 has four scores, which are externalizing problems, internalizing problems, behavioral symptom index, and adaptive skill. The BRIEF has three scores, which are behavioral regulation index, metacognition index, and general executive composite. The CADS-IV has four scores, which are ADHD index, inattentive, hyperactive-impulsive, and total score.
Data analysis
We calculated the Pearson correlation between the cumulative lead exposure and current blood lead level. We also examined the association between the neurodevelopmental scores and lead exposure with CBLI, peak blood lead level, and current blood lead level as biomarkers. We then adjusted for the children’s age and parental education which may be correlated with both the predictor and outcomes (Tellez-Rojo et al., 2006; Roy et al., 2009). Single and multiple regression models were used for these analyses. The basic model used is:
Where neurotest score refers to one of the scores from the neurodevelopmental assessment, and [Pb] refers to CBLI, current blood Pb, or peak blood Pb.
All analyses were conducted using SYSTAT Version 13 software package. An association with a p-value less 0.05 is considered significant, and an association with a p-value less than 0.2 is considered marginally significant.
Results
Subjects
The average age of the 11 subjects at the time of this follow-up study was 11.0 ± 1.6 (std). Five of the subjects were female, and 6 were male. The average maternal education was 13.8 ± 3.0 years (missing for one child). The average paternal education was 13.3 ± 4.4 years (missing for 3 children).
Bone lead measurement results, blood lead test results, and CBLI
The average tibia lead level was 0.7 ± 3.8 ppm with a median of 0.4 ppm (range: −5.8–6.2 ppm). The average calcaneus lead level was 2.5 ± 5.8 ppm with a median of 1.6 ppm (range: −6.8–10.4 ppm). The average uncertainty for the tibia lead measurement was 2.6 ppm, and for the calcaneus measurement 4.4 ppm. The average detection limits (DL), calculated as twice the average uncertainties, were 5.2 and 8.8 parts per million (ppm) for the tibia and calcaneus measurements, respectively (McNeill et al., 2000; Herranz et al., 2008). Only one measurement, the tibia measurement for subject number 70, exceeded the detection limit. Therefore, no analyses were carried out relating bone lead levels to neurodevelopmental test scores.
Table 1 lists the peak blood lead levels, current blood lead levels, and CBLI calculated from the formula described in section cumulative blood lead index (CBLI). Including all the data points, the Pearson correlation coefficient between current blood lead level and CBLI was 0.420 (p = 0.198); the correlation coefficient increased to 0.600 (p = 0.067) when one outlier (ID #54) was excluded. The outlier was the subject for whom the fewest blood lead levels from early childhood were available in the medical record and hence who had the least reliable CBLI value. (This child had only 3 visits to the Children’s Hospital Boston, compared to 6–17 visits for the other children). Figure 1 plots current blood lead level versus CBLI.
Table 1.
Blood lead levels for the 11 subjects.
| ID | Peak Blood Pb (µg/dl) |
Current Blood Pb (µg/dl) |
Current blood Pb uncertainty. (µg/dl) |
CBLI 1a ((µg/dl)·years) |
|---|---|---|---|---|
| 13 | 31 | 3.74 | 0.06 | 68.1 |
| 16 | 37 | 1.64 | 0.02 | 45.6 |
| 4 | 34 | 2.17 | 0.04 | 65.8 |
| 88 | 32 | 1.68 | 0.03 | 49.9 |
| 79 | 37 | 0.49 | 0.03 | 52.6 |
| 14 | 36 | 1.23 | 0.05 | 44.6 |
| 59 | 30 | 0.87 | 0.02 | 44.7 |
| 70 | 32 | 3.19 | 0.06 | 49.3 |
| 91 | 41 | 1.95 | 0.03 | 61.6 |
| 45 | 36 | 0.99 | 0.02 | 47.3 |
| 54 | 34 | 1.00 | 0.02 | 66.6 |
Calculated as described in section cumulative blood lead index.
Figure 1.
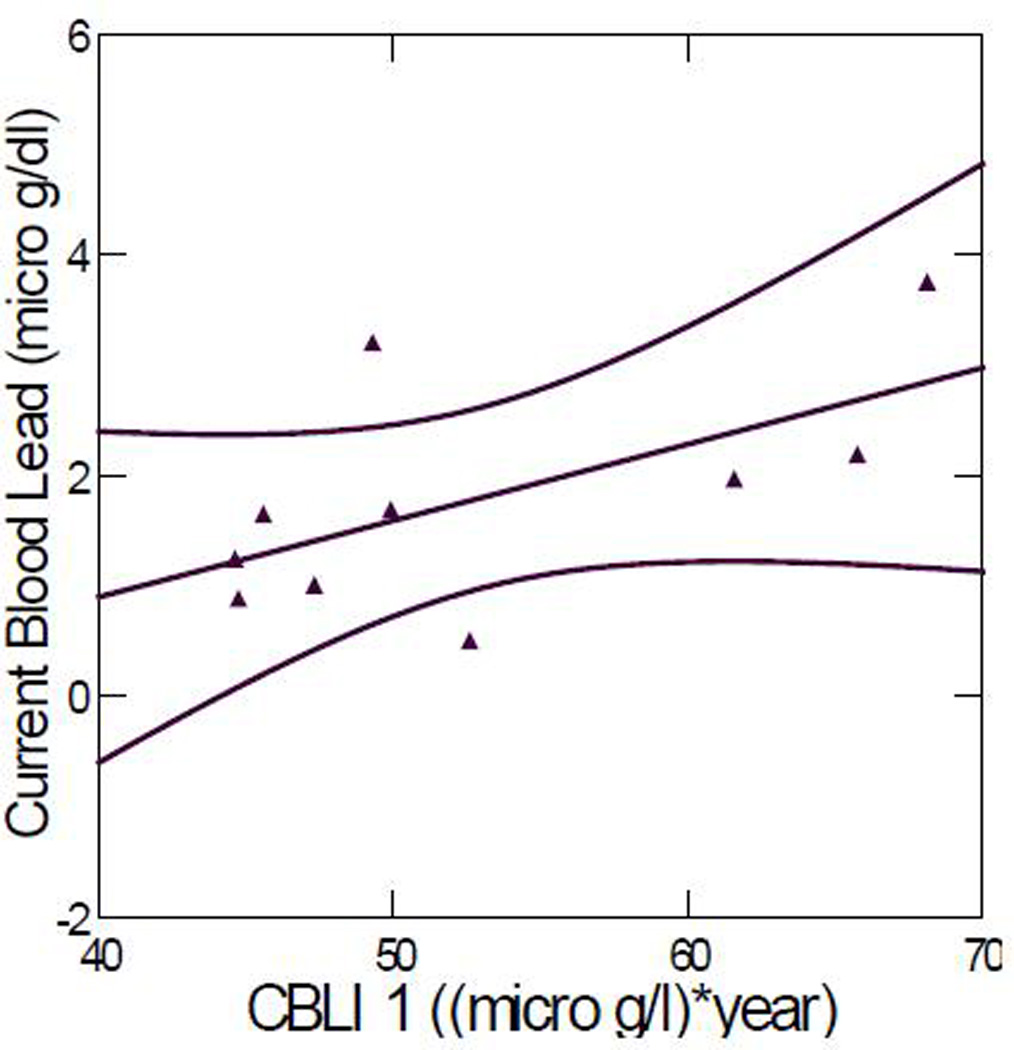
Current blood lead level versus CBLI 1. Correlation coefficient: 0.600 (p = 0.067). (Outlier ID #54 removed). The two curved lines represent the 95% confidence interval.
Neurodevelopmental assessment
The children’s test scores are provided in Table 2. A brief explanation of the scores is provided in the footnote. The WISC-IV test for the first child was performed in error by an inexperienced staff member and the results were considered invalid. The test scores derived from the parent-completed questionnaires for this child were considered valid. The tests for all other children were performed by an experienced neurologist. Among these 11 children, 5 had clinically significant behavioral and/or attention problems, and 9 had scores which were close to clinically significant.
Table 2.
Neurodevelopmental test scoresa.
| ID number | 13 | 16b | 4 | 88 | 79 | 14 | 59 | 70 | 91 | 45 | 54 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wisc-IV | |||||||||||
| Verbal comprehensive | 79 | n.a | 99 | 99 | 98 | 98 | 119 | 112 | 93 | 110 | 121 |
| Perceptual reasoning | 88 | n.a | 100 | 86 | 110 | 106 | 108 | 100 | 96 | 94 | 125 |
| Working memory | 74 | n.a | 88 | 110 | 110 | 116 | 102 | 120 | 71 | 113 | 97 |
| Processing speed | 103 | n.a | 94 | 94 | 97 | 94 | 106 | 112 | 75 | 94 | 118 |
| Full-scale IQ | 81 | n.a | 95 | 96 | 105 | 105 | 113 | 114 | 81 | 105 | 121 |
| BASC-2 | |||||||||||
| Externalizing problems | 79 | 41 | 71 | 59 | 58 | 51 | 48 | 52 | 64 | 48 | 45 |
| Internalizing problems | 68 | 45 | 99 | 79 | 54 | 53 | 52 | 49 | 61 | 60 | 35 |
| Behavioral symptom index | 79 | 51 | 76 | 72 | 58 | 49 | 50 | 48 | 67 | 59 | 38 |
| Adaptive skills | 33 | 35 | 40 | 54 | 43 | 53 | 60 | 47 | 47 | 38 | 66 |
| BRIEF | |||||||||||
| Behavioral regulation index | 93 | 44 | 64 | 81 | 60 | 64 | 57 | 46 | 62 | 58 | 36 |
| Metacognition index | 74 | 53 | 61 | 68 | 63 | 52 | 52 | 49 | 64 | 71 | 35 |
| General executive composite | 83 | 50 | 63 | 74 | 62 | 57 | 54 | 48 | 64 | 67 | 34 |
| CADS-IV | |||||||||||
| ADHD index | 69 | 65 | 60 | 48 | 77 | 58 | 50 | 45 | 63 | 69 | 41 |
| Inattentive | 71 | 62 | 62 | 48 | 71 | 54 | 51 | 53 | 59 | 64 | 41 |
| Hyperactive-impulsive | 85 | 43 | 63 | 51 | 74 | 59 | 57 | 46 | 56 | 64 | 43 |
| Total | 80 | 54 | 68 | 49 | 74 | 57 | 54 | 50 | 59 | 65 | 41 |
WISC-IV: a score of 70 or below are considered clinically significant while scores of 71–75 are considered borderline problematic; BASC-2: For Externalizing, Internalizing, and Behavioral Symptom scales, T-scores of 70 or greater are considered clinically significant, and scores of 66–70 are considered to be of borderline clinical significance; for the Adaptive skills scale, T-scores of 30 or lower are considered clinically significant, and scores of 31–35 are considered to be of borderline clinical significance; BRIEF: T-scores of 65 or greater are considered clinically significant and scores of 60–64 are considered to be of borderline clinical significance; CADS-IV: T-scores of 70 or greater are considered clinically significant, and scores of 66–70 are considered to be of borderline clinical significance.: clinically significant;: borderline clinical significance.
The WISC-IV of one child were not considered valid and were not considered in the analyses.
We evaluated the correlations between neurodevelopmental test scores and CBLI, current blood lead levels, and peak blood lead levels. Table 3 lists the correlation coefficients and the corresponding p values for the relationships between neurodevelopmental test scores and CBLI 1 (the CBLI calculated with blood lead level set to 10 µg/dl at 1.5-year old) and CBLI 2 (the CBLI calculated with blood lead level set to the level for the first visit at 1.5-year old). The correlations adjusted for age and mother’s education are also shown in the table. There are too few data to adjust for father’s education. The data show that there is no significant difference between the associations calculated using the two CBLI values. The associations are somewhat diminished when age and mother’s education were included into the models, however, the majority of the associations remain significant or marginally significant.
Table 3.
Correlation coefficients between neurodevelopmental test scores and CBLI1, CBLI2, and CBLI1 adjusted for age and mother’s education.
| Neuro-test | CBLI 1 | CBLI 2 | CBLI 1 (adjusted for age and mother’s education) |
|---|---|---|---|
| WISC | |||
| Verbal comprehension | a0.746 (p = 0.021) + | a0.710 (p = 0.032) + | b0.682 (p = 0.113) + |
| Perceptual reasoning | 0.406 (p = 0.278) + | b0.478 (p = 0.193) + | 0.161 (p = 0.641) + |
| Working memory | a0.853 (p = 0.003) + | a0.906 (p = 0.001) + | a0.973 (p = 0.015) + |
| Processing speed | 0.283 (p = 0.461) + | 0.368 (p = 0.330) + | 0.260 (p = 0.597) + |
| Full-scale IQ | a0.823 (p = 0.006) + | a0.872 (p = 0.002) + | b0.785 (p = 0.058) + |
| BRIEF | |||
| Behavioral regulation | b0.573 (p = 0.084) + | b0.617 (p = 0.057) + | 0.477 (p = 0.216) + |
| Meta-cognition | b0.560 (p = 0.092) + | b0.517 (p = 0.126) + | 0.355 (p = 0.277) + |
| Global executive composite | b0.614 (p = 0.059) + | b0.614 (p = 0.059) + | b0.450 (p = 0.189) + |
| CADS | |||
| ADHD index | 0.313 (p = 0.378) + | 0.110 (p = 0.762) + | 0.291 (p = 0.484) + |
| DSM-IV inattentive | b0.513 (p = 0.129) + | 0.277 (p = 0.438) + | b0.536 (p = 0.150) + |
| DSM-IV hyperactive | b0.583 (p = 0.077) + | b0.450 (p = 0.192) + | a0.636 (p = 0.050) + |
| DSM-IV total | a0.661 (p = 0.038) + | b0.465 (p = 0.176) + | a0.707 (p = 0.016) + |
| Externalizing problems | a0.943 (p = 0.000) + | a0.928 (p = 0.000) + | a0.937 (p = 0.002) + |
| Internalizing problems | a0.648 (p = 0.043) + | a0.655 (p = 0.040) + | b0.490 (p = 0.156) + |
| Behavioral symptom index | a0.853 (p = 0.002) + | a0.855 (p = 0.002) + | a0.703 (p = 0.006) + |
| Adaptive skills | b0.481 (p = 0.160) + | 0.249 (p = 0.488) + | 0.326 (p = 0.397) + |
Positive sign indicates worse neurodevelopmental performance with increased CBLI, current blood lead, or peak blood lead.
Significant correlation (p < 0.05).
Marginally significant correlation (p < 0.20).
Table 4 summarizes the correlation coefficients and the corresponding p values for the relationship between neurodevelopmental test scores and CBLI 1, current blood lead levels, and peak blood lead levels. The results show that 7 out of 16 test scores are significantly correlated with CBLI (p < 0.05) and an additional 6 are marginally correlated with CBLI (p < 0.20). In all cases, higher CBLI is associated with a worse score. Only 1 out of 16 scores was significantly correlated with current blood lead level (p < 0.05) and an additional 6 were marginally correlated with current blood lead (p < 0.20). For 14 of the 16 correlations, higher current blood lead level was associated with a worse score. Peak blood lead was significantly associated with 1 of 16 test scores and marginally associated with another. Higher peak blood lead level was associated with a worse score for 9 of the 16 correlations.
Table 4.
Correlation coefficients between neurodevelopmental test scores and CBLI, current blood lead level, and peak blood lead level.
| Neuro-test | CBLI 1 (also listed in Table 3) | Current blood lead | Peak blood lead |
|---|---|---|---|
| WISC | |||
| Verbal comprehension | a0.746 (p = 0.021) + | b0.513 (p = 0.129) + | 0.217 (p = 0.547) + |
| Perceptual reasoning | 0.406 (p = 0.278) + | b0.551 (p = 0.099) + | 0.088 (p = 0.810) |
| Working memory | a0.853 (p = 0.003) + | 0.394 (p = 0.260) + | 0.205 (p = 0.571) + |
| Processing speed | 0.283 (p = 0.461) + | 0.076 (p = 0.835) | a0.705 (p = 0.023) + |
| Full-scale IQ | a0.823 (p = 0.006) + | b0.493 (p = 0.147) + | 0.296 (p = 0.407) + |
| BRIEF | |||
| Behavioral regulation | b0.573 (p = 0.084) + | b0.421 (p = 0.197) + | 0.256 (p = 0.447) |
| Meta-cognition | b0.560 (p = 0.092) + | 0.269 (p = 0.423) + | 0.075 (p = 0.826) + |
| Global executive composite | b0.614 (p = 0.059) + | 0.369 (p = 0.265) + | 0.081 (p = 0.813) |
| CADS | |||
| ADHD index | 0.313 (p = 0.378) + | 0.065 (p = 0.850) | b0.473 (p = 0.137) + |
| DSM-IV inattentive | b0.513 (p = 0.129) + | 0.221 (p = 0.514) + | 0.270 (p = 0.423) + |
| DSM-IV hyperactive | b0.583 (p = 0.077) + | 0.209 (p = 0.537) + | 0.089 (p = 0.794) |
| DSM-IV total | a0.661 (p = 0.038) + | 0.253 (p = 0.452) + | 0.085 (p = 0.803) + |
| BASC2 | |||
| Externalizing problems | a0.943 (p = 0.000) + | a0.609 (p = 0.047) + | 0.117 (p = 0.731) |
| Internalizing problems | a0.648 (p = 0.043) + | 0.307 (p = 0.358) + | 0.133 (p = 0.697) |
| Behavioral symptom index | a0.853 (p = 0.002) + | b0.476 (p = 0.139) + | 0.042 (p = 0.902) |
| Adaptive skills | b0.481 (p = 0.160) + | b0.447 (p = 0.169) + | 0.228 (p = 0.500) + |
Positive sign indicates worse neurodevelopmental performance with increased CBLI, current blood lead, or peak blood lead.
Significant correlation (p < 0.05).
Marginally significant correlation (p < 0.20).
Because full scale IQ score is commonly reported in the lead literature, we used this score to illustrate the correlations. Including all the data points, the Pearson correlation coefficient between full-scale IQ scores and CBLI 1 was 0.422 (p = 0.225); the correlation coefficient increased to 0.823 (p = 0.006) when the outlier, the child for whom the least blood lead history data were available (ID #54), was excluded. Figure 2 plots full-scale IQ score versus CBLI 1. The Pearson correlation coefficient between full-scale IQ score and current blood lead level was 0.493 (p = 0.147). There was no significant correlation between full-scale IQ score and peak blood lead level (p = 0.407).
Figure 2.
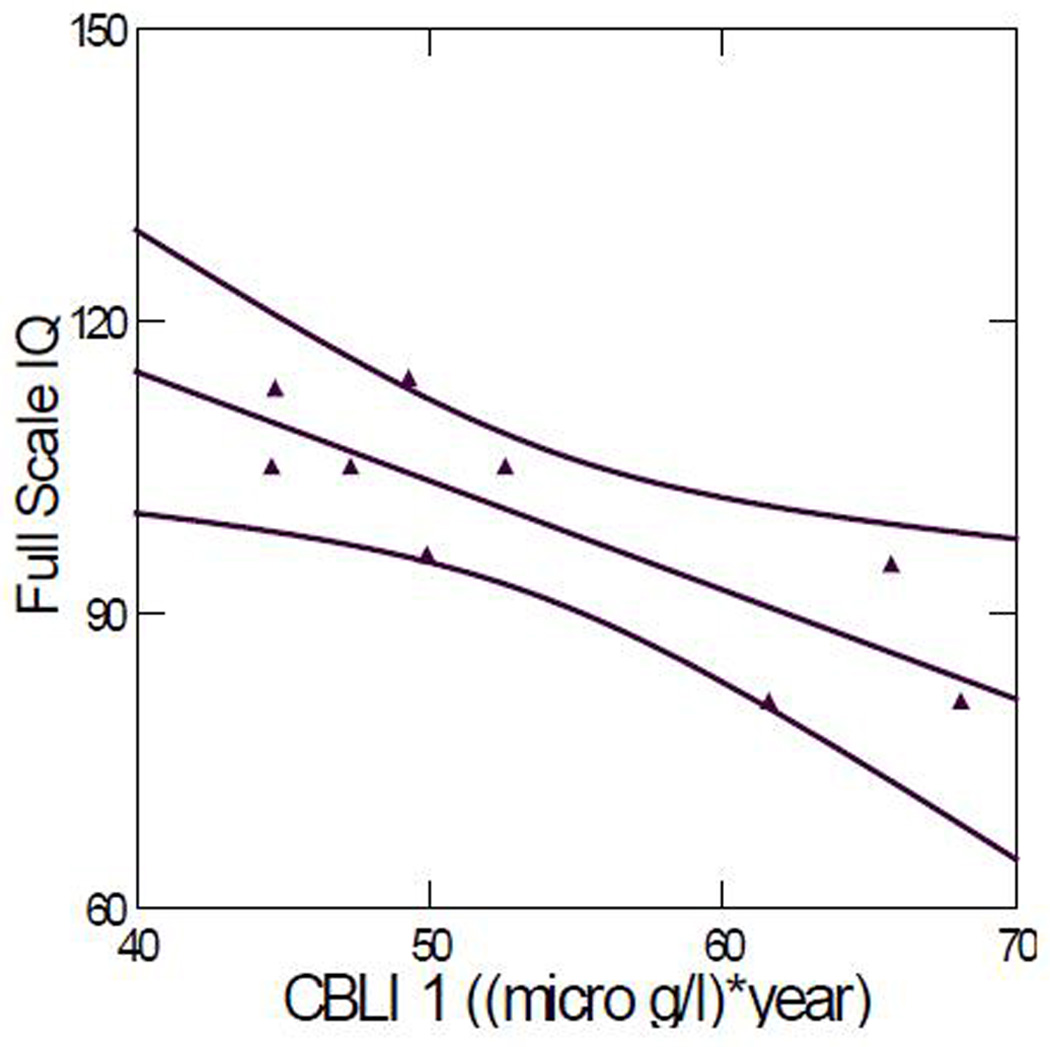
Full-scale IQ score versus CBLI 1. Correlation coefficient: 0.823 (p = 0.006). (Outlier ID #54 removed). The two curved lines represent the 95% confidence interval.
Discussion
Many studies in humans and animals have shown that lead accumulates in bone and returns to the blood during periods when bone remodeling occurs, such as aging. Therefore, lead in bone can elevate blood lead levels even when external lead exposure ceases. This endogenous exposure (Brito et al., 2002; Gwiazda et al., 2005; Fleming et al., 1997) would be expected to have the same health effects as external lead exposure. There have been technological limitations to bone lead measurements which limited the ability to measure lead in the less dense bones of children. Because the density of a child’s bone is less than that of an adult (ICRP, 1996), the KXRF system samples less bone and hence a smaller amount of lead for the same bone lead concentration. So the bone lead detection limit for children is higher than that for adults. Newer generation instruments can overcome this limitation, but our data indicate that bone lead levels are quite low even in children with a history of lead poisoning. The detection limit for our instrument is 2–3 ppm (for adults) compared to 6–10 ppm for the older generation XRF (Nie et al., 2006). We had hoped that this sensitivity would allow us to detect lead in bone even in children. The detection limit we observed in this study is significantly higher than the published value of 2–3 ppm for the adults. There are two main reasons for this discrepancy. First, the density of a child’s bone is less than that of an adult, as we explained above. Second, the activity of the radioisotope (Cd-109) used to excite the lead atoms in this study is lower than that used for the adult study. Against this backdrop, there are toxicokinetic models which predict that bone lead in early childhood is quite low based on two factors (1) dilution due to the rapid growth during childhood and (2) turnover of lead due to both growth and remodeling of the bony matrix. Such models predict that even children with significant lead poisoning would have limited pools of lead in their bones. However, until now there have been no empirical data to support these models. In a previous report (Goldman et al., 1994), a woman who had substantial self-reported lead exposure at childhood had high stores of lead in her bones despite a lack of subsequent environmental or occupational exposure. Our data do not contradict this report as no child in our study had a history of lead exposures as severe as the subject of that report. Needleman et al. reported measurable lead concentrations in the bones of children at age 12 years (Needleman et al., 1996). One possible reason that they detected bone lead in children’s bones with a less sensitive measurement system is that some of the children might have had a continuous moderate or high lead exposure, as opposed to high exposures only at a younger age, which is the case in our study.
Our purpose in conducting this study was to quantify the lead in the children’s bones and to evaluate the association between bone lead levels and late neurodevelopment. For all children but one, bone lead levels were below the detection limit of the most advanced KXRF bone lead quantification system available, preventing us from carrying out these analyses.
We found that a CBLI calculated on the basis of blood lead history data was marginally correlated with current blood lead level (p = 0.067). One interpretation of this finding is that the lead that these children were exposed to still persist in their bodies and is reflected in their current blood lead levels (Lanphear et al., 2005). Because over 80% of the lead in a child’s body stores resides in the bone (Barry and Mossman, 1970), any ‘old’ lead is most likely stored in their bones. Therefore, even at very low levels (i.e. <5 ppm), bones might act as a storage site of lead and cause endogenous exposure in children who were previously exposed to high levels of lead. An alternative interpretation is that previous lead exposure predicts persistent environmental exposure later in life.
CBLI was significantly or marginally correlated with 14 of the16 neurodevelopmental and behavioral test scores, while current blood lead level was significantly or marginally correlated with 7 of the 16 scores. In contrast, the associations between peak blood lead level and the neurodevelopmental scores were much weaker. These findings indicate that elevated lead exposure in early childhood has long-term adverse neurodevelopmental effects on the children. These findings suggest that an index of cumulative lead exposure may be a better predictor of neurodevelopmental outcomes than is current blood lead level or peak blood lead level, which indicates that bone lead level might be a better predictor of late neurodevelopment than is blood lead level. However, the fact that the bone lead levels of all children but one were below the detection limit of the current system suggests that additional increases in sensitivity are needed if this method is to prove useful in future studies of childhood lead exposure, especially for children who were highly exposed to lead only at toddler age. On the other hand, if lead in bone is washed out and hence not detectable, as predicted in O’Flaherty’s model (O’Flaherty, 1998), the correlation between CBLI and current blood lead might reflect only persistent environmental exposure. Whether bone lead represents cumulative lead exposure for children requires further investigation.
The limitations of this study include the small sample size and the lack of information for some covariants such as parental IQ and family income. Despite this, however, we were able to demonstrate remarkably strong associations, in a sample of children with earlylife lead poisoning, between a CBLI and their neurodevelopmental functions later in life, which is consistent with data published in the most recent literature (Mazumdar et al., 2011).
Conclusions
This study adds to the evidence that elevated lead exposure in early childhood has long-term adverse neurodevelopmental and behavioral effects. The study also suggests that in children CBLI is a better predictor of late neurodevelopmental effects than is current or peak blood lead level.
Acknowledgments
The research described in this paper was supported primarily by NIEHS R01-ES013744 and RO1 ES014930. This work was also supported in part by a grant from the Agency for Toxic Substances and Disease Registry (ATSDR) with additional support from the U.S. Environmental Protection Agency (EPA), administered through the Association of Occupational and Environmental Clinics Association (AOEC), Washington D.C. Test subjects were evaluated for bone lead levels, blood lead levels, and neurodevelopmental performance in the Clinical Translational Science Award (CTSA) program of the Children’s Hospital Boston. The authors would like to thank the administrative and clinical staff at the CTSA for their support. The authors would also like to thank Lesley Egden at McMaster University for her assistance with the project.
Footnotes
Declaration of interest
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, ATSDR, or EPA. The authors declare they have no competing financial interests.
References
- Barry PS, Mossman DB. Lead concentrations in human tissues. Br j Ind Med. 1970;27:339–351. doi: 10.1136/oem.27.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito JA, McNeill FE, Webber CE, Wells S, Richard N, Carvalho ML, Chettle DR. Evaluation of a novel structural model to describe the endogenous release of lead from bone. j Environ Monit. 2002;4:194–201. doi: 10.1039/b108817c. [DOI] [PubMed] [Google Scholar]
- Farias P, Echavarria M, Hernandez-Avila M, Villanueva C, Amarasiriwardena C, Hernandez L, Aro A, Hu H. Bone, blood and semen lead in men with environmental and moderate occupational exposure. Int j Environ Health Res. 2005;15:21–31. doi: 10.1080/09603120400018782. [DOI] [PubMed] [Google Scholar]
- Fleming DE, Boulay D, Richard NS, Robin JP, Gordon CL, Webber CE, Chettle DR. Accumulated body burden and endogenous release of lead in employees of a lead smelter. Environ Health Perspect. 1997;105:224–233. doi: 10.1289/ehp.97105224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman RH, White R, Kales SN, Hu H. Lead poisoning from mobilization of bone stores during thyrotoxicosis. Am j Ind Med. 1994;25:417–424. doi: 10.1002/ajim.4700250309. [DOI] [PubMed] [Google Scholar]
- Gwiazda R, Campbell C, Smith D. A noninvasive isotopic approach to estimate the bone lead contribution to blood in children: implications for assessing the efficacy of lead abatement. Environ Health Perspect. 2005;113:104–110. doi: 10.1289/ehp.7241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz M, Idoeta R, Legarda F. Evaluation of Uncertainty and Detection Limits in Radioactivity Measurements. Nuclear Instruments and Methods in Physics Research A. 2008;595:526–534. [Google Scholar]
- Hu H, Aro A, Rotnitzky A. Bone lead measured by X-ray fluorescence: epidemiologic methods. Environ Health Perspect. 1995;103 Suppl 1:105–110. doi: 10.1289/ehp.95103s1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICRP. ICRP publication 70: Basic Anatomical & Physiological Data for Use in Radiological Protection: The Skeleton. Annals of ICRP. 1996;25:36. [PubMed] [Google Scholar]
- Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, Canfield RL, Dietrich KN, Bornschein R, Greene T, Rothenberg SJ, Needleman HL, Schnaas L, Wasserman G, Graziano J, Roberts R. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113:894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggett RW. An age-specific kinetic model of lead metabolism in humans. Environ Health Perspect. 1993;101:598–616. doi: 10.1289/ehp.93101598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar M, Bellinger DC, Gregas M, Abanilla K, Bacic J, Needleman HL. Low-level environmental lead exposure in childhood and adult intellectual function: a follow-up study. Environ Health. 2011;10:24. doi: 10.1186/1476-069X-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill FE, Stokes L, Brito JA, Chettle DR, Kaye WE. 109Cd K x ray fluorescence measurements of tibial lead content in young adults exposed to lead in early childhood. Occup Environ Med. 2000;57:465–471. doi: 10.1136/oem.57.7.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Guallar E, Silbergeld EK, Rothenberg SJ. Lead exposure and cardiovascular disease–a systematic review. Environ Health Perspect. 2007;115:472–482. doi: 10.1289/ehp.9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Schwartz BS, Rothenberg SJ, Hu H, Silbergeld EK, Guallar E. Bone lead levels and blood pressure endpoints: a meta-analysis. Epidemiology. 2008;19:496–504. doi: 10.1097/EDE.0b013e31816a2400. [DOI] [PubMed] [Google Scholar]
- Needleman HL, Riess JA, Tobin MJ, Biesecker GE, Greenhouse JB. Bone lead levels and delinquent behavior. Jama. 1996;275:363–369. [PubMed] [Google Scholar]
- Nie H, Chettle D, Luo L, O’Meara J. In vivo investigation of a new 109Cd gamma-ray induced K-XRF bone lead measurement system. Phys Med Biol. 2006;51:351–360. doi: 10.1088/0031-9155/51/2/011. [DOI] [PubMed] [Google Scholar]
- O’Flaherty EJ. A physiologically based kinetic model for lead in children and adults. Environ Health Perspect. 1998;106 Suppl 6:1495–1503. doi: 10.1289/ehp.98106s61495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz MB. Toxicokinetics of bone lead. Environ Health Perspect. 1991;91:33–37. doi: 10.1289/ehp.919133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Bellinger D, Hu H, Schwartz J, Ettinger AS, Wright RO, Bouchard M, Palaniappan K, Balakrishnan K. Lead exposure and behavior among young children in Chennai, India. Environ Health Perspect. 2009;117:1607–1611. doi: 10.1289/ehp.0900625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih RA, Hu H, Weisskopf MG, Schwartz BS. Cumulative lead dose and cognitive function in adults: a review of studies that measured both blood lead and bone lead. Environ Health Perspect. 2007;115:483–492. doi: 10.1289/ehp.9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somervaille LJ, Chettle DR, Scott MC, Tennant DR, McKiernan MJ, Skilbeck A, Trethowan WN. In vivo tibia lead measurements as an index of cumulative exposure in occupationally exposed subjects. Br j Ind Med. 1988;45:174–181. doi: 10.1136/oem.45.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Téllez-Rojo MM, Bellinger DC, Arroyo-Quiroz C, Lamadrid-Figueroa H, Mercado-García A, Schnaas-Arrieta L, Wright RO, Hernández-Avila M, Hu H. Longitudinal associations between blood lead concentrations lower than 10 microg/dL and neurobehavioral development in environmentally exposed children in Mexico City. Pediatrics. 2006;118:e323–e330. doi: 10.1542/peds.2005-3123. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Jain N, Nie H, Sparrow D, Vokonas P, Schwartz J, Hu H. A prospective study of bone lead concentration and death from all causes, cardiovascular diseases, and cancer in the Department of Veterans Affairs Normative Aging Study. Circulation. 2009;120:1056–1064. doi: 10.1161/CIRCULATIONAHA.108.827121. [DOI] [PMC free article] [PubMed] [Google Scholar]


