Abstract
Turner syndrome (TS) is a model for X-chromosome influences on neurodevelopment because it is most commonly caused by absence of one X-chromosome, and associated with altered brain structure and function. However, all prior in vivo magnetic resonance imaging studies of the brain in TS have either used manual approaches or voxel-based-morphometry (VBM) to measure cortical volume (CV). These methods, unlike surface-based-morphometry (SBM), cannot measure the two neurobiologically distinct determinants of CV– cortical thickness (CT) and surface area (SA) – which have differing genetic determinants, and may be independently altered. Therefore, in 24 adults with X-monosomy and 19 healthy female controls, we used SBM to compare (i) lobar CV, CT and SA, (ii) an index of hemispheric gyrification (iii) CT throughout the cortical sheet, and (iv) CT correlation between cortical regions. Compared to controls, females with TS had (i) significantly increased CT and decreased SA in parietal and occipital lobes (resulting in no significant difference in lobar CV), (ii) reduced hemispheric gyrification bilaterally, (iii) foci of significantly increased CT involving inferior-temporal, lateral-occipital, intraparietal sulcus (IPS), cingulate, and orbito-frontal cortices, (iv) significantly reduced CT correlation between the left IPS and cortical regions including supramarginal and lateral-occipital gyri. Our findings suggest that females with TS have complex, sometimes ‘opposing’, abnormalities in SA/gyrification (decreased) and CT (increased); which can result in no overall detectable differences in CV. Thus haploinsufficiency of X-chromosome genes, may differentially impact the distinct mechanisms shaping SA (e.g. cortical folding) and CT (e.g. dendritic arborization/pruning). CT disruptions are maximal within and between cortical regions previously implicated in the TS cognitive phenotype.
Keywords: Turner syndrome, MRI, cortical thickness, structural covariance
INTRODUCTION
Turner syndrome (TS) is a neurogenetic syndrome seen in approximately 1/2000 live female births (Stochholm et al., 2006). Turner syndrome arises due to partial or complete absence of one X chromosome, with the most common karyotype being X-monosomy [45,XO]. Since, in karyotypically normal [46,XX] females, almost all of one of the X chromosomes in each cell is randomly “silenced” through an epigenetic process known as Lyonization (Lyon, 1961) the TS phenotype may be due to haplo-insufficiency of those X chromosome genes that escape inactivation (Zinn and Ross, 1998). Effective X-linked gene haploinsufficiency in TS is also influenced by the “parent-of-origin” of the intact X chromosome, as the expression of some X chromosome genes differs systematically depending on which parent the chromosome is inherited from through a process known as imprinting (Barlow, 1995). X-chromosome haploinsufficiency in TS is also associated with ovarian failure and related estrogen and androgen deficiency, which may in themselves contribute to the TS phenotype.
Compared to the TS physical phenotype (Ogata and Matsuo, 1995), the behavioral-cognitive and neuroanatomical phenotypes remain less well described. A better understanding of how brain and behavior are altered in TS could shed light on how X-linked genetic and epigenetic influence human neurodevelopment.
Females with TS typically show an uneven cognitive profile with pronounced deficits in visuo-spatial skills alongside verbal abilities within the normal range. Deficits are best documented for tasks involving mental rotation, visual construction, and number manipulation. There are also reports of deficits in other domains such as memory and face processing (Murphy et al., 1994; Ross et al., 2000). Multiple functional magnetic resonance imaging (fMRI) studies have compared brain activity in females with TS to that in controls during the execution of mental rotation and visual construction tasks, and consistently report differences within parieto-frontal systems (especially within the intra-parietal sulcus) (Hart et al., 2006; Kesler et al., 2004; Kesler et al., 2006; Molko et al., 2003). The parietal lobe has also been repeatedly implicated in TS by structural magnetic resonance imaging (sMRI), with the most consistent findings being reduced parietal lobe volumes in TS compared to controls (Brown et al., 2002; Cutter et al., 2006; Murphy et al., 1993; Reiss et al., 1995). It remains unclear however if this reduction relates to white matter volume, grey matter volume, or both. Table 1 summarizes all TS sMRI studies published to date.
TABLE 1.
Summary of all structural magnetic resonance imaging studies comparing cortical anatomy between turner syndrome and typically developing controls, that were available at the time of publication.
| Author (Yr) | Sample Details | Method/Measure | Findings | ||||
|---|---|---|---|---|---|---|---|
| Turner Syndrome (TS) | Controls | TS<C | TS>C | ||||
| Number (XO/other) |
Mean age-yrs (s.d/range) |
Number | Mean age-yrs (s.d/range) |
||||
| Murphy D.G (1993) | 18 (9/9) | 30 (7/-) | 19 | 30(7/-) | Manual | BIL hippocampus, caudate, lenticular nucleus, thalamus, parieto-occipital lobes |
- |
| Reiss A.L (1995) | 30(27/3) | 10(3/6-17) | 30 | 10(3/6-17) | Semi-automated/ “lobar” volumes |
BIL “Parietal region” combined grey and white matter volumes |
R “Pareito-occipital region” combined grey and white matter volumes |
| Brown W.E (2002) | 26(26/-) | 13(-/8-17) | 26 | 13(-/9-17) | Semi-automated/ lobar volumes |
BIL parietal grey matter, BIL occipital white matter volumes |
BIL cerebellar grey matter volumes |
| Freyer S.L (2003) |
27(27/-) | 13(4/7-20) | 27 | 13(4/ 7-20) | Semi-automated mid-sagittal area |
Genu of corpus callosum, pons, cerebellar vermis lobules VI-VII |
- |
| Good C.D (2003) | 21(21/-) | 25(9/-) | 17 | 25(9/-) | VBM | - | BIL amygdala and orbitofrontal cortex grey matter volume |
| Molko N (2003) | 14(10/4) | 25(6/18-36) | 14 | 24(-/20-27) | Automated sulcal morphometry |
Maximal depth R intra-parietal sulcus | - |
| VBM | GREY: L primary motor cortex, R intraparietal sulcus CV. |
GREY: L parieto-occipital region, R primary motor cortex CV |
|||||
| Kesler S.R (2004) | 30(30/-) | 15(6/7-33) | 29 | 15(6/6-32) | Manual - amygdala and hippocampus only |
R hippocampal grey and R hippocampal white matter | L amygdalar grey |
| Molko N (2004) | 14 (10/4) | 25(6/18-36) | 14 | 24(-/20-27) | Automated sulcal morphometry |
Length and depth BIL superior temporal sulcus | - |
| VBM | GREY: CV in BIL anterior cingulate, lingual and orbitofrontal. R insula, postcentral gyrus, intraparietal sulcus, supramarginal gyrus L post-central and superior temporal. WHITE: Adjacent to R head of caudate, post-central sulcus, body of caudate and supramarginal gyrus. Adjacent to L superior temporal sulcus, lingual gyrus, head of caudate, occipito-parietal junction, body of caudate |
GREY: BIL orbitofrontal, lingual, temporal pole CV and caudate grey matter. R post- central and fusiform CV WHITE: Adjacent to R anterior cingulate and intraparietal sulcus. R external capsule and body of corpus callosum. Adjacent to L anterior cingulate, superior temporal gyrus and body of corpus callosum. |
|||||
| Rae C (2004) | 9(9/-) | 27(8/-) | 20 | 27(7/-) | Manual - temporal lobe |
- | CV iin BIL superior temporal sulcus, middle temporal gyrus |
| Cutter W.J (2006) | 27(27/-) | 27(8/-) | 21 | 27(7/-) | Manual region of interest |
BIL parieto-occipital lobes combined (grey and white) matter, R hippocampus and cuadate volumes |
Total cerebellar volume |
| VBM | GREY: BIL parieto-occipital, inferior temporal, caudate and thalamus volumes. L prefrontal CV. WHITE: R occipital, superior parietal, external capsule, body of corpus callosum and cerebellum. L occipitofrontal fasciculus. |
GREY: BIL cerebellar and anterior putamen grey matter volumes. WHITE: BIL middle temporal, orbito-frontal, genu of corpus callosum. R precentral and centrum semi-ovale |
|||||
| Holzapfel M (2006) | 10 (10/-) | 16(5/7-24) | 10 | 15(5/7-24) | VBM of white matter only |
WHITE: BIL internal capsule, L superior temporal gyrus, superior frontal gyrus, inferior and middle temporal gyri, inferior parietal lobule and temporoparietal junction |
- |
Abbreviations: TS (Turner Syndrome), C(Controls), BIL(Bilateral), L(Left), R(Right), CV(Cortical Volume).
Compared to the findings from sMRI studies of lobar volumes in TS, there is much less consistency in the findings of sMRI studies that examined brain anatomy at finer spatial resolution using hand-tracing of regions of interest (Rae et al., 2004), or automated and spatially non-biased voxel-based morphometry (VBM) (Cutter et al., 2006; Good et al., 2003; Molko et al., 2004). This is especially true for studies of the cerebral cortex. For example, CV in the left parieto-occipital junction, right primary motor and superior temporal cortex of TS individuals have been reported as both increased (Molko et al., 2003; Rae et al., 2004) and decreased (Cutter et al., 2006) as compared to controls. Such contrasting findings may be due to true neurostructural heterogeneity in TS, or differences in the clinical and controls samples used across studies with respect to demographic, genetic or endocrine features. Alternatively, the fact that most conflicting findings occur in those cortical regions where people with TS also have significant differences from controls in sulcal morphology (Molko et al., 2003; Molko et al., 2004), suggests that group differences in cortical shape may be confounding group differences in regional CV. However, cortical shape has not been directly modeled in any of the existent studies of regional CV in TS. To date, such studies have used volume-based registration to align sMRI scans across individuals and then measured CV in a voxel-by-voxel manner (VBM). This approach has three important limitations.
Firstly, compared to surface-based methods, volume-based alignment of scans is not able to take into account the complex and highly variable folding patterns of the cortical sheet. Because these folding patterns are known to vary between females with TS and controls (Molko et al., 2003), prior studies may not have been comparing like with like. That is, unless an attempt is made to “line-up” surface features of the cortex across scans before comparing CV between groups, then systematic differences in sulcal position between groups could result in one group’s sulcus being compared to another group’s gyrus.
Secondly, VBM studies only compare regional CV. However, CV within a given region of the cortical sheet is itself a product of two lower-order spatial properties -– cortical thickness (CT) and surface area (SA). Unless CV is broken down into CT and SA there is a risk of falsely concluding that a given cortical region is structurally unaltered in TS when in fact CT and SA for that regions may show marked alterations in “opposing” directions (such that their product - CV - does not show group differences). Conversely, group differences in CV may reflect abnormalities of CT only, SA only, or both these properties. It is important to distinguish these possibilities because measures of CT and SA capture very different sets of biological processes as evidenced by their differing evolutionary histories (Rakic, 1995), developmental trajectories (Armstrong et al., 1995; Sowell et al., 2007), and genetic determinants (Panizzoni et al., 2007). Furthermore, SA is determined by overall brain size and the degreee of cortical folding (gyrification), which are shaped by distinct influences. Decomposing cortical alterations in TS into these distinct components would help to focus investigation of the molecular and developmental pathways that lie between genetic and neurobiological alterations in TS.
Thirdly, there is emerging evidence that compared to a VBM assessment of CV across the cortex, a vertex-based assessment of CT after surface-based registration provides a more sensitive and informative marker of cortical differences between clinical groups (Bermundez P, 2008; Park HJ, 2009).
Furthermore, by inter-relating measures of CT at different vertices, it is possible to describe patterns of structural co-variance across the cortex. The extent to which cortical regions are structurally similar to each other (as indexed by CT co-variance) is neurobiologically meaningful because in typical development (i) cortical regions that show high CT co-variance also show strong shared genetic influences on CT (Schmitt et al., 2008), and (ii) maps of CT co-variance between cortical regions have been found to strongly resemble maps of cortico-cortical connectivity derived from other techniques such as diffusion-tensor imaging (DTI) of white matter tracts (Lerch et al., 2006) and fMRI studies of “functional connectivity” (Chen et al., 2008; Toro et al., 2008). Maps of CT co-variance also vary as a function of disease processes (He et al., 2008), developmental stage, cognitive ability and skill proficiency (Bermundez P, 2008; Lerch et al., 2006). Therefore, if a comparison of CT-covariance amongst females with TS to CT-covariance amongst typically developing controls identifies regional differences - then such regions would represent strong candidate networks for further investigation of structural and functional dysconnectivity in TS with DTI and fMRI.
In summary, surface-based methods for measuring cortical morphometry have multiple potential advantages. However they have not yet been exploited in the study of the TS neuroanatomical phenotype. Therefore we applied a fully automated and well-validated (Kabani et al., 2001; Shaw et al., 2008) method for measuring cortical morphometry (MacDonald et al., 2000) to process brain sMRI data acquired from 24 females with TS and 19 healthy female controls matched for age and verbal intelligence quotient. We have previously characterized this sample using Voxel-Based and Region of Interest methods (Cutter et al., 2006). This allows us to qualitatively compare the findings of volume-based vs surface-based approaches to cortical morphometry. In order to better contextualize our findings, we conducted a systematic review of all sMRI studies conducted in TS to date, and summarize the main findings of these studies in Table 1.
In this paper, we apply surface-based morphometry to address the following questions. Firstly, is the well-replicated reduction of parietal lobe volume in TS contributed to by reduced parietal lobe CV? Secondly, if lobar CV alterations are present in TS - are these due to alterations in CT, SA or both? Thirdly, is there any evidence for alterations in SA and cortical folding in TS? Fourthly, can a spatially non-biased assessment of CT across the cortical sheet in TS using surface-based registration of scans clarify some of the inconsistencies in VBM findings? Fifthly, is there any evidence of selective alterations in co-coordinated CT development in TS compared to controls as indexed by the proxy measure of disrupted inter-regional correlations in CT?
MATERIALS AND METHODS
Systematic Literature Review
We identified all available studies using sMRI to compare brain anatomy in people with clinically and karyotypically confirmed TS, and controls. We excluded case studies, and reviews. Publications were identified by searching MEDLINE, EMBASE and PsychINFO (up to September 8th 2009) using combinations of the following search terms; turner*, syndrome, brain, magnetic, resonance, imaging. This was further supplemented by hand-searches of bibliographies, and repeat database searches using the names of all authors in included studies. The fields of information extracted are detailed in Table 1.
Participants, Karyotyping and Scan Acquisition
These methodological aspects have been detailed previously in our earlier report (Cutter et al., 2006). The sample included in the present study is unchanged except for the exclusion of one control and three TS participants in whom derived models of the cortex did not pass our quality control procedures. Therefore 24 females with TS and 19 healthy controls were included in this study. Briefly, participants with TS were recruited through a university-based behavioural genetics research programme run in collaboration with the South London and Maudsley NHS Foundation Trust, and typically developing controls through local advertisement. Karyotype was determined for each participant with TS by analyzing thirty metaphase spreads using conventional cytogenetic techniques. No participants suffered from any psychiatric or medical disorders that would grossly affect brain function (e.g. epilepsy, neurosurgery, head injury, hypertension, schizophrenia) as determined by structured clinical interview and examination, as well as review of medical notes. Structural MRI data was acquired using a GE Signa 1.5T Neuro-optimised MR system (General Electric, Milwaukee, Wisconsin). Whole head coronal (3D) SPGR images (TR=14msec, TE=3msec, 256×192 acquisition matrix, 124mm × 1.5mm slices) were obtained from all subjects. Ethical approval was obtained from the local Ethics Committee, and informed written consent was obtained from all participants. Participant characteristics are detailed in Table 2.
TABLE 2.
Characteristics of participants in the study.
| Characteristic | Group | |
|---|---|---|
| Controls (n=19) | Turner Syndrome (n=24) | |
| Age yrs [mean(s.d), range] | 27 (7.9), 17-45 | 26 (7.3), 16-40 |
| VIQ [mean(s.d)] | 100 (13.0) | 106 (14.4) |
| PIQ [mean(s.d)] a | 107 (13.9) | 96 (19.2) |
| TBV cm3 [mean(s.d)] | 1176.6 (60.8) | 1204.3 (87.5) |
| Handedness [R/L] | 18/1 | 23/1 |
| X chromosome [parental/maternal] | - | 17/7 |
| Ever taken estrogen replacement | 1 | 24 |
| Ever taken androgen replacement | - | 11 |
| Ever taken growth hormone | - | 12 |
Abbreviations: VIQ(verbal intelligence quotient), PIQ(performance intelligence quotient), TBV(total brain volume)
Significant group difference (p=0.018)
Image Processing
Native MRI scans were submitted to the CIVET pipeline (version 1.1.8) (http://wiki.bic.mni.mcgill.ca/index.php/CIVET) to generate separate cortical models for each hemisphere as described previously (Giedd et al., 2007).
Briefly, this automated set of algorithms begins with linear transformation, correction of non-uniformity artifacts, and segmentation of each image into white matter, grey matter and CSF (Zijdenbos et al., 2002). Next, each image is fitted with 2 deformable mesh models to extract the white/gray and pial surfaces. These surface representations are then used to calculate CT at approximately 45,000 points per hemisphere, and aligned with an atlas to allow automated parcellation of the cortical sheet and comparison of CT at equivalent vertices across different scans. Parcellation generates measures of total lobar cortical volume, total surface area and mean thickness. The middle cortical surface, which lies at the geometric center between the inner and outer cortical surfaces, is used for the calculation of the surface area (Lyttelton, 2009). From these measures we derived estimates of total CV, total SA and mean CT for each lobe (using previously described anatomical definitions of lobar boundaries (Collins et al., 1995)) in each individual. A “gyrification index” (GI) for each hemisphere was defined as the ratio of total area of the cortical sheet in that hemisphere and the “convex hull” of the cortical surface (ie the smallest surface that would enclose the folded cortical sheet) (Zilles et al., 1988). A higher number reflects increased folding.
Statistical Analysis
Lobar Analyses
We first examined how cortical volume (CV), surface area (SA) and thickness (CT) varied between females with TS and controls across four lobes – frontal, parietal, temporal and occipital. The effect of group (TS vs controls) was examined for left and right lobes separately. Neither mean age nor mean TBV differed between groups. However, as both these factors account for a large proportion of variance in cortical anatomy they were included as co-variates in all analyses. As eight (one in each lobe) group comparisons were made for each measure of interest (CV, SA, or CT), a Bonferroni corrected p value of 0.004 was adopted as the threshold for statistical significance. We used linear regression to compare GI for each hemisphere between females with TS and controls with age and TBV entered as co-variates.
Vertex-based Analyses
Next, we complemented lobar analyses by an assessment of the effect of group (TS vs controls) on CT at each of approximately 45,000 points (vertices) on each hemisphere. The results for these analyses were visualized by projecting the effect of group as a t-statistic at each vertex on an examplar cortical surface in MNI space. For the linear model [thickness= intercept + β(Group) + error], the t-statistic is the ratio of β and the standard error of the slope. The t-statistic map was then thresholded using a false discovery rate (FDR) correction with the probability of a rejected null hypothesis being falsely rejected (q) set at 5%.
Differences between females with TS and controls in CT-covariance were studied by first selecting a “seed-voxel” (details of this selection are provided in the results section below), and then determining how group status influenced the correlation between CT at this vertex, and that at every other vertex on the cortical surface. A general linear model was run at every vertex, with “Group”, “Seed-Voxel CT” and the interaction between these two terms as predictor variables. The t-statistic associated with the “group by seed-vertex” interaction term at any given vertex represents the magnitude of “slope” difference between groups in the correlation of CT at that vertex and CT at the seed-vertex. The map of t-statistics for this interaction term was then thresholded using a FDR correction with q=0.05 – to identify cortical regions which were significantly more or less structurally similar to the seed vertex in females with TS compared to controls.
RESULTS
Details of all sMRI studies in TS identified by systematic literature review can be found in Table 1.
Subject characteristics are shown in Table 2. As expected, performance intelligence quotient (PIQ) was significantly lower in the group of individuals with TS than in controls. There were no other statistically significant psychometric or demographic differences between the two groups. Mean TBV was not significantly greater in TS than in controls.
Lobar Analyses (see Table 3)
TABLE 3.
Summary of differences between females with Turner Syndrome and healthy controls in total cortical volume (CV), total cortical surface area (SA), and mean cortical thickness (CT) for each cerebral lobe, by hemisphere.
| LEFT HEMISPHERE |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FRONTAL | PARIETAL | TEMPORAL | OCCIPITAL | |||||||||
| CV(mm3) | SA(mm2) | CT(mm) | CV(mm3) | SA(mm2) | CT(mm) | CV(mm3) | SA(mm2) | CT(mm) | CV(mm3) | SA(mm2) | CT(mm) | |
| CONTROLS | 97375.90 | 31880.50 | 3.55 | 47926.51 | 16881.41 | 3.26 | 83188.52 | 25612.39 | 3.59 | 29225.06 | 10447.05 | 3.27 |
| TURNERS SYNDROME | 99188.87 | 31044.61 | 3.68 | 48205.35 | 16155.36 | 3.45 | 90695.16 | 25405.65 | 3.70 | 31298.80 | 9889.74 | 3.52 |
| DIFFERENCE | 1812.97 | −835.89 | 0.13 | 278.84 | −726.06 | 0.19 | 7506.64 | −206.74 | 0.11 | 2073.74 | −557.31 | 0.25 |
| INCREASED (+) /DECREASED (−) IN TS | (+) | (−) | (+) | (+) | (−) | (+) | (+) | (−) | (+) | (+) | (−) | (+) |
| ANOVA/ANCOVA F STATISTIC | 0.12 | 5.17 | 0.18 | 1.35 | 6.72 | 11.78 | 15.94 | 5.05 | 3.82 | 0.98 | 9.38 | 14.95 |
| P VALUE | 0.75 | 0.03 | 0.07 | 0.25 | 0.01 | 0.001 | 0.0005 | 0.03 | 0.06 | 0.33 | 0.004 | 0.0005 |
|
RIGHT HEMISPHERE |
||||||||||||
| FRONTAL | PARIETAL | TEMPORAL | OCCIPITAL | |||||||||
|
|
||||||||||||
| CV(mm3) | SA(mm2) | CT(mm) | CV(mm3) | SA(mm2) | CT(mm) | CV(mm3) | SA(mm2) | CT(mm) | CV(mm3) | SA(mm2) | CT(mm) | |
|
|
|
|
|
|||||||||
| CONTROLS | 93658.09 | 31150.49 | 3.54 | 43125.81 | 15926.63 | 3.23 | 88417.63 | 27406.77 | 3.56 | 28074.00 | 10334.79 | 3.23 |
| TURNERS SYNDROME | 95164.96 | 30521.95 | 3.65 | 42457.73 | 14986.91 | 3.39 | 96400.49 | 27636.91 | 3.67 | 30079.67 | 10000.20 | 3.49 |
| DIFFERENCE | 1506.87 | −628.54 | 0.11 | −668.08 | −939.71 | 0.17 | 7982.86 | 230.14 | 0.11 | 2005.66 | −334.59 | 0.26 |
| INCREASED (+) /DECREASED (−) IN TS | (+) | (−) | (+) | (−) | (−) | (+) | (+) | (+) | (+) | (+) | (−) | (+) |
| ANOVA/ANCOVA F STATISTIC | 0.17 | 3.91 | 3.11 | 2.99 | 10.31 | 8.63 | 14.35 | 0.82 | 3.54 | 2.39 | 3.14 | 18.83 |
| P VALUE | 0.68 | 0.06 | 0.09 | 0.09 | 0.003 | 0.006 | 0.001 | 0.37 | 0.07 | 0.13 | 0.08 | 0.0005 |
The pattern of differences between females with TS and controls varied for each of the lobar measures of interest (SA, CT and CV).
Statistically significant differences between females with TS and controls in both SA and CT were found in the right parietal and left occipital lobes - but in opposite directions (i.e. SA was reduced but CT was increased in TS). Moreover in these regions there were no significant differences in CV (the product of SA and CT) – suggesting that opposite effects in SA and CT made differences in CV undetectable. Cortical thickness was also greater in females with TS than controls in the left parietal and right occipital lobe. Cortical volume on the other hand showed significant group differences in the temporal lobes bilaterally, where CV was greater in females with TS than controls. Here, CV increases were associated with CT increases [although these did not reach the threshold for statistical significance (left p= 0.06, right p=0.07)].
Consistent with our finding that SA was less in females with TS compared to controls in almost all lobes, both left (F=11.22, p=0.002) and right hemispheric (F= 7.78, p=0.008) GI were significantly reduced in TS.
Cortex-wide Assessment of Cortical Thickness (Fig 1 and Table 4)
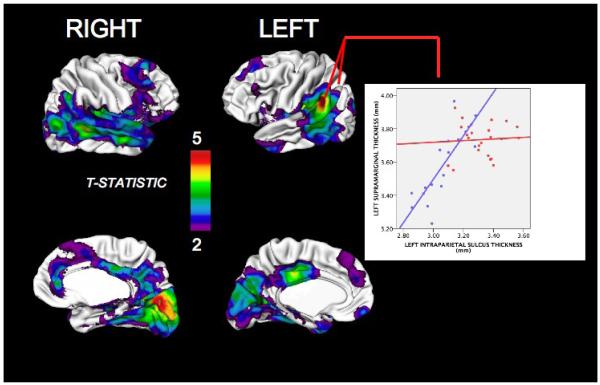
FIGURE 1.
Map of regions where females with Turner Syndrome (TS) show statistically significant (after false discovery rate corrections for multiple comparisons) cortical thickening compared to healthy controls. “Warmer” colors indicate greater difference in cortical thickness (CT) between groups. The accompanying plot details mean CT (+/− 95% confidence intervals for the mean) for females with TS and controls at a region of highly significant group difference in CT – the left intraparietal sulcus. Regions of increased cortical thickness in females with TS compared to controls were restricted to the parahippicampal gyri bilaterally, and are not shown here.
TABLE 4.
Talairach co-ordinates for statistically significant vertices of peak cortical thickness difference between between females with Turner Syndrome and healthy controls after false discovery rate correction for multiple comparisons (q<0.05).
|
TALAIRACH CO-ORDINATES |
|||
|---|---|---|---|
| X | Y | Z | |
| TURNER SYNDROME > CONTROLS | |||
| LEFT | |||
| Intraparietal sulcus | −28 | −69 | 26 |
| Inferior temporal gyrus | −52 | −42 | −24 |
| Middle frontal gyrus | −32 | 51 | 8 |
| RIGHT | |||
| Inferior temporal gyrus | 51 | −40 | −25 |
| Lateral occipital gyrus | 29 | −95 | −9 |
| Cingulate gyrus | 1 | 17 | −19 |
| Rectal gyrus | 2 | 38 | −11 |
| CONTROLS > TURNER SYNDROME | |||
| LEFT | |||
| Parahippocampal gyrus | −17 | −18 | −23 |
| RIGHT | |||
| Parahippocampal gyrus | 28 | −24 | −17 |
| Cingulate gyrus | 2 | −4 | 31 |
Our vertex-based analysis of CT replicated the lobar findings for CT; but also identified additional pre-frontal regions of CT increase in TS. Regardless of whether or not age was included as a covariate, CT was significantly increased bilaterally throughout lateral occipital, parietal, temporal and inferio-lateral frontal cortices in females with TS compared to controls. Occipital and parietal CT increases also partially extended into the medial cortical surface. Within this confluent region of significant CT increase, peaks were evident in bilateral inferior temporal gyri, left intraparietal sulcus and right lateral occipital gyrus. Here, the t-statistic for group difference in CT was especially elevated (e.g. surviving FDR correction at q=0.0005) - reflecting up to 15% increased CT in TS. Females with TS also showed a significant increase in CT in three distinct pre-frontal regions; right orbitofrontal, right anterior cingulate and left anterior middle frontal gyri.
In contrast, cortical thickness decreases in females with TS compared to controls were limited to the parahippocampal cortex bilaterally, and the right isthmus of the cingulate. Talairach co-ordinates for vertices of peak group difference are provided in Table 4.
Cortico-cortical structural co-variance
Of the three peak parieto-occipital foci of CT increase in TS, that located in the left intraparietal sulcus (lPS) was selected as the “seed voxel” with which to examine structural co-variance in CT across the cortical sheet. This was because; (i) it was one of the three most thickened cortical regions in TS compared to controls; (ii) the IPS has been reported as functionally abnormal in all TS fMRI studies conducted to date (Hart et al., 2006; Kesler et al., 2004; Kesler et al., 2006; Molko et al., 2003) and; (iii) its pattern of connectivity with the rest of the cortex in typically developing subjects has been extensively described (Borra et al., 2008; Takahashi et al., 2008; Toro et al., 2008).
There were no cortical regions that showed increased CT-covariance with the left IPS in females with TS compared to controls. However, there were several cortical regions that were significantly less structurally similar to the left IPS in people with TS. This reduction in structural similarity was most pronounced in TS between the left IPS and left supramarginal, left cingulate, and right medial occipital gyri. Structural covariance was also significantly reduced in TS between the left IPS and (i) a continuous occipito-temporal region extending bilaterally and including the cuneus, precuneus, fusiform, temporal pole, temporo-occipital junction, middle and superior temporal cortices, and (ii) bilateral frontal regions including the orbitofrontal cortex, anterior cingulate and dorsolateral prefrontal cortices.
DISCUSSION
Lobar Findings
Our lobar findings that are most directly comparable with existing sMRI studies in TS relate to CV alterations. We identified significantly increased temporal lobe CV bilaterally in TS, and non-significant CV increases in all other lobes (except the right parietal lobe where CV was non-significantly reduced in TS). Significant CV increases within both temporal lobes in TS compared to controls were also found in one (Rae et al., 2004) of the two available studies of temporal CV in TS (Brown et al., 2002; Rae et al., 2004). The fact that we did not identify significant group differences in occipital or parietal CV, suggests that our previous finding, in the same sample (using hand-tracing), of significantly reduced total right parieto-occipital lobe volume (Cutter et al., 2006), was probably driven by reductions in white matter volume in these regions. Our findings differ from those of Brown et al (Brown et al., 2002), who studied a younger sample than ours and found significant lobar CV abnormalities in TS that were restricted to both parietal lobes - where CV was reduced in TS compared to age-matched controls. Therefore, it is possible that lobar CV differences between individuals with TS and controls in temporal and parietal lobes vary across the lifespan. Specifically, reduced parietal CV in TS during childhood relative to controls may no longer be evident by adulthood, whereas increased temporal lobe CV in TS may only emerge after childhood. Testing these hypotheses will require longitudinal sMRI study designs.
Our analytic approach allowed us to move beyond CV in the assessment of lobar cortical anatomy, by simultaneously measuring the two determinants of CV – CT and SA. We identified significantly increased CT in TS compared to controls within the occipital and parietal lobes bilaterally, and significantly decreased SA (when controlling for total brain volume) within the right parietal lobe and left occipital lobe. Moreover, our findings within the right parietal lobe, for example, highlight how marked alterations in CT and SA within a region can ‘cancel each-other out’ – so that measurement of CV alone fails to identify the significant group differences present in the neuroanatomy of that region. Given this, and the fact that alterations of CT and SA are likely to be due to different sets of genetic (Panizzoni et al., 2007), hormonal and environmental factors, acting at different developmental stages (Park HJ, 2009), it will be crucial to disambiguate these two properties of the cortex in future sMRI studies in TS.
The decreased parietal SA we found in TS as compared to controls could be due to; (i) a generalized reduction in brain “radius” which would manifest as a lowered total brain volume (which was not found in our sample); (ii) locally reduced brain “radius” within the parietal lobe (which is consistent with our previous finding of reduced total parietal lobe volume in this sample (Cutter et al., 2006)); or (iii) reduced cortical gyrification within the parietal lobe in TS (which is consistent with reports of reduced sulcal depth within the right parietal lobe in TS (Molko et al., 2003). Our finding of reduced hemispheric gyrification in TS strongly suggests that abnormalities of cortical folding underlie SA reductions in TS, and points towards a role for X-linked genes or sex-steroids in cortical folding. The hypothesis that differences in SA reflect regionally altered cortical folding in TS could be directly tested in future studies using automated methods of sMRI analyses. Such methods have been successfully applied to other neurogenetic syndromes (e.g. William and velocardiofacial syndrome) and established that chromosomal abnormalities in humans can lead to focal alterations in cortical folding that coincide with altered CT (Bearden et al., 2008; Gaser et al., 2006).
Mean CT was greater in TS compared to controls in all lobes, and significantly so within parietal and occipital lobes bilaterally. The possible mechanisms underlying these CT increases are considered below. Vertex-based analyses of CT enabled us to establish if increased mean lobar CT in these regions was due to a generalized or more heterogenous pattern of parieto-occipital cortical thickening, and if there were more localized regions of CT alteration in other lobes that did not translate into significant group differences in mean lobar CT.
Vertex-based assessment of CT in TS
Our lobar CT findings were validated and extended by our vertex-based findings. Our previous VBM analysis of the cortex within the same sample (Cutter et al., 2006) found widespread CV reductions in a distribution that closely matches the CT increases we now report. This suggests that CV reductions identified by VBM in TS may be driven by reduced SA, as has been found in parallel VBM/surface based analyses of other clinical groups such as the congenitally blind (Hae-Jong Park, 2009). Another possibility is that group differences in CT are a result of group differences in sMRI tissue-contrast at the grey-white tissue interface (e.g. due to group differences in intracortical myelination (Sowell et al., 2004)) that is used to identify the inner cortical surface during surface-based morphometry (but which is not explicitly modeled in VBM).
However, the distribution of CT alterations in TS that we identified is highly consistent with the findings of other studies regarding (i) the functional neuroanatomy in typically developing individuals of cognitive tasks that are known to be impaired in TS, and (ii) the regional distribution of differences in cortical activity between people with TS and controls during the execution of these tasks. Thus, we found significantly increased CT in TS within regions that are recruited during mental rotation tasks in healthy controls [(Zacks, 2008) - bilateral intraparietal, temporal-parietal and lateral occipital sulci, and left precentral and posterior temporal sulci]. Impaired mental rotation is commonly reported in TS (Ross et al., 2000). Furthermore, we also find CT to be increased in many of the cortical regions where others have identified functional differences between people with TS and controls using fMRI [(Molko et al., 2003) – bilateral intraparietal sulci, (Tamm et al., 2003) - lateral frontal and primary sensory cortices, (Good et al., 2003) - anterior cingulate, inferior frontal, and temporal cortices]. This suggests that differences in CT and functional alterations of the cortex in TS are inter-related, although the direction of any putative causal relationship remains unclear. A practical consequence of this, however, is that future fMRI studies in TS would benefit from image analysis approaches that allow cortical activation and structure to be interrelated (Lu et al., 2009).
Cortical Thickness Co-variance Analyses
These analyses indicated that the structural similarity (as indexed by CT covariance) between the left IPS and other cortical regions is reduced in people with TS compared to controls. Structural similarity with the left IPS was most markedly reduced in TS within left supramarginal, left cingulate, right medial occipital gyri, and large parts of the lateral and inferior temporal cortices bilaterally. These regions are notable in that (i) they are known to be connected to the IPS by white matter tracts which are thought to be disrupted in TS (e.g. inferior and superior longitudinal fasciculus) based on diffusion tensor imaging (DTI) and sMRI data (Cutter et al., 2006; Holzapfel et al., 2006; Molko et al., 2004), and (ii) simultaneous functional abnormalities within many of these regions have been previously documented in TS during fMRI studies [(Kesler et al., 2006; Molko et al., 2003) - left intra-parietal sulcus, left supramarginal gyrus, superior and middle temporal cortices, cingulate and right inferior and middle frontal cortices during numerical reasoning].
This convergence of these findings suggests that reductions in the structural connectivity, and co-ordinated functioning, between the left IPS and a number of other cortical regions in TS may be associated with a disruption in their co-ordinated structural development – resulting in reduced structural similarity between the left IPS and these regions. Thus, multimodal neuroimaging studies are required in TS in order simultaneously assess the functional and structural connectivity between cortical regions and relate this to CT co-variance.
Mechanisms for altered cortical anatomy in TS
Our study design and sample size did not allow us to examine how cortical anatomy in TS might vary as a function of developmental stage, karyotype, previous estrogen or androgen hormone treatment history, X chromosome parent-of-origin, cognitive profile or psychiatric co-morbidity. Also, we were not able to directly examine the cellular correlates of the neuroimaging differences we identified. However, these findings further refine our understanding of the neuroanatomical phenotype that must be explained in relation to the genetic and environmental factors that are associated with X monosomy.
An obvious candidate genetic factor is the direct effect of X chromosome gene-dosage abnormalities on brain development (Davies and Wilkinson, 2006). A recent study related karyotype and chromosome structure in TS to cognition, and identified altered gene-dosage within Xp22.3 as a strong candidate for the distinctive phenotype of impaired visuo-spatial cognition (Zinn et al., 2007). Of the approximately 30 genes within this region, two that escape X-inactivation are of particular interest - steroid sulfatase (STS) and neuroligin 4 (NLGN4X).
Steroid sulfatase is involved in neurosteroid metabolism, is expressed in the frontal cortex, and has been linked to anxiety-like-traits within a mouse model of TS (Davies et al., 2007). Neurologin 4 is involved in synaptic formation and maintenance (Craig and Kang, 2007), and has been linked to autism spectrum disorders (Jamain et al., 2003), which some have suggested have partially overlapping behavioural phenotypes with TS (Marco and Skuse, 2006). The early neurodevelopmental effects of haploinsufficiency in these and other X chromosome genes would be best examined in animal models (Probst et al., 2008) which would allow phenotypes of interest such as CT and SA to be examined developmentally at macroscopic, cellular and molecular levels.
It is unlikely, however, that direct effects of haploinsufficiency of X chromosome genes in TS are the sole determinants of the neurobiological phenotype. Brain development in TS could be shaped by several aspects of the disorder that are themselves a consequence of gene-dosage abnormalities. For example altered hormonal milieu is a consequence of ovarian failure (and its treatment) in TS, and this is likely to influence the neurobiological phenotype in TS given the important role gonadal steroids play in neurodevelopment (McCarthy, 2008) and perhaps also brain aging (Craig et al 2008). Hormonal treatments for short stature frequently used by TS women may also influence brain anatomy (Cutter et al., 2006). A further possibility to be considered is that that genetically determined aspects of the cognitive and behavioural phenotype in TS phenotype (e.g. altered sampling of the social environment (Mazzola et al., 2006)) can also shape brain development in their own right by impacting on experience-dependent neuronal plasticity.
In summary, our findings indicate that X chromosome monosomy in humans is associated with regional increases in CT and decreases in SA/gyrification, so that studies of CV alone will fail to properly capture the neurostructural phenotype. Thus, haploinsufficiency of X chromosome genes is associated with alterations in the mechanistically distinct pathways that shape SA/gyrification (eg cell-cycle dynamics of neural progenitor cell pool) and CT (e.g. denderitic arborization/pruning) - although further research is required to establish the cellular correlates of our neuroimaging findings. Finally, by fine-mapping CT, and CT co-variance across the cortex in TS, we further implicate a number of cortical regions known to be relevant to the domains of cognition that are impaired in TS, and established as showing altered function and structural inter-connectivity in TS.
FIGURE 2.
Map showing regions where cortical thickness (CT) in females with Turner Syndrome (TS) shows significantly less co-variance with left intra-parietal sulcus CT than is seen in healthy controls. All colored regions are where significant group differences in CT co-variance survived false discovery rate correction for multiple comparisons (q<0.05). The accompanying plot illustrates group differences in CT co-variance between the left intraparietal sulcus and the left supramarginal gyrus. There is a strong relationship between cortical thickness in these two regions amongst healthy controls, that is not present in females with TS.
ACKNOWLEDGMENTS
This work was supported by a UK Medical Research Council Clinical Research Training Fellowship (A.R – G0701370), and partially supported by the MRC UK AIMS network, the Psychiatry Research Trust, and the South London and Maudsley NHS Foundation Trust (National Division). The authors also wish to thank the participants who took part in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K. The ontogeny of human gyrification. Cerebral Cortex. 1995;5:56–63. doi: 10.1093/cercor/5.1.56. [DOI] [PubMed] [Google Scholar]
- Barlow DP. Gametic imprinting in mammals. Science. 1995;270:1610–1613. doi: 10.1126/science.270.5242.1610. [DOI] [PubMed] [Google Scholar]
- Bearden CE, van Erp TG, Dutton RA, Lee AD, Simon TJ, Cannon TD, Emanuel BS, McDonald-McGinn D, Zackai EH, Thompson PM. Alterations in Midline Cortical Thickness and Gyrification Patterns Mapped in Children with 22q11.2 Deletions. Cerebral Cortex. 2008 doi: 10.1093/cercor/bhn064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermundez P, L.J., Evans A, Zatorre RJ. Neuroanatomical Correlates of Musicianship as Revealed by Cortical Thickness and Voxel-Based Morphometry. Cerebral Cortex. 2008 doi: 10.1093/cercor/bhn196. Epub ahead of print, Dec 10th. [DOI] [PubMed] [Google Scholar]
- Borra E, Belmalih A, Calzavara R, Gerbella M, Murata A, Rozzi S, Luppino G. Cortical connections of the macaque anterior intraparietal (AIP) area. Cerebral Cortex. 2008;18:1094–1111. doi: 10.1093/cercor/bhm146. [DOI] [PubMed] [Google Scholar]
- Brown WE, Kesler SR, Eliez S, Warsofsky IS, Haberecht M, Patwardhan A, Ross JL, Neely EK, Zeng SM, Yankowitz J, Reiss AL. Brain development in Turner syndrome: a magnetic resonance imaging study. Psychiatry Research. 2002;116:187–196. doi: 10.1016/s0925-4927(02)00086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ, He Y, Rosa-Neto P, Germann J, Evans AC. Revealing Modular Architecture of Human Brain Structural Networks by Using Cortical Thickness from MRI. Cerebral Cortex. 2008 doi: 10.1093/cercor/bhn003. bhn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DL, Holmes CJ, Peters TM, Evans AC. Automatic 3-D model-based neuroanatomical segmentation. 1995. pp. 190–208.
- Craig AM, Kang Y. Neurexin-neuroligin signaling in synapse development. Current Opinion in Neurobiology. 2007;17:43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutter WJ, Daly EM, Robertson DM, Chitnis XA, van Amelsvoort TA, Simmons A, Ng VW, Williams BS, Shaw P, Conway GS, Skuse DH, Collier DA, Craig M, Murphy DG. Influence of X chromosome and hormones on human brain development: a magnetic resonance imaging and proton magnetic resonance spectroscopy study of Turner syndrome. Biological Psychiatry. 2006;59:273–283. doi: 10.1016/j.biopsych.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Davies W, Humby T, Isles AR, Burgoyne PS, Wilkinson LS. X-monosomy effects on visuospatial attention in mice: a candidate gene and implications for Turner syndrome and attention deficit hyperactivity disorder. Biological Psychiatry. 2007;61:1351–1360. doi: 10.1016/j.biopsych.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Davies W, Wilkinson LS. It is not all hormones: alternative explanations for sexual differentiation of the brain. Brain Research. 2006;1126:36–45. doi: 10.1016/j.brainres.2006.09.105. [DOI] [PubMed] [Google Scholar]
- Gaser C, Luders E, Thompson PM, Lee AD, Dutton RA, Geaga JA, Hayashi KM, Bellugi U, Galaburda AM, Korenberg JR, Mills DL, Toga AW, Reiss AL. Increased local gyrification mapped in Williams syndrome. Neuroimage. 2006;33(1):46–54. doi: 10.1016/j.neuroimage.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Wallace GL, Lenroot RK, Lerch JP, Wells EM, Blumenthal JD, Nelson JE, Tossell JW, Stayer C, Evans AC, Samango-Sprouse CA. XXY (Klinefelter syndrome): a pediatric quantitative brain magnetic resonance imaging case-control study. Pediatrics. 2007;119:e232–240. doi: 10.1542/peds.2005-2969. [DOI] [PubMed] [Google Scholar]
- Good CD, Lawrence K, Thomas NS, Price CJ, Ashburner J, Friston KJ, Frackowiak RS, Oreland L, Skuse DH. Dosage-sensitive X-linked locus influences the development of amygdala and orbitofrontal cortex, and fear recognition in humans. Brain. 2003;126:2431–2446. doi: 10.1093/brain/awg242. [DOI] [PubMed] [Google Scholar]
- Hae-Jong Park JDL, Kim Eung Yeop, PArk Bumhee, Oh Maeng-Keun, Lee SungChul, Kim Jae-Jin. Morphological alterations in the congenital blind based on the analysis of cortical thickness and surface area. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.03.076. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Hart SJ, Davenport ML, Hooper SR, Belger A. Visuospatial executive function in Turner syndrome: functional MRI and neurocognitive findings. Brain. 2006;129:1125–1136. doi: 10.1093/brain/awl046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Chen Z, Evans A. Structural insights into aberrant topological patterns of large-scale cortical networks in Alzheimer’s disease. Journal of Neuroscience. 2008;28:4756–4766. doi: 10.1523/JNEUROSCI.0141-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzapfel M, Barnea-Goraly N, Eckert MA, Kesler SR, Reiss AL. Selective alterations of white matter associated with visuospatial and sensorimotor dysfunction in turner syndrome. Journal of Neuroscience. 2006;26:7007–7013. doi: 10.1523/JNEUROSCI.1764-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nature Genetics. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabani N, Le GG, MacDonald D, Evans AC. Measurement of cortical thickness using an automated 3-D algorithm: a validation study. Neuroimage. 2001;13(2):375–80. doi: 10.1006/nimg.2000.0652. [DOI] [PubMed] [Google Scholar]
- Kesler SR, Haberecht MF, Menon V, Warsofsky IS, Dyer-Friedman J, Neely EK, Reiss AL. Functional neuroanatomy of spatial orientation processing in Turner syndrome. Cerebral Cortex. 2004;14:174–180. doi: 10.1093/cercor/bhg116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Menon V, Reiss AL. Neuro-functional differences associated with arithmetic processing in Turner syndrome. Cerebral Cortex. 2006;16:849–856. doi: 10.1093/cercor/bhj028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch JP, Worsley K, Shaw WP, Greenstein DK, Lenroot RK, Giedd J, Evans AC. Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. Neuroimage. 2006;31:993–1003. doi: 10.1016/j.neuroimage.2006.01.042. [DOI] [PubMed] [Google Scholar]
- Lu LH, Dapretto M, O’Hare ED, Kan E, McCourt ST, Thompson PM, Toga AW, Bookheimer SY, Sowell ER. Relationships between Brain Activation and Brain Structure in Normally Developing Children. Cerebral Cortex. 2009 doi: 10.1093/cercor/bhp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon MF. Gene action in the X-chromosome of the mouse (Mus musculus L.) Nature. 1961;190:372–373. doi: 10.1038/190372a0. [DOI] [PubMed] [Google Scholar]
- Lyttelton O, Karama S, Ad-Dab’bagh Y, Zatorre RJ, CArbonell F, Worsley K, Evans AC. Positional and surface area asymmetry of the human cerebral cortex. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.03.063. Epub ahead of print Apr 2nd. [DOI] [PubMed] [Google Scholar]
- MacDonald D, Kabani N, Avis D, Evans AC. Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage. 2000;12:340–356. doi: 10.1006/nimg.1999.0534. [DOI] [PubMed] [Google Scholar]
- Marco EJ, Skuse DH. Autism-lessons from the X chromosome. Soc Cogn Affect Neurosci. 2006;1:183–193. doi: 10.1093/scan/nsl028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzola F, Seigal A, MacAskill A, Corden B, Lawrence K, Skuse DH. Eye tracking and fear recognition deficits in Turner syndrome. Soc Neurosci. 2006;1:259–269. doi: 10.1080/17470910600989912. [DOI] [PubMed] [Google Scholar]
- McCarthy MM. Estradiol and the developing brain. Physiological Reviews. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molko N, Cachia A, Riviere D, Mangin JF, Bruandet M, Le Bihan D, Cohen L, Dehaene S. Functional and structural alterations of the intraparietal sulcus in a developmental dyscalculia of genetic origin. Neuron. 2003;40:847–858. doi: 10.1016/s0896-6273(03)00670-6. [DOI] [PubMed] [Google Scholar]
- Molko N, Cachia A, Riviere D, Mangin JF, Bruandet M, LeBihan D, Cohen L, Dehaene S. Brain anatomy in Turner syndrome: evidence for impaired social and spatial-numerical networks. Cerebral Cortex. 2004;14:840–850. doi: 10.1093/cercor/bhh042. [DOI] [PubMed] [Google Scholar]
- Murphy DG, Allen G, Haxby JV, Largay KA, Daly E, White BJ, Powell CM, Schapiro MB. The effects of sex steroids, and the X chromosome, on female brain function: a study of the neuropsychology of adult Turner syndrome. Neuropsychologia. 1994;32:1309–1323. doi: 10.1016/0028-3932(94)00065-4. [DOI] [PubMed] [Google Scholar]
- Murphy DG, DeCarli C, Daly E, Haxby JV, Allen G, White BJ, McIntosh AR, Powell CM, Horwitz B, Rapoport SI, et al. X-chromosome effects on female brain: a magnetic resonance imaging study of Turner’s syndrome. Lancet. 1993;342:1197–1200. doi: 10.1016/0140-6736(93)92184-u. [DOI] [PubMed] [Google Scholar]
- Ogata T, Matsuo N. Turner syndrome and female sex chromosome aberrations: deduction of the principal factors involved in the development of clinical features. Human Genetics. 1995;95:607–629. doi: 10.1007/BF00209476. [DOI] [PubMed] [Google Scholar]
- Panizzoni CF-N, Eyer L, Jerningan T, Prom-Wormley E, Neale M, Jacobsen K, Lyons MJ, Grant MD, Franz CE, Xian H, Tsuang M, Fischl B, Seidman L, Dale A, Kremen W. Distinct Genetic Influence on Cortical Surface Area and Cortical Thickness. Cerebral Cortex. 2007 doi: 10.1093/cercor/bhp026. Advanced Access published online March 18th. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, L.J., Kim EY, Park B, Oh MK, Kim JJ. Morphological alterations in the congenital blind based on the analysis of cortical thickness and surface area. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.03.076. Epub ahead of print Apr 8th. [DOI] [PubMed] [Google Scholar]
- Probst FJ, Cooper ML, Cheung SW, Justice MJ. Genotype, phenotype, and karyotype correlation in the XO mouse model of Turner Syndrome. Journal of Heredity. 2008;99:512–517. doi: 10.1093/jhered/esn027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae C, Joy P, Harasty J, Kemp A, Kuan S, Christodoulou J, Cowell CT, Coltheart M. Enlarged temporal lobes in Turner syndrome: an X-chromosome effect? Cerebral Cortex. 2004;14:156–164. doi: 10.1093/cercor/bhg114. [DOI] [PubMed] [Google Scholar]
- Rakic P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends in Neurosciences. 1995;18:383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Mazzocco MM, Greenlaw R, Freund LS, Ross JL. Neurodevelopmental effects of X monosomy: a volumetric imaging study. Annals of Neurology. 1995;38:731–738. doi: 10.1002/ana.410380507. [DOI] [PubMed] [Google Scholar]
- Ross J, Zinn A, McCauley E. Neurodevelopmental and psychosocial aspects of Turner syndrome. Ment Retard Dev Disabil Res Rev. 2000;6:135–141. doi: 10.1002/1098-2779(2000)6:2<135::AID-MRDD8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Schmitt JE, Lenroot R, Ordaz SE, Wallace GL, Lerch JP, Evans AC, Prom EC, Kendler KS, Neale MC, Giedd JN. Variance decomposition of MRI-based covariance maps using genetically informative samples and structural equation modeling. Neuroimage. 2008 doi: 10.1016/j.neuroimage.2008.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. Journal of Neuroscience. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, Xu D, Zhu H, Thompson PM, Toga AW. Sex Differences in Cortical Thickness Mapped in 176 Healthy Individuals between 7 and 87 Years of Age. Cerebral Cortex. 2007;17:1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stochholm K, Juul S, Juel K, Naeraa RW, Gravholt CH. Prevalence, incidence, diagnostic delay, and mortality in Turner syndrome. Journal of Clinical Endocrinology and Metabolism. 2006;91:3897–3902. doi: 10.1210/jc.2006-0558. [DOI] [PubMed] [Google Scholar]
- Takahashi E, Ohki K, Kim DS. Dissociated pathways for successful memory retrieval from the human parietal cortex: anatomical and functional connectivity analyses. Cerebral Cortex. 2008;18:1771–1778. doi: 10.1093/cercor/bhm204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Abnormal prefrontal cortex function during response inhibition in Turner syndrome: functional magnetic resonance imaging evidence. Biological Psychiatry. 2003;53:107–111. doi: 10.1016/s0006-3223(02)01488-9. [DOI] [PubMed] [Google Scholar]
- Toro R, Fox PT, Paus T. Functional Coactivation Map of the Human Brain. Cerebral Cortex. 2008 doi: 10.1093/cercor/bhn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacks JM. Neuroimaging studies of mental rotation: a meta-analysis and review. Journal of Cognitive Neuroscience. 2008;20:1–19. doi: 10.1162/jocn.2008.20013. [DOI] [PubMed] [Google Scholar]
- Zijdenbos AP, Forghani R, Evans AC. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Transactions on Medical Imaging. 2002;21:1280–1291. doi: 10.1109/TMI.2002.806283. [DOI] [PubMed] [Google Scholar]
- Zilles K, Armstrong E, Schleicher A, Kretschmann HJ. The human pattern of gyrification in the cerebral cortex. Anatomy and Embryology. 1988;179:173–179. doi: 10.1007/BF00304699. [DOI] [PubMed] [Google Scholar]
- Zinn AR, Roeltgen D, Stefanatos G, Ramos P, Elder FF, Kushner H, Kowal K, Ross JL. A Turner syndrome neurocognitive phenotype maps to Xp22.3. Behav Brain Funct. 2007;3:24. doi: 10.1186/1744-9081-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn AR, Ross JL. Turner syndrome and haploinsufficiency. Current Opinion in Genetics and Development. 1998;8:322–327. doi: 10.1016/s0959-437x(98)80089-0. [DOI] [PubMed] [Google Scholar]