Abstract
Subject performance, scanner hardware, or biological factors can affect single session neuroimaging measures. Stability studies using calibrated blood oxygenation level dependent functional magnetic resonance imaging (BOLD-fMRI) have been performed in health but not disease. We utilized calibrated BOLD-fMRI to determine the effects of HIV on neurovascular coupling. 6 clinically stable HIV-infected patients (HIV+) and 10 seronegative controls (HIV−) were scanned at two separate sessions approximately 3 months apart. Both mild hypercapnia (5% CO2) exposure and a visual functional activation task were performed. Intra-class correlation coefficients (ICC) and inter-subject variance were determined for calibrated BOLD-fMRI measures (baseline cerebral blood flow (CBF), functional CBF, BOLD, and cerebral metabolic rate of oxygen consumption (CMRO2) changes) for HIV+ and HIV− subjects. The two groups did not differ in age, sex, or education. HIV+ subjects had lower mean baseline CBF (p <0.04, Cohen’s d=−1.07) and functional BOLD responses (p< 0.001, Cohen’s d=−2.47) and a trend towards a decrease in mean functional CBF responses (p= 0.07, Cohen’s d=−0.92) despite similar mean functional CMRO2 changes (p= 0.71, Cohen’s d=0.19). The stability of each calibrated BOLD-fMRI measure, as assessed by ICC, was significantly lower for HIV+ subjects. In addition, HIV+ participants had greater inter-subject variability for baseline CBF (p <0.02), functional BOLD (p< 0.001), CBF (p< 0.001), and CMRO2 (p< 0.002) responses. Our results demonstrate that calibrated BOLD-fMRI measures have excellent stability within healthy controls. In contrast, these values have greater variability in clinically stable HIV+ subjects and may reflect alterations in coupling between CBF and CMRO2 with disease.
Keywords: cerebral blood flow, cerebral metabolic rate of oxygen consumption, stability, functional magnetic resonance imaging, blood oxygenation level dependent imaging
Introduction
While the brain comprises approximately 2% of total body weight, it consumes more than 20% of its total energy. These energy demands are primarily met by aerobic metabolism (Raichle and Mintun, 2006). Cerebral metabolic rate of oxygen consumption (CMRO2) can indicate not only brain function but also tissue viability.
CMRO2 increases by a smaller fraction than cerebral blood flow (CBF) during functional activation. This imbalance is the basis of most functional magnetic resonance imaging (fMRI) mapping studies that use the blood oxygen level dependent (BOLD) effect (Bandettini, 2009; Buxton et al., 2004). However, how disease affects the coupling between functional changes in CBF and CMRO2 remains poorly characterized.
It remains difficult to quantitatively interpret differences in the magnitude of the BOLD response between patients with a particular disease and healthy controls (D'Esposito et al., 2003; Jezzard and Buxton, 2006; Pineiro et al., 2002). While observed distinctions could reflect differences in the evoked neural activity due to the disease process, they could also arise from changes in CBF/CMRO2 coupling during the task, or even chronic changes in the baseline state (Ances et al., 2008; Brown et al., 2003; Buxton et al., 2004; Fleisher et al., 2009). The ability to discriminate between these possible conditions could influence the choice of therapeutic strategies for a particular disease -–e.g., medications that are specifically targeted to treat neuronal dysfunction as opposed to therapy that improves vasculature function.
Our current lack of understanding of brain oxygen metabolism, in both health and disease, is partly due to difficulties in measuring CMRO2(Buxton, 2010). Techniques for determining CMRO2 have usually required the infusion /injection/ inhalation of labeled O2. For example, positron emission tomography (PET) uses radioactively-labeled 15O compounds, but requires multiple tracer studies to acquire sufficient information to discriminate functional CMRO2 changes from CBF and cerebral blood volume (CBV) effects (Mintun et al., 1984; Raichle and Mintun, 2006). Application of the above methods to the clinical setting remains challenging as they: 1) are invasive and require repeated exposure to an exogenous agent; 2) are time consuming to perform (including preparation of tracers, scanning, and clearance of agents); and 3) are quite expensive and require special facilities (e.g., cyclotron).
fMRI provides an alternative non-invasive method for calculating relative CMRO2 changes. Calibrated BOLD fMRI combines BOLD-weighted imaging and arterial spin labeling (ASL), a technique that quantitatively measures CBF, during separate periods of mild hypercapnia or hyperoxia and functional activation (Brown et al., 2007; Buxton, 2010). The administration of mild hypercapnia or hyperoxia allows for determination of a local BOLD scaling parameter M. From this measure functional CMRO2 changes can be calculated using mathematical models of the BOLD effect (Davis et al., 1998). From these calculations, n -the ratio of the fractional changes in CBF to CMRO2- can be determined.
While a growing number of studies have utilized calibrated BOLD fMRI, only one has been performed in a patient population (Stefanovic et al., 2005). In order for calibrated BOLD fMRI to have more widespread applications it must be studied in multiple clinical conditions. Many brain disorders are associated with abnormalities in coupling between CBF and CMRO2. HIV serves as a model system as the virus crosses the blood brain barrier soon after infection and indirectly affects both neurons and the vasculature. Calibrated BOLD fMRI therefore provides an excellent method for disentangling the effects of disease on the BOLD response.
A simple “one subject, one scan” principle may not be applicable to disease states. A single “snapshot” may not epitomize a subject’s true response as he/she could fail to perform during a scanning session. Slight variations in random non-physiological processes (changes in the position of the subject within the scanner, field inhomogeneities during the scan, shimming differences, changes in cognitive strategy over time, or scanner instability) can influence acquired data (McGonigle et al., 2000; Veltman et al., 2000). While the stability of anatomical measures has been studied and used as a biomarker of disease (Urs et al., 2009; Wonderlick et al., 2009), attempts to characterize the stability of fMRI measures have been limited. To date, no stability studies using calibrated BOLD have been performed in a disease population.
Most BOLD fMRI studies have used the magnitude of the response (Aguirre et al., 1998) or the number of overlapping activated voxels (Ramsey et al., 1996) to assess stability. One method to estimate the relative contributions of both within subject and between-subject variance for a group of participants is the intra-class correlation coefficient (ICC) (Shrout and Fleiss, 1979). The ICC provides a quantifiable estimate of inter-scan variability of a particular group in relation to the inter-subject variability. ICC is an ideal choice for quantifying the stability of physiological measurements (baseline CBF, functional CBF, and functional CMRO2) obtained by calibrated BOLD-fMRI. As an initial step towards determining whether changes in calibrated BOLD-fMRI measurements might be used as an indicator of disease activity or to monitor treatment efficacy, we assessed the stability of repeated calibrated BOLD-fMRI values in clinically stable HIV infected (HIV+) subjects and seronegative healthy controls (HIV−).
Methods
Participants
10 HIV− controls and 6 HIV+ subjects were scanned on the same 3 Tesla whole body system (3-T GE Excite, Milwaukee, WI). The Human Research Protections Program at the University of California San Diego (UCSD) approved this study. Individuals with a previous history of neurological illness, psychiatric disorders, or substance or alcohol abuse in the past three months were excluded. Written informed consent was obtained after a complete description of the study was provided to all subjects.
We selected community HIV− controls and clinically stable HIV+ patients followed at the HIV Neurobehavioral Research Center at UCSD. All HIV+ subjects received a clinical examination and neuropsychological performance testing. All HIV+ participants had neuropsychological performance testing that assessed verbal fluency, psychomotor skills, motor skills and praxis, learning and recall, speed of information processing, and executive functioning (Carey et al., 2004). For all HIV+ subjects raw test scores were converted to demographically corrected t scores minimizing the influence of age, education, sex, and ethnicity. An overall global deficit score (GDS) was determined with impairment deemed significant if the GDS ≥ 0.5. All subjects had two calibrated BOLD fMRI sessions performed within 3 months of each other. At each scanning sessions subjects were offered MR compatible eyeglasses if needed. Scans were performed at similar times of day to minimize diurnal variations.
Experimental Design
Each calibrated BOLD fMRI session consisted of: 1) a mild hypercapnia experiment and 2) a functional activation task. In the first part, subjects breathed a hypercapnic air mixture (5% CO2, 21%O2, and 74% N2) through a non-rebreathing face mask (Hans Rudolph 2700 Series, St. Louis, MI). A white square centered on an isoluminant gray background was used as a fixation point during these hypercapnic runs. In the second portion of the calibrated BOLD fMRI session, a black and white radial checkerboard flickered at 8 Hz during activation periods while an isoluminant gray screen with a center fixation square was used for rest portions. The grayscale mid-point between pure black and white on the displayed image was determined to be a red green blue level of 99, corresponding to a luminance of 518cd/m2. Two functional runs were acquired using a block design paradigm (20 s on, 60 s off). A block design paradigm was used as it has greater power and stability compared to event-related designs (Zarahn et al., 1997).
During all scans pulse oximetry (Invivo Millennia 3500; Invivo Research, Orlando, FL) and respiratory effort (BIOPAC Systems, Goleta, CA) were measured. Physiological data were sampled at 40 samples per second using a multi-channel data acquisition board (National Instruments, Austin, TX).
Image Acquisition
A single-shot PICORE QUIPSS II (Wong et al., 1999) pulse sequence (TR=2.5 s, TI1=700 ms, TI2=1500 ms, 20-cm tag width, and a 1-cm tag-slice gap) with a dual-echo gradient echo (GRE) readout and spiral acquisition of k-space (TE1=9.4 ms, TE2=30 ms, flip angle=90°, field of view (FOV)=24 cm, 64 × 64 matrix) method allowed for simultaneous acquisition of ASL and BOLD signals. The difference in the tag and control images from the first echo provided the CBF response, while the average of the tag and control images from the second echo yielded the BOLD response. Four 7 mm-thick slices covering the entire visual cortex (VC) were acquired in a linear fashion from bottom to top. The VC mask was defined by the parietal-occipital sulci, and contained not only the primary visual cortex (V1) but also supplementary regions. Clusters of voxels exhibiting CBF activation within the defined VC boundaries were detected using an overall significance threshold of p=0.05 applied to the first echo data (Ances et al., 2008; Ances et al., 2009a). These activated VC voxels were subsequently used as a region of interest for comparing between sessions.
A high-resolution structural scan was acquired with an inversion recovery prepared 3D fast spoiled GRASS (IR-FSPGR) pulse sequence (TI=450 ms, TR=7.9 ms, TE=3.1 ms, flip angle=12°, FOV=25 × 25 × 16 cm, matrix 256 × 256 × 124). These images were collected after the hypercapnia experiments but before functional activation studies. This scan allowed for additional delays and minimized any possible lingering effects of CO2 on functional activation. Structural images were reviewed to ensure that neurological complications and opportunistic brain disease were not present. The VC mask was applied to the structural scans to determine the volume of this region of interest. The VC volume was obtained from the number of voxels within this manually traced regions and expressed in mm3 (e.g., 1 voxel = 1 mm3) with correction for total intracranial volume.
A baseline CBF scan was acquired to quantify baseline CBF (Wang et al., 2005). A cerebral spinal fluid (CSF) reference scan and a minimum contrast scan were obtained for conversion of the ASL data to quantifiable CBF units (mL/100gm/min). The CSF scan was a single-echo, single repetition scan acquired at full relaxation (TE=2.8 ms). This scan used the same in-plane parameters as the ASL scan except that the number of slices was increased to ensure adequate coverage of the lateral ventricles. A minimum contrast scan (TR=2 s, TE=11 ms) using two 8-interleave repetitions was also acquired with the same slice prescription as the CSF scan (Chalela et al., 2000).
Determination of CMRO2
Calculation of relative CMRO2 changes during functional stimulation were determined using previously described calibrated BOLD fMRI equations (Davis et al., 1998). Fractional BOLD signal change (ΔS/So) is related to the underlying changes in CBF and CMRO2 by:
| [1] |
The parameter M reflects baseline deoxyhemoglobin content and defines the maximum possible BOLD signal change. This value is proportional to the baseline blood volume fraction and O2 extraction fraction. The parameter α is the exponent of the empirical power law relationship between CBV and CBF. It is assumed to be equal to 0.38 (Grubb et al., 1974) while β is taken to be 1.5 based on numerical simulations for a 1.5 T field (Boxerman et al., 1995b; Davis et al., 1998). The parameters α and β are assumed to be the same not only throughout the brain but also for both 1.5 T and 3.0 T scanners.
Mild hypercapnia (5% CO2) allowed for determination of M If mild hypercpania is assumed to not alter CMRO2 (Chen and Pike, 2010; Hafkenschiel et al., 1954; Jones et al., 2005; Kety and Schmidt, 1948; Kim and Ugurbil, 1997; Kliefoth et al., 1979; Novack et al., 1953; Sicard and Duong, 2005) then the ratio CMRO2/CMRO2o equals one, and the measured CBF and BOLD changes during mild hypercapnia can be combined in equation [1] to calculate M. The derived value of M can then be applied to the functional activation data to calculate stimulus-evoked CMRO2/CMRO2o.
Statistical Analysis
Each calibrated BOLD- fMRI variable was natural logarithmically (ln) transformed to improve normality and homoscedasticity. ICC provides a quantifiable measure of inter-scan stability by using estimates of variance components within a linear mixed-effects model (Bartko, 1966; Shrout and Fleiss, 1979). Briefly, for each calibrated BOLD-fMRI measure, if the inter-scan and inter-subject variances are σ2 and τ2 respectively, then the proportion of the total variability due to the subject is:
| [2] |
An ICC close to 1 corresponds to a relatively small inter-scan variability and therefore reflects greater stability. Since only a single scanner was used, a scanner effect was not included in the model. For each calibrated BOLD-fMRI response variable (Y) the analysis was conducted as follows.
Step 1: Comparing means of calibrated BOLD-fMRI measures between HIV+ and HIV− groups
We fitted the following mixed-effects models:
| [3] |
| [4] |
where i stands for subject identification, and j stands for day (j= 1 or 2). For both models the term eij represents the inter-scan error, and is assumed to have a normal distribution, with variance dependent on the group, i.e. var(eij)=σ+2 for the HIV+ group, and σ−2 for the HIV-controls. Similarly, bi stands for the inter-subject error, with normal variances τ+2 and τ−2 for the HIV+ and HIV− groups respectively. The ICC was then determined for the HIV+ and HIV-groups, i.e. ICC+=τ+2/(σ+2+τ+2), and ICC−=τ−2/(σ−2+σ−2). In equation [3] the [group] term is an HIV group effect, and it modifies the mean for the HIV+ subjects. Equation [3] and Equation [4] were then compared using a likelihood ratio test (LRT). If a p-value ≤0.05 was observed using the LRT, the mean responses therefore differed between HIV− and HIV+ groups and model [3] was used. Conversely, if a p>0.05 was observed for the LRT then no difference in mean responses was observed between HIV− and HIV+ groups and model [4] was used.
Step 2: Comparing ICC for calibrated BOLD-fMRI measures between HIV+ and HIV− groups
The ICC for each of the groups, ICC+=τ+2/(σ+2±τ+2), and ICC−=τ−2/(σ−2±τ−2), are equal if the variance components are equal, i.e. σ+ 2=σ−2 and_τ+2=τ−2. We used a LRT to assess if ICC values were different for the two groups In order to keep the inter-scan variance constant for the two groups, the observations were re-scaled so that σ+2 = σ−2. Subsequently, the model derived from Step 1 was compared to a model with same mean structure and variance components that did not depend on group. A statistically significant difference (LRT p-value ≤0.05) indicated a difference in ICC values between the HIV+ and HIV− groups. As an additional precaution we tested for the possible presence of a systematic difference between the first and second scanning sessions for each group by comparing model [3] to one that included a mean term for the time of the scan (first versus second day).
Step 3: Comparing inter-subject variances between HIV+ and HIV− groups
The model from Step 1 (without rescaling the observations) was compared with a similar model in which the constraint of equal inter-subject variances were imposed, i.e., σ+2=σ−2 using the LRT. A significant p-value indicated that the inter-subject variances were different between the HIV+ and HIV− groups.
The differences between the HIV+ and HIV− groups tested in Steps 1–3 can be interpreted in the following manner: (i) Step 1, difference in mean response: The mean levels of one groups was higher than the mean levels of the other group, at both visits; (ii) Step 2, difference in ICC: The group with higher ICC values had trajectories that tended to be more stable, while the group with lower ICC values had less stable trajectories; (iii) Step 3, difference in inter-subject variances: The group with higher inter-subject variance had larger vertical dispersion of the measured values than the group with smaller inter-subject variance, at both visits.
Cohen’s d effect sizes were also calculated for each of the calibrated BOLD parameters to evaluate the degree of difference between groups. Demographic variables were compared between the two groups using a t-test and Pearson’s chi-square test.
Results
Table 1 shows the demographic characteristics of the two groups. No significant differences existed between the groups in relation to sex, age, or education, or previous history of substance or alcohol abuse. Most HIV+ participants were taking antiretroviral medications (83%) and none had changed their regimens between the two scanning sessions. All HIV+ patients remained clinically stable between the scanning sessions and were compliant with their medications. Blood HIV viral load and CD4 counts presented in Table 1 were from laboratory values obtained prior to the first scan for HIV+ patients. These values remained stable for a subsequent draw closest to the second scan (data not shown). The time interval between repeated measurements was similar for both groups (15 ± 1 week for HIV− controls and 16 ± 2 week for HIV+ participants). All subjects tolerated the calibrated BOLD-fMRI studies.
Table 1.
Demographic values for HIV− and HIV+ subjects
| HIV+ Subjects (n=6) |
HIV− Controls (n=10) |
p value | |
|---|---|---|---|
| Demographics | |||
| Mean Age (years old) (SD) | 30 (7) | 30 (6) | 0.93 |
| Sex (% male) | 100% | 60% | 0.08 |
| Mean Education (years) (SD) | 15 (2) | 18 (3) | 0.07 |
| Global Deficit Score (GDS) (SD) | 0.34 (0.16) | NA | - |
| History of Previous Substance Abuse (%) | 16 % | 0 % | 0.20 |
| History of Previous Alcohol Abuse | 0 % | 0 % | 1.0 |
| Laboratory Values | |||
| Mean Current CD4 (cells/mm3) (Interquartiles) | 757 (424,900) | NA | - |
| Mean Nadir CD4 (cells/mm3) (Interquartiles) | 588 (438,750) | NA | - |
| Mean Plasma viral load (log10) (SD) | 2.20 (1.22) | NA | - |
| % taking antiretrovirals | 83 % | NA | - |
| Central Nervous System Penetration Score | 1.0 (0.9) | NA | - |
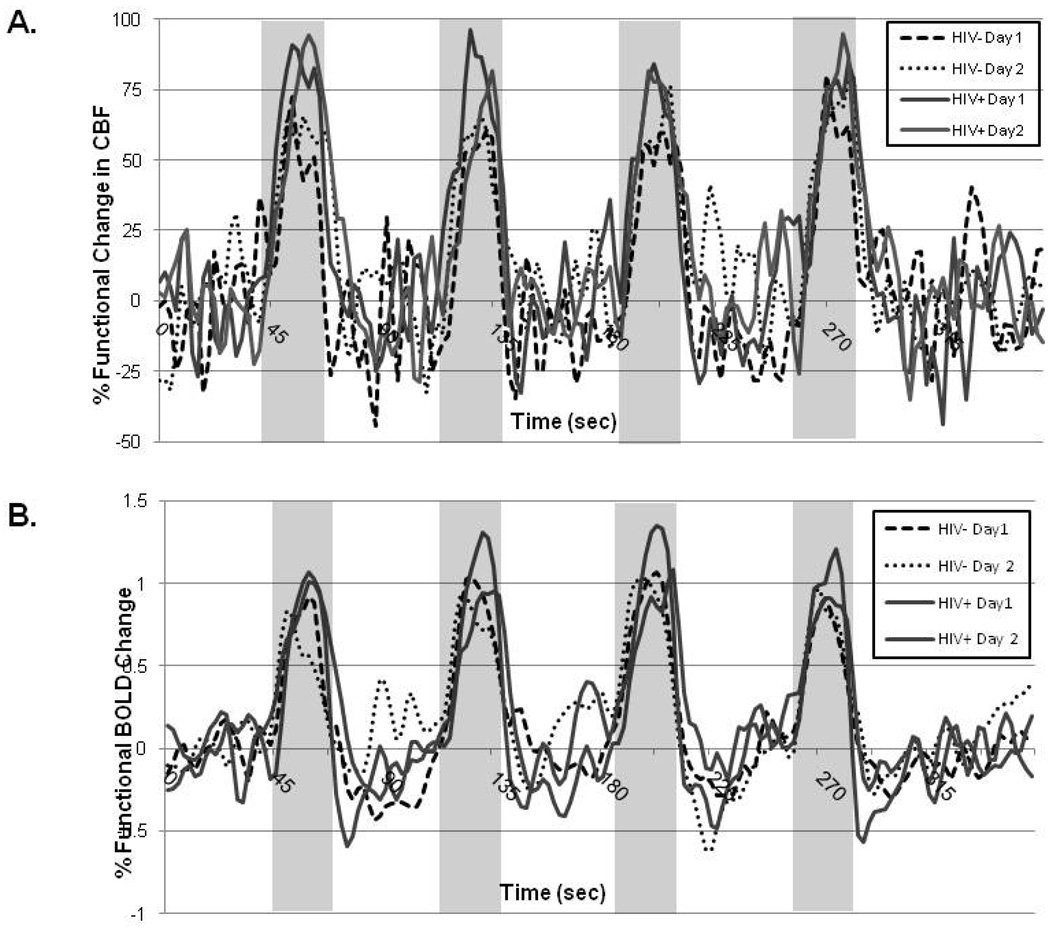
Figure 1 demonstrates a representative time course curve for a control subject and an HIV+ participant for the sessions. The shape of both the BOLD and CBF time courses were similar for the two days for the two groups. Overall, HIV+ participants had significantly larger functional CBF and BOLD responses than HIV− control subjects. However, because a block design method was utilized, we were unable to resolve if significant differences existed in the temporal dynamic of the hemodynamic response between the two groups.
Figure 1.
Time series of functional changes in cerebral blood flow (A) and blood oxygen level dependent (BOLD) responses for a representative seronegative (HIV−) control and HIV infected (HIV+) patient at the two sessions.
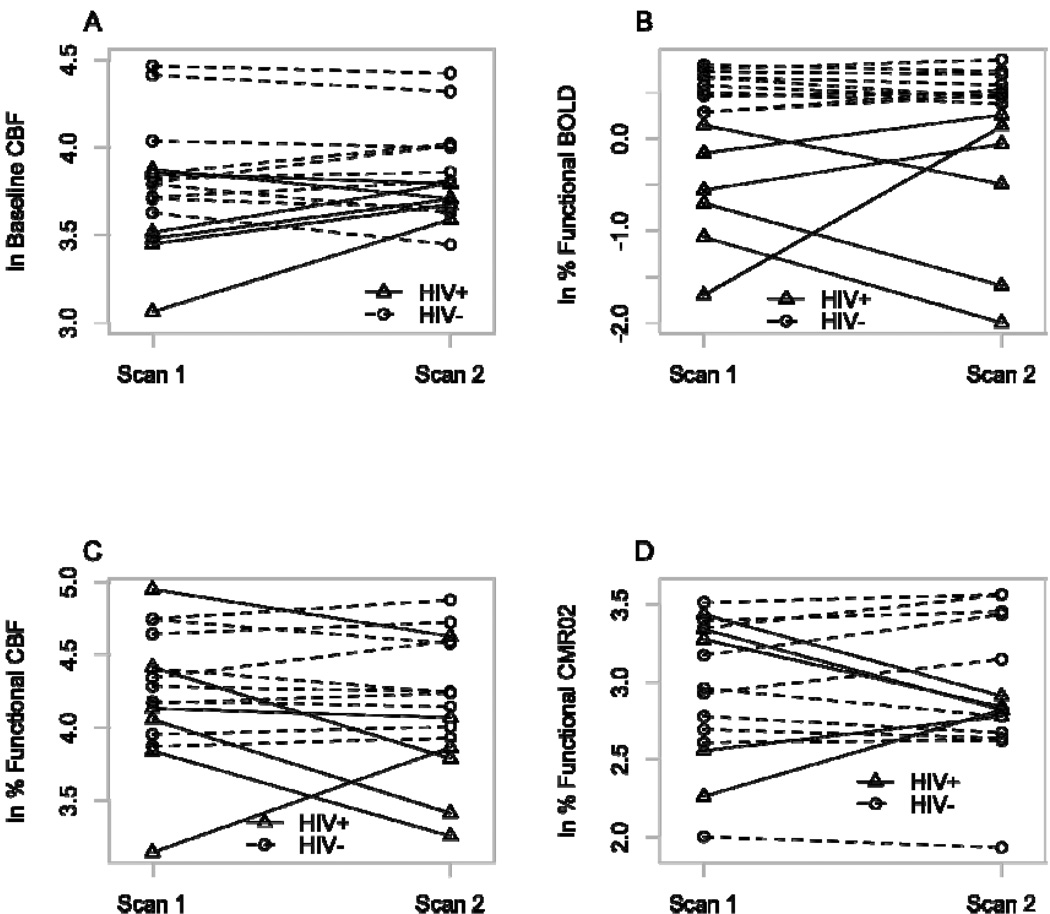
Figure 2 shows the values for each of the natural logarithm (ln) transformed calibrated BOLD-fMRI measures at the first and second MRI scans. Overall, there were no appreciable differences between the first and second scanning sessions for each of the calibrated BOLD parameters for the two groups. Plots at the first and second time point for the two groups are presented for each of the calibrated BOLD measures (Figure 2). As seen in Table 2, HIV+ participants had reduction in mean ln functional BOLD changes (p<0.001, Cohen’s d=−2.47), lower mean ln baseline CBF (p=0.04, Cohen’s d=−1.07), and a trend towards smaller mean ln functional CBF changes (p=0.07, Cohen’s d=−0.92) and ln M values (p=0.09, Cohen’s d=−0.91). In contrast, calculated mean ln CMRO2 requirements for the functional task were similar for the two groups (p=0.71, Cohen’s d=−0.05). The coupling ratio, n, was reduced in HIV+ patients (p=0.006, Cohen’s d=−1.63). Observed decreases in calibrated BOLD-fMRI measures for HIV+ patients were not associated with volumetric loss as region of interest values were similar for both groups (p=0.32).
Figure 2.
Spaghetti plots of natural logarithm transformed calibrated blood oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI) measures for seronegative (HIV−) controls and HIV infected (HIV+) patients at both 1st and 2nd scans. There were no systematic changes in a) baseline cerebral blood flow (CBF) b) functional BOLD c) functional CBF changes or d) functional cerebral metabolic rate of oxygen consumption (CMRO2) between scanning sessions for each group.
Table 2.
Comparison of calibrated blood oxygen level dependent functional magnetic resonance imaging (BOLD-fMRI) values between HIV+ and HIV− subjects.
| HIV+ Subjects (n=6) |
HIV− Controls (n=10) |
p value | Effect size |
|
|---|---|---|---|---|
| Mean ln Baseline CBF | 3.62 | 3.92 | 0.04 | −1.07 |
| Mean ln % Functional CBF change | 3.96 | 4.35 | 0.07 | −0.92 |
| Mean ln % Functional BOLD change |
−0.65 | 0.57 | <0.001 | −2.47 |
| Mean ln % Functional CMRO2 change |
2.99 | 2.96 | 0.71 | 0.19 |
| Mean ln M | −3.09 | −2.79 | 0.09 | −0.91 |
| Mean ln n | 0.75 | 1.38 | 0.006 | −1.63 |
ln= natural log
CBF= cerebral blood flow
CMRO2 = cerebral metabolic rate of oxygen consumption
M= maximum possible BOLD signal change
n= ratio of fractional changes in CBF to CMRO2
Table 3 illustrates the ICC values for both HIV− controls and HIV+ participants. In general, HIV+ subjects had significantly lower ICC values for each of the calibrated BOLD-fMRI measures except for M. Observed decreases in stability, as assessed by ICC, were accompanied by increases in inter-subject variability (Table 4). For each of the calibrated BOLD-fMRI measures, except for M, HIV+ subjects displayed greater inter-subject variance.
Table 3.
Comparison of natural logarithmic (ln) transformations of intraclass coefficient (ICC) values between HIV+ and HIV− subjects.
| HIV+ Subjects (n=6) |
HIV− Controls (n=10) |
p value | |
|---|---|---|---|
| ln Baseline CBF (mL/100gm/min) | 0.140 | 0.901 | 0.002 |
| ln % Functional CBF change | 0.603 | 0.915 | 0.012 |
| ln % Functional BOLD change | 0.125 | 0.609 | 0.14 |
| ln % Functional CMRO2 change | 0.226 | 0.951 | 0.001 |
| ln M Value | 0.866 | 0.879 | 0.58 |
| ln n | 0.489 | 0.968 | 0.001 |
Table 4.
Comparison of ln inter-subject standard deviations for HIV+ and HIV− subjects.
| HIV+ Subjects (n=6) |
HIV− Controls (n=10) |
p value | |
|---|---|---|---|
| ln Baseline CBF (mL/100gm/min) | 0.20 | 0.09 | 0.023 |
| ln % Functional CBF change | 0.38 | 0.09 | <0.001 |
| ln % Functional BOLD change | 0.70 | 0.10 | <0.001 |
| ln % Functional CMRO2 change | 0.38 | 0.11 | 0.002 |
| ln M | 0.14 | 0.11 | 0.58 |
| ln n | 0.20 | 0.07 | 0.004 |
Discussion
The longitudinal assessment of changes in regional brain activity using BOLD-fMRI is becoming increasingly important for the preclinical diagnosis and treatment of clinical disorders. This study is the first to evaluate the utility of calibrated BOLD-fMRI to generate functional response data that have direct physiological significance in disease states rather than simply evaluating the robustness of BOLD-fMRI in the context of brain mapping. We have shown that calibrated BOLD-fMRI measures have excellent stability, as assessed by ICC, within healthy HIV− controls but not in HIV+ patients.
It is often the assumption that observed differences in the BOLD response between patients with a particular disease and healthy controls reflects underlying pathophysiological changes in metabolic demands. Calibrated BOLD-fMRI can begin to tease apart the origin of possible distinctions in the BOLD response. We observed fMRI divergences with HIV+ patients having significantly reduced BOLD functional changes (Table 2). However, these BOLD differences were in fact not due to disparities in functional CMRO2 requirements as similar results were seen for both groups. Instead, the reduced BOLD response in HIV+ patients was due to a combination of a significant reduction in the fractional CBF response, a significant reduction in the ratio of the CBF and CMRO2 responses (n), and a trends towards a decrease in baseline deoxyhemoglobin (as reflected in the measured scaling parameter (M)) (Table 2). The reduction in baseline CBF due to HIV is in good agreement with previous studies by our group and others (Ances et al., 2006; Ances et al., 2009b; Ances et al., 2010; Ernst et al., 2000). Observed decreases in resting CBF with HIV may reflect a reduction in metabolic demands due to synaptodendritic dysfunction and pruning. Baseline CBF could be a good quantitative biomarker to discriminate the effects of HIV disease in the brain (Ances et al., 2009b).
ICC has quickly become one of the most common methods for assessing fMRI stability. One advantage of this method is that it can be easily interpreted with a value of 1.0 indicating near-perfect agreement between the test and retest sessions. In contrast, a value of 0.0 indicates no agreement between the test and retest sessions. Currently there is no consensus regarding what constitutes an acceptable stability level for fMRI studies. Using previously defined benchmarks (Landis and Koch, 1977) stability results can be categorized in the following manner: 0.4–0.59 (moderate stability), 0.6–0.79 (good stability), 0.8–0.99 (high stability), and 1.0 (perfect stability) (Cicchetti and Sparrow, 1981; Eaton et al., 2008). Across a survey of fMRI test-retest stability of healthy controls the average ICC value for BOLD block designed experiments has been determined to be approximately 0.50 (Bennett and Miller, in press). In this study we observed a similar value with an ICC equal to 0.61 for a BOLD block designed task in healthy HIV− controls. What was somewhat surprising to us was that BOLD functional measures appeared to be the least reliable of all of the measured or calculated calibrated BOLD-fMRI values for healthy controls (Table 3).
Currently, studies of test-retest stability of BOLD-fMRI in individuals with clinical disorders have been limited. Mixed results have been observed with some showing decreased stability in certain clinical disorders such as epilepsy and schizophrenia (Chiarelli et al., 2007a; Fernandez et al., 2003; Manoach et al., 2001) while others have seen greater stability in aphasic stroke patients who have recovered (Kimberley et al., 2008). In this study we observed a significant reduction in the stability of all calibrated BOLD fMRI measures, except for M, in HIV+ patients compared to HIV− controls (Table 3). Observed decreases in the ICC values for HIV+ subjects were accompanied by increased inter-subject variance (Table 4). In general, the HIV+ group had between 2–7 times more variability compared to HIV− controls. In summary, the HIV+ group had lower mean values, larger inter-subject variability, and smaller intra-subject autocorrelation (as measured by ICC) than the HIV− group. These results suggest that HIV infection may have a complex disruptive effect on brain processes and can affect the stability of brain signals, both between individuals and within a single individual.
When presented a functional task, HIV+ patients and HIV− controls had similar functional CMRO2 changes despite differences in functional CBF changes (Table 2). Our results suggest an alteration in the basic neurovascular coupling with HIV infection. In particular, the coupling between CBF and CMRO2 (n), was reduced in HIV+ patients on stable HAART. This difference in n was due to a decrease in functional CBF changes in HIV+ patients. Medications specifically targeted to increase CBF may of great benefit to HIV+ patients. However, longitudinal studies of HIV+ participants both prior to and after starting medications using calibrated BOLD-fMRI experiments are required.
Limitations exist in regards to both the patient population studied and technique employed. In this study we investigated a relatively homogenous sample of HIV+ participants as most were receiving antiretroviral therapy and had well controlled viral loads. Even greater inter-subject variability may exist between HIV+ patients on or off therapy with poorly controlled viral loads.
Mild hypercapnia that is administered during the calibrated BOLD-fMRI technique is thought to produce a large change in CBF without changing CMRO2 (Sicard and Duong, 2005). However, there still remains some controversy concerning this assumption (Zappe et al., 2008). A more recent study has confirmed that mild hypercapnia using computerized end-tidal CO2 modulation does not lead to significant changes in global CMRO2 in awake humans (Chen and Pike, 2010). In addition, hyperoxia calibrated BOLD-fMRI methods have been previously performed (Chiarelli et al., 2007b; Chiarelli et al., 2007c; Goodwin et al., 2009). The hyperoxia approach appears to be a very promising alternative, and as future studies systematically compare the two calibrated BOLD methods it may well become the preferred method (Buxton, 2010). Overall, the hyperoxia method has been shown to have relatively small errors for both individual and group data (Chiarelli et al., 2007c) and has very good reliability (Goodwin et al., 2009). For now, though, the hypercapnia approach preserved consistency with our previous HIV− control studies (Ances et al., 2009b).
Limitations also exist in the ASL technique. If transit delays from the tagging region to the imaged slice were longer for HIV+ patients we could underestimate baseline perfusion as not all tagged blood was delivered at the time of measurement. If the transit delay was shortened with activation, we would also overestimate functional CBF change. Future studies that investigate the labeling efficiency in HIV+ patients are currently being performed.
Conclusions
The stability of calibrated BOLD-fMRI measurements was determined for both HIV− controls and HIV+ participants. Overall, excellent stability values, as assessed by ICC, were seen in HIV-controls. For HIV− subjects, functional BOLD responses had the poorest stability of any of the calibrated BOLD-fMRI measures. Overall, HIV+ patients had significantly lower stability values for each of the calibrated BOLD-fMRI parameters compared to HIV− controls. Observed decreases in stability in HIV+ patients, as assessed by ICC, were accompanied by increases in inter-subject variability.
HIV+ subjects had significantly lower mean ln baseline CBF (p <0.04, Cohen’s d=−1.07) and mean ln functional BOLD responses (p< 0.001, Cohen’s d=−2.47) compared to HIV− controls despite similar mean functional CMRO2 (p= 0.71, Cohen’s d=0.19) changes. HIV infection may have a complex disruptive effect on the brain processes leading to an uncoupling between CBF and CMRO2. Our results support the use of calibrated BOLD fMRI as a quantitative probe to study the coupling of CBF and CMRO2. The addition of mild hypercapnia as a calibration technique can allow us to begin to tease apart the complexities of the BOLD-fMRI response.
Acknowledgments
This work was supported by a Dana Foundation Brain Immuno Imaging Award (DF3857-41880) (BA) and NIH grants (1K23MH081786) (BA), (NS-36722 and NS-42069) (RB). In addition, support was provided by AI27670, AI43638, and the UCSD Center for AIDS Research AI 36214, AI29164, AI47745, AI57167, AI55276, MH62512 from the National Institutes of Health (R.E.) and MH22005, AI47033 (F.V.).
Abbreviation Key
- fMRI
functional magnetic resonance imaging
- CBF
cerebral blood flow (CBF)
- CMRO2
cerebral metabolic rate of oxygen consumption
- BOLD
Blood Oxygenation Level Dependent imaging
- ASL
arterial spin labeling
- ICC
intra-class correlation coefficients
- VC
visual cortex
- CSF
cerebral spinal fluid
- CBV
cerebral blood volume
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interest
The authors declare no competing interest.
References
- Aguirre GK, Zarahn E, D’Esposito M. The variability of human, BOLD hemodynamic responses. Neuroimage. 1998;8:360–369. doi: 10.1006/nimg.1998.0369. [DOI] [PubMed] [Google Scholar]
- Ances BM, Leontiev O, Perthen JE, Liang C, Lansing AE, Buxton RB. Regional differences in the coupling of cerebral blood flow and oxygen metabolism changes in response to activation: implications for BOLD-fMRI. Neuroimage. 2008;39:1510–1521. doi: 10.1016/j.neuroimage.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Liang CL, Leontiev O, Perthen JE, Fleisher AS, Lansing AE, Buxton RB. Effects of aging on cerebral blood flow, oxygen metabolism, and blood oxygenation level dependent responses to visual stimulation. Hum Brain Mapp. 2009a;30:1120–1132. doi: 10.1002/hbm.20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Roc AC, Wang J, Korczykowski M, Okawa J, Stern J, Kim J, Wolf R, Lawler K, Kolson DL, Detre JA. Caudate blood flow and volume are reduced in HIV+ neurocognitively impaired patients. Neurology. 2006;66:862–866. doi: 10.1212/01.wnl.0000203524.57993.e2. [DOI] [PubMed] [Google Scholar]
- Ances BM, Sisti D, Vaida F, Liang CL, Leontiev O, Perthen JE, Buxton RB, Benson D, Smith DM, Little SJ, Richman DD, Moore DJ, Ellis RJ. Resting cerebral blood flow: a potential biomarker of the effects of HIV in the brain. Neurology. 2009b;73:702–708. doi: 10.1212/WNL.0b013e3181b59a97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Vaida F, Yeh MJ, Liang CL, Buxton RB, Letendre S, McCutchan JA, Ellis RJ. HIV infection and aging independently affect brain function as measured by functional magnetic resonance imaging. J Infect Dis. 2010;201:336–340. doi: 10.1086/649899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandettini PA. What's new in neuroimaging methods? Ann N Y Acad Sci. 2009;1156:260–293. doi: 10.1111/j.1749-6632.2009.04420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartko JJ. The intraclass correlation coefficient as a measure of reliability. Psychol Rep. 1966;19:3–11. doi: 10.2466/pr0.1966.19.1.3. [DOI] [PubMed] [Google Scholar]
- Bennett CM, Miller MB. How reliable are the results from functional magnetic resonance imaging. New York Adacemy of Sciences. doi: 10.1111/j.1749-6632.2010.05446.x. in press. [DOI] [PubMed] [Google Scholar]
- Boxerman JL, Hamberg LM, Rosen BR, Weisskoff RM. MR contrast due to intravascular magnetic susceptibility perturbations. Magn. Reson. Med. 1995b;34:555–566. doi: 10.1002/mrm.1910340412. [DOI] [PubMed] [Google Scholar]
- Brown GG, Eyler Zorrilla LT, Georgy B, Kindermann SS, Wong EC, Buxton RB. BOLD and perfusion response to finger-thumb apposition after acetazolamide administration: differential relationship to global perfusion. J Cereb Blood Flow Metab. 2003;23:829–837. doi: 10.1097/01.WCB.0000071887.63724.B2. [DOI] [PubMed] [Google Scholar]
- Brown GG, Perthen JE, Liu TT, Buxton RB. A primer on functional magnetic resonance imaging. Neuropsychol Rev. 2007;17:107–125. doi: 10.1007/s11065-007-9028-8. [DOI] [PubMed] [Google Scholar]
- Buxton RB. Interpreting oxygenation-based neuroimaging signals: the importance and the challenge of understanding brain oxygen metabolism. Front Neuroenergetics. 2010;2:8. doi: 10.3389/fnene.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton RB, Uludag K, Dubowitz DJ, Liu TT. Modeling the hemodynamic response to brain activation. Neuroimage. 2004;23 Suppl 1:S220–S233. doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004;26:307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- Chalela JA, Alsop DC, Gonzalez-Atavales JB, Maldjian JA, Kasner SE, Detre JA. Magnetic resonance perfusion imaging in acute ischemic stroke using continuous arterial spin labeling. Stroke. 2000;31:680–687. doi: 10.1161/01.str.31.3.680. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Pike GB. Global cerebral oxidative metabolism during hypercapnia and hypocapnia in humans: implications for BOLD fMRI. J Cereb Blood Flow Metab. 2010;30:1094–1099. doi: 10.1038/jcbfm.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarelli PA, Bulte DP, Gallichan D, Piechnik SK, Wise R, Jezzard P. Flow-metabolism coupling in human visual, motor, and supplementary motor areas assessed by magnetic resonance imaging. Magn Reson Med. 2007a;57:538–547. doi: 10.1002/mrm.21171. [DOI] [PubMed] [Google Scholar]
- Chiarelli PA, Bulte DP, Piechnik S, Jezzard P. Sources of systematic bias in hypercapnia-calibrated functional MRI estimation of oxygen metabolism. Neuroimage. 2007b;34:35–43. doi: 10.1016/j.neuroimage.2006.08.033. [DOI] [PubMed] [Google Scholar]
- Chiarelli PA, Bulte DP, Wise R, Gallichan D, Jezzard P. A calibration method for quantitative BOLD fMRI based on hyperoxia. Neuroimage. 2007c;37:808–820. doi: 10.1016/j.neuroimage.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Cicchetti DV, Sparrow SA. Developing criteria for establishing interrater reliability of specific items: applications to assessment of adaptive behavior. Am J Ment Defic. 1981;86:127–137. [PubMed] [Google Scholar]
- D'Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci U S A. 1998;95:1834–1839. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton KP, Szaflarski JP, Altaye M, Ball AL, Kissela BM, Banks C, Holland SK. Reliability of fMRI for studies of language in post-stroke aphasia subjects. Neuroimage. 2008;41:311–322. doi: 10.1016/j.neuroimage.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Itti E, Itti L, Chang L. Changes in cerebral metabolism are detected prior to perfusion changes in early HIV-CMC: A coregistered (1)H MRS and SPECT study. J Magn Reson Imaging. 2000;12:859–865. doi: 10.1002/1522-2586(200012)12:6<859::aid-jmri8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Fernandez G, Specht K, Weis S, Tendolkar I, Reuber M, Fell J, Klaver P, Ruhlmann J, Reul J, Elger CE. Intrasubject reproducibility of presurgical language lateralization and mapping using fMRI. Neurology. 2003;60:969–975. doi: 10.1212/01.wnl.0000049934.34209.2e. [DOI] [PubMed] [Google Scholar]
- Fleisher AS, Podraza KM, Bangen KJ, Taylor C, Sherzai A, Sidhar K, Liu TT, Dale AM, Buxton RB. Cerebral perfusion and oxygenation differences in Alzheimer's disease risk. Neurobiol Aging. 2009;30:1737–1748. doi: 10.1016/j.neurobiolaging.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin JA, Vidyasagar R, Balanos GM, Bulte D, Parkes LM. Quantitative fMRI using hyperoxia calibration: Reproducibility during a cognitive Stroop task. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.04.064. [DOI] [PubMed] [Google Scholar]
- Grubb RL, Raichle ME, Eichling JO, Ter-Pogossian MM. The effects of changes in PaCO2 on cerebral blood volume, blood flow, and vascular mean transit time. Stroke. 1974;5:630–639. doi: 10.1161/01.str.5.5.630. [DOI] [PubMed] [Google Scholar]
- Hafkenschiel JH, Friedland CK, Zintel HA. The blood flow and oxygen consumption of the brain in patients with essential hypertension before and after adrenalectomy. J Clin Invest. 1954;33:57–62. doi: 10.1172/JCI102870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezzard P, Buxton RB. The clinical potential of functional magnetic resonance imaging. J Magn Reson Imaging. 2006;23:787–793. doi: 10.1002/jmri.20581. [DOI] [PubMed] [Google Scholar]
- Jones M, Berwick J, Hewson-Stoate N, Gias C, Mayhew J. The effect of hypercapnia on the neural and hemodynamic responses to somatosensory stimulation. Neuroimage. 2005;27:609–623. doi: 10.1016/j.neuroimage.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Kety SS, Schmidt CF. The Effects Of Altered Arterial Tensions Of Carbon Dioxide And Oxygen On Cerebral Blood Flow And Cerebral Oxygen Consumption Of Normal Young Men. J Clin Invest. 1948;27:484–492. doi: 10.1172/JCI101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-G, Ugurbil K. Comparison of blood oxygenation and cerebral blood flow effects in fMRI: estimation of relative oxygen consumption change. Magn. Reson. Med. 1997;38:59–65. doi: 10.1002/mrm.1910380110. [DOI] [PubMed] [Google Scholar]
- Kimberley TJ, Khandekar G, Borich M. fMRI reliability in subjects with stroke. Exp Brain Res. 2008;186:183–190. doi: 10.1007/s00221-007-1221-8. [DOI] [PubMed] [Google Scholar]
- Kliefoth AB, Grubb RL, Jr, Raichle ME. Depression of cerebral oxygen utilization by hypercapnia in the rhesus monkey. J Neurochem. 1979;32:661–663. doi: 10.1111/j.1471-4159.1979.tb00404.x. [DOI] [PubMed] [Google Scholar]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- Leontiev O, Buxton RB. Reproducibility of BOLD, perfusion, and CMRO2 measurements with calibrated-BOLD fMRI. Neuroimage. 2007;35:175–184. doi: 10.1016/j.neuroimage.2006.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Halpern EF, Kramer TS, Chang Y, Goff DC, Rauch SL, Kennedy DN, Gollub RL. Test-retest reliability of a functional MRI working memory paradigm in normal and schizophrenic subjects. Am J Psychiatry. 2001;158:955–958. doi: 10.1176/appi.ajp.158.6.955. [DOI] [PubMed] [Google Scholar]
- McGonigle DJ, Howseman AM, Athwal BS, Friston KJ, Frackowiak RS, Holmes AP. Variability in fMRI: an examination of intersession differences. Neuroimage. 2000;11:708–734. doi: 10.1006/nimg.2000.0562. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Raichle ME, Martin WR, Herscovitch P. Brain oxygen utilization measured with O-15 radiotracers and positron emission tomography. J Nucl Med. 1984;25:177–187. [PubMed] [Google Scholar]
- Novack P, Shenkin HA, Bortin L, Goluboff B, Soffe AM. The effects of carbon dioxide inhalation upon the cerebral blood flow and cerebral oxygen consumption in vascular disease. J Clin Invest. 1953;32:696–702. doi: 10.1172/JCI102783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineiro R, Pendlebury S, Johansen-Berg H, Matthews PM. Altered hemodynamic responses in patients after subcortical stroke measured by functional MRI. Stroke. 2002;33:103–109. doi: 10.1161/hs0102.100482. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- Ramsey NF, Kirkby BS, Van Gelderen P, Berman KF, Duyn JH, Frank JA, Mattay VS, Van Horn JD, Esposito G, Moonen CT, Weinberger DR. Functional mapping of human sensorimotor cortex with 3D BOLD fMRI correlates highly with H2(15)O PET rCBF. J Cereb Blood Flow Metab. 1996;16:755–764. doi: 10.1097/00004647-199609000-00001. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Sicard KM, Duong TQ. Effects of hypoxia, hyperoxia, and hypercapnia on baseline and stimulus-evoked BOLD, CBF, and CMRO2 in spontaneously breathing animals. Neuroimage. 2005;25:850–858. doi: 10.1016/j.neuroimage.2004.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanovic B, Warnking JM, Kobayashi E, Bagshaw AP, Hawco C, Dubeau F, Gotman J, Pike GB. Hemodynamic and metabolic responses to activation, deactivation and epileptic discharges. Neuroimage. 2005;28:205–215. doi: 10.1016/j.neuroimage.2005.05.038. [DOI] [PubMed] [Google Scholar]
- Urs R, Potter E, Barker W, Appel J, Loewenstein DA, Zhao W, Duara R. Visual rating system for assessing magnetic resonance images: a tool in the diagnosis of mild cognitive impairment and Alzheimer disease. J Comput Assist Tomogr. 2009;33:73–78. doi: 10.1097/RCT.0b013e31816373d8. [DOI] [PubMed] [Google Scholar]
- Veltman DJ, Friston KJ, Sanders G, Price CJ. Regionally specific sensitivity differences in fMRI and PET: where do they come from? Neuroimage. 2000;11:575–588. doi: 10.1006/nimg.2000.0581. [DOI] [PubMed] [Google Scholar]
- Wang J, Qiu M, Constable RT. In vivo method for correcting transmit/receive nonuniformities with phased array coils. Magn Reson Med. 2005;53:666–674. doi: 10.1002/mrm.20377. [DOI] [PubMed] [Google Scholar]
- Wonderlick JS, Ziegler DA, Hosseini-Varnamkhasti P, Locascio JJ, Bakkour A, van der Kouwe A, Triantafyllou C, Corkin S, Dickerson BC. Reliability of MRI-derived cortical and subcortical morphometric measures: effects of pulse sequence, voxel geometry, and parallel imaging. Neuroimage. 2009;44:1324–1333. doi: 10.1016/j.neuroimage.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EC, Buxton RB, Frank LR. Quantitative perfusion imaging using arterial spin labeling. Neuroimaging Clin N Am. 1999;9:333–342. [PubMed] [Google Scholar]
- Zappe AC, Uludag K, Oeltermann A, Ugurbil K, Logothetis NK. The influence of moderate hypercapnia on neural activity in the anesthetized nonhuman primate. Cereb Cortex. 2008;18:2666–2673. doi: 10.1093/cercor/bhn023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarahn E, Aguirre G, D'Esposito M. A trial-based experimental design for fMRI. Neuroimage. 1997;6:122–138. doi: 10.1006/nimg.1997.0279. [DOI] [PubMed] [Google Scholar]