Summary
The bacterial tubulin homolog FtsZ forms a ring-like structure called the Z ring that drives cytokinesis. We showed previously that FtsZ-YFP-mts, which has a short amphipathic helix (mts) on its C terminus that inserts into the membrane, can assemble contractile Z rings in tubular liposomes without any other protein. Here we study mts-FtsZ-YFP, where the membrane tether is switched to the opposite side of the protofilament. This assembled “inside-out” Z rings that wrapped around the outside surface of tubular liposomes. The inside-out Z rings were highly dynamic, and generated a constriction force that squeezed the tubular liposomes from outside. This is consistent with models where the constriction force is generated by curved protofilaments bending the membrane. We used this system to test how GTP hydrolysis by FtsZ is involved in Z-ring constriction. Without GTP hydrolysis, Z rings could still assemble and generate an initial constriction. However, the constriction quickly stopped, suggesting that Z rings became rigidly stabilized in the absence of GTP hydrolysis. We propose that remodeling of the Z ring, mediated by GTP hydrolysis and exchange of subunits, is necessary for the continuous constriction.
Introduction
FtsZ, a tubulin homolog, is the major cell division molecule in most bacteria and many archaea(Vaughan et al., 2004). FtsZ polymerizes into short one-stranded protofilaments in the presence of GTP, and these undergo a constant disassembly and reassembly as long as GTP is present. In vivo the protofilaments associate further to make the Z ring, which is about 2-4 protofilaments thick on average in several species. The protofilaments are tethered to the cytoplasmic membrane through membrane proteins FtsA and ZipA. This tether involves a 50 amino acid (aa) flexible spacer on the C terminus of FtsZ, tipped by a 17 aa highly conserved peptide that binds to either FtsA or ZipA. See (Erickson et al., 2010) for a recent review of many aspects of FtsZ, and (Adams & Errington, 2009) for a review of the regulating proteins that interact with FtsZ.
We have recently reconstituted Z ring assembly in vitro in a liposome system. (Osawa et al., 2008) For this we constructed a membrane targeted FtsZ (FtsZ-YFP-mts), in which we replaced the 17 aa FtsA-binding peptide with YFP followed by an amphipathic helix (mts = membrane targeted sequence). FtsZ-YFP-mts protofilaments can tether themselves directly to the membrane. We produced multilamellar tubular liposomes, which were frequently leaky to proteins (Osawa & Erickson, 2009). When incorporated inside these tubular liposomes, FtsZ-YFP-mts plus GTP assembled numerous Z rings and helical variants. Moreover the Z rings generated a constriction force on the membrane. This demonstrates that FtsZ can assemble Z rings and generate a constriction force without any other cell division proteins.
Several possible mechanisms have been proposed for how FtsZ might generate a constriction force, reviewed and discussed in (Erickson, 2009). One postulates a continuous Z ring that encircles the cell, with overlapping ends that form lateral bonds. If the overlapping ends could slide, constriction would be favored by increasing the number of lateral bonds (Lan et al., 2009, Horger et al., 2010). A very different mechanism is based on the observation that FtsZ protofilaments can adopt a curved conformation, and when tethered to the membrane they could impose a bending force on the membrane (Lu & Erickson, 1999, Erickson, 1997, Erickson & Stoffler, 1996, Li et al., 2007, Allard & Cytrynbaum, 2009).
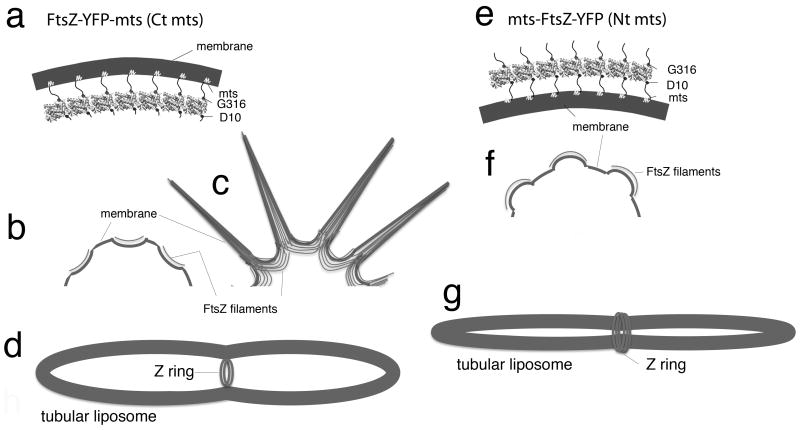
We have previously reported that membrane targeted FtsZ can deform liposomes in two directions. The results are summarized in Fig. 1(Osawa et al., 2008, Osawa et al., 2009). When FtsZ-YFP-mts was externally applied to large thin (or unilamellar) spherical liposomes, it distributed as patches that bent the membrane into concave depressions (Fig. 1b). This bending direction is the same as the Z ring constriction (Fig. 1a, d). Increasing the concentration of FtsZ-YFP-mts caused liposomes to extrude thin tubes, which appear to arise from the vertices between the concave depressions (Fig. 1c). (These tubes have patches of protofilaments aligned parallel to the axis of the tube. The curvature of these tubes is therefore perpendicular to the bending direction of the protofilaments, and is probably due to a balance of geometrical forces not yet understood.) The most important observation was that when FtsZ-YFP-mts was incorporated inside multilamellar tubular liposomes, it assembled Z rings that generated constriction force (Fig. 1d).
Fig. 1.
Schematic summary of membrane deformation by FtsZ. (a) FtsZ-YFP-mts subunits assembled into a curved protofilament, with the C-terminal membrane tether on the outside of the curve. (b) FtsZ-YFP-mts applied to the outside of thin, spherical liposomes, generated concave depressions on the membrane surface. (c) Formation of thin phospholipid tubes. When the concentration of FtsZ-YFP-mts was more than 4 μM, many thin lipid tubes appeared. These tubes were covered by FtsZ filaments which aligned parallel to the long axis of tubes. The curvature of these thin tubes in not easily related to that of the bending protofilaments. (d) Z rings formed on the inside of a tubular liposome by FtsZ-YFP-mts.. When FtsZ-YFP-mts was taken up by multilamellar tubular liposomes, it formed Z rings that generated a constriction force. (e) The membrane tether of mts-FtsZ-YFP is switched to the N-terminal D10, which is on the opposite side of the subunit. This membrane attachment is on the inside of the curved protofilament. (f) On thin spherical liposomes, it generates convex protrusions of the membrane surface. (g) The present study shows that when mts-FtsZ-YFP is applied to the outside of multilamellar tubular liposomes, it assembles inside-out Z rings that wrap around the outside and generate a constriction force by squeezing.
The opposite direction of bending was obtained by switching the membrane attachment to the opposite side of the FtsZ subunit (Osawa et al., 2009). The normal C-terminal attachment is on the “front” face of FtsZ, corresponding to the outside of a microtubule. The N terminus is on the “back” face, approximately 180 degrees away. When the switched construct, mts-FtsZ-YFP, was applied to the outside of spherical liposomes it produced convex protrusions (Fig. 1f), the opposite of the concave depressions produced by FtsZ-YFP-mts. This is consistent with the model that the constriction force is generated by a curved conformation of FtsZ protofilaments, with the “front,” C-terminal attachment on the outside of the curve. The bending of the membrane depends on which side the mts is attached to (Fig. 1a, b, e, f).
We have now extended these observations to show that the N-terminal membrane-targeted FtsZ can also form Z rings, but these wrap around the outside of tubular liposomes. The constriction force generated by these Z rings confirms the model that the force is generated by curved protofilaments bending the membrane. These inside-out Z rings are much easer to find than those inside liposomes, and this has allowed us to explore the ability of FtsZ to generate constriction forces with and without GTP hydrolysis.
Results
Inside-out Z rings
In our previous study of mts-FtsZ-YFP (Osawa et al., 2009) we first tested a construct that had a 1-aa insert between the mts and the N terminus of FtsZ. This gave convex protrusions on the outside of spherical liposomes, but produced no structures on tubular liposomes, either inside or outside. We then tested a 43-aa insert (a sequence predicted to be unstructured) and found that it produced similar convex protrusions on spherical liposomes. Only later did we test this 43-aa construct with tubular liposomes. We found that it assembled abundant Z rings wrapping around the outside of the liposomes; when incorporated inside tubular liposomes no structures were found. We later tested a 15-aa insert (HMGAELTQASGTTSH) and found that it gave even more rings on the outside (Fig. 2a). All the studies reported here used the 15-aa insert. It should be noted that the first 9 aa's of the E. coli FtsZ are apparently unstructured, so the actual link between the mts and the globular domain of FtsZ is 24 aa's, with a contour length of 8.2 nm. We can therefore conclude that a flexible tether of 10 aa's between the globular domain of FtsZ and the mts is sufficient to form convex protrusions on the outside of spherical liposomes. A tether of 24 aa's also supports these patches of convex protrusions, and additionally supports assembly of Z rings around the outside of tubular liposomes.
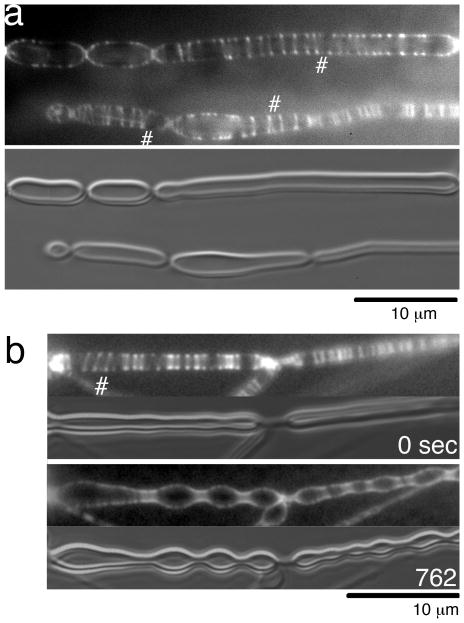
Fig. 2.
Inside-out Z rings on tubular liposomes. (a) Tubular liposomes with typical inside-out Z rings. These liposomes appeared to be attached to the glass slide along most of their length, although most later images show liposomes that are more lightly attached. The # symbols indicate spiral structures. The bottom panel shows the liposomes in DIC. (b) Inside-out Z rings constrict the tubular liposomes. The upper two frames (fluorescence and DIC) were taken about 5 min after the FtsZ was added to the liposomes, and show gentle constrictions. The lower frames were 12 min later, and show deep constrictions. Supporting Information Movie S1 shows the full 12 min period.
Fig. 2a shows an example of Z-rings assembled by mts-FtsZ-YFP wrapping around the outside of tubular liposomes. We call these “inside-out” Z rings to distinguish them from the normal Z rings on the inside of cells or liposomes. It is important to keep in mind that the curvature of the protofilaments is the same, and the term “inside-out” refers to the site of tethering to the membrane, as shown in Fig. 1a,e. The diameter of the inside-out Z rings corresponds to the outer diameter of the thick-walled multilamellar liposomes, placing them clearly on the outside. They bear a striking resemblance to normal Z rings, typically appearing as two bright dots on each side of the Z ring (this is due to the extra brightness when viewing the ring down the edges). In addition, the rings frequently separate into short helical structures, seen when one of the dots separates into two closely spaced dots. Helical structures were especially prominent on large diameter tubular liposomes (# in Fig. 2).
The tubular liposomes in Fig. 2a appeared to be attached to the glass over most of their length. These showed no movements of the liposome itself, and Z rings on it showed restricted movements. However, we could also find tubular liposomes that appeared to be attached only at one or both ends, to either the glass or another liposome. These showed movements of the whole liposome around the attachment site, and importantly showed rapid back and forth movements of the Z rings. This suggests that the structure of Z ring is not highly discontinuous, because fixing one part of the Z ring to the glass surface arrests the movement of the whole ring. To see the free movement of Z rings, we focused our studies on these lightly tethered liposomes.
The inside-out Z rings generated constriction forces that squeezed the liposomes in narrow zones. As shown in Fig. 2b and Movie S1, liposomes initially had smooth walls with Z rings scattered somewhat randomly. Over a period of about ten min the Z-rings moved laterally over the outer surface of tubular liposomes and coalesced into clusters. As these clusters became denser they appeared to constrict the liposome. Between these constrictions the liposome developed prominent bulges. Once constrictions developed, the Z rings appeared trapped there and remained concentrated (Fig. S1, Movie S2). This process is very similar to the Z rings formed inside tubular liposomes (Osawa et al., 2008).
New Z rings could be seen forming over the bulges between the constrictions. They are conspicuous here because these bulges were mostly devoid of Z rings (Fig. S1 and Movie S2). There appears to be two ways to form a new Z ring. In one pathway the new Z ring appears to initiate as a dot on one side of the tubular liposome (Fig. S1 238 s) and grow to the other side (Fig. S1 245 s). It took less than 10 s for the Z ring to grow from the initial dot to uniformly encircle the liposome. In the second pathway a small filamentous fragment seemed to separate from a mature Z ring (Fig. S1 266-294s). In either case, the Z rings appear to form primarily at the center of the bulge where the liposome wall has minimal slope. Once the Z ring has grown to partially or fully surround the bulge, it may be stable enough to constrict as it slides down the slope.
Z-ring assembly and constriction does not require GTP hydrolysis
GTP hydrolysis appears to be unnecessary for Z-ring assembly and cell division in vivo. This was originally suggested from the FtsZ mutant D212G, which was determined to have less than 0.5 % of the hydrolysis activity of wild type FtsZ (Trusca et al., 1998). We have confirmed that the GTPase activity of D212G is 0.3 % of wild type (David E. Anderson and HPE, unpublished results). However, this mutation is not lethal, and cells can divide using only FtsZ-D212G (Dajkovic & Lutkenhaus, 2006, Mukherjee et al., 2001, Osawa & Erickson, 2006). We discovered that these surviving cells need a suppressor mutation somewhere in the genome (Osawa & Erickson, 2006), which has not yet been identified. Nevertheless, the surprising conclusion is that this GTPase-dead mutant can assemble Z rings and function for cell division in vivo (Dajkovic & Lutkenhaus, 2006, Mukherjee et al., 2001, Osawa & Erickson, 2006). The mutant FtsZ84, which has only 10-30% of wild type GTPase, functions normally for division at the permissive temperature. Another mutant, FtsZ(L68D), which hydrolyzes GTP at 3% the rate of wild type FtsZ, could complement the FtsZ null strain(Redick et al., 2005). Although the colonies grew very slowly, they probably did not require a suppressor mutation. This suggests that GTP hydrolysis may not be essential for cell division itself in vivo.
We have shown previously that GMPCPP, a slowly hydrolysable GTP analogue (the hydrolysis rate is about 2% that of GTP for wild type FtsZ (Sontag et al., 2009)), can form Z rings inside tubular liposomes (Osawa et al., 2008). In the present study we looked for assembly of Z rings inside tubular liposomes using FtsZ(D212G)-YFP-mts. Fig. 3a shows a clear example of a tubular liposome with Z rings assembled inside; moreover the Z rings appear to be generating prominent constrictions. We also created mts-FtsZ(D212G)-YFP, and showed that this GTPase-dead mutant assembled inside-out Z rings. Fig. 3b shows that these Z rings generated constrictions.
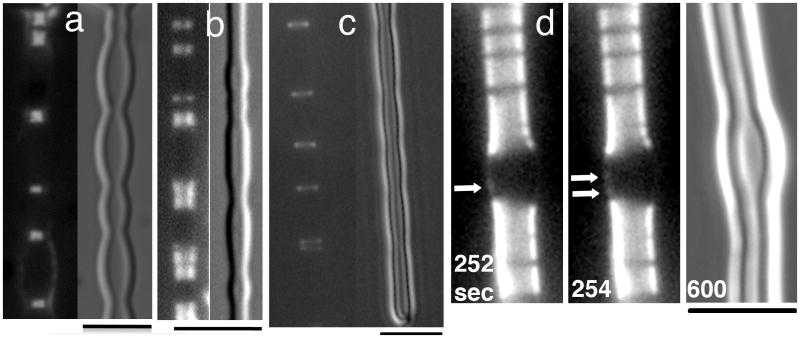
Fig. 3.
Z rings without GTP hydrolysis, inside and outside tubular liposomes. (a) GTPase dead FtsZ(D212G)-YFP-mts assembled Z rings inside tubular liposomes. 8 μM FtsZ was mixed with liposomes and 1 mM GTP, followed by the application of a coverslip. Z rings are located at constriction sites. (b) The mutant mts-FtsZ(D212G)-YFP (N terminal mts), assembled inside-out Z rings on the outside of tubular liposomes. Again Z rings are seen at sites of constriction. (c) Inside-out Z rings were assembled with 8 μM mts-FtsZ-YFP plus 1 mM GMPCPP. The inside-out Z rings were observed at sites of constriction. The rings assembled in GMPCPP showed small rocking movements but were much more static than rings assembled in GTP (Supporting Information Movie S3). (d) Another example of inside-out Z rings assembled from mts-FtsZ-YFP plus 1 mM GMPCPP. This liposome is densely coated with inside-out Z rings at the top and bottom, but devoid of Z rings in the slightly bulged middle segment. The arrows indicate the appearance of new rings (as small dots on one side of the bulge), which slide down to the narrower regions. Supporting Information Movie S4 shows the initiation of new Z rings more clearly. Bars indicate 5 μm.
The experiments with Z rings inside liposomes were technically difficult because Z rings inside liposomes, without GTP hydrolysis, were very rare. To extend these results we used the system of the inside-out Z rings, which gives a much higher frequency of Z rings. We used the wild type mts-FtsZ-YFP with GMPCPP which showed essentially same results as the GTPase-dead mutant D212G but more Z rings on the tubular liposomes. Fig. 3c shows that this formed typical inside-out Z rings with GMPCPP, and they generated constrictions. The major difference compared to GTP rings was that the GMPCPP rings were almost static. Whereas GTP rings showed vigorous sliding movements along the liposome, with new ones appearing on the bulge and sliding toward the constrictions (Movie S1 and S2), the GMPCPP rings rocked back and forth but showed negligible lateral motion (Movie S3). For example, the double ring at the far right in Movie S3 remained separated at a distance of ∼400 nm, and did not coalesce. The double ring on the left started at the same separation, and the rings then approached each other. However in the last frame the upper dot appears to separate, as if this is still two separate rings rocking back and forth.
Whereas inside-out Z rings in GTP are frequently seen further constricting the liposome, we have not been able to see GMPCPP rings in the act of further constricting the membrane. When first found they mostly appear localized at sites of constriction (Fig. 3c) but the constrictions were never seen to advance. Fig. 3d and Movie S4 show a liposome with long segments of packed Z rings at the top and bottom, separated by a gap. The packed Z rings correspond to constricted segments, and the gap is a bulge between them. One can occasionally see new Z rings initiated (as a dot on one side, 252 and 254 s; newly formed Z rings and helices are better seen in Movie S4), but they are mostly too dim to see. However the gap is almost completely filled in at 400 s showing that many new Z rings have formed, slid down the bulge and piled up on the constricted segments (Movie S4). This sliding toward the smaller diameter involves a constriction of the Z ring, even if it does not decrease the diameter of the membrane. This sliding would be produced by the same constriction force, but instead of constricting the membrane, a Z ring on a sloping wall just slides to the smaller diameter.
The initiation of new Z rings in GMPCPP is seen much better in Movie S5 (Fig. S2 shows isolated frames). These persist as partial Z rings, with a dot on one side only, and show restricted sliding toward the constrictions, perhaps because this liposome is stuck to the glass. Over time the bulges, initially devoid of Z rings, are mostly covered with Z rings.
Subunit turnover in inside-out Z rings with and without GTP hydrolysis
We used FRAP (fluorescence recovery after photobleaching) assays to determine the rate at which FtsZ subunits are exchanging between inside-out Z rings and the solution. With GTP the Z rings were exchanging subunits rapidly, with a recovery half time of about 30 s (Fig. 4a). In contrast, with GMPCPP there was no recovery over 9 min (Fig. 4b). Both of these results are similar to our previous study of subunit exchange of FtsZ-YFP-mts on tubular projections on the outside of liposomes (Osawa et al., 2009).
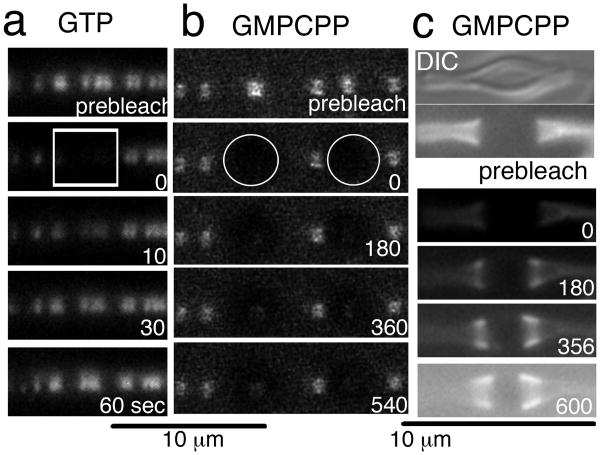
Fig. 4.
Subunit exchange in inside-out Z rings with or without GTP hydrolysis FRAP assays of inside-out Z rings with GTP (a) or GMPCPP (b, c). The bleached areas are indicated by a white rectangle (a) or circles (b). The recovery half time was about 30 s for (a); there was essentially no recovery during 9 min for (b). In the case of (c), the whole area of the image was bleached. There was no recovery over the constricted ends, but new Z rings piled up adjacent to the constrictions. The first image of (c) is a DIC image and the rest of them are fluorescence images.
While existing Z rings showed no exchange in GMPCPP, we did find that new Z rings could be initiated and accumulate along the sides of the existing Z rings, as shown in Fig. 3d and Movie S4. Fig. 4c shows a tubular liposome with a dense accumulation of Z rings on its constricted ends, and a relatively bare bulge in the middle. When this liposome was bleached entirely, the dense FtsZ on the ends did not recover, but new FtsZ began accumulating at the edges of the bare bulge. After ten min the bare zone had narrowed considerably as the newly initiated Z rings built up along the borders. The newly initiated Z rings did not mix with the older (bleached) Z rings at the constricted ends, but piled up beside them to fill in the bare zone. This is essentially the same scenario seen in Fig. 3d and Movie S4.
Discussion
We have demonstrated that FtsZ-YFP-mts forms Z rings only on the inside of tubular liposomes (Osawa et al., 2008), and mts-FtsZ-YFP forms structurally similar Z rings only on the outside. The constriction force of the normal, inside Z rings pulls the concave membrane to a smaller diameter, while the inside-out Z rings squeeze the convex membrane into similar narrow constrictions. We conclude that FtsZ filaments have a unidirectional curvature, and constriction force is generated by bending the membrane.
There are two possible reasons why mts-FtsZ-YFP prefers to form Z rings on tubular liposomes instead of the convex bulge previously described on spherical liposomes (Osawa et al., 2009). First, the tubular liposomes have thick, multilamellar walls, while the spherical liposomes were selected for thin walls. The rigidity of the thick wall may prevent the rising of convex protrusions Second, the geometry of the tubular liposome may be essential to stabilize FtsZ filaments as the ring structure. If a ring formed on a spherical liposome, constriction would cause it to slide down the wall and collapse into a patch. The cylindrical geometry traps the ring structure at the site of constriction, or at most permits it to slide back and forth without constriction.
FtsZ protofilaments have at least two curved conformations – highly curved (24 nm diameter) and intermediate curved (200 nm diameter) (Erickson et al., 2010). The highly curved conformation is linked to GTP hydrolysis(Erickson et al., 2010), although the linkage is not as straightforward as originally thought (Erickson et al., 2010). The intermediate curvature can occur without GTP hydrolysis. Our observation of Z-ring assembly and constriction in the absence of GTP hydrolysis suggests that the intermediate curved conformation may be the most important for force generation, because this bent conformation definitely does not require GTP hydrolysis.
Z rings that are hydrolyzing GTP show a constant turnover of subunits with a half time of 30 s, and these Z rings have been visualized in the process of constricting the membrane. Z rings assembled in GMPCPP are static, i.e., their subunits are not exchanging. These Z rings are typically located at sites of constriction when first found, but we have not found them progressing to smaller constrictions. This suggests that they generated a constriction as they were initially forming, but once stabilized in a cluster they are not be able to constrict further. We have observed that new Z rings that formed over a bulge were able to slide down to the constriction (Movie S4), demonstrating that the Z ring itself can constrict to a smaller diameter without GTP hydrolysis. These new Z rings mostly appear to be incomplete, as indicated by a dot on only one side of the liposome (Movie S4).
While the basic architectural unit of the Z ring seems to be the short protofilament, annealing might be favored when protofilaments are concentrated in the Z ring (Surovtsev et al., 2008, Erickson et al., 2010). This would lead to a long filament, and eventually a closed circle. This seems inconsistent with fast subunit exchange rate in Z rings (Stricker et al., 2002, Anderson et al., 2004), but we will suggest below that the subunit exchange may be a mechanism for creating gaps. Alternatively, the Z ring may be assembled from shorter protofilaments associated by lateral connections to form a ribbon a few protofilaments wide. At least one example of apparent lateral association has been seen by EM of sheets of protofilaments assembled on the membrane of liposomes (Osawa et al., 2009).
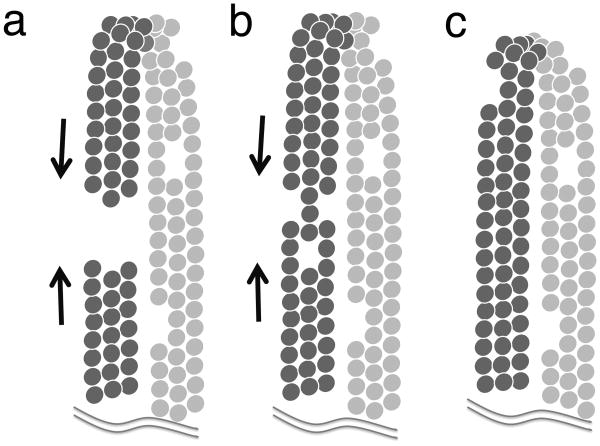
The observation that Z rings appear to arrest their constriction in the absence of GTP hydrolysis leads us to speculate that Z rings can only constrict when they have a gap. The Z ring illustrated in Fig. 5a can constrict until the ends come together. In Fig. 5c the protofilaments have formed closed circles, which block further constriction. Fig. 5b shows an intermediate case, where gaps in different protofilaments may permit constriction if the lateral connections between protofilaments are weak enough to permit sliding. We suggest that in the absence of GTP hydrolysis the newly formed Z rings can constrict as long as they have gaps, or perhaps as long as they have a helical structure where the ends do not connect. Eventually, however, if the ends meet and seal, the closed circles will block further constriction (Fig. 5c).
Fig. 5.
A model of the Z ring as three protofilaments in some kind of lateral contact. The ring in (a) has a gap, and the bending force of the protofilaments can constrict it until the gap is closed. In (b) the gaps are at different locations on the three protofilaments. This ring might also constrict if the lateral connections are loose enough to permit sliding of protofilaments relative to each other. In (c) the gaps have all closed, and this Z ring cannot constrict further until subunit exchange opens new gaps.
In the normal Z ring, hydrolyzing GTP, there is constant exchange of subunits, and this must generate openings in the circle. (How subunits could dissociate from the middle of a protofilament is a question for the future.) Once open, the circle can constrict further until it closes again. The exchange may produce gaps across the entire Z ring (three protofilaments in Fig. 5a), or multiple gaps in individual protofilaments (Fig. 5b). Little is known about the possible lateral connections (Erickson et al., 2010), but this mechanism would suggest that they are weak enough to permit dissociation of subunits and also sliding of protofilaments relative to each other. In the absence of GTP hydrolysis the initial arcs can generate a constriction, but once the circle is closed they cannot constrict further (Fig. 5c). This model is consistent with several examples showing constriction force generated by arcs of FtsZ in mutant bacteria and cyanelles (Addinall & Lutkenhaus, 1996, Bendezu et al., 2009, Sato et al., 2009) (reviewed in (Erickson et al., 2010)).
How can the GTPase-dead mutant D212G function for cell division in the suppressor strains (Dajkovic & Lutkenhaus, 2006, Mukherjee et al., 2001, Osawa & Erickson, 2006). One possibility is that Z-ring regulators in the cell may contribute to subunit turnover, lateral contacts of FtsZ filaments, membrane association of FtsZ filaments and opening of the ring. Alternatively, the Z ring may only need to generate a partial constriction, with division being completed by growth of the cell wall, or by fluctuations of excess membrane, as discussed elsewhere(Erickson & Osawa, 2010).
Other proteins have been well established to generate bending forces on membranes. (i) BAR domain proteins are banana-shaped proteins that insert into the membrane and form helical arrays that extrude membrane tubules of 50-200 nm diameter (Frost et al., 2009). Normal BAR domains wrap around the outside of the tubules, like inside-out Z rings, while members of the inverse-BAR family constrict membrane tubules from the inside. BAR domains do not hydrolyze nucleotides and their membrane bending activity apparently operates through their rigid curvature and reversible binding to membranes. (ii) Dynamin forms helical rings around the neck of invaginating vesicles, and scissions the neck to release the vesicle (Pawlowski, 2010). Although dynamin is a GTPase, GTP hydrolysis is not needed for it to wrap around the membrane and squeeze it into tubules. Hydrolysis is needed for the final scission event, in particular by driving disassembly of the dynamin helix. (iii) The multi-protein ESCRT-III system mediates a variety of membrane budding events. Its operation was spectacularly demonstrated by an in vitro reconstitution with liposomes (Wollert et al., 2009). When the proteins were added to the outside of giant unilamellar vesicles, they caused small membrane vesicles to invaginate to the inside, and eventually pinch off. This is similar to the concave depressions formed by FtsZ-YFP-mts on the outside of spherical liposomes, which occasionally led to vesicle invagination (Osawa et al., 2009). ATP hydrolysis was not needed for one round of vesicle invagination, but permitted new rounds, apparently by disassembling membrane-bound complexes. A particular interest of ESCRT-III is that a homologue of this eukaryotic system is used for cell division in crenarchaea, which do not have FtsZ (Lindas et al., 2008, Samson et al., 2008).
In light of these systems, it is perhaps not surprising that FtsZ can constrict membranes and function for cell division without GTP hydrolysis. The constriction force is generated by the relatively rigid protofilaments striving to achieve their curved conformation. The role of GTP hydrolysis may be to recycle subunits, which would serve two purposes. First, the constant remodeling may allow the Z ring to stay optimally positioned and structured. Second, the gaps generated by remodeling would prevent the Z ring from forming a closed circle, which cannot constrict. We suggest that the active constriction force may be generated by the resulting open arcs, rather than closed circles.
Experimental procedure
Reconstitution of inside-out Z rings on tubular liposomes
FtsZ-YFP-mts (C terminal membrane targeted FtsZ) and mts-FtsZ-YFP (N terminal membrane targeted FtsZ) were expressed from pET11b vector in an E. coli strain C41 (Miroux & Walker, 1996), which is derived from BL21. Detailed procedures of the protein expression and purification were previously described (Osawa & Erickson, 2009). The linker sequence of mts-FtsZ-YFP between mts and FtsZ in this study is HMGAELTQASGTTSH, which is predicted to be unstructured. The liposome preparation was also the same as described before (Osawa & Erickson, 2009). Briefly, a mixture of egg lecithin (Avanti) and DOPG (1,2-dioleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] (Avanti)) at an 8 to 2 ratio was dried on the Teflon disk and was rehydrated with HMKCG buffer (50 mM HEPES/KOH, pH 7.7, 5 mM MgAc, 300 mM KAc, 50 mM KCl 10% (v/v) glycerol) for 12 hrs at 37 °C. The thick-walled multilamellar liposomes were floated off the Teflon disk after gentle agitation. These multilamellar liposomes went to the surface of the HMKCG solution after several hours and were collected from there. To reconstitute Z rings inside or outside of tubular liposomes, we mixed the multi-lamellar liposomes with 1-2 mM GTP and 6-12 μM of the appropriate membrane-targeted FtsZ. When 5-10 μl of the mixture was spread in the narrow space between the slide and cover slip, some multi-lamellar liposomes turned into a tubular shape, probably due to shear forces as a result of the rapid spreading of solution.
In our previous study of FtsZ on the outside of liposomes, we focused on spherical liposomes, which showed either concave depressions or convex protrusions, depending on the orientation of the tether (Osawa et al., 2009). These membrane-bending events were seen best on thinner-walled liposomes, which were obtained in 100 mM KAc. The tubular liposomes studied here were best obtained from thicker-walled multilamellar vesicles, which are produced in 350 mM KAc.
Microscopy
Differential interference contrast and fluorescence images of liposomes were obtained with a Leica microscope DMI4000B with a 100× lens (NA 1.4) and a CCD camera (CoolSNAP HQ2, Roper). The YFP signal was captured through a YFP filter cube (BP500/20 515 BP535/30). Some images were obtained with a Zeiss Axiophot microscope with 100× lens (NA 1.3) and a CCD camera (CoolSNAP HQ, Roper). A Zeiss LSM Live DuoScan confocal microscope with a dedicated bleaching laser was used for the FRAP assay in Fig. 4a, b. In the case of fig. 4c, we used a Leica microscope DMI4000B and globally bleached the YFP fluorescence by illumination with intensified YFP excitation light through the BP500/20 filter for 20 sec.
Supplementary Material
Acknowledgments
The work was supported by NIH grant GM66014 to HPE. We thank David E. Anderson for assistance with the FRAP assays and assay of the GTPase of D212G.
Footnotes
Contributions. M.O. designed the project and carried out the experiments, M.O and H.P.E. analyzed the data and wrote the manuscript.
References
- Adams DW, Errington J. Bacterial cell division: assembly, maintenance and disassembly of the Z ring. Nat Rev Microbiol. 2009;7:642–653. doi: 10.1038/nrmicro2198. [DOI] [PubMed] [Google Scholar]
- Addinall SG, Lutkenhaus J. FtsZ-spirals and -arcs determine the shape of the invaginating septa in some mutants of Escherichia coli. Mol Microbiol. 1996;22:231–237. doi: 10.1046/j.1365-2958.1996.00100.x. [DOI] [PubMed] [Google Scholar]
- Allard JF, Cytrynbaum EN. Force generation by a dynamic Z-ring in Escherichia coli cell division. Proc Natl Acad Sci U S A. 2009;106:145–150. doi: 10.1073/pnas.0808657106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DE, Gueiros-Filho FJ, Erickson HP. Assembly Dynamics of FtsZ Rings in Bacillus subtilis and Escherichia coli and Effects of FtsZ-Regulating Proteins. J Bacteriol. 2004;186:5775–5781. doi: 10.1128/JB.186.17.5775-5781.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendezu FO, Hale CA, Bernhardt TG, de Boer PA. RodZ (YfgA) is required for proper assembly of the MreB actin cytoskeleton and cell shape in E. coli. EMBO J. 2009;28:193–204. doi: 10.1038/emboj.2008.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajkovic A, Lutkenhaus J. Z ring as executor of bacterial cell division. J Mol Microbiol Biotechnol. 2006;11:140–151. doi: 10.1159/000094050. [DOI] [PubMed] [Google Scholar]
- Erickson HP. FtsZ, a tubulin homolog, in prokaryote cell division. Trends Cell Biol. 1997;7:362–367. doi: 10.1016/S0962-8924(97)01108-2. [DOI] [PubMed] [Google Scholar]
- Erickson HP. Modeling the physics of FtsZ assembly and force generation. Proc Natl Acad Sci U S A. 2009;106:9238–9243. doi: 10.1073/pnas.0902258106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson HP, Anderson DE, Osawa M. FtsZ in Bacterial Cytokinesis: Cytoskeleton and Force Generator All in One. Microbiol Mol Biol Rev. 2010;74:504–528. doi: 10.1128/MMBR.00021-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson HP, Osawa M. Cell division without FtsZ--a variety of redundant mechanisms. Mol Microbiol. 2010;78:267–270. doi: 10.1111/j.1365-2958.2010.07321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson HP, Stoffler D. Protofilaments and rings, two conformations of the tubulin family conserved from bacterial FtsZ to alpha/beta and gamma tubulin. Journal of Cell Biology. 1996;135:5–8. doi: 10.1083/jcb.135.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost A, Unger VM, De Camilli P. The BAR domain superfamily: membrane-molding macromolecules. Cell. 2009;137:191–196. doi: 10.1016/j.cell.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horger I, Campelo F, Hernandez-Machado A, Tarazona P. Constricting force of filamentary protein rings evaluated from experimental results. Phys Rev E Stat Nonlin Soft Matter Phys. 2010;81:031922. doi: 10.1103/PhysRevE.81.031922. [DOI] [PubMed] [Google Scholar]
- Lan G, Daniels BR, Dobrowsky TM, Wirtz D, Sun SX. Condensation of FtsZ filaments can drive bacterial cell division. Proc Natl Acad Sci U S A. 2009;106:121–126. doi: 10.1073/pnas.0807963106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Trimble MJ, Brun YV, Jensen GJ. The structure of FtsZ filaments in vivo suggests a force-generating role in cell division. Embo J. 2007;26:4694–4708. doi: 10.1038/sj.emboj.7601895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindas AC, Karlsson EA, Lindgren MT, Ettema TJ, Bernander R. A unique cell division machinery in the Archaea. Proc Natl Acad Sci U S A. 2008;105:18942–18946. doi: 10.1073/pnas.0809467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CL, Erickson HP. The straight and curved conformation of FtsZ protofilaments-evidence for rapid exchange of GTP into the curved protofilament. Cell Struct Funct. 1999;24:285–290. doi: 10.1247/csf.24.285. [DOI] [PubMed] [Google Scholar]
- Miroux B, Walker JE. Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J Mol Biol. 1996;260:289–298. doi: 10.1006/jmbi.1996.0399. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Saez C, Lutkenhaus J. Assembly of an FtsZ Mutant Deficient in GTPase Activity Has Implications for FtsZ Assembly and the Role of the Z Ring in Cell Division. J Bacteriol. 2001;183:7190–7197. doi: 10.1128/JB.183.24.7190-7197.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa M, Anderson DE, Erickson HP. Reconstitution of contractile FtsZ rings in liposomes. Science. 2008;320:792–794. doi: 10.1126/science.1154520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa M, Anderson DE, Erickson HP. Curved FtsZ protofilaments generate bending forces on liposome membranes. EMBO J. 2009;28:3476–3484. doi: 10.1038/emboj.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa M, Erickson HP. FtsZ from divergent foreign bacteria can function for cell division in Escherichia coli. J Bacteriol. 2006;188:7132–7140. doi: 10.1128/JB.00647-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa M, Erickson HP. Tubular liposomes with variable permeability for reconstitution of FtsZ rings. Methods Enzymol. 2009;464:3–17. doi: 10.1016/S0076-6879(09)64001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowski N. Dynamin self-assembly and the vesicle scission mechanism: how dynamin oligomers cleave the membrane neck of clathrin-coated pits during endocytosis. Bioessays. 2010 doi: 10.1002/bies.201000086. [DOI] [PubMed] [Google Scholar]
- Redick SD, Stricker J, Briscoe G, Erickson HP. Mutants of FtsZ Targeting the Protofilament Interface: Effects on Cell Division and GTPase Activity. J Bacteriol. 2005;187:2727–2736. doi: 10.1128/JB.187.8.2727-2736.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson RY, Obita T, Freund SM, Williams RL, Bell SD. A role for the ESCRT system in cell division in archaea. Science. 2008;322:1710–1713. doi: 10.1126/science.1165322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Mogi Y, Nishikawa T, Miyamura S, Nagumo T, Kawano S. The dynamic surface of dividing cyanelles and ultrastructure of the region directly below the surface in Cyanophora paradoxa. Planta. 2009;229:781–791. doi: 10.1007/s00425-008-0872-4. [DOI] [PubMed] [Google Scholar]
- Sontag CA, Sage H, Erickson HP. BtubA-BtubB heterodimer is an essential intermediate in protofilament assembly. PLoS One. 2009;4:e7253. doi: 10.1371/journal.pone.0007253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker J, Maddox P, Salmon ED, Erickson HP. Rapid assembly dynamics of the Escherichia coli FtsZ-ring demonstrated by fluorescence recovery after photobleaching. Proc Natl Acad Sci U S A. 2002;99:3171–3175. doi: 10.1073/pnas.052595099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surovtsev IV, Morgan JJ, Lindahl PA. Kinetic modeling of the assembly, dynamic steady state, and contraction of the FtsZ ring in prokaryotic cytokinesis. PLoS Comput Biol. 2008;4:e1000102. doi: 10.1371/journal.pcbi.1000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trusca D, Scott S, Thompson C, Bramhill D. Bacterial SOS checkpoint protein SulA inhibits polymerization of purified FtsZ cell division protein. J Bacteriol. 1998;180:3946–3953. doi: 10.1128/jb.180.15.3946-3953.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan S, Wickstead B, Gull K, Addinall SG. Molecular evolution of FtsZ protein sequences encoded within the genomes of archaea, bacteria, and eukaryota. J Mol Evol. 2004;58:19–29. doi: 10.1007/s00239-003-2523-5. [DOI] [PubMed] [Google Scholar]
- Wollert T, Wunder C, Lippincott-Schwartz J, Hurley JH. Membrane scission by the ESCRT-III complex. Nature. 2009;458:172–177. doi: 10.1038/nature07836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.