Abstract
FGF signaling and PAX6 transcription are required for neuroectoderm specification of human embryonic stem cells (hESCs). In this study we asked how FGF signaling leads to PAX6 transcription and neuroectoderm specification from hESCs. Under a chemically defined medium, FGF inhibition blocked phosphorylation of ERK1/2 with a significant reduction of PAX6-expressing neuroepithelia, indicating that FGF regulates neural induction through ERK1/2 activation. Activation of FGF-ERK1/2 pathway was necessary for the activity of PARP-1, a conserved nuclear protein catalyzing polymerization of ADP-ribose units. Pharmacological inhibition and genetic ablation of PARP-1 inhibited neural induction from hESCs, suggesting that FGF-ERK1/2 signal pathway regulates neuroectoderm specification through regulating PARP-1 activity. Furthermore, FGF-ERK1/2-PARP-1 cascade regulated the expression of PAX6, a transcription determinant of human neuroectoderm. Together, we propose that FGF regulates hESC neural specification through the ERK1/2-PARP-1 signaling pathway.
Keywords: Human embryonic stem cells, Neural differentiation, Fibroblast Growth Factor, ERK1/2, PARP-1, PAX6
Introduction
Specification of the neuroectoderm from embryonic ectoderm, also known as neural induction, is the first and critical step toward the formation of the complex nervous system. Historical studies employing Xenopus and chick embryos have lead to the “default model” of neural induction1-5. In this model, bone morphogenetic proteins (BMPs), produced by ectoderm cells, prevent them from becoming neural tissues. During gastrulation, inhibitors of BMPs, such as Noggin, Chordin, and follistatin secreted from the embryonic organizer, remove the inhibition. The ectoderm cells hence become neural tissue by default. Nevertheless, BMP inhibition alone is not sufficient for neural induction in ectopic ectoderm regions. Growing evidences indicate that other signaling molecules, especially fibroblast growth factors (FGFs), also play a crucial role in early steps of neural differentiation2, 6-10. In chick embryos, the organizer produces FGFs that are required for neural induction9. In Xenopus, inhibition of FGF signaling suppresses neural induction even in the presence of BMP antagonists11. These findings raised a question of whether the role of FGFs in neural induction is dependent on BMP signaling. Analysis of signaling pathways initiated by BMPs and FGFs during Xenopus development revealed that the main effectors of the FGF pathway, MAPKs, phosphorylate SMAD1, a key downstream transcription factor of BMP signaling, at the linker region. Such modification prevents nuclear translocation of SMAD1 and promotes degradation of SMAD1 through proteosomes 8, 12-15. This discovery brings together FGF and BMP signalings to the default model of neural induction. Whether such a molecular model of neural induction applies to mammals is presently not clear.
Examinations on mammalian neural induction are performed largely using in vitro models of neuroepithelial differentiation from embryonic stem cells (ESCs). These studies confirm the requirement of BMP inhibition and FGF activation in neural differentiation of mouse ESCs. However, FGF signaling appears independent of its inhibitory function on BMP signaling6, 16, 17. For example, the negative effect of FGF inhibition on neural specification of mouse ESCs could not be rescued by BMP inhibitors18, similar to the phenomenon in early chick embryo development in which FGF works independently of BMP activity in neural induction9. Under similar culture conditions, neuroepithelial differentiation of human ESCs also seems to be positively and negatively regulated by FGFs and BMPs, respectively19-21. We found that the protein level and subcellular localization of p-SMAD1 was not altered in neural differentiating human ESCs in the presence of FGF signaling inhibitors, suggesting that FGFs regulate hESC neural differentiation independent of BMP-SMAD signaling21. This raised the question: what would be the alternative pathway that mediates FGF effects on neuroectoderm specification in mammals•
FGFs are known to exert their multiple functions, including neural induction, through the MAPK/ERK pathway5, 22-25. While there are many downstream effectors of ERK, Poly (ADP-ribose) polymerase-1 (PARP-1), which mediates ERK1/2-initiated transcriptional regulation 26, 27, may be one of the candidates. PARP-1 is a highly conserved DNA binding nuclear protein that catalyzes the covalent attachment of Poly (ADP-ribose) units (PAR) to itself and other nuclear target proteins. This leads to modification of target proteins, such as protein-DNA or –protein interactions28-31. Although it has originally been identified as a DNA repair-associated protein that is activated by DNA breakage32, 33, PARP-1 has recently been shown to regulate growth, proliferation and differentiation of a variety of cells29, 30 through transcriptional regulation 34-37. Whether it also mediates the effect of FGFs in neural induction, however, remains unexplored.
In the present study, we found that ERK1/2 is a predominant downstream kinase activated by FGF during neural differentiation of hESCs. Importantly, we found that PARP-1 activity is regulated by FGF-ERK1/2 in neural differentiating hESCs. Suppression of PARP-1 activity using chemical inhibitors or genetic ablation blocks neural differentiation of hESCs, suggesting that PARP-1 is a downstream effector of FGF-ERK1/2 pathway in hESC neural differentiation. Furthermore, we found that PARP-1 bound to the promoter of PAX6, a transcription determinant of human neuroectoderm38, suggesting that FGF-ERK1/2-PARP-1 cascade regulates neuroectoderm specification of hESCs through modulation of PAX6 transcription.
Material and Methods
Reagents
BMP4 and antibodies against SMAD1, SOX2 were obtained from R&D systems Inc (Minneapolis, MN). PAX6 mAb was from the Developmental studies Hybridoma Bank (Iowa City, IW), Abs against p-SMAD1 and ERK1/2 from cell signaling technology (Beverly, MA), actin from Sigma (St. Louis, MO), α-tubulin from Abcam (Cambridge, MA).
hESCs differentiation
The hESCs, H9 (p16-35), H1 (p20-35) were cultured on irradiated mouse embryonic fibroblasts (MEFs) and neural differentiation of hESCs was performed as previously described39, 40. Briefly, neural differentiation was initiated by detaching hESCs from MEF with 1 mg/ml dispase (Invitrogen, Carlsbad, CA), and grown in suspension in the ESC growth medium consisting of Dulbecco's modified eagles medium (DMEM/F12, 20% Knockout replacement serum, 1× nonessential amino acids, 2mM glutamine and 100μM β-mercaptoethanol, all from Invitrogen) to form aggregates for 4 days. The aggregates (day 4) were then transferred to a serum-free minimal (SFM) medium consisting of DMEM/F12, 1× nonessential amino acids and 2 μg/ml heparin (all from Invitrogen), and grown in suspension. After 3 days in suspension (day 7), they were attached to laminin-coated (Invitrogen) coverslips for immunostaining. At day-10 cells were harvested and used for further analysis. For inhibitor treatment, SU5402, U0126 and PJ34 were added to cultures between day 4 to day 6 of differentiation.
Plasmids and viral transduction
PARP-1-specific shRNA and control vectors were obtained from Open Biosystems (RHS4430-99147879 and RHS4346, respectively). We followed the protocol for lentiviral transduction as previously described41. Briefly, 20 μg of transfer vector, 15 μg of lentiviral vector pCMVΔ8.91 and 6μg of vesicular stomatitis virus G protein (VSV-G) were co-transfected to HEK293FT cells (Invitrogen) with calcium phosphate precipitation method. Six hours later, the medium was changed to hESC media, and 2 days later, the virus containing medium was collected and cleared by centrifugation (3000 rpm for 5min) and filtration (0.45μM), and immediately used. For transduction of hESCs, the cells were treated with ROCK inhibitor, (Calbiochem, NJ) for overnight before viral infection. Trypsinized cells were incubated with viral-containing media for 1 hour at 37 °C, and then plated on MEF cells and cultured overnight in the presence of ROCK inhibitor before changing media in the next day. 48 hors after infection, puromycin (5μg/ml) was treated for selection of drug-resistant clones. Clones expressing viral marker GFP were selected and further analyzed.
Mircoarray analysis
The experiments were described in previous study21. Affymetrix Human Genome U133 plus 2.0 microarrays were used to analyze gene expression levels of key components of the FGF signaling pathway at days 0, 6, 10 of hESC neural differentiation. hESC expression profiles were used as a reference. cRNA probe synthesis and array hybridizations were carried out at the NIH Neuroscience Microarray Consortium (http://arrayconsortium.tgen.org/np2/home.do).
Preliminary analysis was performed using Affymetrix Microarray Suite 5.0 (MAS 5.0) and Data Mining Tool softwares. The data were deposited at the NIH Neuroscience Microarray Consortium (http://arrayconsortium.tgen.org/np2/home.do) and in the ArrayExpress database (accession number E-MEXP-2426).
Immunocytochemistry
Neuroepithelial cells from hESCs were stained as described in previous studies39, 40.
Flowcytometry
Harvested cells were filtered through a 70-μM cell strainer, and fixed with 0.1% paraformaldehyde for 15 minutes on ice and permeabilized in 90% methanol for 20 minutes. Cells resuspended in FACS buffer (PBS with 2% donkey serum) were incubated with primary antibodies or normal IgG (as control) overnight at 4 °C, followed by washing and staining with secondary Abs. for 1 hour. Cells were analyzed using a Becton Dickinson FACSCaliber and CellQuest Pro (BD Biosciences, San Diego, http://www.bdbiosciences.com).
Immunoblotting
Cells were lysed with RIPA buffer as described in previous studies 21. Lysates were cleared by centrifugation for 20 min. and the concentration was determined by BCA protein assay kit (BIO-RAD, CA). The lysates were resolved by SDS-PAGE and western blotting was carried out using horse-radish peroxidase-conjugated IgG as a secondary antibody and ECL system for detection.
RT-PCR
Total RNA was isolated using the Trizol kit (Invitrogen, CA) by manufacturer's manual. 1μg/ml of total RNA was used for reverse transcription, followed by real-time PCR using Power SYBR kit (Applied Biosystem, UK). Primers used for real-time PCR were listed in Table S1.
ChIP analysis
After cross-linking with 1 % formaldehyde at 37 °C for 10 min., cells were harvested and washed, followed by lysis with EZ-Chip™ kit according to the manufacturer's manual (Millipore, CA). The chromatin was sheared by sonication and immunoprecipitated with anti-PARP-1 Ab (Abcam, MA). The immune complex was then washed 5 times and reversed. Chromatin DNA was subjected to quantitative PCR using primers for control and PAX6/ SOX2 promoters. (Table S1)
Results
ERK1/2 is a downstream effector of FGF signaling in neuroectoderm specification from hESCs
We have previously shown that under a chemically defined system, hESCs differentiate to a nearly uniform population of neuroepithelial cells at around day 8-10 by expression of neuroectoderm transcription factors PAX6 and SOX2 40. This efficient neural differentiation correlates with expression of FGF signaling components by the differentiating cells (Fig. S1) and is not effectively blocked by BMP21. To delineate how FGF regulates neuroectoderm specification of hESCs, we first investigated which downstream pathways are involved in the FGF-mediated neural differentiation. At day 6 of neural differentiation, right before the appearance of PAX6-expressing neuroepithelial cells, 30 minutes of treatment of cultures with SU5402, an inhibitor of FGF receptors, at the concentration that effectively blocks neural differentiation without causing apparent cell death 18, 21, blocked the phosphorylation of ERK1/2 (Fig. 1A). However, the phosphorylation of other related serine/threonine kinases, p38 and JNK, were not affected. pAKT level in inhibitor-treated cells was also weakly reduced. These results indicate that ERK1/2 is a predominant downstream kinase activated by FGF signaling in neural differentiating hESCs and suggests that ERK1/2 activation is responsible for the FGF-mediated regulation of neural differentiation.
Figure 1.
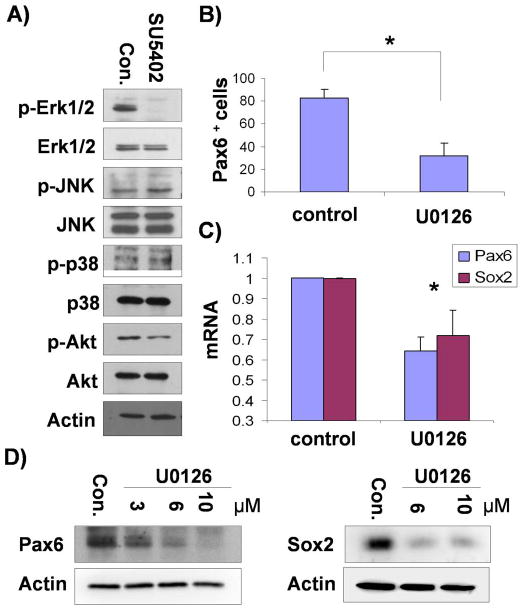
ERK1/2 is a downstream molecule of FGFs in hESC neural differentiation. A) Differentiating hESCs at day 6 were treated with SU5402 (5μM) for 30 min and cell lysates were subjected to immunoblotting with Abs as indicated. B) Differentiating hESCs were treated with U0126 (10μM) or DMSO (as a control) between day 4 and day 6, and cells at day 10 were analyzed for PAX6 expression by flowcytometry. The mean ± S.E. value of the percentage of PAX6+ cells from three independent experiments are shown. *, P<0.05 in comparison with the value from control cells. C) Total RNAs were extracted from cells that were prepared in the same way as B) and the expression of PAX6 and SOX2 were assessed by real time PCR. The mean ± S.E. value from the three independent experiments are shown after normalization to that of control cells. *, P<0.05 in comparison with the value from control cells. D) Differentiating hESCs were treated with U0126 between day 4 and day 6 and cell lysates from day 10 were subjected to immunoblotting with Ab-PAX6 or SOX2 as indicated.
To confirm whether FGF-ERK1/2 signal cascade underlies neural differentiation of hESCs, differentiating cells were treated with an ERK1/2 specific inhibitor, U0126 between day4 and day6 of differentiation and neural differentiation was measured at day 10. FACS analysis, verified by immunostaining on coverslip cultures, revealed that only 30% of the cells were PAX6+ neuroepithelial cells in the presence of U0126 as compared to more than 80% in the control group without U0126 (Fig. 1B). Real time PCR showed that PAX6 and SOX2 expression was decreased in U0126-treated cells (Fig. 1C). Immunoblotting assay showed that the expression of PAX6, and another neural marker, SOX2 were significantly decreased in the cells treated with U0126, compared with control cells (Fig. 1D). Collectively, these data suggest that ERK1/2 works as a downstream effector of FGF signaling to regulate neural induction from hESCs.
FGF-ERK regulates PARP-1 activity during hESC neural differentiation
How FGF-ERK regulates neural induction is not clear. Using the specific antibody targeting the MAPK phosphorylation sites of SMAD, developed by Dr. DeRobertis, we found that activation or inhibition of FGF-ERK did not alter SMAD activity21. This suggests that alternative pathways may mediate the effect of FGF-ERK in neural differentiation. PARP-1 protein, a conserved nuclear enzyme catalyzing polymerization of ADP-ribose units to target proteins, has been shown to be regulated by ERK226, 27. In nerve growth factor-induced differentiation of PC-12 cells, which involves ERK activation, PARP-1 activity is increased42, 43. We therefore hypothesized that PARP-1 may mediate the FGF effect on hESC neural differentiation. Immunoblotting analysis indicated that the PARP-1 activity, revealed by an antibody against PAR, was increased during neural differentiation, whereas the level of PARP-1 protein was not altered (Fig. 2A). Immunostaining of differentiating cells also indicated that PAR formation was increased during neural differentiation (Fig. 2B). This phenomenon was observed with another hES cell line, H1 that was tested. In addition, the temporal change of PARP-1 activity correlated with that of p-ERK1/2 during neural differentiation of hESCs (Fig. S2). This temporal course of PAR activity along hESC neural differentiation corresponds well with expression of FGF and ERK1/2, suggesting that PARP-1 may be a downstream of FGF-ERK pathway.
Figure 2.
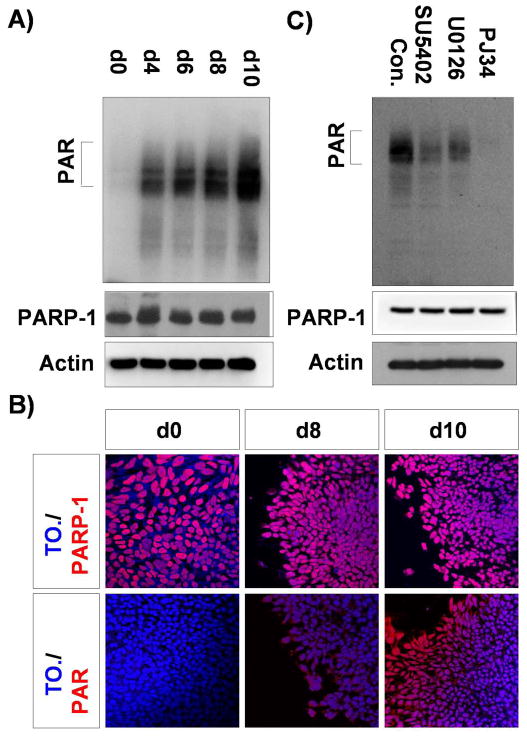
PARP-1 is regulated by FGF-ERK1/2 in the neural differentiating hESCs. A) hESCs (d0) and differentiating cells at day 4 (d4), d6, d8, and d10 were subjected to immunoblotting assay with Ab-PAR and Ab-PARP-1 as indicated. Auto-PARylated PARP-1 is marked on the left. B) hESCs (d0) and differentiating cells at day8 (d8) and day 10 (d10) were subjected to immunostaining with Ab-PARP-1 and Ab-PAR as indicated. Nuclei were shown by To-pro-3 staining. C) Differentiating cells at day 6 were treated with SU5402 (5μM), U0126 and PJ34 (10μM each) for 2 hours at 37 °C, and the cell lysates were immunoblotted with Ab-PAR and PARP-1 as indicated.
To determine whether PARP-1 activity is regulated by FGF-ERK1/2 pathway in the neural differentiating hESCs, we measured the level of enzymatic product of PARP-1, PAR, in the presence of inhibitors of FGF and ERK1/2. Differentiating cells at day 6 were treated with SU5402, U0126, or a PARP-1 specific inhibitor, PJ34 and the cell lysates were subjected to immunoblotting assay using an anti-PAR antibody. Treatment with PJ34 removed most of the auto-PARylating activity from PARP-1. Interestingly, SU5402 and U0126 treatment significantly reduced the level of PAR formation (Fig. 2C). However, the amount of PARP-1 protein was not altered by the above treatments. Taken together, these results suggest that PARP-1 activity is regulated by FGF-ERK1/2 signal pathway during hESC neural differentiation.
PARP-1 activity is required for hESC neural differentiation
Since FGF-ERK1/2 signaling is critical for hESC neural differentiation and PARP-1 is regulated by ERK, we then asked whether PARP-1 is required for hESC neural induction. Neural differentiating cells were treated with PJ34 between day4 and day6 of differentiation and neural differentiation was analyzed at day10. FACS analysis, as well as immunostaining of coverslip cultures, showed that PJ34 treatment significantly reduced the PAX6-expressing neuroepithelial population (Fig. 3A, B). Immunoblotting assay using lysates from the same cell preparations indicated that the expression of PAX6 and SOX2 were decreased in the cells treated with PJ34 in a dose dependent manner (Fig. 3C). Similarly, real time PCR indicated that PARP-1 inhibition resulted in the decrease of PAX6 and SOX2 expression (Fig. 3D). These findings strongly suggest that PARP-1 acts as a downstream effector of FGF-ERK1/2 signal pathway to regulate neural differentiation of hESCs.
Figure 3.
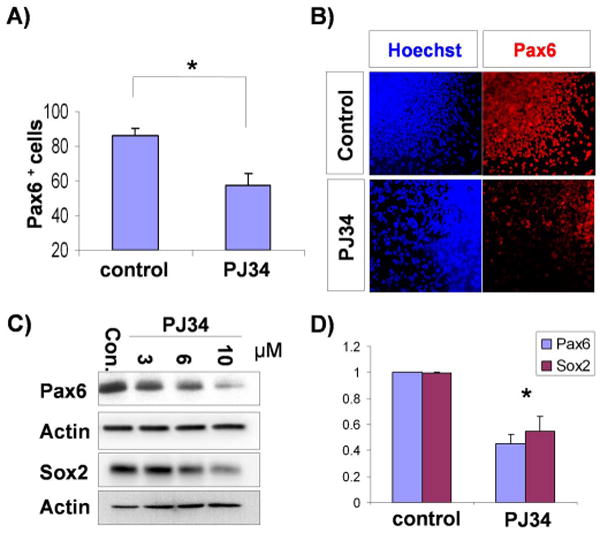
PARP-1 is required for neuroectoderm specification of hESCs. A) Neural differentiating cells were treated with PJ34 (10μM) between day4 and day6, and cells at day 10 were analyzed for the expression of PAX6 by flowcytometry. The mean ± S.E. value of the percentage of PAX6+ cells from three independent experiments are shown. *, P<0.05 in comparison with the value from control cells. B) Differentiating cells were treated with PJ34 (10μM) between day4 and day6 and cells at day 10 were subjected to immunostaining with Ab-PAX6 (red). Nuclei were shown by Hoechst staining. C) Differentiating cells were treated with PJ34 at different concentrations between day4 and day6 and the lysates from day 10 cells were subjected to immunoblotting with Ab-PAX6 or SOX2 as indicated. D) Total RNAs were extracted from cells that were prepared in the same way as A) and the expression of PAX6 and SOX2 were analyzed by real time PCR. The mean ± S.E. value from three independent experiments are shown after normalization to that of control cells. *, P<0.05 in comparison with the value from control cells.
To further confirm the functional role of PARP-1 in neuroectoderm specification of hESCs at the genetic level, we generated PARP-1 knockdown hESC lines by lenti-virus-mediated constitutive expression of PARP-1 specific shRNA. By using GFP as a transgenic marker, we selected one control hESC line and three PARP-1 shRNA-expressing cell lines, two of which highly expressed GFP (#3 and #6), and one of which showed a lower level of GFP expression (#2) (Fig. S3). Consistent with the GFP expression level, PARP-1 protein is largely knocked down in #3 and #6 shRNA lines, while moderately downregulated in #2 line. Expression of control or PARP-1 shRNA did not affect the growth of hESCs, consistent with expression of PARP-1 but without high level of PAR activity at the ESC stage (Fig. 2). The expression of SOX2, OTX2, PAX6 and pluripotent marker, OCT3/4 in the cells expressing PARP-1 shRNA was not changed in ESC stage, compared with control cells (Fig. 4A, left panel). After the transgenic hESCs were differentiated to neuroepithelia for 10 days, the expression of PARP-1 remained highly inhibited in high-GFP expression cell line (#3 and #6), whereas moderately inhibited in the low-GFP expression cell line (#2), as compared to the control cell line (Fig. 4A, right panel). Corresponding to the genetic ablation of PARP-1, the expression of neuroepithelial markers, PAX6, SOX2 and OTX2, was greatly reduced in high-GFP expression cell lines, in comparison to the low-GFP expression cell line and the control cell line (Fig. 4A). OCT3/4 is not expressed in differentiating cells, suggesting that the cells are not proliferating ESCs. The expression of mRNAs for the above neural transcription factors, revealed by real time PCR, showed a similar pattern (Fig. 4B). Consistent with the above observations, immunostaining, confirmed by FACS analysis (Fig. S4), showed that the expression of PAX6 and SOX2 at day10 was suppressed in PARP-1 knockdown cells (Fig. 4C and Fig. S5). Together, data from pharmacological intervention and genetic ablation studies strongly support that PARP-1 is required for efficient neuroectoderm specification of hESCs.
Figure 4.
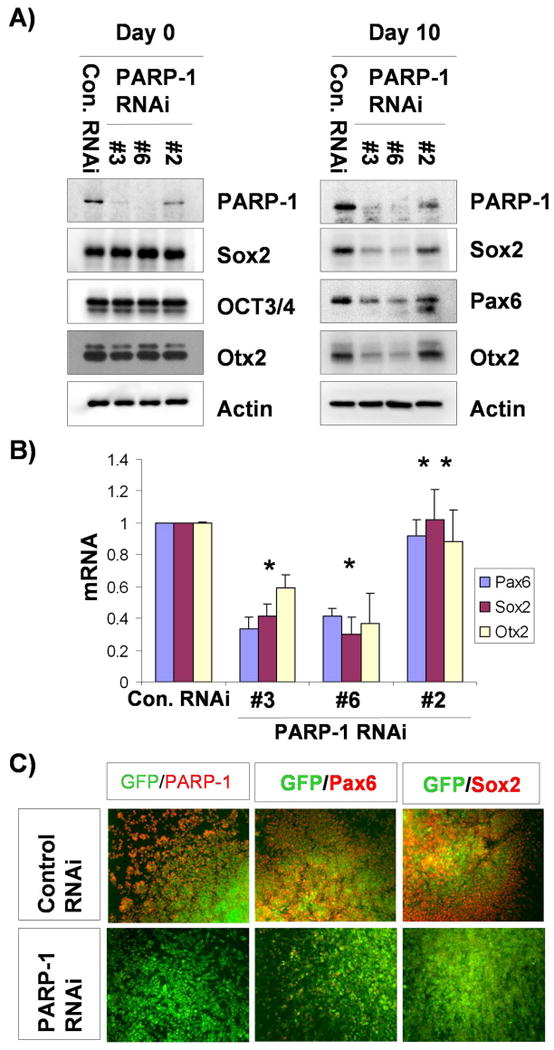
Genetic ablation of PARP-1 blocks neuroectoderm specification of hESCs. A) Lysates from cell lines expressing PARP-1-shRNA and control shRNA at ESC stage (d0) and differentiating stage at day 10 (d10) were subjected to immunoblotting with Ab- PARP-1, PAX6, SOX2, OTX2 or OCT3/4 as indicated. B) Total RNAs were extracted from cells prepared in the same way as A), and the expression of PAX6, SOX2 and OTX2 were analyzed by real-time PCR. The mean ± S.E. value from three independent experiments was shown after normalization to that of control cells. *, P<0.05. **, P>0.05 in comparison with the value from control RNAi cells. C) Neural differentiating cells at day 10 which have mixed population of viral-infected (GFP-positive) and non-infected (GFP-negative) were subjected to immunostaining with Ab-PARP-1, -PAX6 or -SOX2 as indicated. GFP was used as a viral marker.
PARP-1 regulates PAX6 transcription during hESC neural differentiation
PAX6 has recently been shown to be a transcriptional determinant of the human neuroectoderm38. Since PARP-1 regulates hESC neural differentiation, we investigated whether FGF-ERK1/2-PARP-1 pathway directly regulates PAX6 transcription. We first analyzed the protein level of PAX6 in the presence of inhibitors of FGF and ERK1/2. PAX6 protein stability is not changed by treatment with either FGF or ERK1/2 inhibitors. We next tested whether this pathway regulates transcription of PAX6. A direct connection between a signal pathway and the expression of its target gene can be demonstrated by assessing corresponding transcriptional responses in a short term. Neural differentiating cells at day 5 were treated with inhibitors for 2 hrs, and transcription of PAX6 was measured by real time PCR. As shown in Fig. 5A, PJ34 alone significantly suppressed the transcription of PAX6, as did SU5402 and U0126, suggesting that FGF-ERK1/2-PARP-1 cascade regulates the transcription of PAX6. PARP-1 regulates its target gene generally by its enzymatic activity36, 44-48. It may also bind to DNA as a member of a protein complex, such as in the case that PARP-1 binds to the enhancer region where it regulates the expression of quail PAX6 gene 49. We therefore examined whether regulation of PAX6 expression is mediated by direct PAX6 promoter occupancy of PARP-1 by ChIP-qPCR with anti-PARP-1 antibody and lysates from self-renewing (day 0) and neural differentiating (day 10) hESCs. Interestingly, PARP-1 was localized specifically at the promoter region of PAX6, but not at SOX2 promoter (Fig. 5B). Since the activity of PARP-1 is regulated by FGF-ERK along neural differentiation, the localization of PARP-1 in the PAX6 promoter strongly suggests that FGF-ERK1/2-PARP-1 signal pathway regulates neural induction of hESCs at least in part, by controlling the transcription of neural transcription factor, PAX6.
Figure 5.
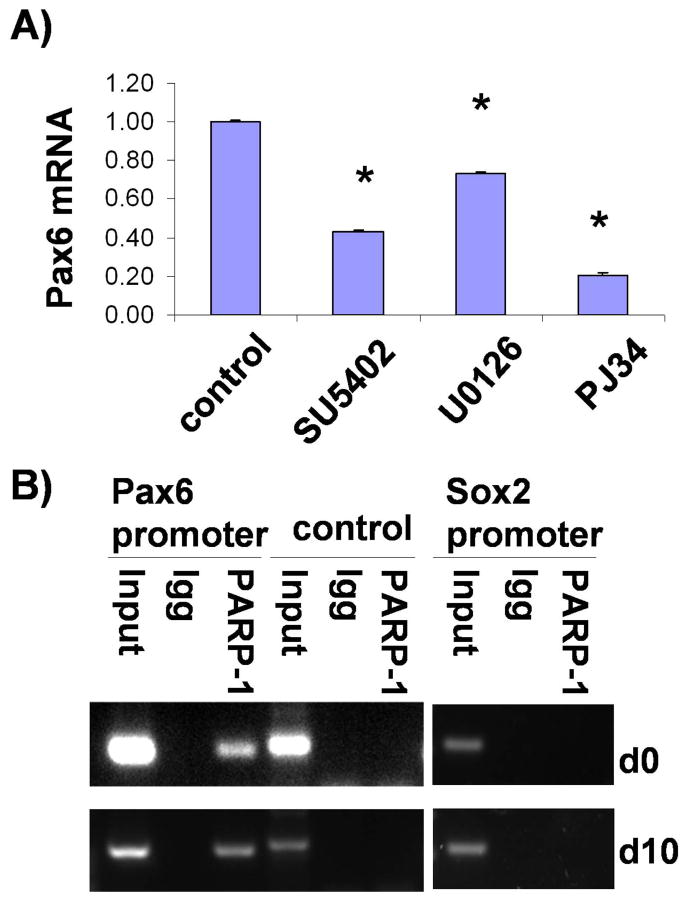
FGF-ERK1/2-PARP signaling regulates PAX6 transcription. A) Differentiating cells at day 5 were treated with SU5402, U0126 or PJ34 for 2 hours, and the expression of PAX6 mRNA was analyzed by real-time PCR. The mean ± S.E. value was shown after normalization to that of control cells. *, P<0.05 in comparison with the value from control cells. B) Chromatin samples were prepared from ESCs (day0) and neural differentiating cells (day10), and subjected to ChIP analysis using Ab-PARP-1 or control IgG. The precipitated chromatin DNA was amplified with primers for the PAX6 promoter, control region and the SOX2 promoter as indicated.
Discussion
Embryogenesis, including neuroectoderm specification, is orchestrated by multiple signaling pathways 50. FGFs and BMPs, the two major and opposing families of extracellular molecules, regulate Xenopus neuroectoderm induction through integration at the SMAD level 8, 12-15, 38. Recent analyses of neuroectoderm specification using ESC models, especially human ESCs, suggest that FGFs affect neuroectoderm specification independent of BMP-SMAD pathway21. Our present study indicates that ERK1/2 is a predominant downstream molecule activated by FGF signaling and responsible for the regulation of neuroectoderm specification. Importantly, we discovered that PARP-1 is directly regulated by the FGF-ERK1/2 pathway during hESC neural differentiation. Furthermore, we show that PARP-1 is localized to the promoter region of PAX6, the human neuroectoderm transcriptional determinant, whereas the PAR activity is regulated by FGF-ERK along neural differentiation. We propose that FGFs regulate neuroectoderm specification of hESCs through the ERK1/2-PARP-1 pathway to control the transcription of PAX6 and hence neuroectoderm specification (Fig. 6).
Figure 6.
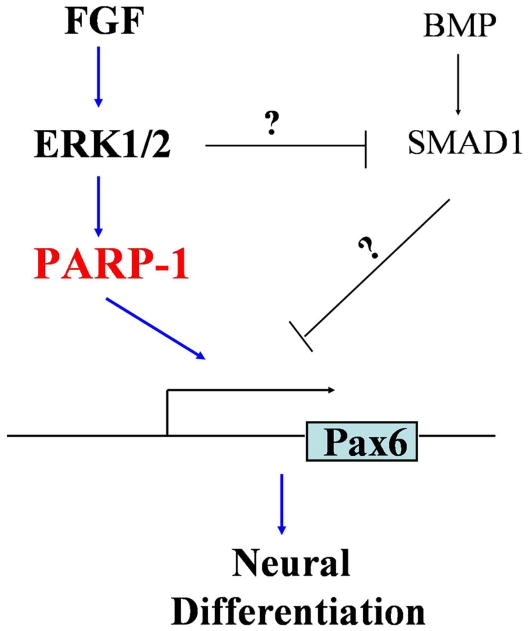
Scheme of the functional role of FGF signaling in neural specification of hESCs. Along neuroectoderm specification of hESCs, FGFs and BMPs regulate PAX6 transcription through parallel pathways besides potential convergence on SMAD1. FGFs mainly activate the ERK1/2-PARP-1 pathway to activate the transcription of PAX6, hence neural specification.
Under multiple biological systems, FGFs exert their function through activation of MAPK/ERK1/224, 51-55. Our present study confirms that ERK1/2 is the predominant effector of FGF signaling during hESC neural differentiation. How FGF-ERK regulates neuroectoderm transcription factors, for that matter also for BMP-SMAD, remains an unresolved issue in the field of neural induction. PARP-1 emerges as a potential mediator as it is regulated by ERK1/2 during cell proliferation and differentiation besides its role in DNA repair26, 27, 29. PARP-1 activation is required for nerve growth factor-mediated neuronal differentiation of PC12 cells, a process that is mediated by ERK1/242, 43. Our present study demonstrated that PAR activity of PARP-1 during hESC neural differentiation is regulated by FGF-ERK1/2 activation. Inhibition of FGF receptors by SU5402 or blockage of ERK1/2 activation by U0126 results in corresponding downregulation of PARP-1. The net outcome of FGF-ERK inhibition is similar to that of PARP-1 inhibition by PJ34, suggesting that PARP-1 activity is required for hESC neural differentiation. This conclusion is validated by our genetic knockdown of PARP-1 in hESCs. In this case, knockdown of PARP-1 blocked hESCs from differentiating to neuroepithelia without favoring alternative fates as meso- and endo-derm markers are not increased (Fig. S6). This inhibition of hESC differentiation occurs even in the presence of (endogenous) FGFs. One may question why the growth of hESCs, which are also affected by FGF256, is not affected. One possibility is that other cellular PARylating activity from members of the PARP family may compensate and rescue the effect of PARP-1 knockdown. Indeed, mice with null mutation of PARP-1 or PARP-2, another member of the PARP family, are viable, but double knockout embryos of PARP-1 and PARP-2 die early in development at the onset of gastrulation 33. Similarly, PARP-1(-/-) mESCs are viable and expandable 57, 58. Alternatively, these effects are mediated by different FGFs. As shown in gene expression profiling data (Fig. S1), FGF8 and FGF9 are highly expressed in neural differentiating hESCs, suggesting that these are the potential molecules that activate endogenous FGF signaling pathway in neural differentiating hESCs, at least in our system. Another possibility is that the effect of FGF2 on hESC proliferation is not mediated by PARP-1. This is suggested by the fact that even though PARP-1 is expressed at the ESC stage, PAR activity is not detected at that stage (Fig. 2). Therefore, PARP-1 appears to mediate the FGF effect on neuroectoderm specification rather than on hESC proliferation.
PARP-1 regulates gene transcription in diverse manners. It binds to promoter regions of target genes in collaboration with its coactivators 59. While its enzymatic activity is dispensable in some cases 35, 60, it is often required, in which other components of the coregulatory complex become the target of PARP-1 enzyme activity 36, 45-48. During our hESC neural differentiation, PARP-1 expression remains constant, whereas the PAR activity increases along differentiation, corresponding to changes in FGF expression. Inhibition of FGF signaling does not alter the expression of PARP-1 but it does indeed block the PAR activity. Therefore, FGF-ERK regulates hESC neural differentiation by modulating the PAR activity. This is further suggested by the fact that PARP-1 is localized to the promoter of PAX6 along hESC differentiation but PAX6 is not expressed at the ESC stage, possibly because of lack of PAR activity. Along hESC differentiation, FGF increases, so are the PAR activity and the transcription of PAX6. This may be similar to the scenario of the PC12 proliferation/differentiation model that PARP-1-mediated PARylation is involved in NGF-induced differentiation 43. Since PARP-1 appears to directly regulate the transcription of PAX6 (Fig. 5), we propose that FGFs regulate neuroectoderm specification from hESCs by regulating PAR activity. This proposition also explains why the growth of hESCs depends on FGF2 but does not require PAR activity.
Supplementary Material
Acknowledgments
This study was supported by the National Institute of Neurological Disorders and Stroke (R01 NS045926), and partly by a core grant to the Waisman Center from the National Institute of Child Health and Human Development (P30 HD03352).
Footnotes
Author Contribution: Youngdong Yoo: Conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing
Cindy Huang: collection and/or assembly of data, data analysis and interpretation
Xiaoqing Zhang: Data analysis and interpretation
Timothy M. LaVaute: Collection and/or assembly of data, data analysis and interpretation
Su-Chun Zhang: Conception and design, financial support, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript
References
- 1.De Robertis EM, Larrain J, Oelgeschlager M, et al. The establishment of Spemann's organizer and patterning of the vertebrate embryo. Nat Rev Genet. 2000;1:171–181. doi: 10.1038/35042039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delaune E, Lemaire P, Kodjabachian L. Neural induction in Xenopus requires early FGF signalling in addition to BMP inhibition. Development. 2005;132:299–310. doi: 10.1242/dev.01582. [DOI] [PubMed] [Google Scholar]
- 3.Harland R, Gerhart J. Formation and function of Spemann's organizer. Annu Rev Cell Dev Biol. 1997;13:611–667. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- 4.Munoz-Sanjuan I, Brivanlou AH. Neural induction, the default model and embryonic stem cells. Nat Rev Neurosci. 2002;3:271–280. doi: 10.1038/nrn786. [DOI] [PubMed] [Google Scholar]
- 5.Stern CD. Neural induction: old problem, new findings, yet more questions. Development. 2005;132:2007–2021. doi: 10.1242/dev.01794. [DOI] [PubMed] [Google Scholar]
- 6.Bertrand V, Hudson C, Caillol D, et al. Neural tissue in ascidian embryos is induced by FGF9/16/20, acting via a combination of maternal GATA and Ets transcription factors. Cell. 2003;115:615–627. doi: 10.1016/s0092-8674(03)00928-0. [DOI] [PubMed] [Google Scholar]
- 7.Cebria F, Kobayashi C, Umesono Y, et al. FGFR-related gene nou-darake restricts brain tissues to the head region of planarians. Nature. 2002;419:620–624. doi: 10.1038/nature01042. [DOI] [PubMed] [Google Scholar]
- 8.Kuroda H, Fuentealba L, Ikeda A, et al. Default neural induction: neuralization of dissociated Xenopus cells is mediated by Ras/MAPK activation. Genes Dev. 2005;19:1022–1027. doi: 10.1101/gad.1306605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Streit A, Berliner AJ, Papanayotou C, et al. Initiation of neural induction by FGF signalling before gastrulation. Nature. 2000;406:74–78. doi: 10.1038/35017617. [DOI] [PubMed] [Google Scholar]
- 10.Wilson SI, Graziano E, Harland R, et al. An early requirement for FGF signalling in the acquisition of neural cell fate in the chick embryo. Curr Biol. 2000;10:421–429. doi: 10.1016/s0960-9822(00)00431-0. [DOI] [PubMed] [Google Scholar]
- 11.Launay C, Fromentoux V, Shi DL, et al. A truncated FGF receptor blocks neural induction by endogenous Xenopus inducers. Development. 1996;122:869–880. doi: 10.1242/dev.122.3.869. [DOI] [PubMed] [Google Scholar]
- 12.Aubin J, Davy A, Soriano P. In vivo convergence of BMP and MAPK signaling pathways: impact of differential Smad1 phosphorylation on development and homeostasis. Genes Dev. 2004;18:1482–1494. doi: 10.1101/gad.1202604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massague J. Integration of Smad and MAPK pathways: a link and a linker revisited. Genes Dev. 2003;17:2993–2997. doi: 10.1101/gad.1167003. [DOI] [PubMed] [Google Scholar]
- 14.Pera EM, Ikeda A, Eivers E, et al. Integration of IGF, FGF, and anti-BMP signals via Smad1 phosphorylation in neural induction. Genes Dev. 2003;17:3023–3028. doi: 10.1101/gad.1153603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sapkota G, Alarcon C, Spagnoli FM, et al. Balancing BMP signaling through integrated inputs into the Smad1 linker. Mol Cell. 2007;25:441–454. doi: 10.1016/j.molcel.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Kunath T, Saba-El-Leil MK, Almousailleakh M, et al. FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development. 2007;134:2895–2902. doi: 10.1242/dev.02880. [DOI] [PubMed] [Google Scholar]
- 17.Stavridis MP, Lunn JS, Collins BJ, et al. A discrete period of FGF-induced Erk1/2 signalling is required for vertebrate neural specification. Development. 2007;134:2889–2894. doi: 10.1242/dev.02858. [DOI] [PubMed] [Google Scholar]
- 18.Ying QL, Stavridis M, Griffiths D, et al. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol. 2003;21:183–186. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
- 19.Gerrard L, Rodgers L, Cui W. Differentiation of human embryonic stem cells to neural lineages in adherent culture by blocking bone morphogenetic protein signaling. Stem Cells. 2005;23:1234–1241. doi: 10.1634/stemcells.2005-0110. [DOI] [PubMed] [Google Scholar]
- 20.Itsykson P, Ilouz N, Turetsky T, et al. Derivation of neural precursors from human embryonic stem cells in the presence of noggin. Mol Cell Neurosci. 2005;30:24–36. doi: 10.1016/j.mcn.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 21.LaVaute TM, Yoo YD, Pankratz MT, et al. Regulation of neural specification from human embryonic stem cells by BMP and FGF. Stem Cells. 2009;27:1741–1749. doi: 10.1002/stem.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Robertis EM, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thisse B, Thisse C. Functions and regulations of fibroblast growth factor signaling during embryonic development. Dev Biol. 2005;287:390–402. doi: 10.1016/j.ydbio.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Wilson SI, Edlund T. Neural induction: toward a unifying mechanism. Nat Neurosci. 2001;4(Suppl):1161–1168. doi: 10.1038/nn747. [DOI] [PubMed] [Google Scholar]
- 26.Cohen-Armon M, Visochek L, Rozensal D, et al. DNA-independent PARP-1 activation by phosphorylated ERK2 increases Elk1 activity: a link to histone acetylation. Mol Cell. 2007;25:297–308. doi: 10.1016/j.molcel.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Kauppinen TM, Chan WY, Suh SW, et al. Direct phosphorylation and regulation of poly(ADP-ribose) polymerase-1 by extracellular signal-regulated kinases 1/2. Proc Natl Acad Sci U S A. 2006;103:7136–7141. doi: 10.1073/pnas.0508606103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ame JC, Spenlehauer C, de Murcia G. The PARP superfamily. Bioessays. 2004;26:882–893. doi: 10.1002/bies.20085. [DOI] [PubMed] [Google Scholar]
- 29.Cohen-Armon M. PARP-1 activation in the ERK signaling pathway. Trends Pharmacol Sci. 2007;28:556–560. doi: 10.1016/j.tips.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Kim MY, Zhang T, Kraus WL. Poly(ADP-ribosyl)ation by PARP-1: ‘PAR-laying’ NAD+ into a nuclear signal. Genes Dev. 2005;19:1951–1967. doi: 10.1101/gad.1331805. [DOI] [PubMed] [Google Scholar]
- 31.Rouleau M, Aubin RA, Poirier GG. Poly(ADP-ribosyl)ated chromatin domains: access granted. J Cell Sci. 2004;117:815–825. doi: 10.1242/jcs.01080. [DOI] [PubMed] [Google Scholar]
- 32.Malanga M, Althaus FR. The role of poly(ADP-ribose) in the DNA damage signaling network. Biochem Cell Biol. 2005;83:354–364. doi: 10.1139/o05-038. [DOI] [PubMed] [Google Scholar]
- 33.Menissier de Murcia J, Ricoul M, Tartier L, et al. Functional interaction between PARP-1 and PARP-2 in chromosome stability and embryonic development in mouse. EMBO J. 2003;22:2255–2263. doi: 10.1093/emboj/cdg206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ha HC, Hester LD, Snyder SH. Poly(ADP-ribose) polymerase-1 dependence of stress-induced transcription factors and associated gene expression in glia. Proc Natl Acad Sci U S A. 2002;99:3270–3275. doi: 10.1073/pnas.052712399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hassa PO, Buerki C, Lombardi C, et al. Transcriptional coactivation of nuclear factor-kappaB-dependent gene expression by p300 is regulated by poly(ADP)-ribose polymerase-1. J Biol Chem. 2003;278:45145–45153. doi: 10.1074/jbc.M307957200. [DOI] [PubMed] [Google Scholar]
- 36.Ju BG, Solum D, Song EJ, et al. Activating the PARP-1 sensor component of the groucho/ TLE1 corepressor complex mediates a CaMKinase IIdelta-dependent neurogenic gene activation pathway. Cell. 2004;119:815–829. doi: 10.1016/j.cell.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 37.Kraus WL, Lis JT. PARP goes transcription. Cell. 2003;113:677–683. doi: 10.1016/s0092-8674(03)00433-1. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Huang C, Chen J, et al. PAX6 is a human neuroectoderm cell fate determinant. Cell Stem Cell. 2010 doi: 10.1016/j.stem.2010.04.017. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li XJ, Du ZW, Zarnowska ED, et al. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- 40.Pankratz MT, Li XJ, Lavaute TM, et al. Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage. Stem Cells. 2007;25:1511–1520. doi: 10.1634/stemcells.2006-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xia X, Ayala M, Thiede BR, et al. In vitro- and in vivo-induced transgene expression in human embryonic stem cells and derivatives. Stem Cells. 2008;26:525–533. doi: 10.1634/stemcells.2007-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pellegrino MJ, Stork PJ. Sustained activation of extracellular signal-regulated kinase by nerve growth factor regulates c-fos protein stabilization and transactivation in PC12 cells. J Neurochem. 2006;99:1480–1493. doi: 10.1111/j.1471-4159.2006.04250.x. [DOI] [PubMed] [Google Scholar]
- 43.Visochek L, Steingart RA, Vulih-Shultzman I, et al. PolyADP-ribosylation is involved in neurotrophic activity. J Neurosci. 2005;25:7420–7428. doi: 10.1523/JNEUROSCI.0333-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frizzell KM, Gamble MJ, Berrocal JG, et al. Global analysis of transcriptional regulation by poly(ADP-ribose) polymerase-1 and poly(ADP-ribose) glycohydrolase in MCF-7 human breast cancer cells. J Biol Chem. 2009;284:33926–33938. doi: 10.1074/jbc.M109.023879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin N, Schwamborn K, Schreiber V, et al. PARP-1 transcriptional activity is regulated by sumoylation upon heat shock. EMBO J. 2009;28:3534–3548. doi: 10.1038/emboj.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olabisi OA, Soto-Nieves N, Nieves E, et al. Regulation of transcription factor NFAT by ADP-ribosylation. Mol Cell Biol. 2008;28:2860–2871. doi: 10.1128/MCB.01746-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu W, Ginjala V, Pant V, et al. Poly(ADP-ribosyl)ation regulates CTCF-dependent chromatin insulation. Nat Genet. 2004;36:1105–1110. doi: 10.1038/ng1426. [DOI] [PubMed] [Google Scholar]
- 48.Zaniolo K, Desnoyers S, Leclerc S, et al. Regulation of poly(ADP-ribose) polymerase-1 (PARP-1) gene expression through the post-translational modification of Sp1: a nuclear target protein of PARP-1. BMC Mol Biol. 2007;8:96. doi: 10.1186/1471-2199-8-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plaza S, Aumercier M, Bailly M, et al. Involvement of poly (ADP-ribose)-polymerase in the PAX-6 gene regulation in neuroretina. Oncogene. 1999;18:1041–1051. doi: 10.1038/sj.onc.1202406. [DOI] [PubMed] [Google Scholar]
- 50.Stern CD. Neural induction: 10 years on since the ‘default model’. Curr Opin Cell Biol. 2006;18:692–697. doi: 10.1016/j.ceb.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 51.Boilly B, Vercoutter-Edouart AS, Hondermarck H, et al. FGF signals for cell proliferation and migration through different pathways. Cytokine Growth Factor Rev. 2000;11:295–302. doi: 10.1016/s1359-6101(00)00014-9. [DOI] [PubMed] [Google Scholar]
- 52.Bottcher RT, Niehrs C. Fibroblast growth factor signaling during early vertebrate development. Endocr Rev. 2005;26:63–77. doi: 10.1210/er.2003-0040. [DOI] [PubMed] [Google Scholar]
- 53.Dvorak P, Dvorakova D, Hampl A. Fibroblast growth factor signaling in embryonic and cancer stem cells. FEBS Lett. 2006;580:2869–2874. doi: 10.1016/j.febslet.2006.01.095. [DOI] [PubMed] [Google Scholar]
- 54.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 55.Green PJ, Walsh FS, Doherty P. Promiscuity of fibroblast growth factor receptors. Bioessays. 1996;18:639–646. doi: 10.1002/bies.950180807. [DOI] [PubMed] [Google Scholar]
- 56.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 57.Hemberger M, Nozaki T, Winterhager E, et al. Parp1-deficiency induces differentiation of ES cells into trophoblast derivatives. Dev Biol. 2003;257:371–381. doi: 10.1016/s0012-1606(03)00097-6. [DOI] [PubMed] [Google Scholar]
- 58.Nozaki T, Masutani M, Watanabe M, et al. Syncytiotrophoblastic giant cells in teratocarcinoma-like tumors derived from Parp-disrupted mouse embryonic stem cells. Proc Natl Acad Sci U S A. 1999;96:13345–13350. doi: 10.1073/pnas.96.23.13345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kraus WL. Transcriptional control by PARP-1: chromatin modulation, enhancer-binding, coregulation, and insulation. Curr Opin Cell Biol. 2008;20:294–302. doi: 10.1016/j.ceb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simbulan-Rosenthal CM, Rosenthal DS, Luo R, et al. PARP-1 binds E2F-1 independently of its DNA binding and catalytic domains, and acts as a novel coactivator of E2F-1-mediated transcription during re-entry of quiescent cells into S phase. Oncogene. 2003;22:8460–8471. doi: 10.1038/sj.onc.1206897. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


