Abstract
Background
Severe pruritus is the primary symptom in atopic dermatitis (AD). Recently, the novel cytokine IL-31 has been implicated in the itching associated with AD.
Objective
We performed this study to determine whether IL-31 serum levels are elevated in AD patients and to better characterize the relationship between serum IL-31 level and other established laboratory parameters.
Methods
We recruited 55 AD patients, 34 with allergic type AD and 21 with non-allergic type AD, and 38 healthy, non-atopic controls. We checked the laboratory values, severity score, and serum IL-31 levels in all patients and controls, and IL-31 mRNA levels in lesion skin were measured in 13 subjects with AD and in four controls.
Results
AD patients displayed significantly higher levels of serum IL-31 that were associated with serum IgE, disease severity, and subjective itch intensity. In AD patients, IL-31 mRNA levels from the lesional skin samples also correlated with serum IL-31 level.
Conclusion
IL-31 is likely one of the many mediators inducing inflammation and pruritus in AD. Although our limited sample size prevents us from making any definitive conclusions, our data demonstrate a strong correlation between IL-31 mRNA level and serum IL-31 protein level, which has never been reported before. Moreover, we found correlations between serum IL-31 level and serum IgE, eosinophil cationic protein, disease severity, and subject itch intensity in certain degrees in AD patients.
Keywords: Atopic dermatitis, IL-31 mRNA, Itching, SCORAD, Serum IL-31
INTRODUCTION
Pruritus is arguably the most important symptom in atopic dermatitis (AD), as it significantly impairs patient's quality of life. Accordingly, one of the main goals in AD treatment is itch management, although AD-associated pruritis is often refractory to most traditional H1-antihistamine therapies. The etiology of pruritus in AD is not yet fully understood, however, several endogenous mediators-neuropeptides, neurotransmitters, proteinases, arachidonic derivatives, and cytokines have been implicated in itch pathophysiology1.
Recently, a novel cytokine, IL-31, was linked to the pathogenesis of pruritis in several chronic inflammatory skin diseases. IL-31 mRNA and protein expression is largely restricted to CD4+ T cells, particularly skin-homing CD45RO+ (memory) cutaneous lymphocyte-associated antigen-positive T cells2 found in the peripheral blood of AD patients and normal controls. To exert its physiologic effect, IL-31 binds to a heterodimeric receptor composed of IL-31 receptor A and oncostatin M receptor.
When compared with healthy controls, AD patients express increased IL-31 mRNA levels in both lesional and nonlesional skin3. In addition to AD, IL-31 mRNA expression is also highly upregulated in allergic contact dermatitis, but not in plaque psoriasis4. Moreover, IL-31 expression has been associated with IL-4 and IL-13 in both types of pruritic eczema.
Several studies have provided further evidence suggesting a possible etiologic role for IL-31 in AD. IL-31 transgenic mice were found to express a phenotype closely mimicking AD5. Specifically, the mice displayed many clinical hallmarks of AD but had normal serum IgE levels5. These data seem to indicate that IL-31 is predominantly produced by Th2 cells, although additional amounts of the cytokine may be also produced by Th1 cells5. In human AD patients, IL-31 mRNA was found to be elevated regardless of disease severity or serum IgE4. Conversely, the common haplotype of the IL-31 gene was strongly linked to non-atopic eczema (or non-allergic AD), indicating that an altered regulation of IL-31 gene expression could be the disease-causing factor in AD6. Grimstad et al. used an AD-like murine model to demonstrate that monoclonal IL-31 rat-anti-mouse antibody was able to effectively reduce scratching behavior when administered during the onset of clinical skin manifestations7.
With this in mind, IL-31 seems to be closely linked to AD pathophysiology and the pruritic symptoms. Here, we quantify serum IL-31 levels and IL-31 mRNA from lesional skin biopsies from AD patients and controls. Laboratory parameters were also examined to identify any other biochemical variances associated with increases in IL-31. Additionally, serum IL-31 levels were compared with objective disease severity and patient-reported subjective itch and sleep loss to determine if increases in IL-31 correlated with worsening patient symptoms.
MATERIALS AND METHODS
Patients
Fifty-five patients (mean age: 12.64 years) with AD were recruited from the Samsung Medical Center between March 2008 and February 2009. The diagnosis of AD, the criteria for AD stratification into ADe (allergic or extrinsic type of AD) and ADi (non-allergic or intrinsic type of AD), and the laboratory blood tests and allergen prick tests were the same as in our previous study8,9. All patients fulfilled the Hanifin and Rajka criteria10. Based on clinical history and total and specific serum IgE, all subjects with AD were classified as either having ADe or ADi. Patients with a total serum IgE level ≥200 U/ml and positive specific serum IgE antibodies toward multiple common inhalant and food allergens were classified as having ADe.
Conversely, individuals were classified as having ADi if they exhibited the clinical phenotype of AD but lacked any history of other atopic disease, had total serum IgE levels within the normal range, and did not exhibit any detectable specific serum IgE response. In total, 34 subjects with ADe, 21 subjects with ADi, and 38 non-atopic controls (mean age 22.8±2.5 years) were included in the study.
This study was conducted according to the Declaration of Helsinki Principles, and written informed consent was obtained from all participants. The Samsung Medical Centre Ethics Committee approved this study.
Clinical and laboratory measurement
AD severity was calculated using the SCORAD index11, and eosinophil count, serum eosinophil cationic protein (ECP), total serum IgE, and specific serum IgE response to a panel of food allergens and aeroallergens were measured using a CAP System (Pharmacia, Uppsala, Sweden). Subjective itch and sleep loss intensity were measured using a patient-reported 0~10 scale (0: no pruritus/sleep loss at all, 10: severe pruritus/sleep loss during the last three nights).
Protein assay using ELISA
Serum IL-31 protein levels were measured using ELISA with the DuoSet human IL-31 ELISA Development kit (Human IL-31 DuoSet; R&D Systems; Minneapolis, MN, USA). All analyses were performed according to the manufacturer's protocol.
RNA extraction
Punch biopsies from lesional skin of patients with ADe (n=8) and ADi (n=5) were collected for mRNA analysis. Similar biopsies were also collected from control individuals without any history of atopic disease (n=4). Isolation of total RNA from human skin biopsy was performed using an easy-BLUE™ Total RNA Extraction Kit (Intron, Seoul, Korea).
Reverse transcriptase polymerase chain reaction (RT-PCR)
cDNA synthesis was performed using the M-MLV RT kit (Intron) according to the manufacturer's instructions. The primer sequences for the target genes were designed using the GENBANK accession sequences with the Primer 3 program (http://frodo.wi.mit.edu/). The forward and reverse primers for IL-31 were 5'-TCGAGGAATTACAGTCCCTCTC-3' and 5'-TGTCGAGGTGCTCTATGATCTC-3', respectively. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used for quantification of the control specimens. The reaction mixture for each gene PCR contained 1µl aliquot from the RT reaction, 1µl of each primer, and 17µl of i-Master mix PCR solution (Intron), producing a final reaction volume of 20µl. The amplification products were resolved in 1.5% agarose gel with ethidium bromide. To quantitate the PCR products, the gels were scanned and the pixel intensity for each band was assessed using the ImageJ program (NIH Image). All results were subsequently normalized to account for the amount of GAPDH present.
Statistical analyses
All statistical analyses were performed with the statistical software package SigmaStat for Windows (Jandel Scientific Erkrath, Wiesbaden, Germany). To test statistical significance, the data were analyzed with the nonparametric method, as our results did not display a normal distribution. To compare the characteristics of the subgroups, the Wilcoxon two-sample test was employed. Correlations between serum IL-31 level and laboratory parameters were assessed using Pearson's correlation. To assess for correlation between serum IL-31 and IL-31 mRNA, Spearman' rank correlation, Kendall's tau coefficient, and Pearson's correlation were used. In all cases, p<0.05 was defined as statistically significant.
RESULTS
IL-31 serum levels are significantly higher in AD patients
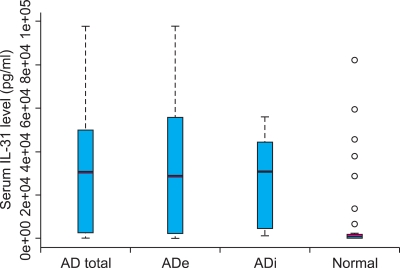
Mean serum IL-31 level was significantly elevated (p<0.05) in AD patients (43,142.8±66,981.6 pg/ml) compared to those of the normal controls (7,881.8±1,842.7 pg/ml). In AD subtypes, serum IL-31 levels were still significantly higher (p<0.05) in both ADe (44,757±68,436.3 pg/ml) and ADi (40,529.45±66,138 pg/ml) groups compared to those of the healthy controls. Interestingly, no statistically significant difference in serum IL-31 levels was found between subgroups (Fig. 1). Therefore, IL-31 may possibly be valuable for the diagnosis of AD.
Fig. 1.
IL-31 serum levels in patients with extrinsic and intrinsic types of AD versus those of non-atopic controls. AD: atopic dermatitis, ADe: allergic or extrinsic type of AD, ADi: non-allergic or intrinsic type of AD.
Serum IL-31 level is correlated with total serum IgE and ECP
In AD patients, the mean total serum IgE, ECP, and eosinophil count were 1,079.3±1,511.4 U/ml, 32.21±29.0 ng/ml, and 432.9±344.8/ul, respectively. The relationship between serum total IgE (p-value; 0.041), ECP (p-value; 0.04) and serum IL-31 level was the only marginally statistically significant association.
Serum IL-31 level is correlated with SCORAD index and subjective itch intensity
In AD patients, the mean SCORAD index, subjective pruritus intensity, and sleep loss were 40.4±13.8, 5.7±2.0, and 4.44±2.1, respectively. The serum IL-31 level was statistically correlated with SCORAD index (p-value; 0.003) and subjective itch intensity (p-value; 0.03) in AD patients, however, there was no correlation between serum IL-31 levels and sleep loss (p-value; 0.13).
Serum IL-31 level is significantly correlated with cutaneous IL-31 mRNA
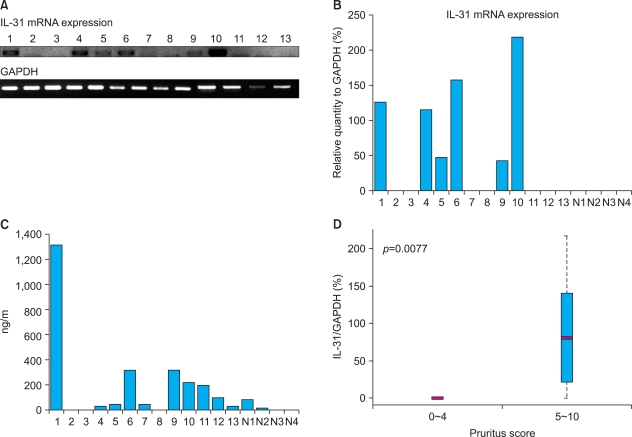
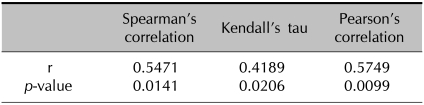
Skin biopsies from AD patients (8 ADe and 5 ADi) and controls (n=4) were analyzed for IL-31 mRNA using RT-PCR. Punch biopsies were obtained from lesional skin of patients with AD and normal skin from controls and were compared with the previously obtained serum IL-31 levels using ELISA (Fig. 2A). IL-31 transcripts were detected in AD patients but not in normal groups. The mean ratio of IL-31/GAPDH mRNA band intensity in AD patients was clearly higher than that in the controls (Fig. 2B). The IL-31 mRNA levels in biopsies from AD patients were consistent with serum IL-31 levels (Fig. 2C). These data confirm that, among AD patients, the levels of IL-31 protein and IL-31 mRNA increased correspondingly, and, when compared with normal subjects, this difference was statistically significant and useful for the diagnosis of AD patients (Table 1, p=0.0141 and 0.0206, respectively, using Spearman's correlation and Kendall's tau). In addition, there was statistical significance to alternative hypothesis that mean of IL31-mRNA in pruritus score 5~10 is greater than that in pruritus score 0~4 via two sample t-test (Fig. 2D, p=0.0077). This result is supportive to other conclusions that serum IL-31 level is positively correlated with subjective itch intensity and IL-31 mRNA level.
Fig. 2.
(A) RT-PCR analysis of IL-31 expression using RNA extracted from skin biopsies. This analysis showed an increase in IL-13 mRNA in some atopic patients (1~13). (B) The band intensity was quantified with ImageJ software (NIH), and the percentages were the ratio of optical intensity normalized to the GAPDH signal. The level of IL-31 mRNA in the controls (N1~N4) was undetectable. (C) The IL-31 protein level in the serum from the patients in the RT-PCR experiment. Patients 1~4, 9~12 are ADe and patients 5~8, and 13 are ADi. (D) Boxplot of IL-31/GAPDH mRNA(%) in pruritus score 0~4 and 5~10 levels. RT-PCR: reverse trascriptase polymerase chain reaction, GAPDH: glyceraldehyde-3-phosphate dehydrogenase, ADe: allergic or extrinsic type of AD, ADi: non-allergic or intrinsic type of AD.
Table 1.
Correlation between serum IL-31 protein level and IL-31 mRNA level in atopic dermatitis patients versus those of the controls
DISCUSSION
AD is a highly pruritic inflammatory skin disease that is believed to alter the intrinsic immunologic response of those afflicted. Acute AD skin lesions are characterized by a predominant infiltration of CD45RO+ activated memory T cells and increased local expression of Th2 type cytokines, especially IL-4 and IL-13, and chemokines12,13.
Pruritus is defined as a sensation leading to the desire to scratch. Both central and peripheral mediators are important in the etiology of pruritus. Specifically, histamine, proteinase, substance P, neurotrophin, prostanoids, and interleukins are all compounds believed to play a role in peripherally mediated pruritis14. Emerging research suggests that many additional intrinsic biochemical compounds may also contribute to pruritis in AD patients. Most notably, IL-2 is known to produce itching in individuals with AD as well as cancer patients receiving exogenous recombinant IL-215. Immunomodulators like tacrolimus and pimecrolimus seem to inhibit the production of IL-2 and are thus very effective in treating AD. Lee et al.16 implicates transepidermal water loss, serum IgE, and β-endorphin as important and independent biologic markers involved in the development of itch intensity in AD.
Although IL-31 transgenic mice exhibit clinical features similar to AD, they did not express the increased serum levels of IgE5 seen in IL-4 transgenic mice17. Accordingly, IL-31 seems to have a different pathophysiologic mechanism than IL-4 in the etiology of AD. Notably, skin levels of IL-31 mRNA were found to be higher in NC/Nga mice with scratching behavior than in similar mice without scratching behavior. These results imply that IL-31 expression may precede the appearance of dermatitis, and that IL-31-induced excessive scratching may play an important role in the early stages of dermatitis in mice18.
We show serum IL-31 levels to be elevated in AD patients regardless of AD subtype. Moreover, our data indicate that, in AD patients, serum IL-31 levels are marginally associated with serum IgE level, ECP, disease severity, and subjective itch intensity. In previous study, Raap et al.19 found that serum IL-31 levels were significantly higher in both ADe and ADi patients, without any difference between AD subgroups, and also showed that SCORAD score was significantly correlated with serum IL-31 level in all AD patients. On the other hands, Neis et al.4 reported no correlations between IL-31 mRNA level and either disease severity or serum IgE level in AD patients as the IL-31 transgenic mice showed intrinsic type of AD. These findings suggest an association between itching and IL-31 level and imply that such itching could induce the sleep loss that was observed in our experiment.
We also found that the IL-31 mRNA levels in skin biopsies from AD patients were higher than those from controls (undetectable in our experiments), and demonstrated significant correlations between IL-31 mRNA and serum IL-31 level in AD patients, and also demonstrated significant correlations between IL-31 mRNA and pruritus score in AD patients. Previously, IL-31 mRNA levels were quantified in both lesional and nonlesional skin biopsies from AD patients and other skin diseases2-4. Data regarding serum IL-31 levels in AD patients has also been reported19. However, there has been no report to date correlating IL-31 mRNA levels with serum IL-31 protein levels in humans.
In conclusion, our results suggest that IL-31 is one of the mediators inducing inflammation and pruritus in AD. Although our limited sample size prevents us from making any definitive conclusions, we demonstrate a strong correlation between IL-31 mRNA level and serum IL-31 protein level, which was never reported before. Moreover, we found correlations between serum IL-31 level and serum IgE, ECP, disease severity and subject itch intensity in certain degrees in AD patients. To better elucidate these relationships, further studies are warranted for a better understanding of the complex etiology of AD-associated pruritus.
Footnotes
This work was funded by a grant (01-PJ3-PG6-01GN12-0001: J-M Yang) from the 2001 Good Health R & D Project, Ministry of Health and Welfare, Republic of Korea, and by a grant (C-A6-216-3) from Samsung Biomedical Research Institute.
References
- 1.Ständer S, Steinhoff M. Pathophysiology of pruritus in atopic dermatitis: an overview. Exp Dermatol. 2002;11:12–24. doi: 10.1034/j.1600-0625.2002.110102.x. [DOI] [PubMed] [Google Scholar]
- 2.Bilsborough J, Leung DY, Maurer M, Howell M, Boguniewicz M, Yao L, et al. IL-31 is associated with cutaneous lymphocyte antigen-positive skin homing T cells in patients with atopic dermatitis. J Allergy Clin Immunol. 2006;117:418–425. doi: 10.1016/j.jaci.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 3.Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. 2006;117:411–407. doi: 10.1016/j.jaci.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 4.Neis MM, Peters B, Dreuw A, Wenzel J, Bieber T, Mauch C, et al. Enhanced expression levels of IL-31 correlate with IL-4 and IL-13 in atopic and allergic contact dermatitis. J Allergy Clin Immunol. 2006;118:930–937. doi: 10.1016/j.jaci.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, Presnell SR, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5:752–760. doi: 10.1038/ni1084. [DOI] [PubMed] [Google Scholar]
- 6.Schulz F, Marenholz I, Fölster-Holst R, Chen C, Sternjak A, Baumgrass R, et al. A common haplotype of the IL-31 gene influencing gene expression is associated with nonatopic eczema. J Allergy Clin Immunol. 2007;120:1097–1102. doi: 10.1016/j.jaci.2007.07.065. [DOI] [PubMed] [Google Scholar]
- 7.Grimstad O, Sawanobori Y, Vestergaard C, Bilsborough J, Olsen UB, Grønhøj-Larsen C, et al. Anti-interleukin-31-anti-bodies ameliorate scratching behaviour in NC/Nga mice: a model of atopic dermatitis. Exp Dermatol. 2009;18:35–43. doi: 10.1111/j.1600-0625.2008.00766.x. [DOI] [PubMed] [Google Scholar]
- 8.Jeong CW, Ahn KS, Rho NK, Park YD, Lee DY, Lee JH, et al. Differential in vivo cytokine mRNA expression in lesional skin of intrinsic vs. extrinsic atopic dermatitis patients using semiquantitative RT-PCR. Clin Exp Allergy. 2003;33:1717–1724. doi: 10.1111/j.1365-2222.2003.01782.x. [DOI] [PubMed] [Google Scholar]
- 9.Rho NK, Kim WS, Lee DY, Lee JH, Lee ES, Yang JM. Immunophenotyping of inflammatory cells in lesional skin of the extrinsic and intrinsic types of atopic dermatitis. Br J Dermatol. 2004;151:119–125. doi: 10.1111/j.1365-2133.2004.06027.x. [DOI] [PubMed] [Google Scholar]
- 10.Hanifin JM, Rajka G. Diagnostic feature of atopic dermatitis. Acta Derma Venerol (Stockh) 1980;92(Suppl):44–47. [Google Scholar]
- 11.Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology. 1993;186:23–31. doi: 10.1159/000247298. [DOI] [PubMed] [Google Scholar]
- 12.Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest. 2004;113:651–657. doi: 10.1172/JCI21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamid Q, Boguniewicz M, Leung DY. Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J Clin Invest. 1994;94:870–876. doi: 10.1172/JCI117408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ständer S, Steinhoff M, Schmelz M, Weisshaar E, Metze D, Luger T. Neurophysiology of pruritus: cutaneous elicitation of itch. Arch Dermatol. 2003;139:1463–1470. doi: 10.1001/archderm.139.11.1463. [DOI] [PubMed] [Google Scholar]
- 15.Chi KH, Myers JN, Chow KC, Chan WK, Tsang YW, Chao Y, et al. Phase II trial of systemic recombinant interleukin-2 in the treatment of refractory nasopharyngeal carcinoma. Oncology. 2001;60:110–115. doi: 10.1159/000055306. [DOI] [PubMed] [Google Scholar]
- 16.Lee CH, Chuang HY, Shih CC, Jong SB, Chang CH, Yu HS. Transepidermal water loss, serum IgE and beta-endorphin as important and independent biological markers for development of itch intensity in atopic dermatitis. Br J Dermatol. 2006;154:1100–1107. doi: 10.1111/j.1365-2133.2006.07191.x. [DOI] [PubMed] [Google Scholar]
- 17.Chan LS, Robinson N, Xu L. Expression of interleukin-4 in the epidermis of transgenic mice results in a pruritic inflammatory skin disease: an experimental animal model to study atopic dermatitis. J Invest Dermatol. 2001;117:977–983. doi: 10.1046/j.0022-202x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- 18.Takaoka A, Arai I, Sugimoto M, Yamaguchi A, Tanaka M, Nakaike S. Expression of IL-31 gene transcripts in NC/Nga mice with atopic dermatitis. Eur J Pharmacol. 2005;516:180–181. doi: 10.1016/j.ejphar.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 19.Raap U, Wichmann K, Bruder M, Stnder S, Wedi B, Kapp A, et al. Correlation of IL-31 serum levels with severity of atopic dermatitis. J Allergy Clin Immunol. 2008;122:421–423. doi: 10.1016/j.jaci.2008.05.047. [DOI] [PubMed] [Google Scholar]