Abstract
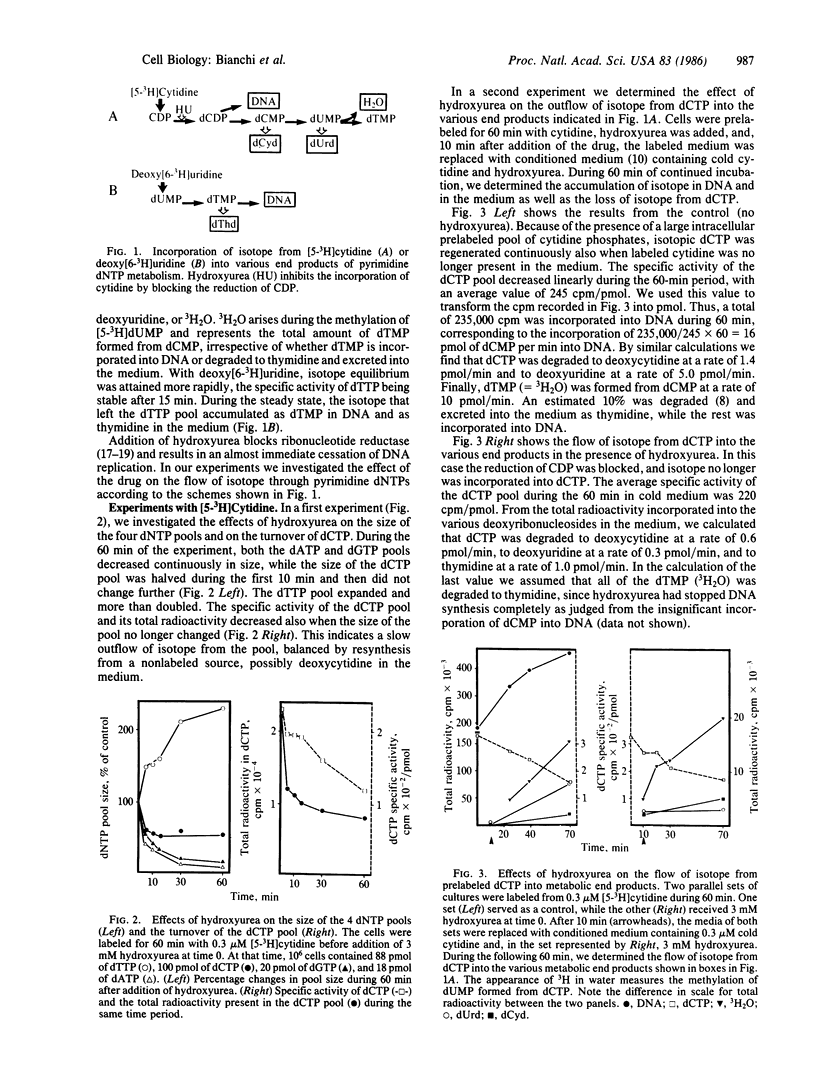
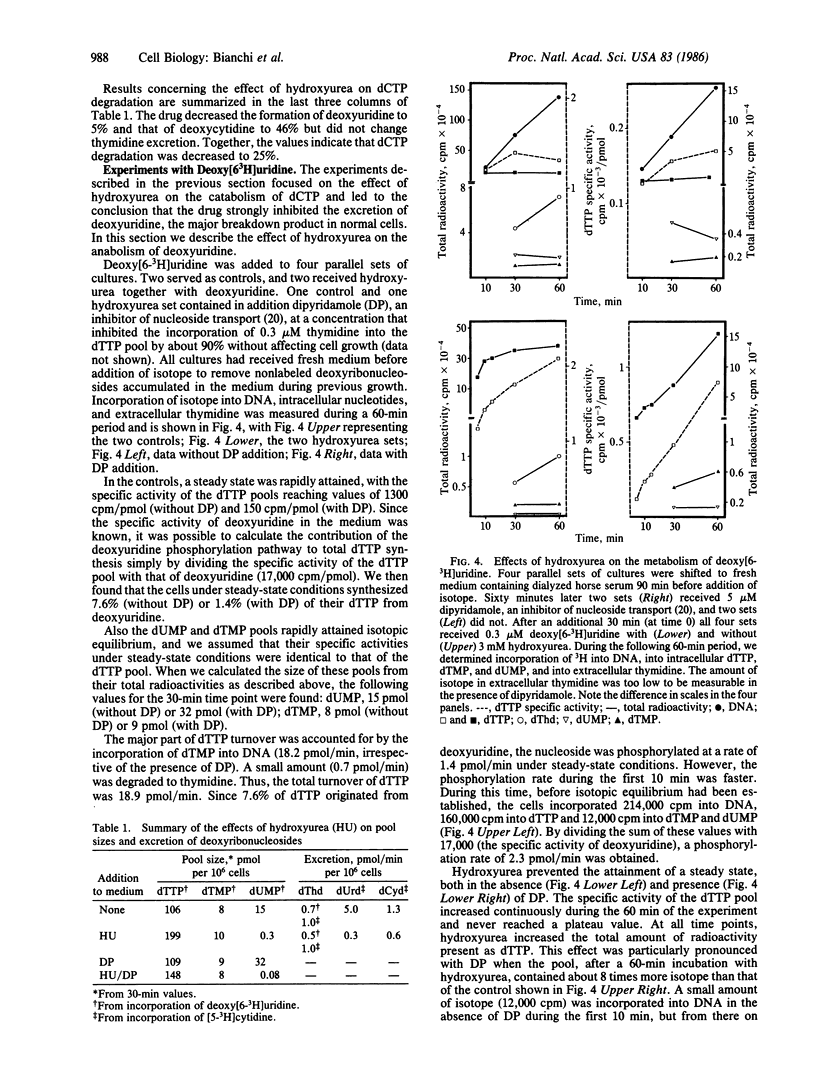
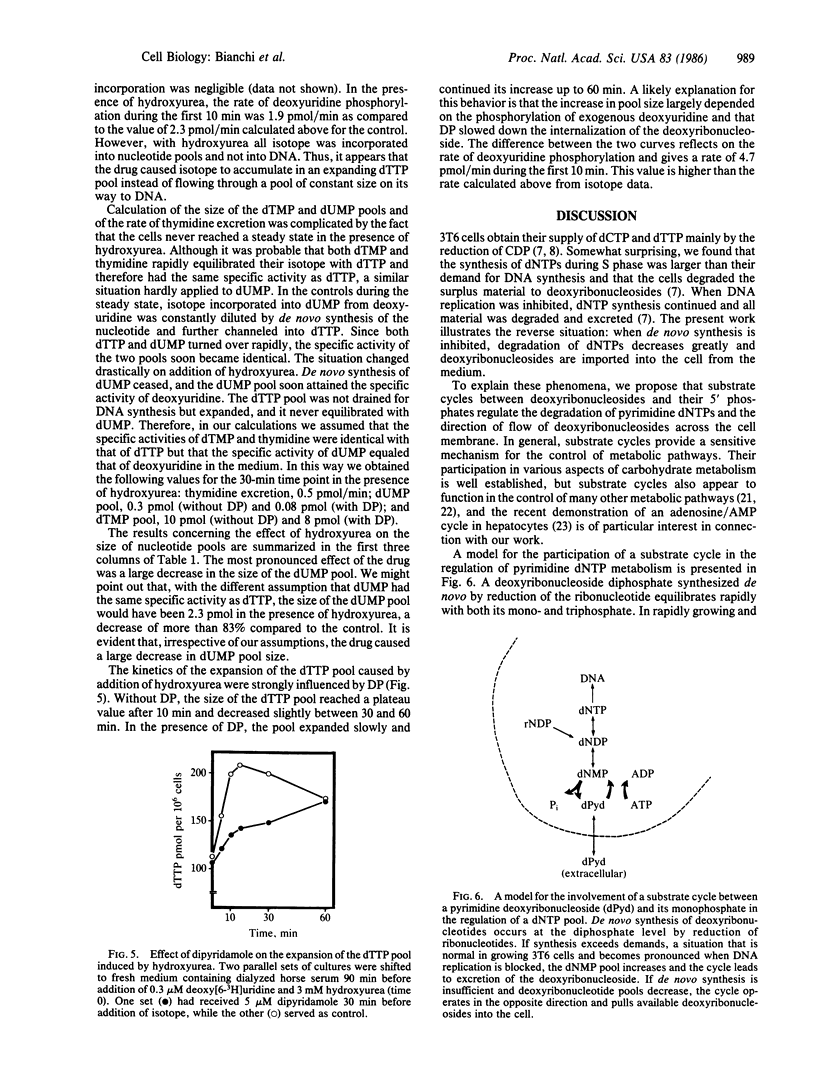
Degradation of pyrimidine deoxyribonucleoside triphosphates plays a major role in the regulation of their pool sizes in 3T6 cells. During normal growth, these cells excrete deoxyribonucleosides (mostly deoxyuridine) into the medium. When DNA strand elongation is inhibited, de novo synthesis of dCTP and dTTP continues, followed by degradation of the deoxyribonucleotides. We now demonstrate that inhibition of de novo synthesis with hydroxyurea stops degradation of deoxyribonucleotides. We now demonstrate that inhibition of de novo synthesis with hydroxyurea stops degradation of deoxyribonucleotides and leads to an influx of deoxyuridine from the medium. This effect appears to be caused by a large drop in the size of the intracellular dUMP pool. We propose that substrate cycles, involving phosphorylation of deoxyribonucleosides by kinases and dephosphorylation of deoxyribonucleoside 5'-phosphates by a nucleotidase, participate in the regulation of the size of pyrimidine deoxyribonucleoside triphosphate pools by directing the flow of deoxyribonucleosides across the cell membrane. While kinases are regulated mainly by allosteric effects, the activity of the nucleotidase appears to be regulated by substrate concentration.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerblom L., Reichard P. Azidocytidine is a specific inhibitor of deoxyribonucleotide synthesis in 3T6 cells. J Biol Chem. 1985 Aug 5;260(16):9197–9202. [PubMed] [Google Scholar]
- BERTANI L. E., HAEGGMARK A., REICHARD P. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEOTIDES. II. FORMATION AND INTERCONVERSION OF DEOXYURIDINE PHOSPHATES. J Biol Chem. 1963 Oct;238:3407–3413. [PubMed] [Google Scholar]
- Bontemps F., Van den Berghe G., Hers H. G. Evidence for a substrate cycle between AMP and adenosine in isolated hepatocytes. Proc Natl Acad Sci U S A. 1983 May;80(10):2829–2833. doi: 10.1073/pnas.80.10.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham J. P., Ives D. H. Deoxycytidine kinase. II. Purification and general properties of the calf thymus enzyme. J Biol Chem. 1970 May 10;245(9):2276–2284. [PubMed] [Google Scholar]
- Hellgren D., Nilsson S., Reichard P. Effects of arabinosyl-cytosine on thymidine triphosphate pools and polyoma DNA replication. Biochem Biophys Res Commun. 1979 May 14;88(1):16–22. doi: 10.1016/0006-291x(79)91690-5. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Regulation of ribonucleotide reductase. Curr Top Cell Regul. 1981;19:47–76. doi: 10.1016/b978-0-12-152819-5.50019-1. [DOI] [PubMed] [Google Scholar]
- Hurley M. C., Palella T. D., Fox I. H. Human placental deoxyadenosine and deoxyguanosine phosphorylating activity. J Biol Chem. 1983 Dec 25;258(24):15021–15027. [PubMed] [Google Scholar]
- Krakoff I. H., Brown N. C., Reichard P. Inhibition of ribonucleoside diphosphate reductase by hydroxyurea. Cancer Res. 1968 Aug;28(8):1559–1565. [PubMed] [Google Scholar]
- Kunz B. A. Genetic effects of deoxyribonucleotide pool imbalances. Environ Mutagen. 1982;4(6):695–725. doi: 10.1002/em.2860040609. [DOI] [PubMed] [Google Scholar]
- Lindberg U., Skoog L. A method for the determination of dATP and dTTP in picomole amounts. Anal Biochem. 1970 Mar;34:152–160. doi: 10.1016/0003-2697(70)90096-5. [DOI] [PubMed] [Google Scholar]
- MALEY F., MALEY G. F. On the nature of a sparing effect by thymidine on the utilization of deoxycytidine. Biochemistry. 1962 Sep;1:847–851. doi: 10.1021/bi00911a017. [DOI] [PubMed] [Google Scholar]
- Magnusson G. Deoxyribonucleotide phosphatase from rat liver and cultured mouse fibroblasts. Eur J Biochem. 1971 May 28;20(2):225–230. doi: 10.1111/j.1432-1033.1971.tb01384.x. [DOI] [PubMed] [Google Scholar]
- Martin D. W., Jr, Gelfand E. W. Biochemistry of diseases of immunodevelopment. Annu Rev Biochem. 1981;50:845–877. doi: 10.1146/annurev.bi.50.070181.004213. [DOI] [PubMed] [Google Scholar]
- Meuth M. The genetic consequences of nucleotide precursor pool imbalance in mammalian cells. Mutat Res. 1984 Apr;126(2):107–112. doi: 10.1016/0027-5107(84)90051-4. [DOI] [PubMed] [Google Scholar]
- Nicander B., Reichard P. Dynamics of pyrimidine deoxynucleoside triphosphate pools in relationship to DNA synthesis in 3T6 mouse fibroblasts. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1347–1351. doi: 10.1073/pnas.80.5.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicander B., Reichard P. Evidence for the involvement of substrate cycles in the regulation of deoxyribonucleoside triphosphate pools in 3T6 cells. J Biol Chem. 1985 Aug 5;260(16):9216–9222. [PubMed] [Google Scholar]
- Nicander B., Reichard P. Relations between synthesis of deoxyribonucleotides and DNA replication in 3T6 fibroblasts. J Biol Chem. 1985 May 10;260(9):5376–5381. [PubMed] [Google Scholar]
- OKAZAKI R., KORNBERG A. DEOXYTHYMIDINE KINASE OF ESCHERICHIA COLI. II. KINETICS AND FEEDBACK CONTROL. J Biol Chem. 1964 Jan;239:275–284. [PubMed] [Google Scholar]
- Rode W., Scanlon K. J., Moroson B. A., Bertino J. R. Regulation of thymidylate synthetase in mouse leukemia cells (L1210). J Biol Chem. 1980 Feb 25;255(4):1305–1311. [PubMed] [Google Scholar]
- Scholtissek C. Studies on the uptake of nucleic acid precursors into cells in tissue culture. Biochim Biophys Acta. 1968 Jun 24;158(3):435–447. doi: 10.1016/0304-4165(68)90297-3. [DOI] [PubMed] [Google Scholar]
- Skoog L. An enzymatic method for the determination of dCTP and dGTP in picomole amounts. Eur J Biochem. 1970 Dec;17(2):202–208. doi: 10.1111/j.1432-1033.1970.tb01154.x. [DOI] [PubMed] [Google Scholar]
- Stimac E., Housman D., Huberman J. A. Effects of inhibition of protein synthesis on DNA replication in cultured mammalian cells. J Mol Biol. 1977 Sep 25;115(3):485–511. doi: 10.1016/0022-2836(77)90167-x. [DOI] [PubMed] [Google Scholar]
- Thelander L., Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- Tomich P. K., Chiu C. S., Wovcha M. G., Greenberg G. R. Evidence for a complex regulating the in vivo activities of early enzymes induced by bacteriophage T4. J Biol Chem. 1974 Dec 10;249(23):7613–7622. [PubMed] [Google Scholar]
- Turner M. K., Abrams R., Lieberman I. Meso-alpha, beta-diphenylsuccinate and hydroxyurea as inhibitors of deoxycytidylate synthesis in extracts of Ehrlich ascites and L cells. J Biol Chem. 1966 Dec 25;241(24):5777–5780. [PubMed] [Google Scholar]
- Ullman B., Kaur K., Watts T. Genetic studies on the role of the nucleoside transport function in nucleoside efflux, the inosine cycle, and purine biosynthesis. Mol Cell Biol. 1983 Jul;3(7):1187–1196. doi: 10.1128/mcb.3.7.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. V., Cheng Y. Human deoxyuridine triphosphate nucleotidohydrolase. Purification and characterization of the deoxyuridine triphosphate nucleotidohydrolase from acute lymphocytic leukemia. J Biol Chem. 1979 Apr 25;254(8):2897–2901. [PubMed] [Google Scholar]
- YOUNG C. W., HODAS S. HYDROXYUREA: INHIBITORY EFFECT ON DNA METABOLISM. Science. 1964 Nov 27;146(3648):1172–1174. doi: 10.1126/science.146.3648.1172. [DOI] [PubMed] [Google Scholar]



