Abstract
Primary localized cutaneous nodular amyloidosis (nodular amyloidosis) is a rare and distinct type of amyloidosis, in which amyloid L deposition is limited to the skin and typically manifested as a tumefactive nodule on the acral sites. However, the definite cause of nodular amyloidosis is still unknown. Although it is relatively well known that the amyloid deposits in nodular amyloidosis originate from immunoglobulin light chains secreted by local plasma cells, traumatic injury to the skin has rarely been recognized as a triggering factor of nodular amyloidosis. Herein, we present a case of a 50-year-old male patient with primary localized cutaneous nodular amyloidosis, which occurred after local trauma, and discuss the relationship between traumatic damage and dermal amyloid L deposition.
Keywords: Primary localized cutaneous nodular amyloidosis, Trauma
INTRODUCTION
Primary localized cutaneous amyloidosis is a metabolic disease of the skin that is characterized by extracellular deposition of amyloid proteins in the skin without evidence of systemic involvement. Generally, this disease is subdivided into macular, papular, and nodular type. Among these subcategories, primary localized cutaneous nodular amyloidosis (nodular amyloidosis) is the rarest type, in which dermal amyloid L deposition is presented as a single, or rarely, multiple nodules of varying size1-3. In contrast to macular or papular amyloidosis, epidermal damage is not thought to play a role in nodular amyloidosis, and the amyloid protein in nodular amyloidosis is believed to derive from immunoglobulin light chains (AL protein) produced by local plasma cells, not from degenerated keratinocytes1-6.
However, there have been a few case reports implying that an association between local trauma and deposition of AL protein may exist.
Herein, we describe a case of a 50-year-old Korean male patient with primary localized cutaneous nodular amyloidosis, which occurred following local trauma. To the best of our knowledge, this is the first report in Korea suggesting a possible triggering role of local trauma in the development of nodular amyloidosis.
CASE REPORT
A 50-year-old Korean man with a tumefactive nodule on his frontal scalp that had increased in size over a 2 months' duration came into our clinic. He mentioned that he used to play soccer and repeatedly hit a ball mostly with the lesional frontal scalp. He recalled that he had bumped his frontal scalp against a ball rather strongly a few days before the onset of the nodule. His past medical history was unremarkable. A review of his systems found no specific symptoms. Physical examination showed a 5×5 cm sized, well-demarcated, dome-shaped, salmon-colored, waxy nodule with overlying purpuric plaques on the frontal area of the scalp (Fig. 1). The lesion was accompanied by mild tenderness. Biopsy specimens were obtained from both salmon-colored lesion and purpuric lesion. Histopathologic examination showed similar findings in both specimens. There were follicular plugging, thinning and flattening of stratum malpighii in the epidermis and deposition of acellular amorphous eosinophilicmaterials throughout the entire dermis. There were a mild to moderate perivascular and interstitial lymphocytic infiltrate admixed with plasma cells, mast cells, and a few eosinophils and a little interstitial mucin deposition in the upper and deep dermis (Fig. 2). Congo red staining with polarizing microscopic examination revealed apple-green birefringence throughout the whole dermis (Fig. 3). An electron microscopic examination revealed uniform, long, and straight filaments (Fig. 4).
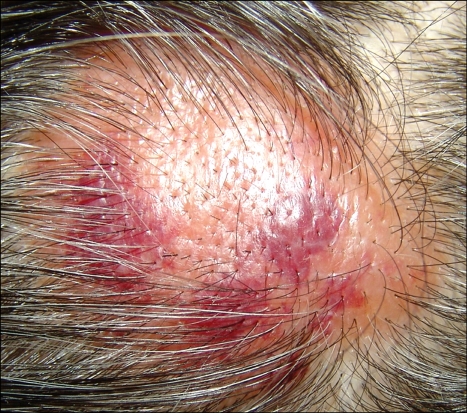
Fig. 1.
A 5×5 cm sized, well-demarcated, dome-shaped, salmon-colored, waxy nodule with overlying purpuric plaques on the frontal scalp.
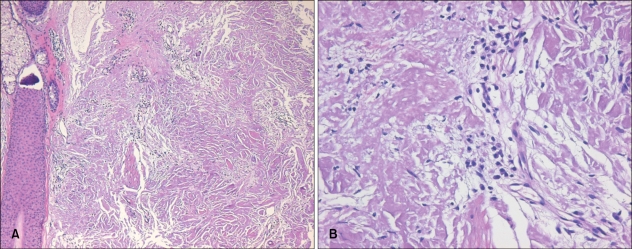
Fig. 2.
(A) Deposition of acellular amorphous eosinophilic materials over the entire dermis (H&E, ×100). (B) Infiltration of numerous plasma cells within the deposits (H&E, ×400).
Fig. 3.
Characteristic apple-green birefringence under polarized light (Congo red staining, ×200).
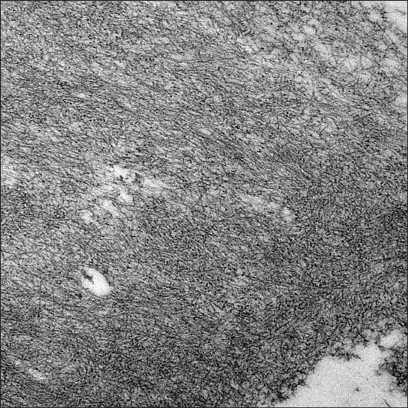
Fig. 4.
Amyloid filaments are straight, long, and of uniform diameter (TEM, ×20,000).
Laboratory studies including complete blood cell with differential cell counts, blood chemistry, and urinalysis were within the normal limits; however, the erythrocyte sedimentation rate w as slightly elevated to 14 mm/hr (reference interval: 0~9 mm/hr). Chest radiograph showed no active lesion in the lung. Electrocardiogram showed normal sinus rhythm. Autoantibodies, including antinuclear antibody, anti-SS-A (Ro) antibody, and anti-SS-B (La) antibody, were all negative. Serum and urine protein electrophoresis analysis also produced normal results.
All these findings were consistent with a diagnosis of primary localized cutaneous nodular amyloidosis. Our patient did not want to take any specific treatment, and has remained in good general health with no evidence of progression to systemic amyloidosis and the nodule remained stable in size during a follow-up of 1 year.
DISCUSSION
Amyloid is an extracellular proteinaceous material that is amorphous, eosinophilic, homogeneous, and hyaline in microscopic appearance. Amyloidosis belongs to a metabolic disease and refers to a spectrum of conditions characterized bythedeposition of amyloid in the tissue. The deposits can be limited in the skin without evidence of systemic involvement (primary localized cutaneous amyloidosis), or ca n be systemic and involve multiple organs andtissues (primary or secondary systemic amyloidosis)2,3.
Primary localized cutaneous amyloidosis is further subdivided into macular, papular, and nodular amyloidosis. Nodular amyloidosis is the rarest type of primary localized cutaneous amyloidosis, and has somewhat distinct characteristics when compared to the other two types. A single, or rarely, multiple nodules occur preferentially on the acral areas, commonly on the face, scalp, or extremities but c an be present anywhere on the skin1-3. The nodules, which usually range from 1 to 3 cm in diameter, have a typically waxy, tumefactive appearance. Patients that are between 50 and 60 years old (mean age: 55 years) are most often affected with no sex predilection5,7.
Histopathologically, the overlying epidermis may show atrophic changes. The entire dermis and sometimes subcutis are filled with amorphous, eosinophilic, and homogeneous amyloid material. Amyloid deposits may be also found in the walls of small blood vessels and around adipocytes. A focal infiltrate of plasma cells is scattered through the deposits8,9. When stained with Congo red, amyloid deposits exhibit characteristic apple-green birefringence under polarized light. However, the above findings cannot completely exclude a diagnosis of nodular colloid degeneration. A transmission electron microscopic examination is useful to differentiate between nodular amyloidosis and nodular colloid degeneration3. Under a transmission electron microscope, amyloid filaments appear straight and long and have a uniform diameter (6 to 7 nm). This is in contrast to colloid in nodular colloid degeneration, which consists of short, wavy, and irregularly arranged filaments that are 3 to 4 nm in diameter3,10,11.
The dermal amyloid L protein is derived from immunoglobulin light chains produced by locally infiltrated plasma cells4,7,12. The precise cause of localized infiltration of plasma cells is unknown. In contrast to macular or papular amyloidosis, traumatic epidermal damage is not thought to play a role in nodular amyloidosis5. In macular or papular amyloidosis, it is strongly believed that chronic rubbing or friction of the skin leads to damage of basal keratinocytes and subsequently results in the deposits of degenerated keratinocytes as amyloid protein2. In a literature review, there are a few articles implying a possible role of local trauma in the deposition of immunoglobulin light chains. Kalajian et al.5 reported a 24-year-old woman with nodular am yloidosis on her chin, which followed local trauma from a thrown full beer can. Symonds et al.13 described a 69-year-old female patient who developed a calcifying amyloidoma (tumoral amyloidosis) on her breast following local trauma to that region. Pasternak et al.14 reported a case of soft tissue amyloidoma on the leg, where a previous traumatic event was suspected. Our case also shows a clear relationship between repeated local trauma on the frontal scalp and the onset of his nodular amyloidosis, and this raises the possibility of local trauma as a potential trigger for the development of nodular amyloidosis. More studies are required to verify the causal relationship between traumatic damage and development of nodular amyloidosis.
Although the amyloid deposits in nodular amyloidosis are composed of AL protein, the same as in primary systemic amyloidosis, by definition, nodular amyloidosis is limited to the skin without any systemic involvement and is generally a benign condition. On the basis of known association of nodular amyloidosis with systemic diseases, however, systemic evaluation and a long-term follow-up evaluation should be performed. Indeed, progression of nodular amyloidosis to systemic amyloidosis has been reported in many articles at the rate of 7% to 50%5,7,15,16. In our case, the patient had no evidence of systemic amyloidosis and laboratory findings including serum and urine protein electrophoresis, chest radiograph, and electrocardiogram were all unremarkable.
In conclusion, we describe a case of a Korean patient who developed primary localized cutaneous nodular amyloidosis after trauma. To the best of our knowledge, this is the first report in Korea suggesting a possible triggering role of traumatic damage in the development of nodular amyloidosis. However, further investigations are needed to clarify the relationship between traumatic damage and dermal amyloid L deposition.
References
- 1.Breathnach SM. Amyloid and amyloidosis. J Am Acad Dermatol. 1988;18:1–16. doi: 10.1016/s0190-9622(88)70001-8. [DOI] [PubMed] [Google Scholar]
- 2.James WD, Berger TG, Elston DM. Andrew's diseases of the skin: clinical dermatology. 10th ed. Philadelphia: Saunders Elsevier; 2006. pp. 519–522. [Google Scholar]
- 3.Elder DE, Elenitsas R, Johnson BL, Jr, Murphy GF, Xu X. Lever's histopathology of the skin. 10th ed. Philadelphia: Lippincott Williams & Wilkins; 2009. pp. 870–876. [Google Scholar]
- 4.Husby G, Sletten K, Blumenkrantz N, Danielsen L. Characterization of an amyloid fibril protein from localized amyloidosis of the skin as lambda immunoglobulin light chains of variable subgroup I (A lambda I) Clin Exp Immunol. 1981;45:90–96. [PMC free article] [PubMed] [Google Scholar]
- 5.Kalajian AH, Waldman M, Knable AL. Nodular primary localized cutaneous amyloidosis after trauma: a case report and discussion of the rate of progression to systemic amyloidosis. J Am Acad Dermatol. 2007;57(2 Suppl):S26–S29. doi: 10.1016/j.jaad.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Huilgol SC, Ramnarain N, Carrington P, Leigh IM, Black MM. Cytokeratins in primary cutaneous amyloidosis. Australas J Dermatol. 1998;39:81–85. doi: 10.1111/j.1440-0960.1998.tb01253.x. [DOI] [PubMed] [Google Scholar]
- 7.Moon AO, Calamia KT, Walsh JS. Nodular amyloidosis: review and long-term follow-up of 16 cases. Arch Dermatol. 2003;139:1157–1159. doi: 10.1001/archderm.139.9.1157. [DOI] [PubMed] [Google Scholar]
- 8.Masuda C, Mohri S, Nakajima H. Histopathological and immunohistochemical study of amyloidosis cutis nodularis atrophicans--comparison with systemic amyloidosis. Br J Dermatol. 1988;119:33–43. doi: 10.1111/j.1365-2133.1988.tb07098.x. [DOI] [PubMed] [Google Scholar]
- 9.Rodermund OE. On amyloidosis cutis nodularis atrophicans (Gottron 1950). At the same time a contribution to the classification of amyloidoses. Arch Klin Exp Dermatol. 1967;230:153–171. [PubMed] [Google Scholar]
- 10.Dupre A, Bonafe JF, Pieraggi MT, Perrot H. Paracolloid of the skin. J Cutan Pathol. 1979;6:304–309. doi: 10.1111/j.1600-0560.1979.tb01138.x. [DOI] [PubMed] [Google Scholar]
- 11.Kawashima Y, Matsubara T, Kinbara T, Hirone T, Kitamura K, Himi A, et al. Colloid degeneration of the skin--a case report. J Dermatol. 1977;4:115–121. doi: 10.1111/j.1346-8138.1977.tb01023.x. [DOI] [PubMed] [Google Scholar]
- 12.Kitajima Y, Seno J, Aoki S, Tada S, Yaoita H. Nodular primary cutaneous amyloidosis. Isolation and characterization of amyloid fibrils. Arch Dermatol. 1986;122:1425–1430. [PubMed] [Google Scholar]
- 13.Symonds DA, Eichelberger MF, Sager GL. Calcifying amyloidoma of the breast. South Med J. 1995;88:1169–1172. doi: 10.1097/00007611-199511000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Pasternak S, Wright BA, Walsh N. Soft tissue amyloidoma of the extremities: report of a case and review of the literature. Am J Dermatopathol. 2007;29:152–155. doi: 10.1097/01.dad.0000211513.98230.74. [DOI] [PubMed] [Google Scholar]
- 15.Brownstein MH, Helwig EB. The cutaneous amyloidoses I Localized forms. Arch Dermatol. 1970;102:8–19. [PubMed] [Google Scholar]
- 16.Woollons A, Black MM. Nodular localized primary cutaneous amyloidosis: a long-term follow-up study. Br J Dermatol. 2001;145:105–109. doi: 10.1046/j.1365-2133.2001.04291.x. [DOI] [PubMed] [Google Scholar]