Abstract
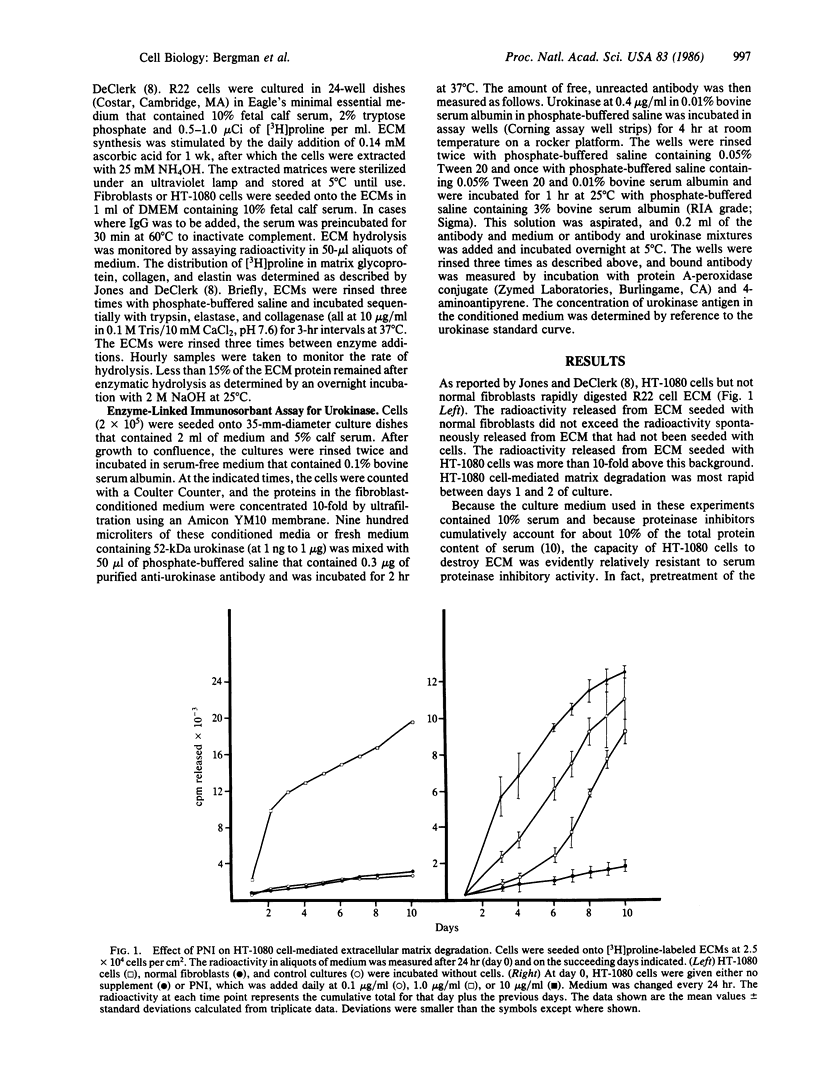
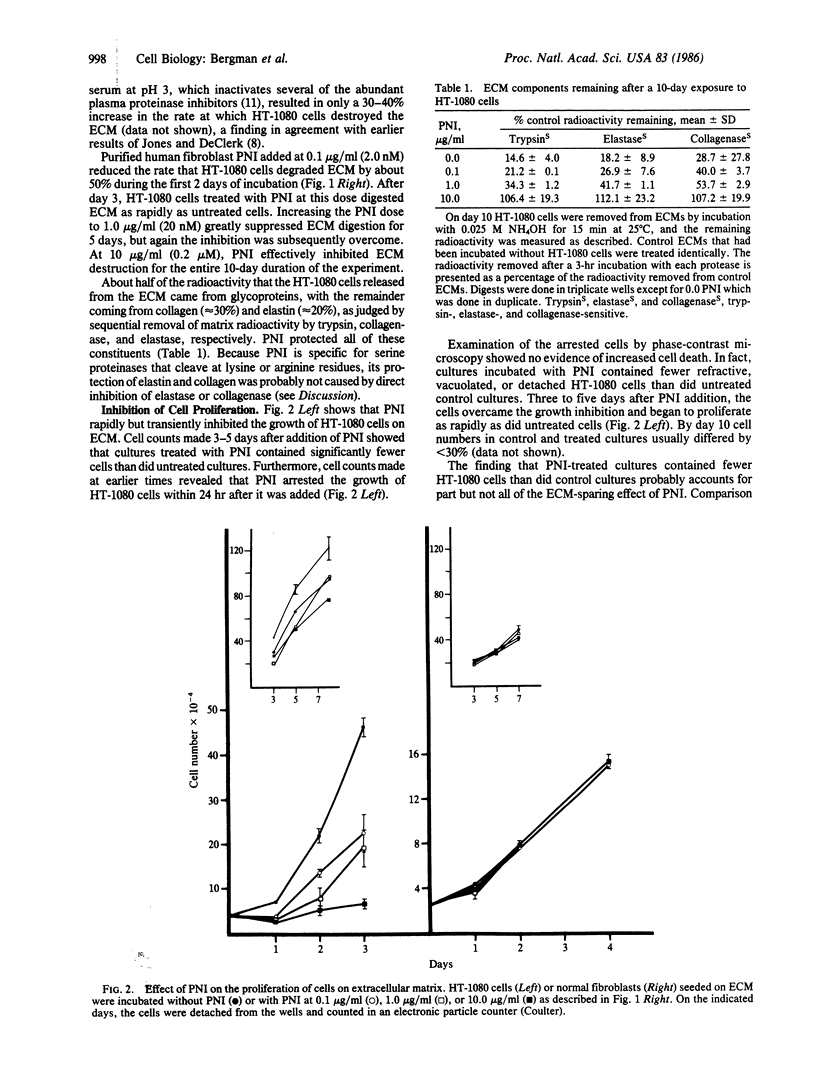
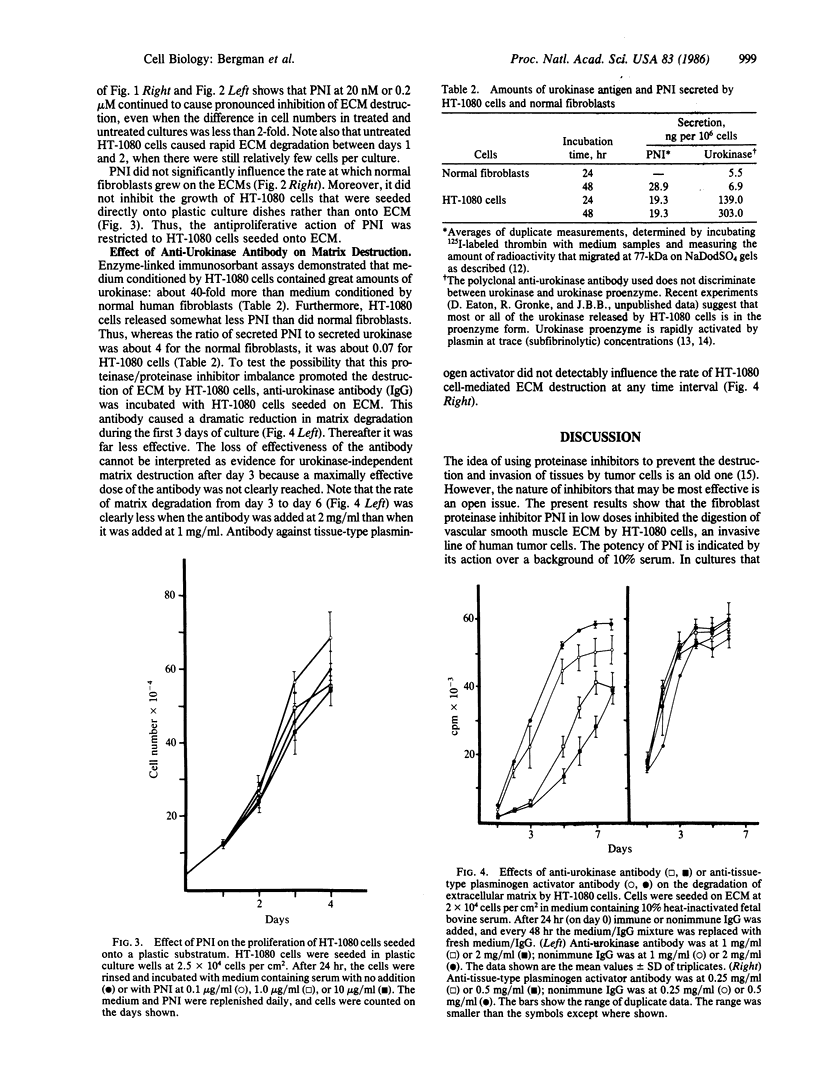
Human fibrosarcoma (HT-1080) cells, in contrast to normal fibroblasts, rapidly hydrolyze the glycoprotein, collagen, and elastin extracellular matrix (ECM) synthesized by cultured rat aortic smooth muscle cells. This degradation occurs at a rapid rate in the presence of serum, indicating that the cellular proteases responsible are relatively insensitive to serum proteinase inhibitors. Here it is shown that protease nexin I (PNI), a fibroblast-secreted inhibitor of urokinase, plasmin, and certain other serine proteinases, effectively inhibited the HT-1080 cell-mediated degradation of this ECM. PNI at 2.0 nM significantly inhibited matrix destruction for 1-2 days and at 0.2 microM caused a virtually complete inhibition that persisted for the entire 10-day period of observation. Inhibition of ECM destruction was accompanied by a transient arrest of HT-1080 cell proliferation that took place during the first 3 days after PNI addition. PNI did not inhibit the growth of normal fibroblasts and also did not inhibit the growth of HT-1080 cells that were seeded onto plastic dishes rather than onto ECM. Like many types of malignant cells, HT-1080 cells release large amounts of urokinase. Antibody against this plasminogen activator partially protected ECM from HT-1080 cell-mediated hydrolysis, indicating that it may have been a target of PNI. One potential physiological function of PNI could be to help maintain the integrity of connective tissue matrices, protection that malignant cells could overcome by secreting proteinases in excessive amounts.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Eaton D. L., Baker J. B. Evidence that a variety of cultured cells secrete protease nexin and produce a distinct cytoplasmic serine protease-binding factor. J Cell Physiol. 1983 Nov;117(2):175–182. doi: 10.1002/jcp.1041170207. [DOI] [PubMed] [Google Scholar]
- Eaton D. L., Scott R. W., Baker J. B. Purification of human fibroblast urokinase proenzyme and analysis of its regulation by proteases and protease nexin. J Biol Chem. 1984 May 25;259(10):6241–6247. [PubMed] [Google Scholar]
- Jones P. A., DeClerck Y. A. Destruction of extracellular matrices containing glycoproteins, elastin, and collagen by metastatic human tumor cells. Cancer Res. 1980 Sep;40(9):3222–3227. [PubMed] [Google Scholar]
- Kohga S., Harvey S. R., Weaver R. M., Markus G. Localization of plasminogen activators in human colon cancer by immunoperoxidase staining. Cancer Res. 1985 Apr;45(4):1787–1796. [PubMed] [Google Scholar]
- Loskutoff D. J., Edgington T. E. Synthesis of a fibrinolytic activator and inhibitor by endothelial cells. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3903–3907. doi: 10.1073/pnas.74.9.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low D. A., Scott R. W., Baker J. B., Cunningham D. D. Cells regulate their mitogenic response to thrombin through release of protease nexin. Nature. 1982 Jul 29;298(5873):476–478. doi: 10.1038/298476a0. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Cancer metastasis. Organ colonization and the cell-surface properties of malignant cells. Biochim Biophys Acta. 1982 Dec 21;695(2):113–176. doi: 10.1016/0304-419x(82)90020-8. [DOI] [PubMed] [Google Scholar]
- Ossowski L., Reich E. Antibodies to plasminogen activator inhibit human tumor metastasis. Cell. 1983 Dec;35(3 Pt 2):611–619. doi: 10.1016/0092-8674(83)90093-4. [DOI] [PubMed] [Google Scholar]
- Scott R. W., Bergman B. L., Bajpai A., Hersh R. T., Rodriguez H., Jones B. N., Barreda C., Watts S., Baker J. B. Protease nexin. Properties and a modified purification procedure. J Biol Chem. 1985 Jun 10;260(11):7029–7034. [PubMed] [Google Scholar]
- Scott R. W., Eaton D. L., Duran N., Baker J. B. Regulation of extracellular plasminogen activator by human fibroblasts. The role of protease nexin. J Biol Chem. 1983 Apr 10;258(7):4397–4403. [PubMed] [Google Scholar]
- Skriver L., Larsson L. I., Kielberg V., Nielsen L. S., Andresen P. B., Kristensen P., Danø K. Immunocytochemical localization of urokinase-type plasminogen activator in Lewis lung carcinoma. J Cell Biol. 1984 Aug;99(2):753–757. doi: 10.1083/jcb.99.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller E. K., Schleuning W. D., Reich E. Complex-formation and inhibition of urokinase by blood plasma proteins. Biochem J. 1983 Oct 1;215(1):123–131. doi: 10.1042/bj2150123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson E. L., Becker M. L., Hoal E. G., Dowdle E. B. Molecular species of plasminogen activators secreted by normal and neoplastic human cells. Cancer Res. 1980 Mar;40(3):933–938. [PubMed] [Google Scholar]
- Wun T. C., Ossowski L., Reich E. A proenzyme form of human urokinase. J Biol Chem. 1982 Jun 25;257(12):7262–7268. [PubMed] [Google Scholar]



