Abstract
In the yeast Saccharomyces cerevisiae triacylglycerols (TAG) are synthesized by the acyl-CoA dependent acyltransferases Dga1p, Are1p, Are2p and the acyl-CoA independent phospholipid:diacylglycerol acyltransferase (PDAT) Lro1p which uses phosphatidylethanolamine (PE) as a preferred acyl donor. In the present study we investigated a possible link between TAG and PE metabolism by analyzing the contribution of the four different PE biosynthetic pathways to TAG formation, namely de novo PE synthesis via Psd1p and Psd2p, the CDP-ethanolamine (CDP-Etn) pathway and lyso-PE acylation by Ale1p. In cells grown on the non-fermentable carbon source lactate supplemented with 5 mM ethanolamine (Etn) the CDP-Etn pathway contributed most to the cellular TAG level, whereas mutations in the other pathways displayed only minor effects. In cki1∆dpl1∆eki1∆ mutants bearing defects in the CDP-Etn pathway both the cellular and the microsomal levels of PE were markedly decreased, whereas in other mutants of PE biosynthetic routes depletion of this aminoglycerophospholipid was less pronounced in microsomes. This observation is important because Lro1p similar to the enzymes of the CDP-Etn pathway is a component of the ER. We conclude from these results that in cki1∆dpl1∆eki1∆ insufficient supply of PE to the PDAT Lro1p was a major reason for the strongly reduced TAG level. Moreover, we found that Lro1p activity was markedly decreased in cki1∆dpl1∆eki1∆, although transcription of LRO1 was not affected. Our findings imply that (i) TAG and PE syntheses in the yeast are tightly linked; and (ii) TAG formation by the PDAT Lro1p strongly depends on PE synthesis through the CDP-Etn pathway. Moreover, it is very likely that local availability of PE in microsomes is crucial for TAG synthesis through the Lro1p reaction.
Abbreviations: CF, cellular fraction; CL, cardiolipin; DAG, diacylglycerol; DGAT, diacylglycerol acyltransferase; DMPE, dimethylphosphatidylethanolamine; ER, endoplasmic reticulum; Etn, ethanolamine; LP, lipid particle; LPL, lysophospholipid(s); MMGlu, minimal glucose media; MMLac, minimal lactate media; MAM, mitochondria associated membrane; PA, phosphatidic acid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PS, phosphatidylserine; PDAT, phospholipid:diacylglycerol acyltransferase; RT-PCR, reverse transcription polymerase chain reaction; SE, steryl ester; TAG, triacylglycerol; TLC, thin-layer chromatography; YPD, complex glucose media; YPLac, complex lactate media
Keywords: Phosphatidylethanolamine, Triacylglycerol, Acyltransferase, CDP-ethanolamine, Yeast, Saccharomyces cerevisiae
Highlights
► CDP-Etn pathway provides PE for Lro1p. ► Depletion of PE in ER causes low TAG. ► Link between phospholipid and TAG metabolism.
1. Introduction
Storage lipids in all types of eukaryotic cells accumulate mostly as triacylglycerols (TAG) and steryl esters (SE) which are the major components of a specialized globular compartment of the cell named lipid particle (LP), lipid droplet, lipid body or oil body [1]. LP consist of a randomly packed TAG core surrounded by several more or less ordered SE shells and a phospholipid surface monolayer with a small set of specific proteins embedded [1–3]. Mechanism(s) of LP biogenesis are still a matter of dispute [4], but the most convincing hypothesis for this process is budding of LP from the endoplasmic reticulum (ER) [2,5–7]. According to this model, TAG and SE formed in the ER accumulate in certain microdomains of the ER membrane. Upon further synthesis of neutral lipids, LP precursors are formed, which at a critical size bud off the ER and are released into the cytosol [2,4].
In Saccharomyces cerevisiae two proteins localized to the ER, Are1p and Are2p, catalyze the synthesis of SE with slightly different substrate specificity. Are2p is the major SE synthase of the yeast and prefers ergosterol as a substrate, whereas Are1p uses ergosterol and its precursors at nearly equal efficiency with a slight preference for lanosterol. Mutants lacking ARE1 and ARE2 are completely devoid of SE and accumulate free sterols [5,8]. In addition, Are1p and Are2p can also catalyze TAG synthesis although with minor efficiency compared to the two major yeast TAG synthesizing enzymes, Dga1p and Lro1p [8–12]. In Saccharomyces cerevisiae two primary mechanisms of TAG formation were identified, namely an acyl-CoA dependent reaction catalyzed by the diacylglycerol acyltransferase (DGAT) Dga1p, and an acyl-CoA independent pathway involving the phospholipid:diacylglycerol acyltransferase (PDAT) Lro1p [9–14]. Dga1p is dually localized to the ER and LP and appears to be more efficient than Lro1p under standard growth conditions when cells are in the stationary phase [3,9–11]. In contrast, Lro1p which requires phospholipids as acyl donor is exclusively localized to the ER [12–15]. Lro1p preferentially uses phosphatidylethanolamine (PE) as co-substrate in vitro and transfers its sn-2 acyl group to diacylglycerol (DAG), resulting in the formation of TAG and lyso-PE [13–15]. This reaction has no counterpart in mammalian cells.
The link between TAG synthesis and PE metabolism through Lro1p as described above led us to investigate metabolic interactions between biosynthesis/degradation of these two lipids in some more detail. In Saccharomyces cerevisiae, PE synthesis occurs by four different pathways. In brief, two of these pathways are accomplished by phosphatidylserine (PS) decarboxylases Psd1p and Psd2p which use PS as a substrate. While Psd1p is localized to mitochondria, Psd2p is a component of a Golgi/vacuolar compartment [16–21]. Yeast PE can also be synthesized through the cytidyldiphosphate ethanolamine (CDP-Etn) branch of the so-called Kennedy pathway using Etn and DAG as substrates [16,22,23]. The CDP-Etn pathway is also linked to sphingolipid metabolism through the action of the dihydrosphingosine phosphate lyase Dpl1p which sets Etn-P free [24,25]. Moreover, the lyso-PE acyltransferase Ale1p which is present in the mitochondria associated ER (MAM) catalyzes an alternative pathway to form PE [26,27]. Surprisingly, Tgl3p, the major yeast TAG lipase, can also act as a lyso-PE acyltransferase [28].
In a series of studies from our laboratory [29–33] we observed preferences in the incorporation of newly formed PE through the different pathways into different cellular compartments. These findings led us to speculate that also distinctions regarding the relative contributions of PE biosynthesis to TAG formation by Lro1p may exist between the different PE synthesizing routes. We used haploid single and multiple deletion strains bearing defects in PE biosynthesis, namely psd1Δ, psd2Δ, psd1Δpsd2Δ, cki1Δdpl1Δeki1Δ (CDP-Etn mutant) and ale1Δ to address this question. Yeast cells used for these experiments were grown on the non-fermentable carbon source lactate because under these conditions the level of PE in the cell becomes more critical for cell growth and viability than in glucose grown cells [31]. Here we report a clear metabolic link between TAG formation in the yeast and PE synthesis via the CDP-Etn branch of the Kennedy pathway, but not by other PE biosynthetic routes. In the light of these findings, the lipid metabolic network of PE and TAG metabolism in the yeast is discussed.
2. Materials and methods
2.1. Strains and culture conditions
Strains used throughout this study are listed in Table 1. Cells were cultivated aerobically in 2 l Erlenmeyer flasks to the stationary growth phase (A600 ~ 4) at 30 °C in minimal lactate medium consisting of 2.66% lactate (Roth), 0.67% yeast nitrogen base without amino acids (USBiological), 0.073% amino acid mix (Roth, Fluka) supplemented with 5 mM Etn (Merck) and adjusted to pH 5.5 with KOH. Main cultures were inoculated to an A600 of 0.1 from precultures grown aerobically for 48 h in YPD medium containing 1% yeast extract (Oxoid), 2% peptone (Oxoid) and 2% glucose (Merck) at 30 °C.
Table 1.
Yeast strains used in this study.
| Strain | Genotype | Source/reference |
|---|---|---|
| Wild type | BY4741 MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Euroscarf |
| psd1Δ | BY4741 MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 psd1Δ::His3MX6 | This study |
| psd2Δ | BY4741 MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 psd2Δ::KanMX4 | Euroscarf |
| ale1Δ | BY4741 MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 ale1Δ::KanMX4 | Euroscarf |
| lro1Δ | BY4741 MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lro1Δ::KanMX4 | Euroscarf |
| dga1Δ | BY4741 MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 dga1Δ::KanMX4 | Euroscarf |
| cki1Δdpl1Δeki1Δ | BY4741 MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 cki1Δ::His3MX6 dpl1Δ::LEU2 eki1Δ::KanMX4 | This study |
| psd1Δpsd2Δ | BY4741 MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 psd1Δ::His3MX6 psd2Δ::KanMX4 | This study |
| dga1Δlro1Δ | BY4741 MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 dga1Δ::His3MX6 lro1Δ::KanMX4 | This study |
For synthesis of radioactively labeled [14C]phospholipids, a dga1Δlro1Δ double deletion strain was grown on minimal glucose media containing 2% glucose (Merck), 0.67% yeast nitrogen base without amino acids (USBiological) and 0.073% amino acid mix (Roth, Fluka). Details of the labeling procedure will be described below.
2.2. Strain construction
Single-step deletion of chromosomal genes was carried out using the PCR-mediated technique described by Longtine et al. [34]. The marker module His3MX6 including the Schizosaccharomyces pombe his5+ gene on the vector pFA6a was used to replace YNL169c encoding Psd1p and YOR245c encoding Dga1p in the single deletion strains psd2Δ::KanMX4 and lro1Δ::KanMX4, respectively [34–36]. Deletion cassettes containing homologous regions to start and stop regions of either PSD1 or DGA1 and the entire HIS5+ gene were constructed with primers listed in Table 2. A 1.4 kbp PCR fragment was generated with ExTaq DNA polymerase (Takara, Otsu, Japan) by using 150 ng of plasmid as a template in a standard PCR mixture containing PCR buffer (20 mM Mg2+), 0.2 mM deoxynucleoside triphosphates, each, and a 1 μM solution of primers in a total volume of 100 μl. After a denaturation step of 2 min at 94 °C, fragments were amplified for 10 cycles of 15 s at 94 °C, 60 s at 55 °C, and 100 s at 72 °C; and for 25 cycles of 15 s at 94 °C, 90 s at 68 °C, and 60 s at 72 °C, followed by a final elongation step for 10 min at 72 °C.
Table 2.
Primers used for the construction of deletion strains and for RT-PCR. The underlined sequences are homologous to the His3MX6 disruption cassette (PSD1-H1, PSD1-H2, DGA1 Del_for, DGA1 Del_rev). Amplified plasmid DNA with the primers PSD1-H1 and PSD1-H2 led to PSD1 deletion, whereas DGA1 Del_for and Del_rev to DGA1 deletion after homologues recombination to the target loci YNL169c and YOR245c, respectively. Lro1 FP, Lro1 RP, Dga1 FP, Dga1 RP, Act1 FP and Act1 RP were used to determine expression levels of LRO1, DGA1 and ACT1.
| Primer | Primer sequence (5′–3′) |
|---|---|
| PSD1-H1 | GCCAGTTAAGAACGCCTTGGCGCAAGGGAGGACGCTCCTCCGGATCCCCGGGTTAATTAA |
| PSD1-H2 | CAGGTATGTGGTTCCAAGTGTTTGTCGCTCTTTGAATTTGGAATTCGAGCTCGTTTAAAC |
| DGA1 Del_for | ATAAGGAAACGCAGAGGCATACAGTTTGAACAGTCACATAACGGATCCCCGGGTTAATTAA |
| DGA1 Del_rev | TTCCTGTAAGTTAATACTCTTACTTAAGATATACAGCCCGAATTCGAGCTCGTTTAAAC |
| Lro1 FP | ATGGGCACACTGTTTCGAAGAAATG |
| Lro1 RP | AACAGGATGTTTGGCCTCGATATTA |
| Dga1 FP | AAGAAGGAAGAAGGAAGCCCTACAG |
| Dga1 RP | CCTGGTTGCGATAGTCAATAGTAGA |
| Act1 FP | GGTCCCAATTGCTCGAGAGAT |
| Act1 RP | GAAGTCCAAGGCGACGTAACA |
Overnight cultures (A600 ~ 0.8) of single mutants psd2Δ::KanMX4 and lro1Δ::KanMX4 were used for transformation with the high-efficiency lithium acetate transformation method [37]. Transformants were grown on plates lacking histidine for 3 days at 30 °C. Plates used for cultivation of psd1Δpsd2Δ transformants contained in addition 5 mM Etn to permit growth. Large colonies were transferred to fresh plates for further selection. Clones yielding colonies were considered as positive transformants and further checked for correct integration of the respective deletion cassette. Verification of the correct replacement of PSD1 and DGA1 by the His3MX6 module was done by colony PCR. In brief, oligonucleotides were designed to bind outside the target locus and within the marker module [36,38]. Correct integration of the marker resulted in the appearance of the respective PCR fragment.
2.3. Isolation and characterization of subcellular fractions
Total cell-free homogenate (3000 × g supernatant) and 100,000 × g microsomes were prepared from cells grown to the stationary growth phase as described previously [21,39]. Proteins from isolated fractions were precipitated with trichloroacetic acid at a final concentration of 10%, the obtained protein pellet was solubilized in 0.1% SDS, 0.1 M NaOH, and proteins were quantified by the method of Lowry et al. [40] with bovine serum albumin as a standard. SDS-polyacrylamide gel electrophoresis was carried out by the method of Laemmli [41], and Western blot analysis by the method of Haid and Suissa [42]. Proteins were detected by enzyme-linked immunosorbent assay using rabbit antisera as the first antibody and peroxidase-conjugated goat anti-rabbit IgG as the second antibody. Antibodies used in this study were directed against the mitochondrial and microsomal marker proteins, Por1p and 40-kDa protein, respectively [21].
2.4. Lipid extraction and analysis
Lipids were extracted from total cell-free homogenate and microsomes of yeast cells grown to the stationary growth phase by the procedure of Folch et al. [43] using chloroform/methanol (2:1; v/v). After washing the organic phase with 0.034% MgCl2 solution (w/v), 2 N KCl/methanol (4:1; v/v), and methanol/water/chloroform (48:47:3; per vol), extracts were taken to dryness, dissolved in 50 μl chloroform/methanol (2:1; v/v) and applied to thin-layer chromatography (TLC) plates (Silica gel 60; Merck, Darmstadt, Germany) with the aid of a sample applicator (CAMAG, Automatic TLC Sampler 4, Muttenz, Switzerland). For analyzing neutral lipids, TAG and SE, chromatograms were developed in an ascending manner to the half-distance of the plate by using light petroleum/diethyl ether/acetic acid (70:30:2; per vol). After brief drying, TLC were further developed to the top of the plate using light petroleum/diethyl ether (49:1; v/v) as the second solvent system. For separating DAG from ergosterol light petroleum/diethyl ether/acetic acid (70:10:2; per vol) were used as a solvent system and chromatograms were developed to the top of the plate. Quantification of ergosteryl esters was accomplished by densitometric scanning at 275 nm using a Shimadzu CS-930 dual-wavelength chromatoscanner and ergosterol (Sigma) as a standard. TAG were visualized by post-chromatographic staining after dipping TLC plates into a solution containing 0.8 g MnCl2 × 4H2O, 120 ml water, 120 ml methanol and 9 ml concentrated sulfuric acid, and charring at 105 °C for 30 min. Quantification of TAG was carried out by densitometric scanning at 400 nm with triolein (NuCheck, Inc., Elysian, Maine) as a standard. For analyzing total phospholipids, the same solvent systems as described above were used to separate phospholipids from neutral lipids. Lipids were visualized by iodine vapor; phospholipids were scraped off the plate and quantified by the method of Broekhuyse [44].
Individual phospholipids were separated by two-dimensional thin-layer chromatography on silica gel 60 plates (Merck) using chloroform/methanol/25% NH3 (68:35:5; per vol) as the first and chloroform/acetone/methanol/acetic acid/water (53:20:10:10:5; per vol) as the second developing solvent system. Phospholipids were visualized by staining with iodine vapor, scraped off the plate and quantified by the method of Broekhuyse [44].
2.5. Preparation of radiolabeled lipid substrates and measurement of TAG synthase activity in vitro
Radioactively labeled [14C]phospholipids were synthesized by incubating dga1Δlro1Δ yeast mutants with 10 μCi [14C]oleic acid (PerkinElmer Life Sciences) for 24 h at 30 °C in 100 ml minimal glucose media (see above). After harvesting and disrupting cells with glass beads (Sartorius, 0.25–0.30 diameter), lipids were extracted and total phospholipids were separated from neutral lipids as described above. Phospholipids were scraped off the plate and extracted from the silica gel with chloroform/methanol (1:4; v/v) for 3 h. The organic phase was collected and the remaining silica gel was again extracted twice for 1 h, each. After combining the organic phases and drying under a steam of nitrogen, phospholipids were dissolved in 1 ml chloroform/methanol (2:1; v/v), and radioactivity was measured by liquid scintillation counting using LSC Safety (Baker, Deventer, The Netherlands) with 5% water as a scintillation cocktail [11]. Additionally, phospholipid concentration was quantified by the method of Broekhuyse [44].
The phospholipid:diacylglycerol acyltransferase (PDAT) assay was performed in a final volume of 200 μl containing phospholipids labeled with [14C]oleic acid (27 nmol; 70,000 dpm), 200 μg protein from 100,000 × g microsomes, 150 mM TrisCl (pH 7.0), 15 mM KCl, 15 mM MgCl2, 0.5 mM CHAPS, and 0.05 mM dioleoylglycerol [3,13]. The acyl-CoA:diacylglycerol acyltransferase (DGAT) assay was performed in a final volume of 200 μl containing 16 nmol unlabeled oleoyl-CoA and 0.68 nmol [14C]oleoyl-CoA (0.02 μCi), 100 μg protein from cell-free homogenate, 150 mM TrisCl (pH 7.0), 15 mM KCl, 15 mM MgCl2, 0.5 mM CHAPS, and 0.025 mM dioleoylglycerol [3,11]. Incubations were carried out for 30 min at 30 °C and terminated by addition of 3 ml chloroform/methanol (2:1; v/v). Lipids were extracted and neutral lipids were separated as described above. After visualization with iodine vapor, TAG were scraped off, and radioactivity was measured by liquid scintillation counting using LSC Safety (Baker, Deventer, The Netherlands) with 5% water as a scintillation cocktail.
2.6. RNA isolation and reverse transcription polymerase chain reaction (RT-PCR)
Total RNA from cultivated cells grown to A600 ~ 2 on minimal lactate media supplemented with 5 mM Etn at 30 °C was isolated by RNeasy kit (Qiagen). Reverse transcription was carried out with 2 μg of RNA after DNAse-I treatment (1 U RQ1, Promega) in a total volume of 15 μl reverse transcription buffer (15 min at 37 °C). Reverse transcriptase from Invitrogen was used, according to the manufacturer's protocol. Amplification of the PCR products was measured during exponential phase of the reaction. PCR was carried out in a final volume of 30 μl containing 3 μl 10× GC buffer (BioTherm), 2 μl cDNA, 0.1 mM dNTP-Mix, 0.5 pM of each primer (Table 2) and 0.6 U Polymerase (BioTherm, GenXpress). For DGA1 expression, a fragment was obtained according to the following program. After a denaturation step of 2 min at 96 °C, fragments were amplified for 27 cycles of 5 s at 96 °C, 10 s at 55 °C, 15 s at 72 °C, a final elongation step for 10 min at 72 °C followed by cooling to 4 °C. For LRO1 expression, the amplification was carried out for 28 cycles of 5 s at 96 °C, 10 s at 52 °C, and 15 s at 72 °C. As a control, the ACT1 expression was tested by amplifying the fragments for 25 cycles of 10 s at 96 °C, 60 s at 58 °C and 120 s at 72 °C.
3. Results
3.1. Growth of strains bearing defects in phosphatidylethanolamine synthesis
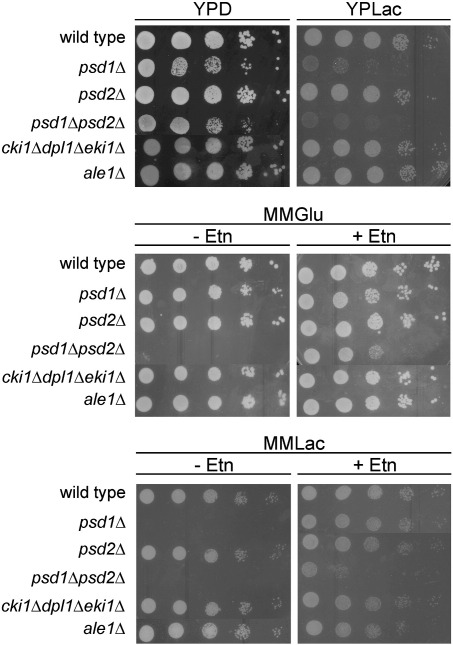
PE is one of the most prominent phospholipids found in yeast membranes. Previous work from our laboratory has shown that the requirement for PE in Saccharomyces cerevisiae is more stringent on non-fermentable carbon sources, i.e. when mitochondria are fully developed, than on fermentable carbon sources [31,45]. Mutants defective in either one of the PS decarboxylases, Psd1p or Psd2p, grow on glucose-containing media like wild type, but lack of both enzymes leads to auxotrophy for Etn (Fig. 1) or choline [31]. In contrast, strains with the psd1Δ mutation alone or in combination with psd2Δ are not viable on lactate as a carbon source unless rescued by addition of 5 mM Etn to the medium as a minimum requirement for viability. Lethality of psd1Δ on non-fermentable carbon sources is also prevented by supply with either serine or choline [31].
Fig. 1.
Growth of yeast strains with defects in phosphatidylethanolamine biosynthesis depends on the carbon sources. Cell suspensions of strains listed in the figure were spotted at dilutions (1, 1/10, 1/100, 1/1000, 1/10,000) on YPD, YPLac, MMGlu and MMLac with or without 5 mM ethanolamine. Incubation was carried out at 30 °C. YPD, complex glucose media; YPLac, complex lactate media; MMGlu, minimal glucose media; MMLac, minimal lactate media; Etn, ethanolamine.
The requirement of a defined PE level for yeast growth and viability raised the questions whether (i) the cellular amount/availability of PE and (ii) different routes for PE biosynthesis affect TAG formation by Lro1p. For this purpose, various strains bearing defects in PE formation, in particular psd1Δ, psd2Δ, psd1Δpsd2Δ, ale1Δ, and the CDP-Etn pathway mutant cki1Δdpl1Δeki1Δ, were grown on minimal lactate media supplemented with Etn creating stringent conditions for PE requirement. For blocking the CDP-Etn branch of the Kennedy pathway efficiently a cki1Δdpl1Δeki1Δ triple mutant was required. CKI1 and EKI1 encode kinases with overlapping specificities for the substrates Etn and choline. Furthermore, the CDP-Etn pathway is linked to sphingolipid catabolism through the action of the dihydrosphingosine phosphate lyase Dpl1p which cleaves phosphorylated sphingoid bases to long chain aldehydes and ethanolamine phosphate (Etn-P) [24,25,31]. In addition, two mutants defective in TAG biosynthesis, lro1Δ and dga1Δ, were used to investigate TAG and phospholipid biosynthesis under these growth conditions. As expected, growth of strains defective in PSD1 showed the longest lag phase compared to the other mutants. Nevertheless, wild type and mutants reached nearly the same A600 values (3–5 units) by entering the stationary growth phase (data not shown). Since neutral lipid accumulation starts at the end of exponential growth phase and reaches its highest level in the stationary phase [3], wild type and mutant cells were analyzed at this growth stage.
3.2. Neutral lipids from mutants bearing defects in phosphatidylethanolamine or triacylglycerol synthesis
To address the question as to the link between the different PE biosynthetic routes and neutral lipid storage in the yeast, we first quantified TAG and ergosteryl esters in wild type and mutant cells grown on minimal lactate media supplemented with Etn. Previous studies had shown that in cells grown on glucose Dga1p and Lro1p contributed differentially to TAG biosynthesis with Dga1p being the more efficient enzyme [3,9]. In lactate grown cells, deletion of DGA1 led to a reduction of the TAG content to ~ 60%, whereas deletion of LRO1 reduced the TAG level to ~ 80% of wild type (Fig. 2). Previously, it was reported that in cells grown on glucose TAG was also synthesized by the two steryl ester synthases, Are1p and Are2p, although with minor efficiency [9,10]. In our experiments with yeast cells grown on lactate, TAG was below the detection limit in a dga1Δlro1Δ double deletion strain indicating that Are1p and Are2p did not contribute to TAG formation.
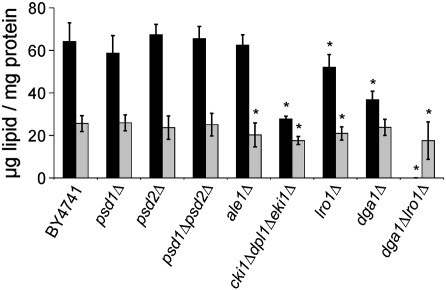
Fig. 2.
Neutral lipid composition of wild type and mutants defective in either phosphatidylethanolamine or triacylglycerol biosynthesis grown to stationary phase. Amounts of triacylglycerols (black bars) and ergosteryl esters (gray bars) in μg lipid per mg protein were measured in strains as indicated. Data are mean values of 3 independent experiments with error bars indicating the standard deviation. Significance was calculated by Student's t-test (two tailed, unpaired). Values indicated by * correspond to P < 0.05 and were defined to be significant.
Deletions of PSD1, PSD2, PSD1PSD2 and ALE1 had no significant effect on TAG biosynthesis (Fig. 2). In contrast, a cki1Δdpl1Δeki1Δ strain bearing defects in the CDP-Etn branch of the Kennedy pathway exhibited a significant reduction of the TAG level to ~ 40% of wild type. This decrease was even more pronounced than in dga1Δ where a reduction to ~ 60% was observed. The striking observation that deletion of LRO1 has less impact on TAG formation than a block in the CDP-Etn pathway can be explained by the compensatory effect of Dga1p. It has been shown that Dga1p is able to restore wild type TAG level in the lro1Δ mutant [9]. When the CDP-Etn pathway is blocked, both TAG biosynthetic routes are active but the availability of PE for Lro1p appears to become paramount and causes the observed decrease of TAG. To prove the hypothesis that the CDP-Etn pathway actively supports TAG formation in yeast, we grew wild type cells on minimal lactate media with or without supplementation of 5 mM Etn. In non-supplemented cultures the TAG level decreased to ~ 60% (38 ± 4 μg TAG/mg protein) of the control cultures supplemented with 5 mM Etn (64 ± 9 μg TAG/mg protein). These data strongly support the view that PE synthesis through the CDP-Etn pathway and TAG biosynthesis by Lro1p are linked metabolic processes.
Ergosteryl ester formation was not affected in mutants devoid of PS decarboxylases. Deletion of LRO1, ALE1 or the genes of the CDP-Etn pathway led to a slight decreased ergosteryl ester level compared to wild type (see Fig. 2). Analysis of DAG levels in wild type and corresponding mutant strains revealed no changes (data not shown).
We concluded from these results that PE biosynthesis through the CDP-Etn pathway and TAG formation were tightly linked and specific. While in cki1Δdpl1Δeki1Δ accumulation of diacylglycerol (DAG) not used as a substrate for PE/PC formation through the Kennedy pathway was not detected and TAG formation was not enhanced, we hypothesized that the reduced level of PE as a donor of fatty acids might have a negative impact on Lro1p catalyzed TAG synthesis. Alternatively, gene regulatory effects could not be ruled out. Therefore, we considered both the availability of PE for channeling fatty acids to the acyl-CoA independent pathway of TAG synthesis and transcriptional control as possible reasons for the decreased amount of TAG in cki1Δdpl1Δeki1Δ.
3.3. Acyl-CoA dependent and acyl-CoA independent triacylglycerol synthase activities in mutants bearing defects in phosphatidylethanolamine synthesis
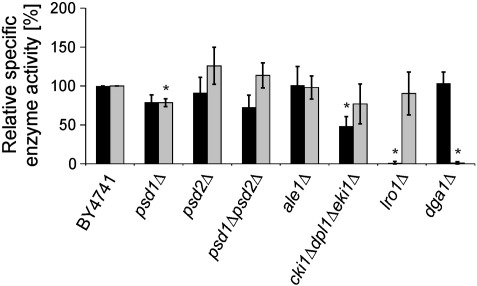
First, we investigated the activities of TAG synthesizing enzymes in mutants compromised in PE biosynthesis. Samples of cell-free homogenate and microsomes were used to analyze acyl-CoA dependent Dga1p and acyl-CoA independent Lro1p activities, respectively. Deletion of PSD2 in psd2Δ and psd1Δpsd2Δ strains led to 10–20% increase, whereas blocking the CDP-Etn pathway resulted in a slightly decreased Dga1p activity to ~ 80% of wild type (Fig. 3). A significant reduction of Dga1p activity to ~ 80% of wild type was only found in psd1Δ. Notably, Dga1p activities in ale1Δ and lro1Δ were not significantly altered.
Fig. 3.
Relative activities of triacylglycerol synthesizing enzymes in vitro. Acyl-CoA:diacylglycerol acyltransferase activity (Dga1p) (gray bars) was measured in vitro using total cell-free homogenate. Phospholipid:diacylglycerol acyltransferase activity (Lro1p) (black bars) was measured using 100,000 × g microsomes. The specific activity of Lro1p and Dga1p in wild type was set to 100%, and data for mutant strains were calculated accordingly. As negative control, phospholipid:diacylglycerol acyltransferase was measured in lro1Δ, and acyl-CoA:diacylglycerol acyltransferase in dga1Δ. Data are mean values of 3 independent experiments with error bars indicating the standard deviation. Significance was calculated by Student's t-test (two tailed, unpaired). Values indicated by * correspond to P < 0.05 and were defined to be significant.
Lro1p activity was slightly reduced in psd1Δ and psd1Δpsd2Δ and largely unaffected in psd2Δ, ale1Δ and dga1Δ (Fig. 3). However, a significant decrease of Lro1p activity to ~ 50% of wild type was observed in the cki1Δdpl1Δeki1Δ triple mutant. Although we have to take into account that enzyme activities measured in vitro need not always reflect the potential of an enzyme in vivo, we can speculate that reduced activity of Lro1p in cki1Δdpl1Δeki1Δ may contribute to the lower TAG content of this strain.
3.4. LRO1 and DGA1 gene expression in mutants bearing defects in phosphatidylethanolamine synthesis
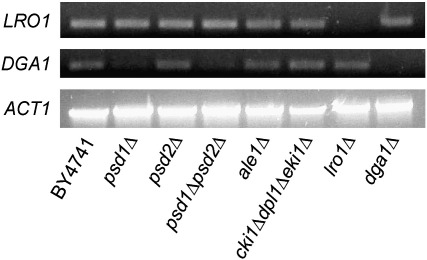
One explanation for the decreased TAG levels (see Fig. 2) in cki1Δdpl1Δeki1Δ may be reduced expression of DGA1 and/or LRO1. Reverse transcription polymerase chain reaction (RT-PCR) was used to analyze the expression levels of these two genes in mutants defective in PE biosynthesis. As can be seen from Fig. 4, expression of LRO1 was not affected in all mutants tested. This result was surprising insofar as Lro1p activity was reduced in cki1Δdpl1Δeki1Δ (see Fig. 3). It has to be taken into account, however, that the activity of this enzyme may be reduced by different effects, e.g., by translational control, post-translational modification or enzyme inhibition. In contrast, expression of DGA1 was dramatically down-regulated in psd1Δ and psd1Δpsd2Δ (Fig. 4). Interestingly, in both strains devoid of PSD1, the enzymatic activity of acyl-CoA dependent TAG formation was only slightly reduced or even increased (see Fig. 3), and TAG levels were similar to wild type (see Fig. 2). Altogether, these results indicate that transcriptional control of TAG synthesizing enzymes appears to have a minor impact, if any, on TAG formation in vivo and does not explain the low level of TAG in cki1Δdpl1Δeki1Δ. Therefore, we tested the effect of subcellular availability of the PDAT substrate PE for TAG synthesis as a possible alternative reason for our findings.
Fig. 4.
Gene expression levels of LRO1, DGA1 and ACT1 from wild type and strains defective in phosphatidylethanolamine and triacylglycerol biosynthesis. Strains listed in the figure were tested by RT-PCR. ACT1 (actin) was used as a loading control and lro1Δ and dga1Δ were used as negative control for LRO1 and DGA1 expression.
3.5. Phospholipid pattern in mutants with defects in phosphatidylethanolamine synthesis
Previous studies from our laboratory had shown that yeast cells require more PE on non-fermentable carbon sources than on fermentable carbon sources, and mitochondrial synthesis of PE by Psd1p becomes paramount [31]. Furthermore, it was concluded that the microsomal PE pool is mainly derived from the CDP-Etn branch of the Kennedy pathway whereas PE formed through Psd2p is preferentially metabolized to PC [29]. Here, we analyzed the cellular and microsomal PE levels of mutants defective in PE biosynthesis when yeast cells were grown on the non-fermentable carbon source lactate. Microsomal fractions were checked for purity by Western Blot (data not shown). Quality of subcellular fractions from all strains tested was identical. Phospholipid analysis revealed a reduction of the cellular PE content in psd1Δ and psd2Δ, and even more pronounced in psd1Δpsd2Δ as an additive effect (Table 3). Notably, the cellular PE level in cki1Δdpl1Δeki1Δ was reduced to 17% of total phospholipids compared to 15% in psd1Δpsd2Δ and 25% in wild type indicating that the CDP-Etn pathway, besides Psd1p, is also an important route for cellular PE formation under these conditions. Analysis of microsomal PE levels from mutants compromised in PE biosynthesis highlighted the importance of the CDP-Etn pathway for the supply of PE to TAG synthesis by Lro1p. The microsomal PE level in cki1Δdpl1Δeki1Δ was dramatically reduced to 12% of total phospholipids compared to 21% in wild type and was even lower than in psd1Δpsd2Δ (16%), whereas deletion of either PSD1 or PSD2 alone had hardly any effect (Table 3). The decrease in cellular and microsomal PE was mainly compensated by elevated levels of PC. Interestingly, an increased level of cellular PE was found in ale1Δ, whereas the microsomal level of PE was similar to wild type. Since Ale1p had been identified as an efficient lyso-PE acyltransferase [26,27] we rather expected a decrease of PE in the deletion strain. The reason for this finding may be that Ale1p has a broad substrate specificity [46–48] and/or other PE forming enzymes might compensate for the deletion of ALE1.
Table 3.
Phospholipid composition of cell-free homogenate and microsomes from cells grown on minimal lactate media supplemented with 5 mM ethanolamine. CF, cellular fraction; H, cell-free homogenate; M, microsomes (100,000 × g); LPL, lysophospholipids; PI, phosphatidylinositol; PS, phosphatidylserine; PE, phosphatidylethanolamine; PC, phosphatidylcholine; CL, cardiolipin; DMPE, dimethylphosphatidylethanolamine; PA, phosphatidic acid. Mean values of at least three measurements and standard deviations are shown.
| Strain | Phospholipids in cell-free homogenate and microsomes (mol%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| CF | LPL | PI | PS | PC | PE | CL | DMPE | PA | |
| Wild type | H | 2.35 ± 1.78 | 11.40 ± 2.65 | 5.09 ± 1.57 | 50.95 ± 4.58 | 24.95 ± 2.35 | 3.73 ± 1.46 | 0.39 ± 0.54 | 1.14 ± 0.95 |
| M | 1.41 ± 0.43 | 9.91 ± 0.82 | 6.30 ± 0.96 | 58.11 ± 2.02 | 20.66 ± 2.23 | 1.45 ± 0.30 | 0.16 ± 0.19 | 2.01 ± 0.67 | |
| psd1Δ | H | 0.73 ± 0.52 | 11.87 ± 2.32 | 6.97 ± 1.17 | 58.83 ± 3.05 | 18.65 ± 1.03 | 2.12 ± 0.60 | 0.24 ± 0.26 | 0.59 ± 0.26 |
| M | 1.72 ± 0.19 | 10.40 ± 2.24 | 6.90 ± 1.37 | 57.23 ± 4.38 | 20.50 ± 1.61 | 1.14 ± 0.24 | 0.67 ± 0.30 | 1.44 ± 0.56 | |
| psd2Δ | H | 0.20 ± 0.34 | 8.55 ± 0.72 | 6.88 ± 2.16 | 59.73 ± 2.92 | 21.44 ± 4.31 | 2.64 ± 1.78 | 0.04 ± 0.07 | 0.52 ± 0.54 |
| M | 1.35 ± 0.13 | 10.56 ± 0.55 | 6.12 ± 0.71 | 57.01 ± 1.64 | 21.10 ± 2.29 | 1.79 ± 0.25 | 0.42 ± 0.16 | 1.65 ± 0.88 | |
| psd1Δpsd2Δ | H | 0.96 ± 0.73 | 5.12 ± 3.27 | 9.01 ± 2.36 | 66.74 ± 5.86 | 14.63 ± 1.05 | 1.97 ± 0.71 | 0.89 ± 1.02 | 0.69 ± 0.47 |
| M | 1.85 ± 0.52 | 8.80 ± 2.23 | 9.71 ± 2.25 | 60.67 ± 3.90 | 16.01 ± 0.43 | 1.19 ± 0.14 | 0.39 ± 0.15 | 1.38 ± 0.40 | |
| ale1Δ | H | 0.52 ± 0.45 | 7.35 ± 3.76 | 6.13 ± 2.66 | 51.71 ± 4.72 | 30.32 ± 2.35 | 2.55 ± 1.01 | 0.38 ± 0.66 | 1.06 ± 0.68 |
| M | 1.61 ± 0.32 | 10.52 ± 1.42 | 5.10 ± 0.36 | 56.94 ± 2.67 | 21.58 ± 2.91 | 1.74 ± 0.33 | 0.65 ± 0.22 | 1.86 ± 0.54 | |
| cki1Δdpl1Δeki1Δ | H | 0.77 ± 0.88 | 11.56 ± 3.66 | 5.34 ± 1.57 | 59.22 ± 4.57 | 16.93 ± 1.12 | 4.40 ± 0.68 | 0.20 ± 0.32 | 1.57 ± 0.58 |
| M | 2.31 ± 1.12 | 10.80 ± 4.15 | 5.14 ± 1.36 | 65.62 ± 5.18 | 12.15 ± 0.24 | 1.67 ± 0.39 | 0.56 ± 0.41 | 1.75 ± 0.98 | |
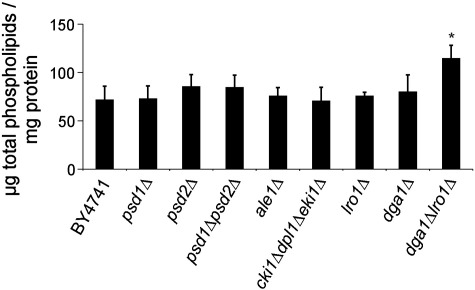
The total cellular amounts of phospholipids (Fig. 5) remained nearly constant in all PE mutants and comparable to wild type. However, the cellular level of total phospholipids was significantly increased in dga1Δlro1Δ. This result can be explained by the utilization of DAG which is not converted to TAG in this strain via CDP-Etn pathway for phospholipid synthesis.
Fig. 5.
Amounts of total phospholipids from wild type and strains defective in phosphatidylethanolamine and triacylglycerol biosynthesis grown to the stationary phase. The amounts of total phospholipids in μg lipid per mg protein were measured in strains as indicated. Data are mean values of 3 independent experiments with error bars indicating the standard deviation. Significance was calculated by Student's t-test (two tailed, unpaired). Values indicated by * correspond to P < 0.05 and were defined to be significant.
4. Discussion
Here, we report a novel physiological link between phospholipid metabolism and TAG formation in the yeast Saccharomyces cerevisiae. At first sight, this finding appears to be not surprising, because both lipid classes recruit their DAG moiety from the same precursor phosphatidic acid. During exponential growth of the yeast, these precursors are rather channeled to phospholipid synthesis than TAG formation for maintaining membrane formation, cell growth and viability. When cells enter the stationary growth phase, phospholipid biosynthesis is decreased and the surplus of DAG and fatty acids are directed towards TAG formation leading to accumulation of this storage lipid in cytosolic LP. Similarities in the fatty acid compositions of TAG and phospholipids, but also the important role of TAG hydrolysis for sustaining the level of major yeast membrane phospholipids, support the idea of a link between TAG and phospholipid metabolism [49,50]. Identification of phospholipid:diacylglycerol acyltransferases (PDATs) refined our understanding of the close relationship between phospholipid and TAG metabolism. Previous studies demonstrated that PE is the preferred acyl-donor for the acyl-CoA independent reaction of TAG biosynthesis catalyzed by Lro1p in yeast and plants [13–15]. Since these data were derived from in vitro studies, the questions as to the relevance of the Lro1p reaction in vivo remained. The usage of PE as a donor of fatty acids for TAG synthesis by Lro1p was of particular interest because this phospholipid is synthesized by four different routes in the yeast (see Introduction). For this purpose, we dissected the relative contributions of the different PE biosynthetic pathways to TAG synthesis using a set of distinct yeast mutants.
To make lipid metabolic conditions for the yeast as stringent as possible, especially with respect to the requirement for PE, we cultivated cells on minimal media containing lactate as a non-fermentable carbon source. We had demonstrated before that on complex media containing lactate Psd1p became paramount as a PE synthesizing enzyme, and even exogenous supply of Etn could not restore the cellular and mitochondrial levels of PE [31]. An unexpected finding, however, was that the CDP-Etn branch of the Kennedy pathway became almost equally important as Psd1p when cells were grown on minimal lactate media (see Table 3) or on complex media containing oleic acid [33]. Thus, culture conditions and especially the use of different carbon sources affect the various PE biosynthetic pathways. Notably, the growth characteristics of psd1Δ and cki1Δdpl1Δeki1Δ on lactate media are different. Whereas the mutant bearing defects in the CDP-Etn branch of PE synthesis can grow very well on minimal lactate media, psd1Δ requires supplementation with Etn (see Fig. 1). The reason for this growth defect might be the specific depletion of mitochondrial PE in the psd1Δ mutant strain.
The second striking effect observed with cki1Δdpl1Δeki1Δ cultivated under the given conditions was the dramatically decreased TAG level (see Fig. 2). This effect was unique among all mutant strains compromised in PE formation and surprising. We had initially speculated that lack of competition for the cellular DAG pool would lead to an increase of TAG in cki1Δdpl1Δeki1Δ. This assumption was in line with results obtained with mice where elimination of the CDP-Etn pathway indeed led to an increase of neutral lipid classes [51,52]. The phosphoethanolamine cytidylyltransferase (ECT)-deficient liver/hepatocytes of these mice displayed 10-fold elevated levels of TAG whereas cholesterol, cholesterol esters, DAG and free fatty acids were about 2-fold higher than controls. Our data, however, demonstrated a profound decrease of cellular TAG when PE formation via the CDP-Etn pathway was blunted. This discrepancy between mammalian cells and the yeast is most likely due to different efficiency of PE supply by the various PE biosynthetic pathways. Whereas in the yeast Psd1p is the major enzyme of cellular PE formation [29,31], in mammalian cells the major route of PE biosynthesis strongly depends on the type of tissue and cell [53]. It was reported that in several cell lines such as CHO cells PE was predominantly supplied by PS decarboxylation, whereas in rat liver/hepatocytes and hamster heart the CDP-Etn pathway was the major route of PE synthesis. The other marked difference is the existence of the PDAT Lro1p in yeast which has no counterpart in mammalian cells [9,12–14].
The third striking observation was that the total cellular level of PE is not crucial for TAG synthesis in the yeast. This can be clearly seen from the lipid profiles of mutant strains (see Table 3 and Fig. 2). While in psd1∆psd2∆ and in cki1∆dpl1∆eki1∆ cellular levels of PE were comparably low, the TAG level was dramatically affected only in cki1∆dpl1∆eki1∆. We assume that subcellular localization of the enzymes of the CDP-Etn pathway and Lro1p in the ER is more relevant for the observed effect than the total amount of PE in cells. This view is confirmed by the strongly decreased microsomal level of PE and the significant reduction of Lro1p activity in cki1∆dpl1∆eki1∆ which is associated with profound reduction of TAG (see Table 3 and Fig. 3).
In summary, the CDP-Etn branch of the Kennedy pathway appears to fulfill two tasks in the yeast. First, it supplies mitochondria with PE which is underlined by the observation that the growth defect of psd1∆ can be rescued by Etn (see Fig. 1). Secondly, the CDP-Etn branch appears to support TAG formation through the acyl-CoA independent pathway catalyzed by Lro1p. The dramatically reduced TAG level in cki1∆dpl1∆eki1∆ (see Fig. 2), the reduced Lro1p activity measured in vitro (see Fig. 3) and the low cellular and microsomal PE content (see Table 3) in this strain support this hypothesis. In summary, we demonstrate that both, subcellular localization of enzymes involved in this process in the ER and availability of PE within this compartment play an important role in the network of phospholipid and TAG metabolism.
Acknowledgements
We thank M. Connerth and R. Nebauer for providing psd1Δ and cki1Δdpl1Δeki1Δ mutant strains. This work was financially supported by the Fonds zur Förderung der wissenschaftlichen Forschung in Österreich (DK Molecular Enzymology W901-B05 and project P21429 to G. D.).
References
- 1.Zweytick D., Athenstaedt K., Daum G. Intracellular lipid particles of eukaryotic cells. Biochim. Biophys. Acta. 2000;1469:101–120. doi: 10.1016/s0005-2736(00)00294-7. [DOI] [PubMed] [Google Scholar]
- 2.Athenstaedt K., Zweytick D., Jandrositz A., Kohlwein S.D., Daum G. Identification and characterization of major lipid particle proteins of the yeast Saccharomyces cerevisiae. J. Bacteriol. 1999;181:6441–6448. doi: 10.1128/jb.181.20.6441-6448.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Czabany T., Wagner A., Zweytick D., Lohner K., Leitner E., Ingolic E., Daum G. Structural and biochemical properties of lipid particles from the yeast Saccharomyces cerevisiae. J. Biol. Chem. 2008;283:17065–17074. doi: 10.1074/jbc.M800401200. [DOI] [PubMed] [Google Scholar]
- 4.Walther T.C., Farese R.V., Jr. The life of lipid droplets. Biochim. Biophys. Acta. 2009;1791:459–466. doi: 10.1016/j.bbalip.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zweytick D., Leitner E., Kohlwein S.D., Yu C., Rothblatt J., Daum G. Contribution of Are1p and Are2p to steryl ester synthesis in the yeast Saccharomyces cerevisiae. Eur. J. Biochem. 2000;267:1075–1082. doi: 10.1046/j.1432-1327.2000.01103.x. [DOI] [PubMed] [Google Scholar]
- 6.Athenstaedt K., Daum G. Biosynthesis of phosphatidic acid in lipid particles and endoplasmic reticulum of Saccharomyces cerevisiae. J. Bacteriol. 1997;179:7611–7616. doi: 10.1128/jb.179.24.7611-7616.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leber R., Landl K., Zinser E., Ahorn H., Spök A., Kohlwein S.D., Turnowsky F., Daum G. Dual localization of squalene epoxidase, Erg1p, in yeast reflects a relationship between the endoplasmic reticulum and lipid particles. Mol. Biol. Cell. 1998;9:375–386. doi: 10.1091/mbc.9.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorger D., Athenstaedt K., Hrastnik C., Daum G. A yeast strain lacking lipid particles bears a defect in ergosterol formation. J. Biol. Chem. 2004;279:31190–31196. doi: 10.1074/jbc.M403251200. [DOI] [PubMed] [Google Scholar]
- 9.Sandager L., Gustavsson M.H., Ståhl U., Dahlqvist A., Wiberg E., Banas A., Lenman M., Ronne H., Stymne S. Storage lipid synthesis is non-essential in yeast. J. Biol. Chem. 2002;277:6478–6482. doi: 10.1074/jbc.M109109200. [DOI] [PubMed] [Google Scholar]
- 10.Oelkers P., Cromley D., Padamsee M., Billheimer J.T., Sturley S.L. The DGA1 gene determines a second triglyceride synthetic pathway in yeast. J. Biol. Chem. 2002;277:8877–8881. doi: 10.1074/jbc.M111646200. [DOI] [PubMed] [Google Scholar]
- 11.Sorger D., Daum G. Synthesis of triacylglycerols by the acyl-coenzyme A:diacyl-glycerol acyltransferase Dga1p in lipid particles of the yeast Saccharomyces cerevisiae. J. Bacteriol. 2002;184:519–524. doi: 10.1128/JB.184.2.519-524.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oelkers P., Tinkelenberg A., Erdeniz N., Cromley D., Billheimer J.T., Sturley S.L. A lecithin cholesterol acyltransferase-like gene mediates diacylglycerol esterification in yeast. J. Biol. Chem. 2000;275:15609–15612. doi: 10.1074/jbc.C000144200. [DOI] [PubMed] [Google Scholar]
- 13.Dahlqvist A., Ståhl U., Lenman M., Banas A., Lee M., Sandager L., Ronne H., Stymne S. Phospholipid:diacylglycerol acyltransferase: an enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc. Natl. Acad. Sci. U. S. A. 2000;97:6487–6492. doi: 10.1073/pnas.120067297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosal A., Banas A., Ståhl U., Dahlqvist A., Lindqvist Y., Stymne S. Saccharomyces cerevisiae phospholipid:diacylglycerol acyl transferase (PDAT) devoid of its membrane anchor region is a soluble and active enzyme retaining its substrate specificities. Biochim. Biophys. Acta. 2007;1771:1457–1463. doi: 10.1016/j.bbalip.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Ståhl U., Carlsson A.S., Lenman M., Dahlqvist A., Huang B., Banaś W., Banaś A., Stymne S. Cloning and functional characterization of a phospholipid:diacylglycerol acyltransferase from Arabidopsis. Plant Physiol. 2004;135:1324–1335. doi: 10.1104/pp.104.044354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daum G., Lees N.D., Bard M., Dickson R. Biochemistry, cell biology and molecular biology of lipids of Saccharomyces cerevisiae. Yeast. 1998;14:1471–1510. doi: 10.1002/(SICI)1097-0061(199812)14:16<1471::AID-YEA353>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 17.Kuchler K., Daum G., Paltauf F. Subcellular and submitochondrial localization of phospholipid-synthesizing enzymes in Saccharomyces cerevisiae. J. Bacteriol. 1986;165:901–910. doi: 10.1128/jb.165.3.901-910.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trotter P.J., Pedretti J., Voelker D.R. Phosphatidylserine decarboxylase from Saccharomyces cerevisiae. Isolation of mutants, cloning of the gene, and creation of a null allele. J. Biol. Chem. 1993;268:21416–21424. [PubMed] [Google Scholar]
- 19.Trotter P.J., Pedretti J., Yates R., Voelker D.R. Phosphatidylserine decarboxylase 2 of Saccharomyces cerevisiae. Cloning and mapping of the gene, heterologous expression, and creation of the null allele. J. Biol. Chem. 1995;270:6071–6080. doi: 10.1074/jbc.270.11.6071. [DOI] [PubMed] [Google Scholar]
- 20.Trotter P.J., Voelker D.R. Identification of a non-mitochondrial phosphatidylserine decarboxylase activity (PSD2) in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 1995;270:6062–6070. doi: 10.1074/jbc.270.11.6062. [DOI] [PubMed] [Google Scholar]
- 21.Zinser E., Sperka-Gottlieb C.D.M., Fasch E.V., Kohlwein S.D., Paltauf F., Daum G. Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote Saccharomyces cerevisiae. J. Bacteriol. 1991;173:2026–2034. doi: 10.1128/jb.173.6.2026-2034.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kennedy E.P., Weiss S.B. The function of cytidine coenzymes in the biosynthesis of phospholipids. J. Biol. Chem. 1956;222:193–214. [PubMed] [Google Scholar]
- 23.Kim K., Kim K.H., Storey M.K., Voelker D.R., Carman G.M. Isolation and characterization of the Saccharomyces cerevisiae EKI1 gene encoding ethanolamine kinase. J. Biol. Chem. 1999;274:14857–14866. doi: 10.1074/jbc.274.21.14857. [DOI] [PubMed] [Google Scholar]
- 24.Saba J.D., Nara F., Bielawska A., Garrett S., Hannun Y.A. The BST1 gene of Saccharomyces cerevisiae is the sphingosine-1-phosphate lyase. J. Biol. Chem. 1997;272:26087–26090. doi: 10.1074/jbc.272.42.26087. [DOI] [PubMed] [Google Scholar]
- 25.Mandala S.M., Thornton R., Tu Z., Kurtz M.B., Nickels J., Broach J., Menzeleev R., Spiegel S. Sphingoid base 1-phosphate phosphatase: a key regulator of sphingolipid metabolism and stress response. Proc. Natl. Acad. Sci. U. S. A. 1998;95:150–155. doi: 10.1073/pnas.95.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riekhof W.R., Voelker D.R. Uptake and utilization of lyso-phosphatidylethanolamine by Saccharomyces cerevisiae. J. Biol. Chem. 2006;281:36588–36596. doi: 10.1074/jbc.M608851200. [DOI] [PubMed] [Google Scholar]
- 27.Riekhof W.R., Wu J., Jones J.L., Voelker D.R. Identification and characterization of the major lysophosphatidylethanolamine acyltransferase in Saccharomyces cerevisiae. J. Biol. Chem. 2007;282:28344–28352. doi: 10.1074/jbc.M705256200. [DOI] [PubMed] [Google Scholar]
- 28.Rajakumari S., Daum G. Janus-faced enzymes yeast Tgl3p and Tgl5p catalyze lipase and acyltransferase reactions. Mol. Biol. Cell. 2010;21:501–510. doi: 10.1091/mbc.E09-09-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bürgermeister M., Birner-Grünberger R., Nebauer R., Daum G. Contribution of different pathways to the supply of phosphatidylethanolamine and phosphatidylcholine to mitochondrial membranes of the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta. 2004;1686:161–168. doi: 10.1016/j.bbalip.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Bürgermeister M., Birner-Grünberger R., Heyn M., Daum G. Contribution of different biosynthetic pathways to species selectivity of aminoglycerophospholipids assembled into mitochondrial membranes of the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta. 2004;1686:148–160. doi: 10.1016/j.bbalip.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Birner R., Bürgermeister M., Schneiter R., Daum G. Roles of phosphatidylethanolamine and of its several biosynthetic pathways in Saccharomyces cerevisiae. Mol. Biol. Cell. 2001;12:997–1007. doi: 10.1091/mbc.12.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuiki I., Schnabl M., Czabany T., Hrastnik C., Daum G. Phosphatidylethanolamine synthesized by four different pathways is supplied to the plasma membrane of the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta. 2010;1801:480–486. doi: 10.1016/j.bbalip.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberger R., Connerth M., Zellnig G., Daum G. Phosphatidylethanolamine synthesized by three different pathways is supplied to peroxisomes of the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta. 2009;1791:379–387. doi: 10.1016/j.bbalip.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 34.Longtine M.S., McKenzie A., III, Demarini D.J., Shah N.G., Wach A., Brachat A., Philippsen P., Pringle J.R. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 35.Wach A., Brachat A., Alberti-Segui C., Rebischung C., Philippsen P. Heterologous HIS3 marker and GFP reporter modules for PCR-targeting in Saccharomyces cerevisiae. Yeast. 1997;13:1065–1075. doi: 10.1002/(SICI)1097-0061(19970915)13:11<1065::AID-YEA159>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 36.Wach A., Brachat A., Pöhlmann R., Philippsen P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- 37.Gietz D., St. Jean A., Woods R.A., Schiestl R.H. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992;20:1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wach A. PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in S. cerevisiae. Yeast. 1996;12:259–265. doi: 10.1002/(SICI)1097-0061(19960315)12:3%3C259::AID-YEA901%3E3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 39.Zinser E., Daum G. Isolation and biochemical characterization of organelles from the yeast, Saccharomyces cerevisiae. Yeast. 1995;11:493–536. doi: 10.1002/yea.320110602. [DOI] [PubMed] [Google Scholar]
- 40.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 41.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 42.Haid A., Suissa M. Immunochemical identification of membrane proteins after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Methods Enzymol. 1983;96:192–205. doi: 10.1016/s0076-6879(83)96017-2. [DOI] [PubMed] [Google Scholar]
- 43.Folch J., Lees M., Sloane Stanley G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 44.Broekhuyse R.M. Phospholipids in tissues of the eye. I. Isolation, characterization and quantitative analysis by two-dimensional thin-layer chromatography of diacyl and vinyl-ether phospholipids. Biochim. Biophys. Acta. 1968;152:307–315. doi: 10.1016/0005-2760(68)90038-6. [DOI] [PubMed] [Google Scholar]
- 45.Birner R., Nebauer R., Schneiter R., Daum G. Synthetic lethal interaction of the mitochondrial phosphatidylethanolamine biosynthetic machinery with the prohibitin complex of Saccharomyces cerevisiae. Mol. Biol. Cell. 2003;14:370–383. doi: 10.1091/mbc.E02-05-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ståhl U., Stålberg K., Stymne S., Ronne H. A family of eukaryotic lysophospholipid acyltransferases with broad specificity. FEBS Lett. 2008;582:305–309. doi: 10.1016/j.febslet.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 47.Jain S., Stanford N., Bhagwat N., Seiler B., Constanzo M., Boone C., Oelkers P. Identification of a novel lysophospholipid acyltransferase in Saccharomyces cerevisiae. J. Biol. Chem. 2007;282:30562–30569. doi: 10.1074/jbc.M706326200. [DOI] [PubMed] [Google Scholar]
- 48.Tamaki H., Shimada A., Ito Y., Ohya M., Takase J., Miyashita M., Miyagawa H., Nozaki H., Nakayama R., Kumagai H. LPT1 encodes a membrane-bound O-acyltransferase involved in the acylation of lysophospholipids in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 2007;282:34288–34298. doi: 10.1074/jbc.M704509200. [DOI] [PubMed] [Google Scholar]
- 49.Taylor F.R., Parks L.W. Triaglycerol metabolism in Saccharomyces cerevisiae relation to phospholipid synthesis. Biochim. Biophys. Acta. 1979;575:204–214. doi: 10.1016/0005-2760(79)90022-5. [DOI] [PubMed] [Google Scholar]
- 50.Rajakumari S., Rajasekharan R., Daum G. Triacylglycerol lipolysis is linked to sphingolipid and phospholipid metabolism of the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta. 2010;1801:1314–1322. doi: 10.1016/j.bbalip.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 51.Leonardi R., Frank M.W., Jackson P.D., Rock C.O., Jackowski S. Elimination of the CDP-ethanolamine Pathway Disrupts Hepatic Lipid Homeostasis. J. Biol. Chem. 2009;284:27077–27089. doi: 10.1074/jbc.M109.031336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fullerton M.D., Hakimuddin F., Bonen A., Bakovic M. The development of a metabolic disease phenotype in CTP:phosphoethanolamine cytidylyltransferase-deficient mice. J. Biol. Chem. 2009;284:25704–25713. doi: 10.1074/jbc.M109.023846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vance J.E., Vance D.E. Phospholipid biosynthesis in mammalian cells. Biochem. Cell Biol. 2004;82:113–128. doi: 10.1139/o03-073. [DOI] [PubMed] [Google Scholar]