Abstract
In the yeast Saccharomyces cerevisiae as in other eukaryotes non-polar lipids are a reservoir of energy and building blocks for membrane lipid synthesis. The yeast non-polar lipids, triacylglycerols (TG) and steryl esters (SE) are stored in so-called lipid particles/droplets (LP) as biologically inert form of fatty acids and sterols. To understand LP structure and function in more detail we investigated the molecular equipment of this compartment making use of mass spectrometric analysis of lipids (TG, SE, phospholipids) and proteins. We addressed the question whether or not lipid and protein composition of LP influence each other and performed analyses of LP from cells grown on two different carbon sources, glucose and oleate. Growth of cells on oleate caused dramatic cellular changes including accumulation of TG at the expense of SE, enhanced the amount of glycerophospholipids and strongly increased the degree of unsaturation in all lipid classes. Most interestingly, oleate as a carbon source led to adaptation of the LP proteome resulting in the appearance of several novel LP proteins. Localization of these new LP proteins was confirmed by cell fractionation. Proteomes of LP variants from cells grown on glucose or oleate, respectively, were compared and are discussed with emphasis on the different groups of proteins detected through this analysis. In summary, we demonstrate flexibility of the yeast LP lipidome and proteome and the ability of LP to adapt to environmental changes.
Abbreviations: TG, triacylglycerols; SE, steryl esters; LP, lipid particles; PA, phosphatidic acid; PI, phosphatidylinositol; PS, phosphatidylserine; PC, phosphatidylcholine; PE, phosphatidylethanolamine; CL, cardiolipin; LP, lysophospholipids; DMPE, dimethyl-PE
Keywords: Triacylglycerol, Phospholipid, Mass spectrometry, Protein, Lipid particle/droplet, Yeast
Highlights
► Molecular analysis of yeast lipid particles/droplets components. ► Combination of lipidome and proteome analysis. ► Effect of oleate stress on lipid particle lipidome and proteome. ► High flexibility of lipid particles on environmental conditions.
1. Introduction
During the last decades, a strong increase of adipositas and obesity mainly related to unbalanced diet has led to intense studies of lipid uptake, biosynthesis, storage and mobilization. Lipid metabolism and especially lipid storage are inevitably linked to an organelle, the so-called lipid particle (LP), lipid droplet or oil body. The baker's yeast Saccharomyces cerevisiae has been used for a long time as a model for LP research addressing structural, functional and metabolic aspects of this compartment [1–4]. One characteristic structural feature of LP is the surface monolayer of phospholipids which protects the highly hydrophobic core of the droplet from the cellular environment [5]. The polar head groups of these phospholipids face the cytosol, and the hydrophobic side chains (fatty acids) associate with the non-polar interior of LP. The hydrophobic core representing more than 95 mass% of LP consists of triacylglycerols (TG) and steryl esters (SE) which lack charged groups and are therefore not suited as constituents of membrane bilayers. As shown recently in our laboratory, SE of LP form several ordered shells below the phospholipid monolayer, whereas TG are more or less randomly packed in the center of the droplet [4].
The surface phospholipid monolayer membrane of LP contains a small but specific set of proteins embedded. Prominent proteins of mammalian LP are perilipin, adipophilin, CGI-58 (comparative gene identification-58), hormone-sensitive lipase (HSL), adipose TG lipase (ATGL), TIP47 (Tail-Interacting Protein 47 kDa), PAT-proteins, S3-12 and OXPAT [6–13]. A functional link of mammalian lipid droplets to the spliceosome and proteasome was also suggested [14,15]. The most prominent proteins of plant oil droplets are the oleosins, which cover the surface of the droplet and prevent them from coalescence [16]. In yeast LP, homologues of perilipins or oleosines have not been detected. However, a number of proteins involved in lipid metabolism were found to be characteristic for this yeast organelle [17]. Several proteins of the yeast LP could be assigned to functional groups such as phosphatidic acid biosynthesis, fatty acid activation, TG and SE metabolism and sterol synthesis. For distinct targeting of proteins to mammalian lipid droplets or plant oil bodies well defined hydrophobic domains are required, that are either sufficient by themselves [18,19], require the presence of a proline knot motif (PKM), e.g. with oleosins [20,21], or contain polar sequences which flank the hydrophobic stretches [22]. In the yeast, hydrophobic regions of the C-terminus were found to have some influence on the correct targeting of LP proteins [23].
Besides storing lipids as source of chemical energy and building blocks of biological membranes, yeast LP were assumed to be important players in lipid homeostasis [24]. However, the mechanism by which proteins are targeted to and associated with the LP surface is still a matter of dispute. A favored model suggests that TG and/or SE synthesizing enzymes form large amounts of non-polar lipids between the two leaflets of the ER which finally results in budding of nascent LP [25–28]. Alternative mechanisms of LP biogenesis have also been discussed [29] but all of them agree on the origin of LP from the ER.
The composition of LP strongly depends on the carbon source used for cell cultivation [30]. A helpful tool to make yeast cells “obese” is growth on oleate. Under these conditions, peroxisome proliferation is induced because this organelle is the only subcellular fraction of S. cerevisiae where β-oxidation of fatty acids occurs [31]. Yeast cells grown on oleate also accumulate large amounts of non-polar lipids with oleic acid as a major constituent resulting in massive formation of LP [32,33]. Moreover, fatty acids may act as signaling molecules in gene expression. As an example, oleic acid binds to upstream promoter elements with the oleate response element (ORE) being the most prominent target [34,35].
The aim of the present study was to re-investigate the molecular equipment of yeast LP and to extend previous proteome and lipid analyses from our laboratory [17]. Highly improved and sophisticated methods of mass spectrometry were employed to address this question. Variation of the carbon source (glucose vs. oleate) used for cultivation of yeast cells allowed us to isolate LP variants which strongly differed in their lipid composition. We also show that these changes of the lipid profile affect the LP proteome in an adaptive process. Finally, our analysis led to the identification of some novel LP proteins which will be described in this study in some detail.
2. Methods and materials
2.1. Yeast strains and culture conditions
Yeast strains used in this study are listed in Table 1. Cells were grown at 30 °C in rich medium containing 1% yeast extract, 2% peptone and 2% glucose (YPD); or 0.3% yeast extract, 0.5% peptone, 0.1% glucose, 0.5% KH2PO4 and 0.1% oleic acid (YPO). For solubilizing fatty acids in YPO 0.2% Tween 80 was added to the media. Oleic acid (grade pure, Merck, Darmstadt, Germany) used routinely as carbon source contained impurities of myristic acid (≤ 5.0%), palmitic acid (≤ 16.0%), palmitoleic acid (≤ 8.0%), margaric acid (≤ 0.2%), stearic acid (6.0%), linoleic acid (≤ 18.0%), linolenic acid (≤ 4.0%) and fatty acids of chain length longer than C18 (≤ 4.0%).
Table 1.
Saccharomyces cerevisiae strains used in this study.
| Strain | Relevant genotype | Source |
|---|---|---|
| BY4741 | Mata lys2Δ0 leu2 Δ0 ura3 Δ0 his3Δ1 | EUROSCARF |
| ATCC 201388 (OSH4-GFP) | Mata his3Δ1 leu2 Δ0 met15Δ0 ura3Δ0 OSH4-GFP::HIS3MX | INVITROGEN |
| ATCC 201388 (VPS66-GFP) | Mata his3Δ1 leu2 Δ0 met15Δ0 ura3Δ0 VPS66-GFP::HIS3MX | INVITROGEN |
| ATCC 201388 (CPR5-GFP) | Mata his3Δ1 leu2 Δ0 met15Δ0 ura3Δ0 CPR5-GFP::HIS3MX | INVITROGEN |
| ATCC 201388 (GTT1-GFP) | Mata his3Δ1 leu2 Δ0 met15Δ0 ura3Δ0 GTT1-GFP::HIS3MX | INVITROGEN |
| ATCC 201388 (UBX2-GFP) | Mata his3Δ1 leu2 Δ0 met15Δ0 ura3Δ0 UBX2-GFP::HIS3MX | INVITROGEN |
| ATCC 201388 (YPT7-GFP) | Mata his3Δ1 leu2 Δ0 met15Δ0 ura3Δ0 YPT7-GFP::HIS3MX | INVITROGEN |
| ATCC 201388 (PDI1-GFP) | Mata his3Δ1 leu2 Δ0 met15Δ0 ura3Δ0 PDI1-GFP::HIS3MX | INVITROGEN |
Pre-cultures were grown for 48 h at 30 °C on YPD. Then, YPD or YPO, respectively, were inoculated to a starting OD600 of 0.1, and cells were grown at 30 °C until they reached the stationary phase, i.e. 24 h for both conditions. At this time point cells were harvested for organelle preparation.
2.2. Subcellular fractionation of yeast cells
Subcellular fractions of yeast cells were prepared by published procedures [2,36]. In brief, cells were grown to the stationary phase, harvested by centrifugation and converted to spheroplasts using zymolyase 20 T (Seikagaku Corporation, Japan). For the isolation of LP, spheroplasts were homogenized in buffer LP-A (12% Ficoll, 10 mM MES/Tris pH 6.9, 0.2 mM EDTA, 1 mM PMSF) with 30 strokes in a Dounce Homogenizer. The suspension was centrifuged at 7,000 rpm for 5 min, the supernatant was collected, and the pellet was re-homogenized and re-centrifuged as described above. Combined homogenates were overlaid with buffer LP-A in an Ultra-Clear Centrifuge Tube (Beckman) and centrifuged at 28,000 rpm for 45 min using a swing out rotor AH-629. The resulting layer on top containing LP was used for loading a new ultracentrifuge tube. The sample was overlaid with buffer LP-B (8% Ficoll, 10 mM MES/Tris pH 6.9, 0.2 mM EDTA, 1 mM PMSF), and after ultracentrifugation at 28,000 rpm for 30 min the floating layer on top was collected. Prior to the last ultracentrifugation step, buffer LP-D (0.25 M sorbitol, 10 mM MES/Tris pH 6.9, 0.2 mM EDTA) was filled into a fresh ultracentrifuge tube, and the homogenized sample was loaded to the bottom of the tube. Ultracentrifugation as described before led to a top layer consisting of highly purified LP. The pellet fraction from the last centrifugation step was collected as well.
For the isolation of mitochondria, microsomes, peroxisomes and cytosol spheroplasts were resuspended in breaking buffer (5 mM MES pH 6.0, 1 mM KCl, 0.5 mM EDTA, 0.6 M sorbitol, 1 mM PMSF) and homogenized by 15 strokes in a Dounce Homogenizer. Homogenates were centrifuged at 5,500 rpm for 5 min. Supernatants were collected and the pellet was re-homogenized and re-centrifuged. Combined supernatants were centrifuged at 13,000 rpm for 30 min. The resulting supernatants containing cytosol and microsomes were well separated from pellets consisting of mitochondria and peroxisomes. The homogenized pellet was loaded onto a sucrose step gradient (35/24/17% sucrose) and centrifuged in a Sorvall AH-629 swing out rotor at 26,000 rpm for 90 min. Mitochondria (upper) and peroxisomes (lower) containing layers were collected from the gradient, and organelles were sedimented at 15,000 rpm for 20 min. Supernatants obtained in the previous step were centrifuged at 18,000 rpm yielding a fraction which contains 30,000 and 40,000 g microsomes. The remaining supernatant was transferred to a T-865 rotor and centrifuged at 45,000 rpm for 45 min. The resulting supernatant was regarded as the cytosolic fraction.
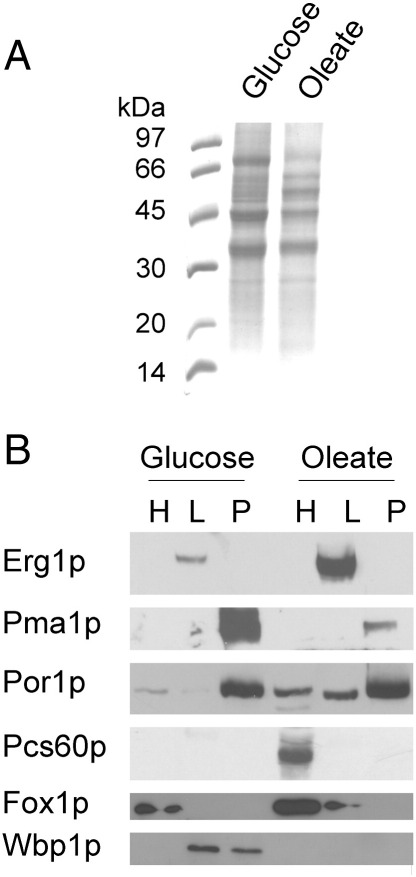
Isolated fractions were routinely tested by Western blot analysis [37] using rabbit antibodies against Erg1p, Por1p, Pma1p, Pcs60p, Fox1p and Wbp1p. Peroxidase conjugated secondary antibody and enhanced chemiluminescent signal detection reagents (SuperSignal™, Pierce Chemical Company, Rockford, IL, USA) were used to visualize immunoreactive bands.
2.3. Protein analysis
Proteins from isolated LP and homogenates were precipitated with trichloroacetic acid (TCA) at a final concentration of 10%. Proteins were quantified by the method of Lowry et al. [38] using bovine serum albumin as standard. Prior to protein analysis, samples of LP were delipidated. For this purpose, non-polar lipids were extracted with two volumes of diethyl ether, the organic phase was withdrawn, residual diethyl ether was removed under a stream of nitrogen, and proteins were analyzed as described above. SDS-PAGE (sodium dodecylsulfate polyacrylamide gel electrophoresis) was carried out as described by Laemmli [39] using 12.5% separation gels.
2.4. Lipid analysis
Lipids from yeast cells grown on YPD or YPO, respectively, were extracted as described by Folch et al. [40]. For quantification of non-polar lipids, extracts were applied to Silica Gel 60 plates (Merck, Darmstadt, Germany) with the aid of a sample applicator (CAMAG, Automatic TLC Sampler 4), and chromatograms were developed in an ascending manner by using the solvent system light petroleum/diethyl ether/acetic acid (25:25:1; per vol.) for the first third of the distance. Then, plates were briefly dried and further developed to the top of the plate using the solvent system light petroleum/diethyl ether (49:1; v/v). Quantification of ergosteryl esters was carried out by densitometric scanning at 275 nm with a Shimadzu dual wavelength chromatoscanner CS-930 using ergosterol as standard. To further analyze TG, TLC plates were dipped into a charring solution consisting of 0.63 g of MnCl2·4H2O, 60 ml of water, 60 ml of methanol, and 4 ml of concentrated sulfuric acid, briefly dried and heated at 100 °C for 30 min. Then, lipids were quantified by densitometric scanning at 400 nm using a Shimadzu dual wavelength chromatoscanner CS-930 with triolein as standard.
For phospholipid analysis, lipid extracts from homogenate and LP were loaded manually onto silica gel 60 plates. Individual phospholipids were separated by two dimensional TLC using chloroform/methanol/25% NH3 (65:35:5; per vol.) as first, and chloroform/acetone/methanol/acetic acid/water (50:20:10:10:5; per vol.) as second dimension solvent. Lipids were stained with iodine vapor, scraped off the plate and quantified by the method of Broekhyuse [41]. This method was also used for quantification of total phospholipids obtained as separate bands from neutral lipid analysis (see above).
2.5. Mass spectrometry of neutral lipids and phospholipids
Lipid extracts were prepared as described above and diluted 1:100 in acetonitrile (ACN)/2-propanol (5:2; v/v), 1% ammonium acetate, 0.1% formic acid. As internal standards 5 nmol TG (species 17:0/17:0/17:0) and PC (species 12:0/12:0) were added. For chromatographic separation a Thermo Hypersil GOLD C18 column (100 × 1 mm, 1.9 mm) was used employing solvent A (water with 1% ammonium acetate, 0.1% formic acid) and solvent B (acetonitrile/2-propanol (5:2; v/v); 1% ammonium acetate; 0.1% formic acid). The gradient changed from 35 to 70% B in 4 min and then to 100% B in another step of 16 min. These conditions were held for 10 min with a flow rate of 250 μl/min. Mass spectrometry was performed by HPLC direct coupling to a FT-ICR-MS hybrid mass spectrometer (LTQ-FT, Thermo Scientific) equipped with an IonMax ESI source. The mass spectrometer was operated at a mass accuracy of < 2 ppm with external calibration and a resolution of 200,000 full width at half height (FWHH) at 400 m/z. The spray voltage was set to 5,000 V, capillary voltage to 35 V, and the tube lens was at 120 V. The capillary temperature was at 250 °C. Peak areas are calculated by QuanBrowser for all lipid species identified previously by exact mass (< 2 ppm) and retention time. Calculated peak areas for each species were expressed as percent of the sum of all peak areas in the respective lipid class.
2.6. Mass spectrometry of proteins
After TCA precipitation, 100 μg of protein pellets were dissolved in 100 μl of 25 mM NH4HCO3 in an Eppendorf tube. Disulfide bridges were reduced in the presence of 45 mM dithiothreitol (DTT) for 1 h at 60 °C and 400 rpm shaking in a Thermomixer comfort (Eppendorf). The solution was then allowed to cool to room temperature, and cysteine residues were alkylated in the presence of 100 mM iodoacetamide for 45 min in the dark at room temperature. To avoid subsequent alkylation of trypsin, the reaction was quenched after 45 min by adding 12.5 μl 45 mM DTT and incubating for another 45 min at room temperature. Then, trypsin was added to the reduced and alkylated samples to obtain a protein to enzyme ratio of 1:50 (w/w). The solution was incubated overnight at 37 °C. The digestion was stopped by addition of 1 μl of a 10% trifluoroacetic acid (TFA) solution.
Prior to nano-liquid chromatographic (nLC) separation, samples were vacuum concentrated to approximately 8 μl and solvent A (8% ACN, 0.1% TFA) was added to a final volume of 15 μl. Separation was performed on a Proxeon Biosystems EASY-nLC™ system (Odense, Denmark) coupled to a SunCollect MALDI spotting device (Sunchrom, Friedrichsdorf, Germany). Peptides were loaded onto an in-house packed 100 μm × 30 mm pre-column (Waters X-Bridge™ BEH 180 C18 300 Å 3.5 μm), desalted with 30 μl solvent A for 15 min and separated on an in-house packed 100 μm × 150 mm column (Waters X-Bridge™ BEH 180 C18 300 Å 3.5 μm) at a flow rate of 400 nl/min. The gradient profile linearly increased from 8 to 45% solvent B (92% ACN, 0.1% TFA) within 100 min, to 90% B within 20 min, 10 min at 90% B, back to 8% B within 5 min and stayed at 8% for another 5 min. The eluent from the LC was pre-mixed with matrix, α-cyano-4-hydroxycinnamic acid, from an auxiliary pump (flow rate, 1.2 μl/min) and spotted every 20 s on a blank 123 × 81 mm Opti-TOF™ LC/MALDI Insert metal target. This matrix solution contained 3.5 mg/ml α-cyano-4-hydroxycinnamic acid (Bruker Daltonics, Bremen, Germany) dissolved in 70% ACN/0.1% TFA, spiked with 60 fmol [Glu1]-Fibrinopeptide B (Bachem, Weil, Germany) for internal calibration.
Mass spectra were acquired using an Applied Biosystems/MDS Sciex 4800 TOF/TOF™ Analyzer (AB SCIEX, Darmstadt, Germany). The instrument is equipped with a Nd:YAG laser, emitting at 355 nm at a frequency of 200 Hz. All spectra were acquired in the positive reflector mode between 700 and 4,500 m/z with fixed laser intensity. A total of 750 laser shots per spot were accumulated. An 8-point plate model external calibration was performed using the Sequazyme™ Peptide Mass Standards Kit (AB Sciex). Fragmentation was performed with collision energy of 1 kV using air as collision gas. To reduce sample consumption during measurement, stop conditions for MS/MS were defined. A minimum number of 15 fragment ions above 45 S/N with at least 12 accumulated sub-spectra; a minimum of 1,250 and maximum of 2,500 laser shots were recorded. To avoid unnecessary multiple selections of identical precursor, MS/MS precursor selection was carried out via the instrument's software job-wide interpretation. A total of 6 precursors per spot with a minimum signal-to-noise-ratio of 80 were selected for fragmentation. Potential matrix signals were removed from precursor selection by excluding potential matrix clusters between 700 and 1,400 m/z (decimal values 0.030 ± 0.1 m/z) as well as the internal calibrants.
Mascot Generic Format (MGF) files were retrieved from each MALDI MS/MS spectra using the built-in Peaks2Mascot feature, exporting up to 65 peaks per MS/MS spectrum, each requiring a minimum signal-to-noise of 5. The MGF files were processed using the Mascot™ database search engine v2.2.03 (Matrix Science Ltd., UK). For the mascot search parameter, trypsin was set as the proteolytic enzyme and a total of 3 miscleavages were allowed. In addition, carboxymethylation of cysteine and oxidation of methionine were set as fixed and variable modification, respectively. MS precursor mass tolerance was set to 50 ppm and MS/MS mass tolerance to 0.5 Da. The search was performed with a custom Saccharomyces Genome Database generated from Saccharomyces Genome Database (http://www.yeastgenome.org) containing 6,717 entries as of November 30th 2008. A decoy database consisting of similar-length random protein sequences was automatically generated and searched. All statistical analyses were based on peptides having Mascot™ MS/MS ions scores exceeding the “identity or extensive homology threshold” (p < 0.05). In the case of multiple fragmentations of identical precursors, due to recurrence in repetitive runs, only data from the highest scoring peptide were kept.
3. Results
3.1. Exogenous oleic acid is preferentially incorporated into triacylglycerols
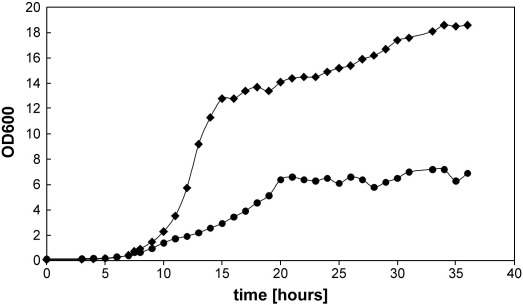
Growth of wild type yeast cells on glucose or oleic acid as the sole carbon source (Fig. 1) shows some marked differences. In the exponential phase cells cultivated on oleate exhibit a lower growth rate than on glucose. On both carbon sources, however, the stationary phase is reached after 24 h.
Fig. 1.
Carbon source dependent growth of BY4741. A yeast pre-culture grown in YPD for 48 h was used to inoculate fresh YPD or YPO media at a starting OD600 of 0.1. Cells were incubated at 30 °C with shaking. At time points indicated aliquots were withdrawn, cells were washed in 0.5% fatty acid-free BSA, and turbidity was measured. ♦, BY4741 grown on glucose; ●, BY4741 grown on oleic acid.
Growth of yeast cells on oleic acid dramatically affects the lipid metabolism and has a major impact on the lipid storage organelle, the lipid particle/droplet (LP). Whereas wild type cells cultivated on glucose (YPD) contain approximately the same amounts of the storage lipids TG and SE, growth on oleate dramatically increased the amount of TG in LP at the expense of SE (Table 2, upper panel). The strong effect caused by the shift of the carbon source from glucose to oleate can also be seen from the values mg lipid per g cell dry weight (Table 2, lower panel).
Table 2.
Lipid composition of Saccharomyces cerevisiae homogenate and lipid particles. Cells were grown on either YPD or YPO for 24 h, and non-polar lipids from lipid particles and phospholipids from homogenate and lipid particles were quantified. The amounts of non-polar lipids and total phospholipids were also estimated from total cell extracts and quantified per g cell dry weight (CDW). Data are from three independent experiments with standard deviation (±). Significance was calculated by Student's t-test (two tailed, unpaired). All values listed correspond to P < 0.05 and were defined to be significant.
| Glucose (YPD) | Oleate (YPO) | |
|---|---|---|
| mg phospholipids/mg protein | ||
| Triacylglycerols | 32.0 ± 4.0 | 97.3 ± 8.9 |
| Steryl esters | 36.7 ± 4.1 | 1.0 ± 0.3 |
| mg phospholipids/mg protein | ||
| Homogenate | 0.047 ± 0.003 | 0.071 ± 0.004 |
| Lipid particles | 0.423 ± 0.048 | 0.889 ± 0.054 |
| mg non-polar lipids/g CDW | ||
| Triacylglycerols | 5.9 ± 0.8 | 30.5 ± 4.8 |
| Steryl esters | 2.6 ± 0.3 | 0.3 ± 0.1 |
| mg phospholipids/g CDW | ||
| Total cell extract | 24.2 ± 3.1 | 31.9 ± 2.0 |
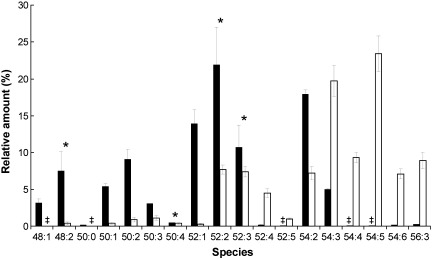
To address the formation of TG in more detail, molecular species of this lipid class from LP were analyzed by mass spectrometry. TG patterns from cells grown on YPD or YPO, respectively, showed marked differences (Fig. 2). In cells grown on glucose, 52:1, 52:2, 52:3 and 54:2 constituted the major TG species. The 52 species contain one C16 and two C18 fatty acids with one, two or all three fatty acids unsaturated (Table 3). Stereospecific positions of fatty acids could not be deduced from this analysis. The four TG species mentioned above comprised approximately 65% of total TG. The remaining portion of TG contained two or three C16 fatty acids, either saturated or unsaturated. Approximately 5% of 54:3 TG was detected suggesting that oleic acid was the exclusive fatty acid in these lipids.
Fig. 2.
Oleate as a carbon source affects the TG species distribution. TG of LP from wild type cells grown on glucose (dark bars) or oleate (white bars) were analyzed by MS. Data are mean values from at least 2 independent experiments. ŧ = values are ≤ 0.1%. Significance was calculated by Student's t-test (two tailed, unpaired). Values indicated by * correspond to P > 0.05 and were defined to be non significant.
Table 3.
Possible species composition of triacylglycerols and phospholipids as identified by mass spectrometry. Possible combinations of acyl chains in triacylglycerol (TG) and phospholipids are based on the fact that major fatty acids in Saccharomyces cerevisiae are 14:0 (myristic acid), 14:1 (myristoic acid), 16:0 (palmitic acid), 16:1 (palmitoleic acids), 18:0 (stearic acid) and 18:1 (oleic acid). Linoleic acid (18:2) and fatty acids of chain length longer than 18 are impurities in commercial oleate used as carbon source and incorporated during cultivation.
| TG species | Possible fatty acids |
|---|---|
| 48:1 | 16:0–16:0–16:1 |
| 48:2 | 16:0–16:1–16:1 |
| 50:0 | 16:0–16:0–18:0 |
| 50:1 | 16:0–16:1–18:0; 16:0–16:0–18:1 |
| 50:2 | 16:0–16:1–18:1; 16:1–16:1–18:0; 16:0–16:0–18:2 |
| 50:3 | 16:1–16:1–18:1; 16:0–16:1–18:2 |
| 50:4 | 16:1–16:1–18:2 |
| 52:1 | 16:0–18:0–18:1; 16:1–18:0–18:0 |
| 52:2 | 16:0–18:1–18:1; 16:1–18:0–18:1; 16:0–18:0–18:2 |
| 52:3 | 16:1–18:1–18:1; 16:1–18:0–18:2; 16:0–18:1–18:2 |
| 52:4 | 16:0–18:2–18:2 |
| 52:5 | 16:1–18:2–18:2 |
| 54:2 | 18:0–18:1–18:1; 18:0–18:0–18:2 |
| 54:3 | 18:1–18:1–18:1; 18:0–18:1–18:2 |
| 54:4 | 18:1–18:1–18:2; 18:0–18:2–18:2 |
| 54:5 | 18:1–18:2–18:2 |
| 54:6 | 18:2–18:2–18:2 |
| 56:3 | 18:1–18:1–20:1; 18:0–18:2–20:1; 18:1–18:2–20:0 |
| Phospholipid species |
Possible fatty acids |
| 28:0 | 14:0–14:0 |
| 32:0 | 16:0–16:0; 14:0–18:0 |
| 32:1 | 16:0–16:1; 14:0–18:1; 14:1–18:0 |
| 32:2 | 16:1–16:1; 14:1–18:1; 14:0–18:2 |
| 34:1 | 16:0–18:1; 16:1–18:0 |
| 34:2 | 16:1–18:1; 16:0–18:2 |
| 36:1 | 18:0–18:1 |
| 36:2 | 18:1–18:1 |
| 36:3 | 18:1–18:2 |
When oleic acid was supplied exogenously as a carbon source the fatty acid pattern of TG changed tremendously. In contrast to cells grown on glucose, C16 was incorporated into TG only to a minor percentage in the presence of oleate in the medium. The vast majority of fatty acids in TG from cells grown on YPO were C18, preferentially C18:1 (see Fig. 2). This result indicated that oleic acid was not only used as a carbon source, but also directly incorporated into complex lipids. Noteworthy, several TG species contained polyunsaturated fatty acids, e.g., 54:5 (18:1/18:2/18:2), and species with longer acyl chains, e.g. 56:3 (C18:1/C18:2/C20:0) (see Table 3). This finding is due to impurities in oleate samples used as carbon source.
3.2. Growth of yeast cells on oleate increases the amount of total phospholipids
To investigate the effect of oleate as carbon source and the composition of phospholipids from total cell extracts and LP, we performed conventional phospholipid analysis based on thin layer chromatographic separation and mass spectrometry. As can be seen from Table 2 (upper panel) cultivation of cells on oleate resulted in a 1.5 fold increase of total phospholipids in the homogenate. An even higher increase of phospholipids was seen with LP from YPO grown cells. The increased level of phospholipids from cells grown on YPO can also be seen from calculations of mg phospholipid per g cell dry weight (see Table 2, lower panel).
To investigate the influence of the carbon source on the phospholipid pattern we subjected homogenates and LP from cells grown on glucose or oleate to glycerophospholipid analysis (Table 4). In YPD grown cells, the major phospholipids in the homogenate were phosphatidylcholine (PC), phosphatidylethanolamine (PE) and phosphatidylinositol (PI) with PC being the most abundant class. These major phospholipids constituted approximately 90% of total cellular phospholipids. Only minor amounts of phosphatidic acid (PA), phosphatidylserine (PS) and cardiolipin (CL) were detected in these samples. Homogenates from cells grown on oleate showed a similar phospholipid pattern with some slight variations. Again, PC, PE and PI were the major phospholipids with even higher amounts of PC. The level of PI was slightly increased at the expense of PE. Amounts of PS and CL did not change much, but PA dropped to levels close to detection limits.
Table 4.
Phospholipid composition of homogenate and lipid particles isolated from cells grown on glucose (YPD) or oleate (YPO). PA, phosphatidic acid; PI, phosphatidylinositol; PS, phosphatidylserine; PC, phosphatidylcholine; PE, phosphatidylethanolamine; CL, cardiolipin; LP, lysophospholipids; DMPE, dimethyl-PE. Significance was calculated by Student's t-test (two tailed, unpaired). Values indicated by * correspond to P < 0.05 and were defined to be significant.
| % of total phospholipids |
||||
|---|---|---|---|---|
| Homogenate |
Lipid particles |
|||
| YPD | YPO | YPD | YPO | |
| PA | 2.8 ± 0.4* | 0.7 ± 0.7* | 1.8 ± 1.3 | 1.3 ± 2.7 |
| PI | 14.5 ± 5.9 | 16.9 ± 3.8 | 21.5 ± 3.4 | 21.5 ± 3.4 |
| PS | 3.8 ± 0.4 | 3.3 ± 0.9 | 2.1 ± 2.6 | 0.8 ± 0.9 |
| PC | 51.1 ± 5.5 | 53.0 ± 1.4 | 57.5 ± 1.7 | 56.4 ± 2.7 |
| PE | 23.6 ± 1.4 | 20.1 ± 3.7 | 16.6 ± 1.9 | 16.9 ± 2.8 |
| CL | 2.3 ± 0.3* | 3.7 ± 0.8* | 0 ± 0 | 1.0 ± 1.2 |
| LP | 0 ± 0* | 0.3 ± 0.6* | 0.3 ± 0.6 | 0.7 ± 0.5 |
| DMPE | 1.9 ± 1.2 | 1.5 ± 1.4 | 0 ± 0 | 1.3 ± 1.9 |
In LP from cells grown on YPD, PC, PE and PI were also the most prominent glycerophospholipids. Interestingly, levels of PC and especially PI were higher whereas PE was lower in LP than in the homogenate. Other phospholipids occurred only at negligible amounts in LP. When cells were grown on oleate, the phospholipid pattern of LP was very similar to YPD with the exception of PS which dropped to ~ 0.8% on YPO.
3.3. Mass spectrometric analysis of phospholipids from homogenates and lipid particles
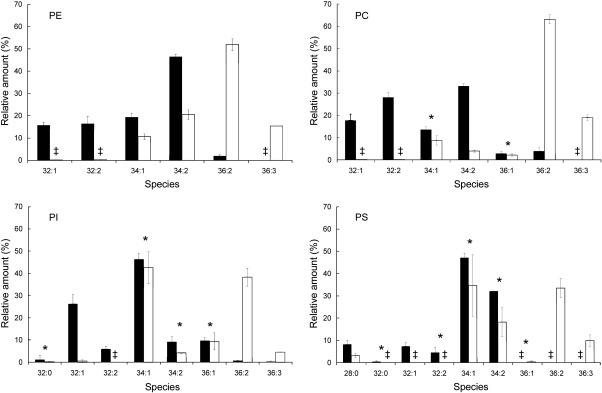
As described above no major differences were observed in the pattern of phospholipid classes of LP from cells grown either on glucose or oleate. For a more detailed analysis of lipid species, however, we subjected phospholipids from homogenate and LP to mass spectrometry. The fact that major fatty acids of the yeast S. cerevisiae are C16 and C18 made the species patterns rather simple. As can be seen from Fig. 3, the most abundant species of PC, PE, PS, and PI from cells grown on glucose contained C16 and C18 in their mono-unsaturated or saturated form (see also Table 3). It has to be noted, however, that species patterns of individual phospholipids varied in a most typical way. In PE, the 34:2 species (C16:1/C18:1) were predominant, whereas in PC 32:2 and 34:1 occurred at a similar level. Only small amounts of 36:2 (C18:1/C18:1) were detected in PE and PC. Contrary to PE and PC, PI and PS showed a strong enrichment of 34:1 species. In PI, the second abundant species was 32:1, whereas in PS 34:2 occurred as the other major species. Another interesting observation was the presence of substantial amounts of 36:1 in PI. This species was also found in PC, but not in PS and PE. PS also contained substantial amounts of 28:0 which is most likely composed of C16:0 and C12:0.
Fig. 3.
Species composition of phospholipids from yeast homogenates. Lipid extracts of homogenate from wild type cells grown on glucose (dark bars) or oleate (white bars) were analyzed by MS for phospholipid species. Data are mean values from at least 2 independent experiments. ŧ = values are ≤ 0.1%. Significance was calculated by Student's t-test (two tailed, unpaired). Values indicated by * correspond to P > 0.05 and were defined to be non significant. For abbreviation see legend of Table 4.
Mass spectrometry of phospholipids from homogenates of cells grown on oleate showed a completely different fatty acid pattern. Not surprisingly, incorporation of oleic acid (C18:1) into phospholipids was high under these cultivation conditions. Interestingly, species of major phospholipids seem to come in as pairs. PC and PE contained oleic acid as a major constituent (see Fig. 3) resulting in large amounts of 36:2 species. This effect was most pronounced in PC, whereas in PE C18:1 was combined with C16:1 (34:2 species). Species patterns of PI and PS from cells grown on oleate looked different. Although in both phospholipids oleic acid was highly present in the di-unsaturated species 36:2, the 34:1 species (C16:0/C18:1 or C16:1/C18:0) occurred at an almost equal level. In PS and PE 34:2 was another prominent combination of fatty acids. The appearance of 36:3 species in all phospholipids was due the impurities of oleate samples used as carbon source as described above.
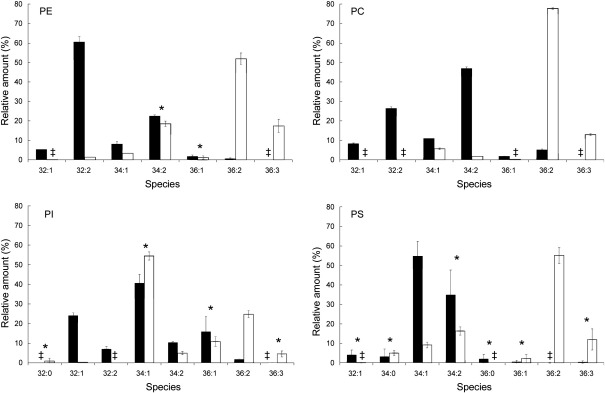
When phospholipid species of LP from cells grown on glucose (Fig. 4) were analyzed we realized that in most cases the species patterns reflected very much those of total cell extracts (see Fig. 3) following an equilibrium rule (“you take what you get”). However, one major exception was the large amount of 32:2 PE in LP which did not occur in the homogenate. Additionally, we found two fully saturated species 34:0 (C16:0/C18:0) and 36:0 (C18:0/C18:0) in PS from the LP fraction, which were obviously below the detection limit in total cell extracts.
Fig. 4.
Species composition of phospholipids from yeast lipid particles. Lipid extracts of LP from wild type cells grown on glucose (dark bars) or oleate (white bars) were analyzed by MS for phospholipid species. Data are mean values from at least 2 independent experiments. ŧ = values are ≤ 0.1%. Significance was calculated by Student's t-test (two tailed, unpaired). Values indicated by * correspond to P > 0.05 and were defined to be non significant. For abbreviation see legend of Table 4.
Differences between the phospholipid species patterns of LP and homogenate from YPO grown cells were mainly in PI and PS fractions. Whereas PE and PC from LP exhibited a species pattern similar to the homogenate, the amount of 34:1 PI in LP was increased at the expense of 36:2, and the level of 36:2 PS in LP was much higher than in the homogenate. Thus, despite the strong influence of exogenous oleic acid on the formation of phospholipid species, individual phospholipid classes retained at least some typical constituents.
Whereas species analysis of phospholipids yielded precise information of molecular components, the ratio of saturated to unsaturated species gave us an insight into the physical status of membranes. Remarkably, the different phospholipid classes exhibited dramatic differences in their degree of saturation (Table 5). As the most striking example, PI is the phospholipid containing most saturated fatty acids followed by PS irrespective of the carbon source used for the cultivation of cells. In LP from cells grown on glucose a slight selectivity seems to occur insofar as more unsaturated PE and PC species accumulated compared to the homogenate. Oleate as a carbon source led to a more than 3-fold reduction of saturated PE and PC species, but only to a moderate although pronounced effect on PI and PS. Also in cells grown on oleate, LP contained more unsaturated PE and PC species than bulk membranes, but this effect was also observed with PS. In contrast, the level of saturated PI species in LP remained high even when oleate was present as carbon source.
Table 5.
Saturation status of major phospholipids from homogenate and lipid particles. Cells were grown on glucose (YPD) or oleic acid (YPO), and subcellular fractions were obtained as described in the Methods and materials section. For abbreviation see legend to Table 4.
| % of saturated fatty acids |
||||
|---|---|---|---|---|
| Homogenate |
Lipid particles |
|||
| Glucose (YPD) | Oleate (YPO) | Glucose (YPD) | Oleate (YPO) | |
| PE | 17.5 | 5.4 | 7.6 | 2.3 |
| PC | 17.2 | 5.6 | 10.6 | 3.0 |
| PI | 42.4 | 26.5 | 40.4 | 33.8 |
| PS | 35.9 | 20.7 | 34.9 | 10.8 |
3.4. Influence of the carbon source on the pattern of lipid particle proteins
During the last decade, the yeast LP proteome was studied based on mass spectrometry and localization of GFP-tagged proteins. Applying these methods, 45 LP proteins were identified (http://www.yeastgenome.org/20112010; http://yeastgfp.yeastgenome.org/20112010; http://ypl.tugraz.at/20112010). Interestingly, most of these proteins are not exclusively localized to the LP but seem to have a second localization either to microsomal and/or mitochondrial fractions. Recent experiments in our laboratory, however, led us to the assumption that the list of LP proteins was not complete most likely due to methodological limitations. Therefore, we re-addressed this issue by employing a novel method of proteome analysis based on nano-LC-MS (see Methods and materials section). With this method we tested whether or not a change of the carbon source (YPD vs. YPO) had an impact on the LP protein pattern. We have to be aware, however, that also in the present experimental effort LP proteins of minor abundance may have escaped detections. At the same time, it has to be noted that contamination of LP with other cell organelles which can never be avoided may lead to false positive hits as will be discussed below.
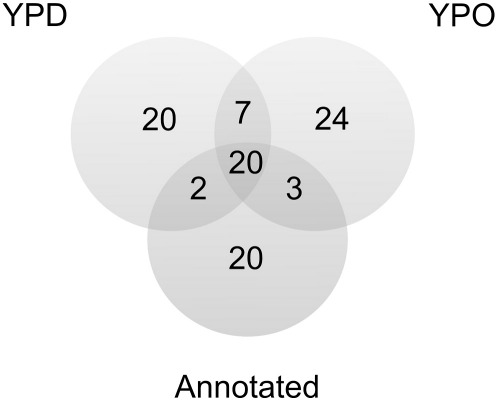
Initial analysis of LP from cells grown either on YPD or YPO by SDS-PAGE (Fig. 5A) revealed already qualitative differences. These differences were not due to different qualities of preparations as shown by Western blot analysis (Fig. 5B). Analysis by nLC-MALDI TOF/TOF of LP proteins from cells grown on glucose led to the identification of 49 proteins as shown by the Venn diagram in Fig. 6. Twenty two of these proteins had already been assigned to LP before. Analysis of LP proteins from cells grown on oleate resulted in the identification of 54 proteins; 23 of these proteins were already known before as LP constituents. A complete list of the identified proteins with more than one peptide detected is shown in Table 6.
Fig. 5.
Protein analysis of yeast lipid particles and quality control. A, Protein patterns of the lipid particle fraction from Saccharomyces cerevisiae grown on glucose or oleate. Low molecular weight standards are shown in the lane on the left. Lanes were loaded with 15 μg total protein, each. Proteins were stained with Coomassie Blue. B, Quality control of subcellular fractions. Western blot analysis was performed with homogenate (H), lipid particle (L) and an organelle pellet fraction (P) obtained as side product of LP isolation from cells grown on glucose or oleate. Antisera used were reactive with Erg1p, squalene monooxygenase (LP marker); Pma1p, plasma membrane H+-ATPase (plasma membrane marker); Por1p, mitochondrial porin (mitochondrial marker); Pcs60p, peroxisomal AMP-binding protein (peroxisomal marker); Fox1p, peroxisomal fatty acyl CoA oxidase (peroxisomal marker); and Wbp1p, beta subunit of the oligosaccharyl transferase glycoprotein complex (microsomal marker).
Fig. 6.
Venn diagram displaying identification of yeast lipid particle proteins. The graph summarizes computer supported analysis of LP proteins. Numbers indicate proteins found in LP fractions from yeast cells grown on glucose (YPD) or oleate (YPO). Annotated LP proteins were assigned to this compartment through previous studies.
Table 6.
Proteome of yeast lipid particles. §novel LP protein, found in cells grown on glucose or oleate, ¥ numbers indicate fragments used for identification of proteins from cells grown on glucose (D) or oleate (O). Proteins identified by only single peptides were excluded. C, cytosol; M, mitochondria; PM, plasma membrane; ER, endoplasmic reticulum; LP, lipid particle; End, endosomes; G, Golgi; Mic, microsomes; V, vacuole; Px, peroxisome; N, nucleus; nEnv, nuclear envelope; ext, extrinsic to membrane; mem, integral to membrane; bud, cellular bud; rib, ribosomal subunit; CW, cell wall; R, ribosome; Retro, retrotransposon; mTub, microtubule. Databases used: SGD (http://www.yeastgenome.org/20112010); GFP (http://yeastgfp.yeastgenome.org/20112010); YPL (http://ypl.tugraz.at/20112010).
| Gene name | Systematic name | SGD | GFP | YPL | This study | D¥ | O¥ | Localization (SGD) | Description |
|---|---|---|---|---|---|---|---|---|---|
| ACH1 | YBL015W | ✓ | 2 | C/M | CoA transferase activity | ||||
| ADH1 | YOL086C | ✓ | 2 | C/PM | Alcohol dehydrogenase | ||||
| ALG9 | YNL219C | ✓ | 2 | ER | Mannosyltransferase | ||||
| ATF1 | YOR377W | ✓ | LP/End | Alcohol acyltransferase | |||||
| ATP2 | YJR121W | ✓ | 9 | M | Subunit of mitochondria ATP synthase | ||||
| AYR1 | YIL124W | ✓ | ✓ | ✓ | 15 | 12 | C/ER/LP/M | NADPH-dependent 1-acyl dihydroxyacetone phosphate reductase | |
| BSC2 | YDR275W | ✓ | ✓ | LP | Unknown | ||||
| COY1 | YKL179C | ✓ | G | Golgi membrane protein | |||||
| CPR5§ | YDR304C | ✓ | 7 | 3 | C/ER | Peptidyl-prolyl cis-trans isomerase | |||
| CSR1 | YLR380W | ✓ | LP/C/M/Mic | Phosphatidylinositol transfer protein | |||||
| CST26 | YBR042C | ✓ | ✓ | ✓ | LP | Required for incorporation of stearic acid into phosphatidylinositol | |||
| CWH43 | YCR017C | ✓ | 2 | PM | Putative sensor/transporter protein | ||||
| DFM1 | YDR411C | ✓ | 2 | ER | ER localized derlin-like family member | ||||
| DGA1 | YOR245C | ✓ | ✓ | 3 | LP | Diacylglycerol acyltransferase | |||
| DPL1 | YDR294C | ✓ | 2 | ER | Dihydrosphingosine phosphate lyase | ||||
| DPM1 | YPR183W | ✓ | 2 | ER/M | Dolichol phosphate mannose synthase | ||||
| EHT1 | YBR177C | ✓ | ✓ | ✓ | 19 | 18 | LP/M | Acyl-coenzymeA:ethanol O-acyltransferase | |
| ENO2 | YHR174W | ✓ | 2 | V/PM/M | Enolase II | ||||
| ERG1 | YGR175C | ✓ | ✓ | ✓ | 5 | 15 | ER/LP | Squalene epoxidase | |
| ERG6 | YML008C | ✓ | ✓ | ✓ | ✓ | 37 | 30 | ER/LP/M | Delta(24)-sterol C-methyltransferase |
| ERG7 | YHR072W | ✓ | ✓ | ✓ | ✓ | 7 | 2 | ER/LP/PM | Lanosterol synthase |
| ERG27 | YLR100W | ✓ | ✓ | ✓ | ✓ | 9 | 4 | ER/M | 3-Keto sterol reductase |
| FAA1 | YOR317W | ✓ | ✓ | 20 | 15 | LP/PM/M | Long chain fatty acyl-CoA synthetase | ||
| FAA3 | YIL009W | ✓ | 3 | unknown | Long chain fatty acyl-CoA synthetase | ||||
| FAA4 | YMR246W | ✓ | ✓ | ✓ | ✓ | 13 | 6 | LP/C | Long chain fatty acyl-CoA synthetase |
| FAS1 | YKL182W | ✓ | LP/C/M | Fatty acid synthetase | |||||
| FAT1 | YBR041W | ✓ | ✓ | ✓ | ✓ | 13 | 9 | PM/LP/Mic/PX | Fatty acid transporter |
| FMP52 | YER004W | ✓ | 4 | ER/M | Unknown | ||||
| GPT2 | YKR067W | ✓ | 12 | C/ER | sn-1 Acyltransferase | ||||
| GTT1§ | YIR038C | ✓ | 2 | 8 | ER/M/PM | Glutathione S-transferase | |||
| GVP36 | YIL041W | ✓ | 3 | C/G | BAR domain-containing protein | ||||
| HFD1 | YMR110C | ✓ | ✓ | ✓ | 7 | 18 | M/LP/End | Putative fatty aldehyde dehydrogenase | |
| HSP12 | YFL014W | ✓ | 6 | C/PM/N | Heat shock protein | ||||
| KAR2 | YJL034W | ✓ | 9 | ER | ATPase | ||||
| LAP4 | YKL103C | ✓ | 3 | V | Vacuolar aminopeptidase | ||||
| LDB16 | YCL005W | ✓ | ✓ | LP/M | Unknown | ||||
| MSC1 | YML128C | ✓ | 2 | M/ER/PM | Unknown | ||||
| NUS1 | YDL193W | ✓ | ✓ | ✓ | 9 | 4 | ER/LP/nEnv | Putative prenyltransferase | |
| OSH4§ | YPL145C | ✓ | 3 | 5 | C/G/ext | Oxysterol binding protein | |||
| OSW5 | YMR148W | ✓ | ✓ | 2 | mem | Unknown | |||
| PDI1§ | YCL043C | ✓ | 6 | 7 | ER | Disulfide isomerase | |||
| PDR16 | YNL231C | ✓ | ✓ | ✓ | 5 | 9 | LP/Mic/PM/C | Phosphatidylinositol transfer protein | |
| PET10 | YKR046C | ✓ | ✓ | ✓ | ✓ | 12 | 24 | LP | Unknown |
| PGC1 | YPL206C | ✓ | ✓ | 9 | LP/M | Phosphatidylglycerol phospholipase C | |||
| PIL1 | YGR086C | ✓ | C/M/PM | Primary component of eisosomes | |||||
| PMA1 | YGL008C | ✓ | 3 | PM/M/mem | Plasma membrane H + − ATPase | ||||
| PMT1 | YDL095W | ✓ | 2 | ER | Protein O-mannosyltransferase | ||||
| PMT2 | YAL023C | ✓ | 3 | ER | Protein O-mannosyltransferase | ||||
| POR1 | YNL055C | ✓ | 2 | M | Mitochondrial porin | ||||
| POX1 | YGL205W | ✓ | 3 | PX | Fatty-acyl coenzyme A oxidase | ||||
| RHO1 | YPR165W | ✓ | 4 | mem/PX/PM/M/bud | GTP-binding protein | ||||
| RPL5 | YPL131W | ✓ | 3 | rib | Protein component of the large (60S) ribosomal subunit | ||||
| RPL10 | YLR075W | ✓ | 2 | rib | Protein component of the large (60S) ribosomal subunit | ||||
| RPS1B | YML063W | ✓ | 2 | rib | Ribosomal protein 10 (rp10) of the small (40S) subunit | ||||
| RPS3 | YNL178W | ✓ | 5 | rib | Protein component of the small (40S) ribosomal subunit | ||||
| RPS19B | YNL302C | ✓ | 2 | rib | Protein component of the small (40S) ribosomal subunit | ||||
| RPS31 | YLR167W | ✓ | 4 | rib/C | Fusion protein that is cleaved to yield a ribosomal protein of the small (40S) subunit and ubiquitin | ||||
| RRT8 | YOL048C | ✓ | ✓ | ✓ | 4 | 2 | LP | Unknown | |
| RTN2 | YDL204W | ✓ | 3 | ER/nEnv | Unknown | ||||
| SEC61 | YLR378C | ✓ | 5 | ER | Essential subunit of Sec61 complex | ||||
| SEC63 | YOR254C | ✓ | 4 | ER/M | Essential subunit of Sec63 complex | ||||
| SHE10 | YGL228W | ✓ | 4 | unknown | Putative glycosylphosphatidylinositol (GPI)-anchored protein of unknown function | ||||
| SLC1 | YDL052C | ✓ | ✓ | ✓ | 2 | 3 | LP | 1-Acyl-sn-glycerol-3-phosphate acyltransferase | |
| SNA2 | YDR525W-a | ✓ | ✓ | Mem/C | Unknown | ||||
| SNX41 | YDR425W | ✓ | End | Sorting nexin | |||||
| SRT1 | YMR101C | ✓ | LP | Cis-prenyltransferase | |||||
| SSA1 | YAL005C | ✓ | 2 | C/PM/N | ATPase | ||||
| SSO1 | YPL232W | ✓ | PM | Plasma membrane t-snare | |||||
| TDH1 | YJL052W | ✓ | ✓ | 4 | C/LP/M/PM/CW | Glyceraldehyde-3-phosphate dehydrogenase, isozyme 1 | |||
| TDH2 | YJR009C | ✓ | C/LP/M/PM/CW | Glyceraldehyde-3-phosphate dehydrogenase, isozyme 2 | |||||
| TDH3 | YGR192C | ✓ | ✓ | 5 | C/LP/M/PM/CW | Glyceraldehyde-3-phosphate dehydrogenase, isozyme 3 | |||
| TEF1 | YPR080W | ✓ | 5 | M/R | Translational elongation factor EF-1 alpha | ||||
| TGL1 | YKL140W | ✓ | ✓ | 12 | 14 | LP/mem | Steryl ester hydrolase | ||
| TGL3 | YMR313C | ✓ | ✓ | ✓ | 4 | 5 | LP | Triacylglycerol lipase | |
| TGL4 | YKR089C | ✓ | ✓ | ✓ | 3 | 4 | LP | Triacylglycerol lipase | |
| TGL5 | YOR081C | ✓ | ✓ | ✓ | 5 | 3 | LP | Triacylglycerol lipase | |
| USE1 | YGL098W | ✓ | ER | SNARE protein | |||||
| TSC10 | YBR265W | ✓ | 2 | C/ER/M | 3-ketosphinganine reductase | ||||
| TUB2 | YFL037W | ✓ | 2 | mTub | Beta-tubulin | ||||
| UBX2§ | YML013W | ✓ | 5 | 3 | ER/M | Protein involved in ER-associated protein degradation | |||
| VPS66§ | YPR139C | ✓ | 6 | 4 | C | Cytoplasmic protein of unknown function involved in vacuolar protein sorting | |||
| WBP1 | YEL002C | ✓ | 3 | ER/nEnv | Beta subunit of the oligosaccharyl transferase (OST) glycoprotein complex | ||||
| YBR204C | YBR204C | ✓ | ✓ | 3 | unknown | Serine hydrolase | |||
| YDR018C | YDR018C | ✓ | unknown | Similarity to acyltransferase | |||||
| YEH1 | YLL012W | ✓ | ✓ | LP/mem | Steryl ester hydrolase | ||||
| YIM1 | YMR152W | ✓ | ✓ | ✓ | 4 | LP/C/M | Unknown | ||
| YGR038C-B | YGR038C-B | ✓ | 2 | Retro | Retrotransposon TYA Gag and TYB Pol genes | ||||
| YJU3 | YKL094W | ✓ | ✓ | ✓ | 3 | 2 | LP/C/M/PM | Serine hydrolase | |
| YNL134C | YNL134C | ✓ | 2 | C/N | Unknown | ||||
| YNL208W | YNL208W | ✓ | 3 | M/R | Unknown | ||||
| YOR059C | YOR059C | ✓ | LP | Unknown | |||||
| YOR246C | YOR246C | ✓ | ✓ | LP | Similarity to oxidoreductase | ||||
| YPT7§ | YML001W | ✓ | 4 | 2 | V/M | GTPase | |||
| ZEO1 | YOL109W | ✓ | 9 | M/PM/ext | Peripheral membrane protein |
Besides the known set of LP proteins some additional proteins were identified as potentially novel components of this compartment. Surprisingly, only 7 proteins (Table 6; proteins marked with §) so far not assigned to LP were identified on both variants of LP (YPD and YPO), namely Pdi1p, Cpr5p, Gtt1p, Osh4p, Ubx2p, Vps66p and Ypt7p. For this reason we considered these proteins most important and selected them for further and more detailed analysis. These proteins had been assigned to other subcellular compartments, mainly the cytosol and the ER, as listed in the Saccharomyces Genome Database (http://www.yeastgenome.org/20112010). To prove or disprove localization of the candidate proteins to LP, cell fractionation and Western blot analyses of isolated organelles were performed (Fig. 7). Two of the candidate proteins, namely Vps66p and Gtt1p, showed a clear and more or less exclusive localization to LP, whereas other proteins were also detected in the ER (Osh4p, Crp5p and Ubx2p) and peroxisomes (Crp5p and Ubx2p). Ypt7p was found in all cellular fractions analyzed. A further interesting aspect was the impact of the carbon source used for cultivating cells onto the localization pattern of these proteins. Localization of Vps66p and Gtt1p to LP remained unaltered irrespective of the carbon source used. Osh4p, Cpr5p and Ubx2p resided in LP and ER and partially in peroxisomes when glucose was used for cultivation. Utilization of oleate as the sole carbon source led to a pronounced enrichment of these polypeptides in the LP fraction and absence from other fractions. This effect was not observed for Ypt7p, which remained widely distributed independent of the carbon source used. The only candidate protein whose localization to the LP was not confirmed by Western blotting was Pdi1p (data not shown).
Fig. 7.
Subcellular localization of newly discovered lipid particle proteins. Cell fractionation was performed with C-terminally tagged proteins expressed under native promoters. Cells were cultivated on glucose or oleate, respectively, and subcellular fractions were prepared as described in the Methods and materials section. H, homogenate; P, organelle pellet fraction obtained as side product of LP isolation; L, lipid particles; ER, endoplasmic reticulum (30,000 g and 40,000 g microsomes); C, cytosol; Px, peroxisomes; Mit, mitochondria.
As described in the introduction, yeast LP proteins belong to certain functional families. Therefore, it was interesting to include the newly identified LP proteins into the functional framework of LP. Functional assignments, however, have to be interpreted with caution because many of them rely on computer based and not on biochemical analysis. Employing the Saccharomyces Genome Database (http://www.yeastgenome.org/20112010) we showed that a large number of LP proteins were involved in lipid metabolism (see Table 6). In LP from cells grown on YPD 21 members of this group were identified, and in cells grown on oleate 17 proteins of this kind were detected. Other functions attributed to LP proteins were protein glycosylation, cell wall organization or ER unfolded protein response. The biological function of a number of proteins was unknown, namely 6 in YPD and 7 in YPO grown cells. Some clusters of proteins were strongly affected by the change of the carbon source, e.g. proteins involved in ER unfolded protein response or energy providing processes.
4. Discussion
Lipid particles (lipid droplets, oil bodies) have gained much attention during the last decade due to their role as lipid buffer, oil storage and their involvement in human diseases. However, we are only at the beginning to understand metabolic functions which are attributed to this compartment. The present study extends our recent knowledge about the molecular equipment of LP through identification and analysis of LP components from the yeast S. cerevisiae. This microorganism is frequently used as a reliable model for multicellular eukaryotes due to homologies and similarities, and due to the ease of manipulation by nutritional and genetic means. An important question addressed in this study was the behavior of yeast LP in response to changes of culture conditions, especially to cultivation of cells on different carbon sources. We show that growth of the yeast either on glucose or oleate resulted in dramatic changes of the lipid pattern of LP, but also in an adaptive response of the LP proteome.
4.1. Lipid particle lipidome
Initial studies of the lipid pattern of LP [2,3,42] mainly addressed the role of this compartment as lipid depot. More recent investigations extended this knowledge to species analysis of TG, SE and phospholipids [33] employing mass spectrometry developed for systematic analysis of the yeast lipids [43,44]. Another important finding [32,33,45,46] was that growth of yeast cells on oleate (YPO medium) greatly stimulated the proliferation of yeast LP and changed the lipid profile dramatically. This aspect is specifically addressed in the present study and extended to changes of cell cultivation.
Our lipidome analyses led to a number of important observations. First, we found that TG contain large amounts of unsaturated fatty acids, especially C18:1, even when cells were grown on glucose (see Fig. 2). This effect although not unexpected was dramatically enhanced when oleate served as a carbon source. The reason for this finding appears to be the preference of TG synthesizing enzymes, Dga1p and Lro1p [47] for specific substrates. As a result of this specificity, TG storage molecules have a high degree of fluidity and accumulate as a soft and non-structured core of LP [4]. The second striking observation of the present study was that oleate as a carbon source selectively stimulated formation of TG, whereas SE synthesis was dramatically decreased. Most recently, we [33] showed that inhibition of Are2p, the major SE synthase of S. cerevisiae, by oleate was the reason for this finding. Surprisingly, in Yarrowia lipolytica oleic acid as a carbon source had the opposite effect causing a decrease of TG but an increase of SE [30]. The molecular reason of this effect is not known. Finally, the strong incorporation of C18:1 into TG of S. cerevisiae grown on oleate clearly demonstrated that the exogenous fatty acid was not only used as source of energy and a substrate for β-oxidation in peroxisomes [48], but also directly incorporated as building block into complex lipids.
Although phospholipids are only minor constituents of LP they may play an important role in this organelle. First, phospholipids of the LP surface monolayer determine the contact of the otherwise highly hydrophobic particle to the hydrophilic environment. For this reason, an appropriate polar shielding is required. Second, the small set of proteins embedded in the surface phospholipid monolayer of LP may require a specific membrane environment for functionality. Third, the nature of the LP surface membrane phospholipid composition may provide a direct link to the biogenesis of this organelle. Taking into consideration that LP are most likely derived from the ER a large portion of the LP surface membrane may be “extracted” from the ER. It is tempting to speculate that a combined selectivity for phospholipids and proteins (see below) leads to the specific surface membrane composition of LP. Finally, the amount of phospholipids available for the synthesis of the LP surface as well as the lipid composition of the monolayer membrane may determine the size of the droplets and thus be critical for LP biogenesis.
As can be seen from Table 4, PC and PI are the most prominent glycerophospholipids in the LP monolayer surface membrane. The enrichment of PC and PI in LP occurred irrespective of the carbon source used in the culture medium. This result suggests that phospholipids with specific headgroups are important for structure, maintenance and/or functionality of the LP surface. Whereas PC is the classical bulk phospholipid in most membranes, PI is a typical member of the group of negatively charged phospholipids. It is known that inositol phospholipids including PI are important for interfacial binding of proteins and regulating protein functions at the cell interface. As PI is polyanionic, it can create unspecific electrostatic interactions with proteins in a very efficient way [49,50] and may thus act as modulator of enzymes localized to the LP surface. The other remarkable observation was that PI in both total cell extracts and LP was highly saturated (see Table 5), even when oleate was present as a carbon source. In both fractions only minor amounts of double unsaturated PI species were detected. Interestingly, PS exhibits a similar degree of unsaturation as PI. The reason for this finding may be biosynthesis of both phospholipids from the same precursor, CDP-DAG, although by two distinct enzymes [51].
Changing the carbon source from glucose to oleate did not dramatically affect the phospholipid pattern in total cell extracts and LP. The only alteration in the phospholipid composition of the homogenate was a slight increase of PI and PC in oleate grown cells at the expense of PE. However, the total amount of phospholipids (see Table 2) in both homogenate and LP was markedly increased when yeast cells were grown on oleate. We assume that the excess of phospholipids in cells grown on YPO [33] leads to enhanced proliferation of intracellular membranes as shown by electron microscopic inspection (our own preliminary and unpublished results).
4.2. Proteome of yeast lipid particles
Two important aspects addressed in this study extended our existing knowledge [17,52] about the yeast LP proteome. First, we were able to detect LP proteins which had escaped previous detection. Secondly, we demonstrate that growth of yeast cells on oleate did not only induce proliferation of peroxisomes [53–56] and lead to massively enhanced LP formation, but also to marked changes of the LP proteome.
Data summarized in Table 6 show that 49 proteins were identified as constituents of LP from YPD grown cells, and 54 proteins of LP from YPO grown cells. Only 20 of the previously annotated proteins occurred in both LP variants (see Fig. 6) suggesting that these proteins represent a “basic equipment” of LP. Most of these proteins are involved in lipid metabolism, such as Erg-proteins, lipases or fatty acid activating proteins [27]. Interestingly, seven proteins in both types of LP had not been attributed to LP before, namely Pdi1p, Cpr5p, Gtt1p, Osh4p, Ubx2p, Ypt7p and Vps66p. Localization of six of these polypeptides to LP as confirmed by Western blotting (see Fig. 7) was an important step to sort out false positive hits of the proteome analysis. Two of these polypeptides, Osh4p and Vps66p, were previously identified as S. cerevisiae orthologs in LP of Yarrowia lipolytica [30]. In S. cerevisiae, Osh4p and Cpr5p were found to be dually located to LP and microsomes (see Fig. 7). This result was not surprising because other LP proteins showed similar properties. As examples, Erg6p, a prominent LP protein [17], has also been localized to the ER [23,57]; and the long chain fatty acyl-CoA synthase Faa1p was found in LP [17], mitochondria [58] and the plasma membrane [59].
Previous studies with yeast LP proteins had suggested that lack of transmembrane spanning domains was a typical feature of these proteins [23]. This finding was explained by the fact that LP contain a surface phospholipid monolayer which makes embedding of proteins containing transmembrane segments difficult. Although our current knowledge about structural properties of the newly detected LP proteins is restricted to computer based predictions (http://www.yeastgenome.org/20112010), it appears that these proteins largely fulfill the above mentioned requirement. Pdi1p, Cpr5p, Gtt1p, Osh4p, Ubx2p and Ypt7p lack putative bilayer membrane spanning domains, and only one transmembrane domain was predicted for Vps66p.
Assignment of novel functions to LP on the basis of proteome analysis is farfetched because it relies only on computer based predictions. Nevertheless, at least two of our newly detected LP proteins can be unequivocally assigned to the large family of LP proteins which are involved in lipid metabolism. Recently, Vps66p was found to be a novel lysophosphatidic acid acyl transferase (LPA-AT) of LP (E. Testet, personal communication). Moreover, Lockshon et al. [60] found that Vps66p was important for normal growth of yeast cells on oleate. Also Osh4p, one of the oxysterol binding protein homologues of the yeast, had been shown before to be involved in the regulation of lipid metabolism [61–63]. Physical interaction of Osh4p (http://www.yeastgenome.org/20112010) with three other LP proteins involved in lipid metabolism, Eht1p, Fat1p and Scl1p, may also be important for Osh4p function. Im et al. [64] showed that Osh4p as other lipid transfer proteins contains a lipid-binding pocket which enables uptake of sterols from a membrane and release at another one. This finding is in line with the observation that Osh4p can transfer sterols between liposomes in vitro [63]. Since LP constitute a large depot of yeast sterols, although in the form of steryl esters, susceptibility of Osh4p to this compartment may be linked to sterol mobilization. Also Gtt1p has a potential interaction partner on LP which is involved in lipid metabolism, namely the dihydroxyacetone phosphate acyltransferase Ayr1p (http://www.yeastgenome.org/20112010). However, a metabolic link between the two proteins has not been demonstrated.
In summary, the lipidome and proteome analyses of yeast LP as presented here are a step forward to understand the biochemistry and cell biology of this organelle. Our view of LP has to be revised insofar as this compartment besides lipid storage may fulfill distinct requirements of cell metabolism. We show here that LP are highly flexible regarding their lipid and protein equipment and appear to adapt quickly to environmental changes. It will be a specific challenge for future LP research to shed light on regulatory aspects which govern the complex network of the LP biosynthetic process.
Acknowledgments
This work was supported by the Fonds zur Förderung der wissenschaftlichen Forschung in Österreich (projects 18857, 23029 and W901-B05 to G.D.).
References
- 1.Clausen M.K., Christiansen K., Jensen P.K., Behnke O. Isolation of lipid particles from baker's yeast. FEBS Lett. 1974;43:176–179. doi: 10.1016/0014-5793(74)80994-4. [DOI] [PubMed] [Google Scholar]
- 2.Leber R., Zinser E., Zellnig G., Paltauf F., Daum G. Characterization of lipid particles of the yeast, Saccharomyces cerevisiae. Yeast. 1994;10:1421–1428. doi: 10.1002/yea.320101105. [DOI] [PubMed] [Google Scholar]
- 3.Zinser E., Paltauf F., Daum G. Sterol composition of yeast organelle membranes and subcellular distribution of enzymes involved in sterol metabolism. J. Bacteriol. 1993;175:2853–2858. doi: 10.1128/jb.175.10.2853-2858.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czabany T., Wagner A., Zweytick D., Lohner K., Leitner E., Ingolic E., Daum G. Structural and biochemical properties of lipid particles from the yeast Saccharomyces cerevisiae. J. Biol. Chem. 2008;283:17065–17074. doi: 10.1074/jbc.M800401200. [DOI] [PubMed] [Google Scholar]
- 5.Tauchi-Sato K., Ozeki S., Houjou T., Taguchi R., Fujimoto T. The surface of lipid droplets is a phospholipid monolayer with a unique fatty acid composition. J. Biol. Chem. 2002;277:44507–44512. doi: 10.1074/jbc.M207712200. [DOI] [PubMed] [Google Scholar]
- 6.Goodman J.M. The gregarious lipid droplet. J. Biol. Chem. 2008;283:28005–28009. doi: 10.1074/jbc.R800042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olofsson S.-O., Bostrom P., Andersson L., Rutberg M., Levin M., Perman J., Boren J. Triglyceride containing lipid droplets and lipid droplet-associated proteins. Curr. Opin. Lipidol. 2008;19:441–447. doi: 10.1097/MOL.0b013e32830dd09b. [DOI] [PubMed] [Google Scholar]
- 8.Thiele C., Spandl J. Cell biology of lipid droplets. Curr. Opin. Cell Biol. 2008;20:378–385. doi: 10.1016/j.ceb.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Farese R.V., Walther T.C. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell. 2009;139:855–860. doi: 10.1016/j.cell.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodman J.M. Demonstrated and inferred metabolism associated with cytosolic lipid droplets. J. Lipid Res. 2009;50:2148–2156. doi: 10.1194/jlr.R001446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bickel P.E., Tansey J.T., Welte M.A. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim. Biophys. Acta. 2009;1791:419–440. doi: 10.1016/j.bbalip.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robenek H., Buers I., Hofnagel O., Robenek M.J., Troyer D., Severs N.J. Compartmentalization of proteins in lipid droplet biogenesis. Biochim. Biophys. Acta. 2009;1791:408–418. doi: 10.1016/j.bbalip.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi T., Omatsu N., Matsushita S., Osumi T. CGI-58 interacts with perilipin and is localized to lipid droplets. Possible involvement of CGI-58 mislocalization in Chanarin–Dorfman syndrome. J. Biol. Chem. 2004;279:30490–30497. doi: 10.1074/jbc.M403920200. [DOI] [PubMed] [Google Scholar]
- 14.Fujimoto T., Ohsaki Y. The proteasomal and autophagic pathways converge on lipid droplets. Autophagy. 2006;2:299–301. doi: 10.4161/auto.2904. [DOI] [PubMed] [Google Scholar]
- 15.Guo Y., Walther T.C., Rao M., Stuurman N., Goshima G., Terayama K., Wong J.S., Vale R.D., Walter P., Farese R.V. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature. 2008;453:657–661. doi: 10.1038/nature06928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang A.H.C. Oleosins and oil bodies in seeds and other organs. Plant Physiol. 1996;110:1055–1061. doi: 10.1104/pp.110.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Athenstaedt K., Zweytick D., Jandrositz A., Kohlwein S.D., Daum G. Identification and characterization of major lipid particle proteins of the yeast Saccharomyces cerevisiae. J. Bacteriol. 1999;181:6441–6448. doi: 10.1128/jb.181.20.6441-6448.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subramanian V., Garcia A., Sekowski A., Brasaemle D.L. Hydrophobic sequences target and anchor perilipin A to lipid droplets. J. Lipid Res. 2004;45:1983–1991. doi: 10.1194/jlr.M400291-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Zehmer J.K., Bartz R., Liu P., Anderson R.G.W. Identification of a novel N-terminal hydrophobic sequence that targets proteins to lipid droplets. J. Cell Sci. 2008;121:1852–1860. doi: 10.1242/jcs.012013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abell B.M., Holbrook L.A., Abenes M., Murphy D.J., Hills M.J., Moloney M.M. Role of the proline knot motif in oleosin endoplasmic reticulum topology and oil body targeting. Plant Cell. 1997;9:1481–1493. doi: 10.1105/tpc.9.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abell B.M., Hahn M., Holbrook L.A., Moloney M.M. Membrane topology and sequence requirements for oil body targeting of oleosin. Plant J. 2004;37:461–470. doi: 10.1111/j.1365-313x.2003.01988.x. [DOI] [PubMed] [Google Scholar]
- 22.Ingelmo-Torres M., González-Moreno E., Kassan A., Hanzal-Bayer M., Tebar F., Herms A., Grewal T., Hancock J.F., Enrich C., Bosch M., Gross S.P., Parton R.G., Pol A. Hydrophobic and basic domains target proteins to lipid droplets. Traffic. 2009;10:1785–1801. doi: 10.1111/j.1600-0854.2009.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Müllner H., Zweytick D., Leber R., Turnowsky F., Daum G. Targeting of proteins involved in sterol biosynthesis to lipid particles of the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta. 2004;1663:9–13. doi: 10.1016/j.bbamem.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Kurat C.F., Natter K., Petschnigg J., Wolinski H., Scheuringer K., Scholz H., Zimmermann R., Leber R., Zechner R., Kohlwein S.D. Obese yeast: triglyceride lipolysis is functionally conserved from mammals to yeast. J. Biol. Chem. 2006;281:491–500. doi: 10.1074/jbc.M508414200. [DOI] [PubMed] [Google Scholar]
- 25.Athenstaedt K., Daum G. The life cycle of neutral lipids: synthesis storage and degradation. Cell. Mol. Life Sci. 2006;63:1355–1369. doi: 10.1007/s00018-006-6016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Czabany T., Athenstaedt K., Daum G. Synthesis, storage and degradation of neutral lipids in yeast. Biochim. Biophys. Acta. 2007;1771:299–309. doi: 10.1016/j.bbalip.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Rajakumari S., Grillitsch K., Daum G. Synthesis and turnover of non-polar lipids in yeast. Prog. Lipid Res. 2008;47:157–171. doi: 10.1016/j.plipres.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Walther T.C., Farese R.V. The life of lipid droplets. Biochim. Biophys. Acta. 2009;1791:459–466. doi: 10.1016/j.bbalip.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robenek H., Hofnagel O., Buers I., Robenek M.J., Troyer D., Severs N.J. Adipophilin-enriched domains in the ER membrane are sites of lipid droplet biogenesis. J. Cell Sci. 2006;119:4215–4224. doi: 10.1242/jcs.03191. [DOI] [PubMed] [Google Scholar]
- 30.Athenstaedt K., Jolivet P., Boulard C., Zivy M., Negroni L., Nicaud J.M., Chardot T. Lipid particle composition of the yeast Yarrowia lipolytica depends on the carbon source. Proteomics. 2006;6:1450–1459. doi: 10.1002/pmic.200500339. [DOI] [PubMed] [Google Scholar]
- 31.Erdmann R., Veenhuis M., Mertens D., Kunau W.H. Isolation of peroxisome-deficient mutants of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 1989;86:5419–5423. doi: 10.1073/pnas.86.14.5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenberger S., Connerth M., Zellnig G., Daum G. Phosphatidylethanolamine synthesized by three different pathways is supplied to peroxisomes of the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta. 2009;1791:379–387. doi: 10.1016/j.bbalip.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 33.Connerth M., Czabany T., Wagner A., Zellnig G., Leitner E., Steyrer E., Daum G. Oleate inhibits steryl ester synthesis and causes liposensitivity in yeast. J. Biol. Chem. 2010;285:26832–26841. doi: 10.1074/jbc.M110.122085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duplus E., Glorian M., Forest C. Fatty acid regulation of gene transcription. J. Biol. Chem. 2000;275:30749–30752. doi: 10.1074/jbc.R000015200. [DOI] [PubMed] [Google Scholar]
- 35.Trotter P.J. The genetics of fatty acid metabolism in Saccharomyces cerevisiae. Annu. Rev. Nutr. 2001;21:97–119. doi: 10.1146/annurev.nutr.21.1.97. [DOI] [PubMed] [Google Scholar]
- 36.Zinser E., Daum G. Isolation and biochemical characterization of organelles from the yeast, Saccharomyces cerevisiae. Yeast. 1995;11:493–536. doi: 10.1002/yea.320110602. [DOI] [PubMed] [Google Scholar]
- 37.Haid A., Suissa M. Immunochemical identification of membrane proteins after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Methods Enzymol. 1983;96:192–205. doi: 10.1016/s0076-6879(83)96017-2. [DOI] [PubMed] [Google Scholar]
- 38.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 39.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 40.Folch J., Lees M., Sloane-Stanley G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 41.Broekhuyse R.M. Phospholipids in tissues of the eye. I. Isolation, characterization and quantitative analysis by two-dimensional thin-layer chromatography of diacyl and vinyl-ether phospholipids. Biochim. Biophys. Acta. 1968;152:307–315. doi: 10.1016/0005-2760(68)90038-6. [DOI] [PubMed] [Google Scholar]
- 42.Schneiter R., Brugger B., Sandhoff R., Zellnig G., Leber A., Lampl M., Athenstaedt K., Hrastnik C., Eder S., Daum G., Paltauf F., Wieland F.T., Kohlwein S.D. Electrospray ionization tandem mass spectrometry (ESI-MS/MS) analysis of the lipid molecular species composition of yeast subcellular membranes reveals acyl chain-based sorting/remodeling of distinct molecular species en route to the plasma membrane. J. Cell Biol. 1999;146:741–754. doi: 10.1083/jcb.146.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ejsing C.S., Sampaio J.L., Surendranath V., Duchoslav E., Ekroos K., Klemm R.W., Simons K., Shevchenko A. Global analysis of the yeast lipidome by quantitative shotgun mass spectrometry. Proc. Natl. Acad. Sci. U.S.A. 2009;106:2136–2141. doi: 10.1073/pnas.0811700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guan X.L., Wenk M.R. Mass spectrometry-based profiling of phospholipids and sphingolipids in extracts from Saccharomyces cerevisiae. Yeast. 2006;23:465–477. doi: 10.1002/yea.1362. [DOI] [PubMed] [Google Scholar]
- 45.Garbarino J., Sturley S.L. Saturated with fat: new perspectives on lipotoxicity. Curr. Opin. Clin. Nutr. Metab. Care. 2009;12:110–116. doi: 10.1097/MCO.0b013e32832182ee. [DOI] [PubMed] [Google Scholar]
- 46.Petschnigg J., Wolinski H., Kolb D., Zellnig G., Kurat C.F., Natter K., Kohlwein S.D. Good fat, essential cellular requirements for triacylglycerol synthesis to maintain membrane homeostasis in yeast. J. Biol. Chem. 2009;284:30981–30993. doi: 10.1074/jbc.M109.024752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oelkers P., Cromley D., Padamsee M., Billheimer J.T., Sturley S.L. The DGA1 gene determines a second triglyceride synthetic pathway in yeast. J. Biol. Chem. 2002;277:8877–8881. doi: 10.1074/jbc.M111646200. [DOI] [PubMed] [Google Scholar]
- 48.Hiltunen J.K., Mursula A.M., Rottensteiner H., Wierenga R.K., Kastaniotis A.J., Gurvitz A. The biochemistry of peroxisomal beta-oxidation in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2003;27:35–64. doi: 10.1016/S0168-6445(03)00017-2. [DOI] [PubMed] [Google Scholar]
- 49.Takei K., Haucke V., Slepnev V., Farsad K., Salazar M., Chen H., De Camilli P. Generation of coated intermediates of clathrin-mediated endocytosis on protein-free liposomes. Cell. 1998;94:131–141. doi: 10.1016/s0092-8674(00)81228-3. [DOI] [PubMed] [Google Scholar]
- 50.Gardocki M.E., Jani N., Lopes J.M. Phosphatidylinositol biosynthesis: biochemistry and regulation. Biochim. Biophys. Acta. 2005;1735:89–100. doi: 10.1016/j.bbalip.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Carman G.M., Henry S.A. Phospholipid biosynthesis in the yeast Saccharomyces cerevisiae and interrelationship with other metabolic processes. Prog. Lipid Res. 1999;38:361–399. doi: 10.1016/s0163-7827(99)00010-7. [DOI] [PubMed] [Google Scholar]
- 52.Zweytick D., Athenstaedt K., Daum G. Intracellular lipid particles of eukaryotic cells. Biochim. Biophys. Acta. 2000;1469:101–120. doi: 10.1016/s0005-2736(00)00294-7. [DOI] [PubMed] [Google Scholar]
- 53.Saleem R.A., Knoblauch B., Mast F.D., Smith J.J., Boyle J., Dobson C.M., Long-O'Donnell R., Rachubinski R.A., Aitchison J.D. Genome-wide analysis of signaling networks regulating fatty acid-induced gene expression and organelle biogenesis. J. Cell Biol. 2008;181:281–292. doi: 10.1083/jcb.200710009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith J.J., Marelli M., Christmas R.H., Vizecoumar F.J., Dilworth d.J., Ideker T., Galitski T., Dimitrov K., Rachubinski R.A., Aitchison J.D. Transcriptome profiling to identify genes involved in peroxisome assembly and function. J. Cell Biol. 2002;158:259–271. doi: 10.1083/jcb.200204059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith J.J., Ramsey S.A., Marelli M., Marzolf B., Hwang D., Saleem R.A., Rachubinski R.A., Aitchison J.D. Transcriptional responses to fatty acid are coordinated by combinatorial control. Mol. Syst. Biol. 2007;3:115. doi: 10.1038/msb4100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gurvitz A., Rottensteiner H. The biochemistry of oleate induction: transcriptional upregulation and peroxisome proliferation. Biochim. Biophys. Acta. 2006;1763:1392–1402. doi: 10.1016/j.bbamcr.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 57.McCammon M.T., Hartmann M.A., Bottema C.D., Parks L.W. Sterol methylation in Saccharomyces cerevisiae. J. Bacteriol. 1984;157:475–483. doi: 10.1128/jb.157.2.475-483.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sickmann A., Reinders J., Wagner Y., Joppich C., Zahedi R., Meyer H.E., Schonfisch B., Perschil I., Chacinska A., Guiard B., Rehling P., Pfanner N., Meisinger C. The proteome of Saccharomyces cerevisiae mitochondria. Proc. Natl. Acad. Sci. U.S.A. 2003;100:13207–13212. doi: 10.1073/pnas.2135385100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Delom F., Szponarski W., Sommerer N., Boyer J.C., Bruneau J.M., Rossignol M., Gibrat R. The plasma membrane proteome of Saccharomyces cerevisiae and its response to the antifungal calcofluor. Proteomics. 2006;6:3029–3039. doi: 10.1002/pmic.200500762. [DOI] [PubMed] [Google Scholar]
- 60.Lockshon D., Surface L.E., Kerr E.O., Kaeberlein M., Kennedy B.K. The sensitivity of yeast mutants to oleic acid implicates the peroxisome and other processes in membrane function. Genetics. 2007;175:77–91. doi: 10.1534/genetics.106.064428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schulz T.A., Choi M.G., Raychaudhuri S., Mears J.A., Ghirlando R., Hinshaw J.E., Prinz W.A. Lipid-regulated sterol transfer between closely apposed membranes by oxysterol-binding protein homologues. J. Cell Biol. 2009;187:889–903. doi: 10.1083/jcb.200905007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raychaudhuri S., Prinz W.A. The diverse functions of oxysterol-binding proteins. Annu. Rev. Cell Dev. Biol. 2010;26:157–177. doi: 10.1146/annurev.cellbio.042308.113334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raychaudhuri S., Im Y.J., Hurley J.H., Prinz W.A. Nonvesicular sterol movement from plasma membrane to ER requires oxysterol-binding protein-related proteins and phosphoinositides. J. Cell Biol. 2006;173:107–119. doi: 10.1083/jcb.200510084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Im Y.J., Raychaudhuri S., Prinz W.A., Hurley J.H. Structural mechanism for sterol sensing and transport by OSBP-related proteins. Nature. 2005;437:154–158. doi: 10.1038/nature03923. [DOI] [PMC free article] [PubMed] [Google Scholar]