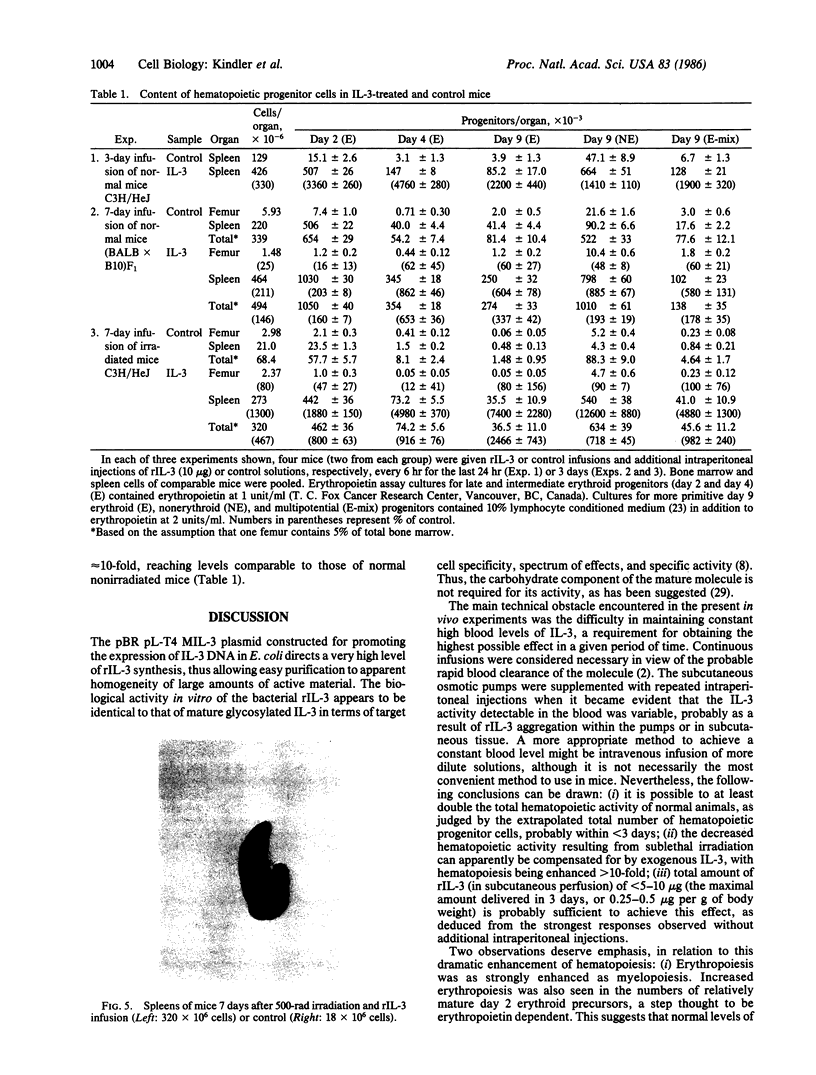
Abstract
Mouse interleukin 3 (IL-3) cDNA was cloned into a plasmid construction, allowing the synthesis of very high quantities of IL-3 in Escherichia coli. The recombinant (r) IL-3, purified to homogeneity, was active in vitro on the proliferation and differentiation of various hematopoietic progenitor cells at 1 pM. To maintain detectable blood levels of IL-3, osmotic pumps containing rIL-3 or control solutions were placed under the skin of normal and irradiated C3H/HeJ and (BALB X B10) F1 mice. The effect of IL-3 on hematopoietic progenitor cell numbers in spleen and bone marrow was evaluated 3 and 7 days later by using an in vitro clonal assay. The results demonstrated the following: (i) Doses of IL-3 infused at the rate of 2.5-5 ng per g of body weight per hr were sufficient to increase the numbers of hematopoietic progenitors in normal mice by at least 2-fold within 3 days. (ii) In mice with progenitor cell levels depressed by sublethal irradiation, 7-day treatment with IL-3 resulted in a 10-fold increase to near normal levels. (iii) The erythroid and myeloid lineages appeared to be enhanced to the same extent. (iv) Enhancement of hematopoiesis occurred primarily in spleen, but hematopoietic foci were also evident in the liver; in contrast, total cell and progenitor cell numbers were decreased in the bone marrow.
Full text
PDF

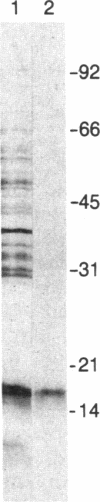
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazill G. W., Haynes M., Garland J., Dexter T. M. Characterization and partial purification of a haemopoietic cell growth factor in WEHI-3 cell conditioned medium. Biochem J. 1983 Mar 15;210(3):747–759. doi: 10.1042/bj2100747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Garland J., Scott D., Scolnick E., Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980 Oct 1;152(4):1036–1047. doi: 10.1084/jem.152.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Kafatos F. C., Maxam A. M., Maniatis T. Enzymatic in vitro synthesis of globin genes. Cell. 1976 Feb;7(2):279–288. doi: 10.1016/0092-8674(76)90027-1. [DOI] [PubMed] [Google Scholar]
- Eliason J. F., Odartchenko N. Colony formation by primitive hemopoietic progenitor cells in serum-free medium. Proc Natl Acad Sci U S A. 1985 Feb;82(3):775–779. doi: 10.1073/pnas.82.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliason J. F. Studies on the role of serum in long-term bone marrow cell cultures. Exp Hematol. 1983 Feb;11(2):98–106. [PubMed] [Google Scholar]
- Fung M. C., Hapel A. J., Ymer S., Cohen D. R., Johnson R. M., Campbell H. D., Young I. G. Molecular cloning of cDNA for murine interleukin-3. Nature. 1984 Jan 19;307(5948):233–237. doi: 10.1038/307233a0. [DOI] [PubMed] [Google Scholar]
- Goodman J. W., Hall E. A., Miller K. L., Shinpock S. G. Interleukin 3 promotes erythroid burst formation in "serum-free" cultures without detectable erythropoietin. Proc Natl Acad Sci U S A. 1985 May;82(10):3291–3295. doi: 10.1073/pnas.82.10.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson G. S., Bradley T. R. Properties of haematopoietic stem cells surviving 5-fluorouracil treatment: evidence for a pre-CFU-S cell? Nature. 1979 Oct 4;281(5730):381–382. doi: 10.1038/281381a0. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Henderson L., Klein F., Palaszynski E. Procedures for the purification of interleukin 3 to homogeneity. J Immunol. 1982 Dec;129(6):2431–2436. [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Oroszlan S., Henderson L. E., Copeland T. D., Fitch F., Prystowsky M. B., Goldwasser E., Schrader J. W., Palaszynski E. Biologic properties of homogeneous interleukin 3. I. Demonstration of WEHI-3 growth factor activity, mast cell growth factor activity, p cell-stimulating factor activity, colony-stimulating factor activity, and histamine-producing cell-stimulating factor activity. J Immunol. 1983 Jul;131(1):282–287. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee F., Bertrand K., Bennett G., Yanofsky C. Comparison of the nucleotide sequences of the initial transcribed regions of the tryptophan operons of Escherichia coli and Salmonella typhimurium. J Mol Biol. 1978 May 15;121(2):193–217. doi: 10.1016/s0022-2836(78)80005-9. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson A. M., Orkin S. H. Characterization of the homopolymer tailing reaction catalyzed by terminal deoxynucleotidyl transferase. Implications for the cloning of cDNA. J Biol Chem. 1982 Dec 25;257(24):14773–14782. [PubMed] [Google Scholar]
- Miyatake S., Yokota T., Lee F., Arai K. Structure of the chromosomal gene for murine interleukin 3. Proc Natl Acad Sci U S A. 1985 Jan;82(2):316–320. doi: 10.1073/pnas.82.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Oostra B. A., Harvey R., Ely B. K., Markham A. F., Smith A. E. Transforming activity of polyoma virus middle-T antigen probed by site-directed mutagenesis. Nature. 1983 Aug 4;304(5925):456–459. doi: 10.1038/304456a0. [DOI] [PubMed] [Google Scholar]
- Palacios R., Henson G., Steinmetz M., McKearn J. P. Interleukin-3 supports growth of mouse pre-B-cell clones in vitro. Nature. 1984 May 10;309(5964):126–131. doi: 10.1038/309126a0. [DOI] [PubMed] [Google Scholar]
- Rennick D. M., Lee F. D., Yokota T., Arai K. I., Cantor H., Nabel G. J. A cloned MCGF cDNA encodes a multilineage hematopoietic growth factor: multiple activities of interleukin 3. J Immunol. 1985 Feb;134(2):910–914. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Simons G., Remaut E., Allet B., Devos R., Fiers W. High-level expression of human interferon gamma in Escherichia coli under control of the pL promoter of bacteriophage lambda. Gene. 1984 Apr;28(1):55–64. doi: 10.1016/0378-1119(84)90087-8. [DOI] [PubMed] [Google Scholar]
- Suggs S. V., Wallace R. B., Hirose T., Kawashima E. H., Itakura K. Use of synthetic oligonucleotides as hybridization probes: isolation of cloned cDNA sequences for human beta 2-microglobulin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6613–6617. doi: 10.1073/pnas.78.11.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzer B. M. Genetic factors in leucocyte responses to endotoxin: further studies in mice. J Immunol. 1969 Jul;103(1):32–38. [PubMed] [Google Scholar]
- Switzer R. C., 3rd, Merril C. R., Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem. 1979 Sep 15;98(1):231–237. doi: 10.1016/0003-2697(79)90732-2. [DOI] [PubMed] [Google Scholar]
- TILL J. E., McCULLOCH E. A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961 Feb;14:213–222. [PubMed] [Google Scholar]
- Wiltrout R. H., Mathieson B. J., Talmadge J. E., Reynolds C. W., Zhang S. R., Herberman R. B., Ortaldo J. R. Augmentation of organ-associated natural killer activity by biological response modifiers. Isolation and characterization of large granular lymphocytes from the liver. J Exp Med. 1984 Nov 1;160(5):1431–1449. doi: 10.1084/jem.160.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Lee F., Rennick D., Hall C., Arai N., Mosmann T., Nabel G., Cantor H., Arai K. Isolation and characterization of a mouse cDNA clone that expresses mast-cell growth-factor activity in monkey cells. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1070–1074. doi: 10.1073/pnas.81.4.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]