Abstract
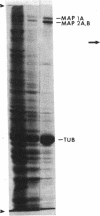
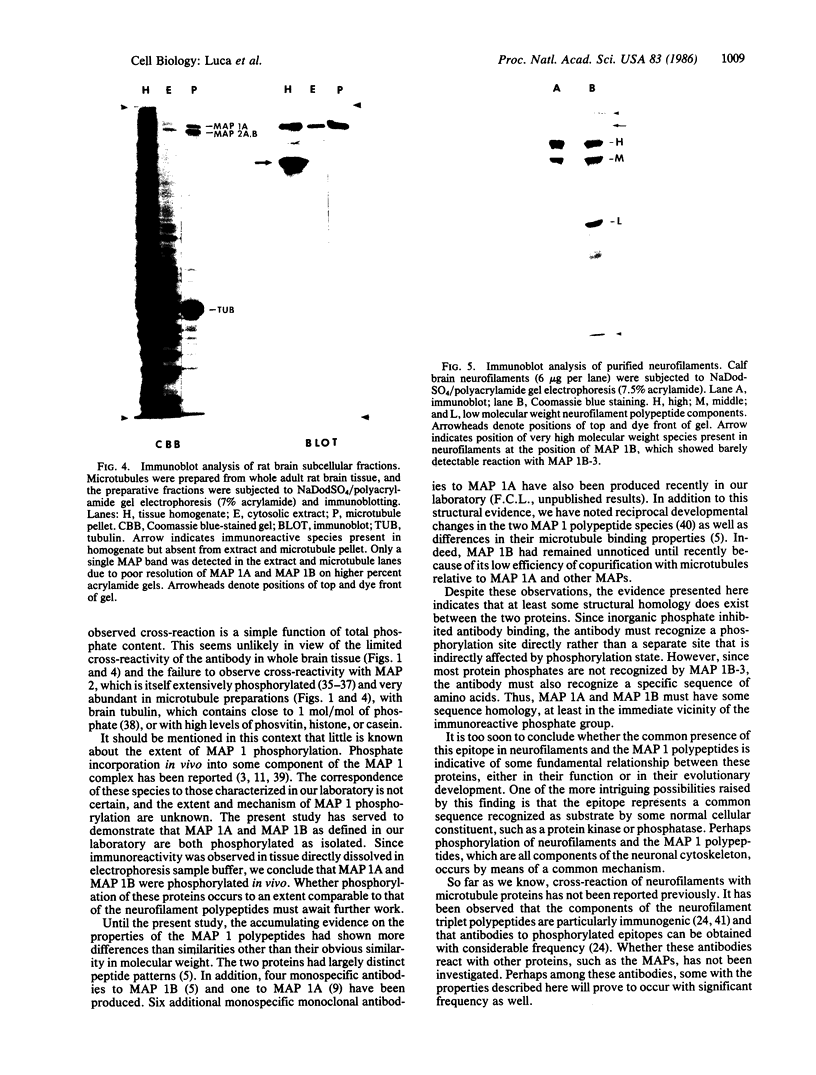
A monoclonal antibody is described that was raised against bovine brain microtubule-associated protein (MAP) 1B. Immunoblot analysis revealed that immunoreactivity was abolished by dephosphorylation of the antigen. The antigen/antibody reaction was also directly inhibited by sodium phosphate. In whole brain tissue, MAP 1B was the primary immunoreactive species. However, the antibody was also found to react with MAP 1A as well as with the high and middle molecular weight neurofilament polypeptides. No cross-reaction with MAP 2, which is known to be extensively phosphorylated, other MAPs, or the low molecular weight neurofilament polypeptide was observed. This evidence suggests at least some sequence homology between these different polypeptide components of the neuronal cytoskeleton and points to a common mechanism for their phosphorylation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asai D. J., Thompson W. C., Wilson L., Dresden C. F., Schulman H., Purich D. L. Microtubule-associated proteins (MAPs): a monoclonal antibody to MAP 1 decorates microtubules in vitro but stains stress fibers and not microtubules in vivo. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1434–1438. doi: 10.1073/pnas.82.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder L. I., Frankfurter A., Kim H., Caceres A., Payne M. R., Rebhun L. I. Heterogeneity of microtubule-associated protein 2 during rat brain development. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5613–5617. doi: 10.1073/pnas.81.17.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom G. S., Luca F. C., Vallee R. B. Microtubule-associated protein 1B: identification of a major component of the neuronal cytoskeleton. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5404–5408. doi: 10.1073/pnas.82.16.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom G. S., Luca F. C., Vallee R. B. Widespread cellular distribution of MAP-1A (microtubule-associated protein 1A) in the mitotic spindle and on interphase microtubules. J Cell Biol. 1984 Jan;98(1):331–340. doi: 10.1083/jcb.98.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom G. S., Schoenfeld T. A., Vallee R. B. Widespread distribution of the major polypeptide component of MAP 1 (microtubule-associated protein 1) in the nervous system. J Cell Biol. 1984 Jan;98(1):320–330. doi: 10.1083/jcb.98.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom G. S., Vallee R. B. Association of microtubule-associated protein 2 (MAP 2) with microtubules and intermediate filaments in cultured brain cells. J Cell Biol. 1983 Jun;96(6):1523–1531. doi: 10.1083/jcb.96.6.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne R. D., Cumming R. Ontogeny of microtubule-associated protein 2 in rat cerebellum: differential expression of the doublet polypeptides. Neuroscience. 1984 Jan;11(1):156–167. doi: 10.1016/0306-4522(84)90220-3. [DOI] [PubMed] [Google Scholar]
- Davis F. M., Tsao T. Y., Fowler S. K., Rao P. N. Monoclonal antibodies to mitotic cells. Proc Natl Acad Sci U S A. 1983 May;80(10):2926–2930. doi: 10.1073/pnas.80.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacourte A., Filliatreau G., Boutteau F., Biserte G., Schrevel J. Study of the 10-nm-filament fraction isolated during the standard microtubule preparation. Biochem J. 1980 Nov 1;191(2):543–546. doi: 10.1042/bj1910543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper B. A. Rat brain microtubule protein: purification and determination of covalently bound phosphate and carbohydrate. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2283–2287. doi: 10.1073/pnas.69.8.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek B., Heldin C. H. Use of an antiserum against phosphotyrosine for the identification of phosphorylated components in human fibroblasts stimulated by platelet-derived growth factor. J Biol Chem. 1984 Sep 10;259(17):11145–11152. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Frackelton A. R., Jr, Ross A. H., Eisen H. N. Characterization and use of monoclonal antibodies for isolation of phosphotyrosyl proteins from retrovirus-transformed cells and growth factor-stimulated cells. Mol Cell Biol. 1983 Aug;3(8):1343–1352. doi: 10.1128/mcb.3.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard D. L., Kirschner M. W. A polymer-dependent increase in phosphorylation of beta-tubulin accompanies differentiation of a mouse neuroblastoma cell line. J Cell Biol. 1985 Mar;100(3):764–774. doi: 10.1083/jcb.100.3.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Liem R. K., Shelanski M. L. Regulation of a high molecular weight microtubule-associated protein in PC12 cells by nerve growth factor. J Cell Biol. 1983 Jan;96(1):76–83. doi: 10.1083/jcb.96.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann H., Dalton J. M., Wiche G. Microheterogeneity of microtubule-associated proteins, MAP-1 and MAP-2, and differential phosphorylation of individual subcomponents. J Biol Chem. 1985 May 10;260(9):5797–5803. [PubMed] [Google Scholar]
- Hirokawa N., Bloom G. S., Vallee R. B. Cytoskeletal architecture and immunocytochemical localization of microtubule-associated proteins in regions of axons associated with rapid axonal transport: the beta,beta'-iminodipropionitrile-intoxicated axon as a model system. J Cell Biol. 1985 Jul;101(1):227–239. doi: 10.1083/jcb.101.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam K., Burns R. Multiple phosphorylation sites of microtubule-associated protein (MAP2) observed at high ATP concentrations. FEBS Lett. 1981 Jan 26;123(2):181–185. doi: 10.1016/0014-5793(81)80282-7. [DOI] [PubMed] [Google Scholar]
- Jones S. M., Williams R. C., Jr Phosphate content of mammalian neurofilaments. J Biol Chem. 1982 Sep 10;257(17):9902–9905. [PubMed] [Google Scholar]
- Julien J. P., Mushynski W. E. Multiple phosphorylation sites in mammalian neurofilament polypeptides. J Biol Chem. 1982 Sep 10;257(17):10467–10470. [PubMed] [Google Scholar]
- Kim H., Binder L. I., Rosenbaum J. L. The periodic association of MAP2 with brain microtubules in vitro. J Cell Biol. 1979 Feb;80(2):266–276. doi: 10.1083/jcb.80.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik K. S., Duffy L. K., Dowling M. M., Abraham C., McCluskey A., Selkoe D. J. Microtubule-associated protein 2: monoclonal antibodies demonstrate the selective incorporation of certain epitopes into Alzheimer neurofibrillary tangles. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7941–7945. doi: 10.1073/pnas.81.24.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai H., Imazawa M., Miyamoto K. Developmental changes in components of chick brain microtubule-associated protein-1 (MAP-1) and tau proteins. J Biochem. 1985 Feb;97(2):529–532. doi: 10.1093/oxfordjournals.jbchem.a135087. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liem R. Simultaneous separation and purification of neurofilament and glial filament proteins from brain. J Neurochem. 1982 Jan;38(1):142–150. doi: 10.1111/j.1471-4159.1982.tb10865.x. [DOI] [PubMed] [Google Scholar]
- Nairn A. C., Detre J. A., Casnellie J. E., Greengard P. Serum antibodies that distinguish between the phospho- and dephospho-forms of a phosphoprotein. Nature. 1982 Oct 21;299(5885):734–736. doi: 10.1038/299734a0. [DOI] [PubMed] [Google Scholar]
- Ross A. H., Baltimore D., Eisen H. N. Phosphotyrosine-containing proteins isolated by affinity chromatography with antibodies to a synthetic hapten. Nature. 1981 Dec 17;294(5842):654–656. doi: 10.1038/294654a0. [DOI] [PubMed] [Google Scholar]
- Sato C., Nishizawa K., Nakayama T., Kobayashi T. Effect upon mitogenic stimulation of calcium-dependent phosphorylation of cytoskeleton-associated 350,000- and 80,000-mol-wt polypeptides in quiescent 3Y1 cells. J Cell Biol. 1985 Mar;100(3):748–753. doi: 10.1083/jcb.100.3.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden S. C., Pollard T. D. Phosphorylation of microtubule-associated proteins regulates their interaction with actin filaments. J Biol Chem. 1983 Jun 10;258(11):7064–7071. [PubMed] [Google Scholar]
- Sloboda R. D., Dentler W. L., Rosenbaum J. L. Microtubule-associated proteins and the stimulation of tubulin assembly in vitro. Biochemistry. 1976 Oct 5;15(20):4497–4505. doi: 10.1021/bi00665a026. [DOI] [PubMed] [Google Scholar]
- Sloboda R. D., Rudolph S. A., Rosenbaum J. L., Greengard P. Cyclic AMP-dependent endogenous phosphorylation of a microtubule-associated protein. Proc Natl Acad Sci U S A. 1975 Jan;72(1):177–181. doi: 10.1073/pnas.72.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson K., Marton L. S., Dieperink M. E., Molnar G. K., Schlaepfer W. W., Helgason C. M. Circulating autoantibodies to the 200,000-dalton protein of neurofilaments in the serum of healthy individuals. Science. 1985 May 31;228(4703):1117–1119. doi: 10.1126/science.4039466. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Sternberger N. H. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurkauf W. E., Vallee R. B. Extensive cAMP-dependent and cAMP-independent phosphorylation of microtubule-associated protein 2. J Biol Chem. 1983 Jun 25;258(12):7883–7886. [PubMed] [Google Scholar]
- Vallee R. B. A taxol-dependent procedure for the isolation of microtubules and microtubule-associated proteins (MAPs). J Cell Biol. 1982 Feb;92(2):435–442. doi: 10.1083/jcb.92.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R. B., Borisy G. G. Removal of the projections from cytoplasmic microtubules in vitro by digestion with trypsin. J Biol Chem. 1977 Jan 10;252(1):377–382. [PubMed] [Google Scholar]
- Vandre D. D., Davis F. M., Rao P. N., Borisy G. G. Phosphoproteins are components of mitotic microtubule organizing centers. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4439–4443. doi: 10.1073/pnas.81.14.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]