Abstract
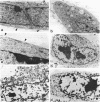
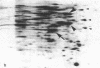
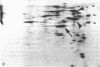
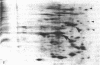
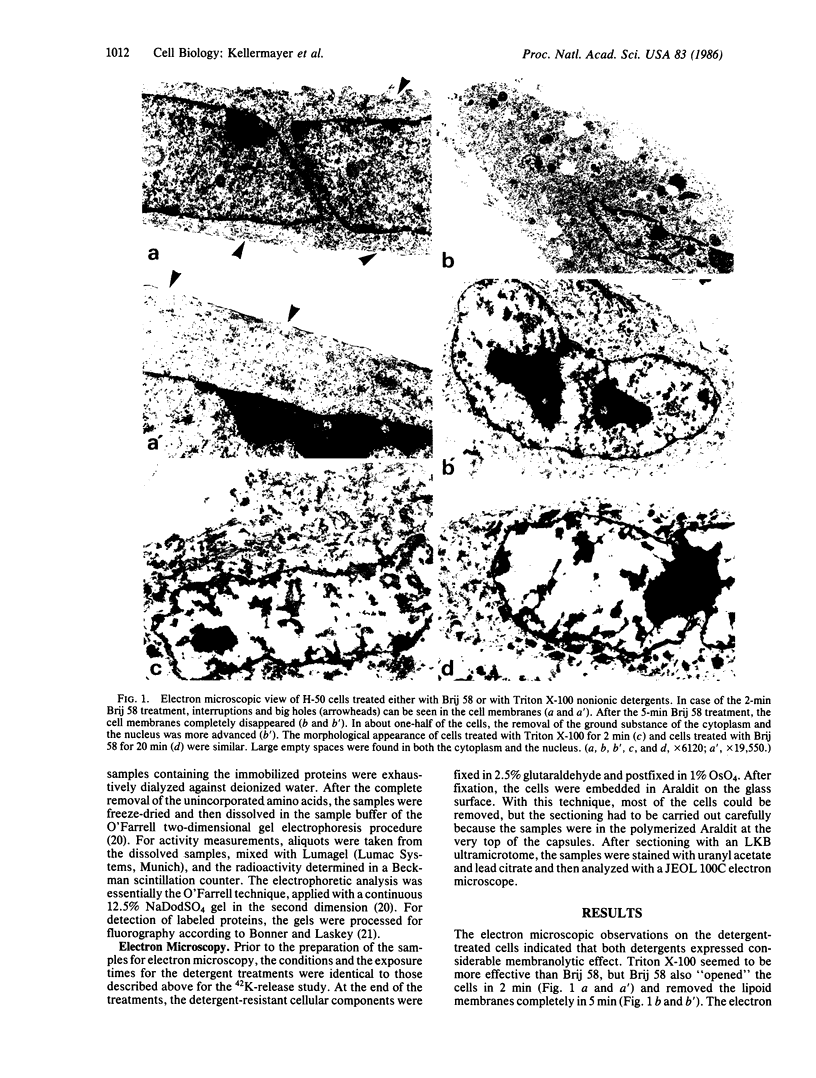
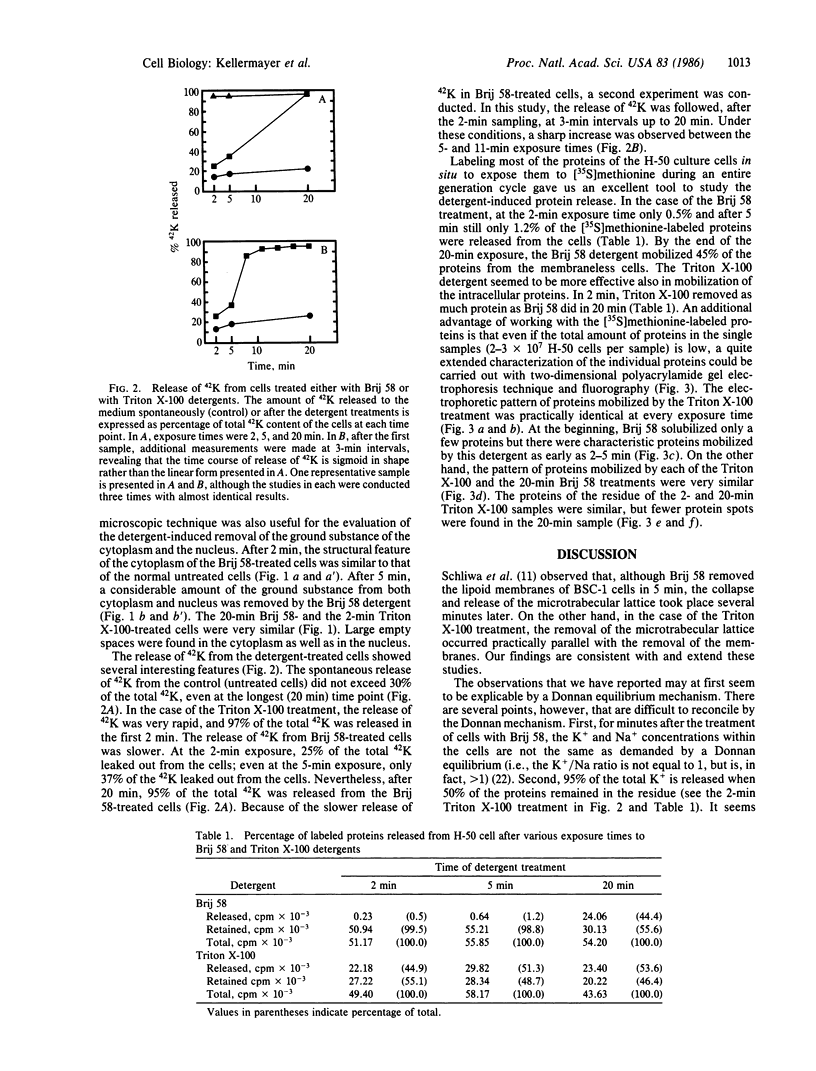
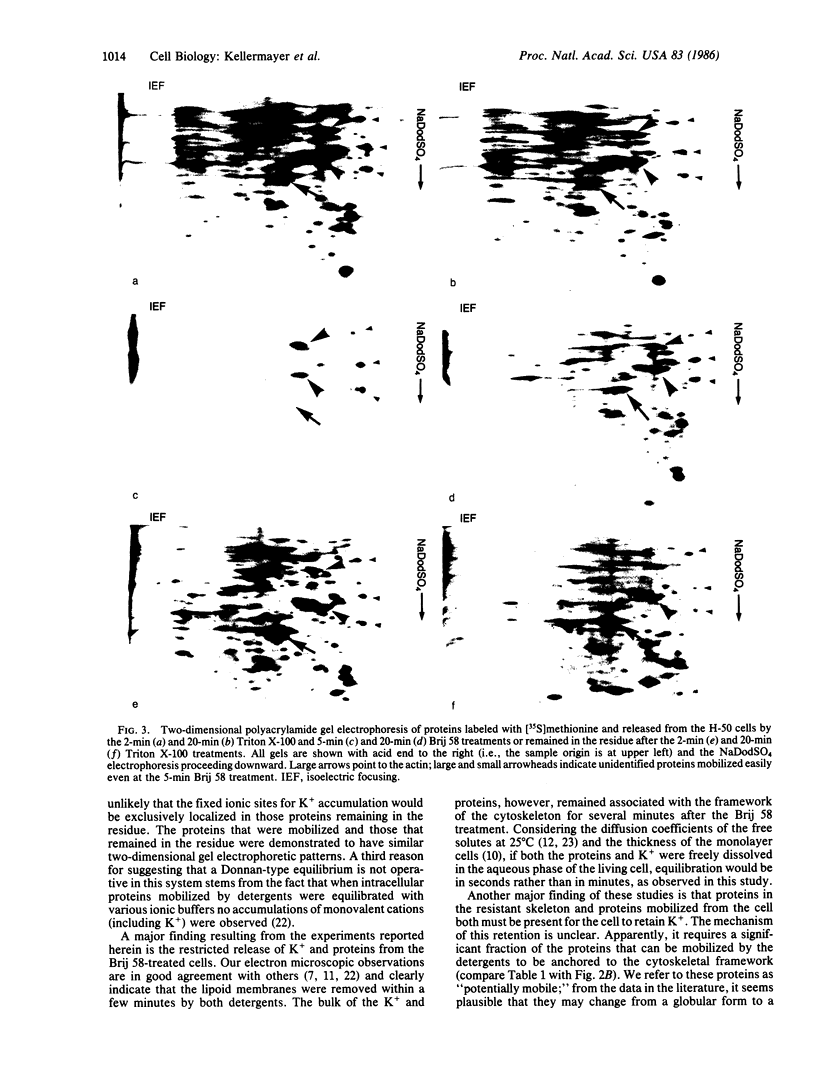
Monolayer H-50 tissue culture cells were treated with Triton X-100 and Brij 58 nonionic detergents, and their electron microscopic morphology along with the release of the intracellular proteins and K+ were studied. Although Triton X-100 was more effective, both detergents removed the lipoid membranes within 5 min. The mobilization and solubilization of the cytoplasmic and nuclear proteins occurred much faster with Triton X-100 than with Brij 58. In Triton X-100-treated cells, the loss of K+ was complete within 2 min. The loss of K+ from the Brij 58-treated cells was complete only after 10 min and the mobilization of K+ showed sigmoid-type release kinetics. These results support the view that most of K+ and "diffusible" proteins not freely dissolved in the cellular water, but they are cocompartmentalized inside the living cell.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cameron I. L., LaBadie D. R., Hunter K. E., Hazlewood C. F. Changes in water proton relaxation times and in nuclear to cytoplasmic element gradients during meiotic maturation of Xenopus oocytes. J Cell Physiol. 1983 Jul;116(1):87–92. doi: 10.1002/jcp.1041160113. [DOI] [PubMed] [Google Scholar]
- Clegg J. S. Properties and metabolism of the aqueous cytoplasm and its boundaries. Am J Physiol. 1984 Feb;246(2 Pt 2):R133–R151. doi: 10.1152/ajpregu.1984.246.2.R133. [DOI] [PubMed] [Google Scholar]
- Cope F. W., Damadian R. Biological ion exchanger resins. IV. Evidence for potassium association with fixed charges in muscle and brain by pulsed nuclear magnetic resonance of 39K. Physiol Chem Phys. 1974;6(1):17–30. [PubMed] [Google Scholar]
- Dean W. L., Suarez C. P. Binding, activation, and solubilization of the Ca2+-ATPase from sarcoplasmic reticulum by nonionic detergents. Membr Biochem. 1984;5(3):181–191. doi: 10.3109/09687688409150277. [DOI] [PubMed] [Google Scholar]
- Edelmann L. Potassium binding sites in muscle: electron microscopic visualization of K, Rb, and Cs in freeze-dried preparations and autoradiography at liquid nitrogen temperature using 86Rb and 134Cs. Histochemistry. 1980;67(3):233–242. doi: 10.1007/BF00692757. [DOI] [PubMed] [Google Scholar]
- Ernst E. Inorganic materials in the striated muscle. Acta Biochim Biophys Acad Sci Hung. 1975;10(1-2):95–99. [PubMed] [Google Scholar]
- Felix H. Permeabilized cells. Anal Biochem. 1982 Mar 1;120(2):211–234. doi: 10.1016/0003-2697(82)90340-2. [DOI] [PubMed] [Google Scholar]
- Fey E. G., Capco D. G., Krochmalnic G., Penman S. Epithelial structure revealed by chemical dissection and unembedded electron microscopy. J Cell Biol. 1984 Jul;99(1 Pt 2):203s–208s. doi: 10.1083/jcb.99.1.203s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton A. B. Assembly associated with the cytomatrix. J Cell Biol. 1984 Jul;99(1 Pt 2):209s–211s. doi: 10.1083/jcb.99.1.209s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallyas F. Physico-chemical mechanism of the argyrophil I reaction. Histochemistry. 1982;74(3):393–407. doi: 10.1007/BF00493439. [DOI] [PubMed] [Google Scholar]
- Gascoyne P. R., Pethig R., Szent-Györgyi A. Water structure-dependent charge transport in proteins. Proc Natl Acad Sci U S A. 1981 Jan;78(1):261–265. doi: 10.1073/pnas.78.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Kirschner M. W. Filament organization revealed in platinum replicas of freeze-dried cytoskeletons. J Cell Biol. 1980 Jul;86(1):212–234. doi: 10.1083/jcb.86.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellermayer M., Hazlewood C. F. Dynamic inorganic ion-protein interactions in structural organization of DNA of living cell nuclei. Cancer Biochem Biophys. 1979;3(4):181–188. [PubMed] [Google Scholar]
- Kellermayer M., Jobst K. Cytoplasmic protein network in HeLa cells. Histochemistry. 1975 Jul 30;44(2):193–195. doi: 10.1007/BF00494081. [DOI] [PubMed] [Google Scholar]
- Kellermayer M., Rouse D., Gyorkey F., Hazlewood C. F. Potassium retention in membraneless thymus lymphocyte nuclei. Physiol Chem Phys Med NMR. 1984;16(6):503–511. [PubMed] [Google Scholar]
- Kellermayer M., Rouse D., Györkey F., Hazlewood C. F. Ionic milieu and volume adjustments in detergent-extracted thymic nuclei. Physiol Chem Phys Med NMR. 1983;15(4):345–354. [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annu Rev Biochem. 1982;51:219–250. doi: 10.1146/annurev.bi.51.070182.001251. [DOI] [PubMed] [Google Scholar]
- Lehto V. P., Virtanen I., Kurki P. Intermediate filaments anchor the nuclei in nuclear monolayers of cultured human fibroblasts. Nature. 1978 Mar 9;272(5649):175–177. doi: 10.1038/272175a0. [DOI] [PubMed] [Google Scholar]
- Ling G. N. The physical state of water and ions in living cells and a new theory of the energization of biological work performance by ATP. Mol Cell Biochem. 1977 May 3;15(3):159–172. doi: 10.1007/BF01734106. [DOI] [PubMed] [Google Scholar]
- Mastro A. M., Keith A. D. Diffusion in the aqueous compartment. J Cell Biol. 1984 Jul;99(1 Pt 2):180s–187s. doi: 10.1083/jcb.99.1.180s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negendank W. Studies of ions and water in human lymphocytes. Biochim Biophys Acta. 1982 Oct 20;694(2):123–161. doi: 10.1016/0304-4157(82)90022-3. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. The detertent-resistant cytoskeleton of tissue culture cells includes the nucleus and the microfilament bundles. Exp Cell Res. 1977 May;106(2):339–349. doi: 10.1016/0014-4827(77)90179-3. [DOI] [PubMed] [Google Scholar]
- Porter K. R., Tucker J. B. The ground substance of the living cell. Sci Am. 1981 Mar;244(3):56–67. doi: 10.1038/scientificamerican0381-56. [DOI] [PubMed] [Google Scholar]
- Schliwa M., van Blerkom J., Porter K. R. Stabilization and the cytoplasmic ground substance in detergent-opened cells and a structural and biochemical analysis of its composition. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4329–4333. doi: 10.1073/pnas.78.7.4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H., Haken H. Co-operative dynamics in organelles. J Theor Biol. 1983 Sep 21;104(2):261–273. doi: 10.1016/0022-5193(83)90414-9. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. Contribution of actin to the structure of the cytoplasmic matrix. J Cell Biol. 1984 Jul;99(1 Pt 2):15s–21s. doi: 10.1083/jcb.99.1.15s. [DOI] [PMC free article] [PubMed] [Google Scholar]