Abstract
Epidermal growth factor receptor (EGFR)-targeted therapies have been effective in some cancers, but not in hepatocellular carcinoma (HCC). The aim of this study was to investigate the drug potential to overcome multi-drug resistance in HCC cells. Thirteen drug-sensitive HCC cells were assessed using the CCK-8 assay. G0-G1 arrest was measured by FACS. Western blot analysis was used to detect the key enzymes in both the Ras/Raf and PI3K pathways. When establishing the IC50 of HCC to several drugs, including EKB-569, sorafenib, erlotinib, gefitinib, pazopanib, and brivanib, SK-Hep1 cells treated with EKB-569 have shown the highest (72.8%-86.4%) G0-G1 arrest and decreased the phosphorylation of AKT and ERK at the protein level. We found that EKB-569 had higher efficacy in HCC, compared to first generation, reversible EGFR-TK inhibitors. Furthermore, the combination of sorafenib and EKB-569 showed a synergistic effect to inhibit proliferation of SNU-475, previously the most resistant cell to EGFR-TKIs. Therefore, novel EKB-569 in combination with sorafenib may be able to overcome HCC resistance to EGFR-TK inhibitors.
Keywords: Epidermal Growth Factor Receptor (EGFR)-Tyrosine Kinase Inhibitors (TKIs), EKB-569, Multi-drug Resistance, Hepatocellular Carcinoma (HCC) Cells
INTRODUCTION
With an annual incidence of over 560,000 deaths, hepatocellular carcinoma (HCC) is the sixth most common malignancy and the third leading cause of cancer-related mortality worldwide (1). Liver cancer accounts for 4% of all cancers and more than 70% of all liver cancers occur in Asia, with high incidence of liver cancer in the East Asian countries, including Korea, China, and Japan (2). Recent research has demonstrated that Ras/Raf/MAPK and PI3K/AKT/mTOR pathways appear to modulate important signaling sequences in the development and progression of HCC. The Ras/Raf/MAPK pathway is activated in the majority of advanced HCCs, as a result of increased signaling induced from upstream growth factors, such as epidermal growth factor (EGF), hepatocyte growth factor (HGF), or insulin-like growth factor (IGF), and also because of inactivation of tumor suppressor genes, including PTEN (3, 4). The PI3K/AKT/mTOR signaling pathway plays a pivotal role in HCC and was found activated in 30%-50% of HCC cases (5). The etiology of HCC tumorigenesis and recurrence is currently poorly understood, and there is urgent need to find effective targets to treat HCC and to prevent tumor recurrence.
Sorafenib is a multi-targeted tyrosine kinase inhibitor acting on vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), raf, c-kit, and flt-3, and has been shown to inhibit HCC-induced proliferation and angiogenesis. Recent clinical trials for sorafenib treatment of advanced HCC demonstrated promising results (6-8). Various other novel drugs are currently under study to enhance efficacy and reduce toxicity in the treatment of advanced HCC. Brivanib has been shown to demonstrate potent and selective inhibition of both VEGFR and FGFR-1 tyrosine kinases (9) and inhibited the growth of HCC xenografts in vivo (10). Multicenter phase III studies involving brivanib in patients with advanced HCC are ongoing. Pazopanib is another potent, multi-target receptor tyrosine kinase inhibitor of VEGFR-1, -2, and -3, PDGFR-α and -β, and c-kit, and has demonstrated in vivo anti-tumor effect in HCC xenografts (11).
The epidermal growth factor receptor (EGFR) signaling pathway is an important mediator of cancer cell oncogenesis, proliferation, maintenance, and survival. For this reason, it has long been an attractive candidate as anticancer drug target (12). Both gefitinib and erlotinib, the first-generation EGFR tyrosine kinase inhibitors (TKIs), have single-agent activity against various cancer cells, including advanced non-small cell lung cancer (NSCLC); thus, erlotinib improved survival when given as salvage treatment after chemotherapy in NSCLC (13, 14), but showed only a minor effect in HCC (15, 16). The second generation of EGFR TKIs, including EKB-569, is now emerging from the developmental pipeline and is being introduced into clinical trials. In addition to blocking EGFR signaling, these novel EGFR TKIs target additional members of the ErbB family, such as HER-2 or other downstream or parallel pathways, including the VEGFR pathway. EKB-569 is a potent, low molecular weight, selective and second-generation irreversibly binding inhibitor of EGFR-TK activity (17).
The purpose of this in vitro study was to investigate the effects of the second-generation compound (EKB-569) in HCC. EKB-569 was evaluated for its potential as part of a chemosensitizing combination treatment with sorafenib, in tailored therapies for resistant tumors.
MATERIALS AND METHODS
Cell culture
Four human hepatoma cell lines (Hep3B, Huh-7, SK-Hep1, and HepG2) were cultured in DMEM medium (Life Technologies, Grand Island, NY, USA). Similarly, SNU-354, SNU-368, SNU-398, SNU-423, SNU-449, SNU-475, SNU-739, SNU-886, and SNU-878 cells were cultured in RPMI-1640 medium, supplemented with 10% fetal bovine serum (FBS) and antibiotics (Life Technologies). The cultured cells were incubated in 5% CO2 at 37℃.
Chemicals and antibodies
Sorafenib, erlotinib, gefitinib, pazopanib, and brivanib were obtained from LC Laboratories (Woburn, MA, USA). EKB-569 was obtained from Wyeth (Pfizer Inc., NY, NY, USA). Primary antibodies against either total or phosphorylated (p) AKT (Ser473), ERK1/2 (Thr 202/204), STAT3, and EGFR (Cell Signaling Technology, Danvers, MA, USA), cyclinD1, p27, and Rb (BD biosciences, San Diego, CA, USA), β-actin (Sigma-Aldrich, St. Louis, MO, USA), CDK4, P21, phospho-Rb, anti-rabbit IgG horseradish peroxidase, and mouse IgG were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Phosphatase inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA), cell counting kit-8 (CCK-8; Dojindo Molecular Technologies Inc., Kumamoto, Japan), and Restore-Stripping Buffer for Western blot were obtained from Pierce (Rockford, IL, USA).
Drug treatment and cell viability
Thirteen exponentially growing HCC cell lines were trypsinized and plated at 4-5 × 103 cells/mL in 96-well culture plates. After 24 hr incubation, cells were treated for 72 hr with serial three-fold dilutions of 10 µM for each drug. Each experiment was repeated at least three times. Cell viability was measured by the CCK-8 assay. The optical density (OD) was measured using a spectrophotometer (Molecular Devices Co., Sunnyvale, CA, USA). Graph and fold was calculated with PRISM Software (GraphPad Software, CA, USA).
Cell cycle analysis by flow cytometry
Approximately 2 × 106 cells were trypsinized, washed twice with PBS, and fixed in 70% ice-cold ethanol for 1 hr. The samples were then concentrated by removing the ethanol and exposed to 100 µg/mL RNase A (8 µL; Sigma-Aldrich) for 30 min, at 37℃. Cellular DNA was stained with propidium iodide. Cell cycle distributions were determined using flow cytometry (BD FACS Caliber; Biosciences, San Jose, CA, USA).
Western blot analysis
Protein extraction was done by protein extraction solution (iNtRON Biotechnology, Seongnam, Korea) with a protease inhibitor cocktail (Sigma-Aldrich) and protein quantification was performed by a BCA Protein Assay kit (Pierce). Equal amounts of protein (50 µg) were electrophoresed on NuPAGE 4%-12% Bis- Tris Gels (Invitrogen, Carlsbad, CA, USA), and transferred to Immobilon-P membrane (Millipore, Billerica, MA, USA). The membranes were then blocked with 5% non-fat milk and probed with the primary antibody (1:1,000) in 1 × TBS with Tween-20 buffer (BIOSESANG Inc., Seongnam, Korea). Then, the blots were visualized under enhanced chemiluminescence (Amersham, Buckinghamshire, UK), according to the manufacturer's instructions.
RESULTS
Response of human hepatoma cell lines to EGFR inhibitor and sorafenib
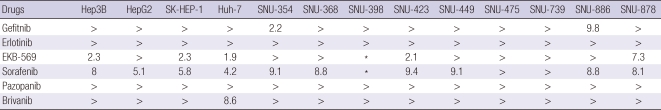
To explore the potential anti-cancer agent on HCC, we examined the effects of multi-targeted tyrosine kinase inhibitors, and first and second-generation EGFR-targeted agents on the growth of human hepatoma cell lines (Table 1). Each test was performed with drug concentration at a serial 3-fold dilution (10 µM to 3.3 nM) and similar results were obtained from three independent experiments. Although we could not find a drug most effective in all 13 cell lines (Table 1), the IC50 values of the cell lines treated with the irreversible ErbB inhibitor (EKB-569) were lower than those of cells treated with gefitinb, erlotinib, brivanib, and pazopanib, except for sorafenib, which seemed the most effective of the drugs. In addition, Hep3B, HepG2, SK-Hep1, Huh-7, SNU-423, SNU-739 and SNU-878 cells treated with EKB-569 showed better results even than sorafenib treatment. All drugs showed IC50 values > 10 µM in SNU-398, SNU-475 and SNU-739. In the SNU-398 cell line in particular, the IC50 values of both sorafenib and EKB-569 were less than 5 µM in replicate independent experiments (data not shown). However, subsequent subculture altered the response to the drugs. Therefore additional experiments were not performed, and EKB-569 and sorafenib were indicated as asteriks in Table 1. SNU-739 cells treated with IGF-1R inhibitor and other second generation EGFR-TKIs (data not shown) showed IC50 < 10 µM. SK-Hp1, Huh-7, SNU-447 and SNU-739 cells treated with brivanib and SNU-449 and SNU-886 cells treated with EKB-569 showed values close to IC50 of 10 µM. However, in cell viability experiments with drugs (including unpublished data), the results obtained for SNU-475 cells could not be reproduced in all 13 HCC cell lines. Therefore, for further study of EKB-569 mechanism, we chose the cell line SK-Hep1 which was sensitive to EKB-569, and the cell line SNU-475, which showed the greatest resistance to the drug.
Table 1.
IC50 of HCC cell lines to several drugs
Effect of sorafenib and EGFR-targeted therapies on the growth of human hepatoma cell lines. Thirteen hepatoma cell lines were treated with sorafenib, VGFR, and EGFR-targeted therapies for 24 hr at concentrations ranging between 3.3 nM and 10 µM. Cellular viability was assessed by the CCK-8 assay. IC50 value was calculated using PRISM Software. In general, values over 10 µM showed significant change, indicated by " > "; asteriks (*) indicates data not shown.
Increase of G0-G1 arrest by EKB-569
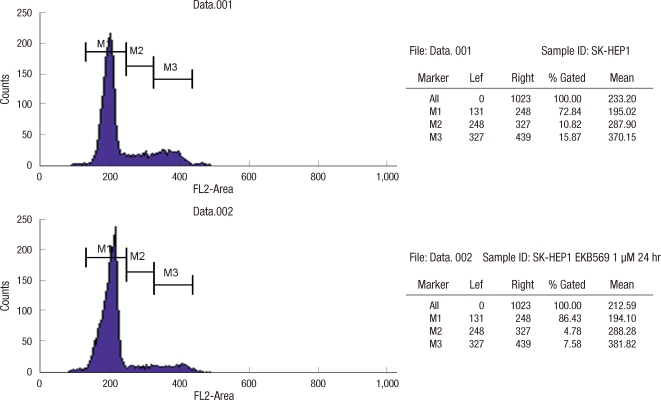
To examine how EKB-569 inhibits cell proliferation, we analyzed G0-G1 arrest by flow cytometry, after SK-Hep1 and SNU-475 cells were treated with EKB-569 for 24 hr. In SK-Hep1 cells, G0-G1 arrest was increased with approximately 13.6% (from 72.8% to 86.4%) with 1 µM EKB-569. However, in SNU-475 cells, G0-G1 arrest only increased 5.4% (from 75.8% to 81.2%) even with 5 µM EKB-569 (Fig. 1).
Fig. 1.
Detection of EKB-569-induced G0-G1 arrest by FACS analysis. SK-Hep1 cells were treated with the indicated concentrations of EKB-569 for 24 hr. EKB-569 induced G0-G1 arrest in SK-Hep1.
EKB-569 decreased the phosphorylation of AKT and ERK at the protein level
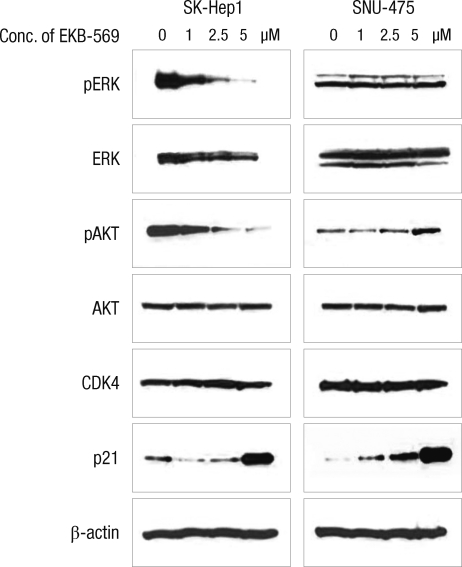
SK-Hep1 and SNU-475 cells were treated with the indicated concentrations of EKB-569 for 24 hr (Fig. 2). In SK-Hep1 cells, after treatment with EKB-569, we found down-regulation of phosphorylated ERK and AKT and up-regulation of p21 in a dose-dependent manner; however, there were no significant differences in the protein levels of cell cycle-related protein, CDK4, and EGFR following treatment with 1, 2.5, or 5 µM EKB-569 (data not shown).
Fig. 2.
EKB-569 decreased the phosphorylation of AKT and ERK at the protein level. SK-Hep1 cells were treated with the indicated concentrations of EKB-569 for 24 hr, and Western blotting of total-, pERK, total-, pAKT, CDK4, p21, and β-actin (loading control) was performed. Immunoblotting for each protein was done at least twice, using independently prepared lysates.
Effect of combined sorafenib and EKB-569 treatment on EGFR-targeted resistant cell line
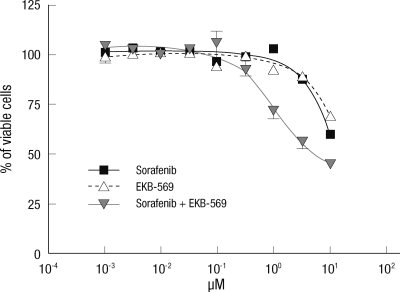
We determined whether sorafenib plus EKB-569 could have an effect on drug-resistant cell lines. SNU-475 cell line was the most resistant to several drugs in our experiments. Therefore, SNU-475 cells were treated with sorafenib alone, EKB-569 alone, or sorafenib plus EKB-569 combination at 0, 0.0033. 0.01, 0.033, 0.1, 0.33, 1, 3.3, and 10 µM concentrations, respectively, for 72 hr (Fig. 3). Cellular viability was measured using the CCK-8 kit. The combination of these two agents had a synergistic effect on SNU-475 cells and the IC50 was 5.1 µM. These results suggest that combined treatment with sorafenib and EKB-569 may have a therapeutic effect on hepatoma cells, which were resistant to many other molecular-targeted agents.
Fig. 3.
Effect of sorafenib/EKB-569 co-treatment on the EGFR-targeted resistant SNU-475 cell line. SNU-475 cells were treated with sorafenib alone, EKB-569 alone, or sorafenib plus EKB-569 at the indicated concentrations, for 72 hr. Cellular viability was measured using the CCK-8 assay.
DISCUSSION
HCC is one of the most resistant tumors for which conventional chemotherapy has shown only a minor effect, with response rates below 10% (18, 19). Sorafenib has been shown to inhibit tumor cell proliferation by blocking the Ras/Raf/MAPK pathway and to inhibit angiogenesis by blocking both VEGFR and PDGFR signaling (6). Therefore, sorafenib was known to be the most effective agent in HCC. To identify the new potential anti-tumor agents on HCC, we screened several drugs, including targeted agents under current ongoing investigation, such as pazopanib, brivanib, EKB-569, elrotinib, gefitinib, and sorafenib (9-11). In our results from 13 HCC cell lines, the novel irreversible EGFR inhibitor EKB-569 showed higher efficacy than first generation, reversible EGFR inhibitors (Table 1). To the best of our knowledge, this is the first report highlighting the effects of EKB-569 on HCC.
Drugs that alter the cell cycle are of particular interest, since cell cycle regulation is the basic mechanism underlying proliferation, differentiation, and death of cancer cells. Progression of cells through the normal cell cycle requires coordinated actions of positive and negative factors. Mitogenic signals stimulate sequential assembly and activation of cyclin D/CDK4/6 and cyclinE/CDK2 in early and late G1, resulting in the phosphorylation of Rb protein, release of E2F transcription factors, and induction of late-G1-specific genes (20, 21). Because we observed inhibition of cell proliferation using a microscope, we examined cell cycle arrest by flow cytometry and Western blot analysis in SK-Hep1 and SNU475 cells treated with EKB-569 for 24 hr. In cells sensitive to novel EGFR-TKI, such as SK-Hep1, only 1 µM EKB-569 inhibited proliferation by increasing G0-G1 arrest (Fig. 1) and caused a dose-dependent decrease in the expression of pAKT and pERK and a dose-dependent increase in the expression of p21 (Fig. 2), but did not change CDK4, cyclin D1, and p27 (data not shown). Because AKT seemed to play an important role in the survival of tumor cells in the present study, AKT may be one of the targets in HCC therapy.
There are some reports showing that tumorigenesis may be related to EGFR mutations, MET amplifications (22), the IGF-1R pathway (23), PTEN (24, 25), PIK3CA (26), and KRAS (27) mutations in various cancer cells, but no EGFR mutations in HCC (28, 29). We also analyzed the PIK3CA exon 20 and KRAS exon 1 in 13 HCC cell lines. Our results were similar to the effects on wildtype cells in all 13 HCC cell lines. We did not find any association with certain mutations of genes or pathways in both EKB-569 sensitive and resistant cell lines. Of all 13 HCC cell lines, SNU-475 was the most resistant to several molecular-targeted agents, including EKB-569. We have shown for the first time that sorafenib plus EKB-569 has an effect on drug-resistant cell lines. Sorafenib (10 µM) or EKB-569 (10 µM) alone could not inhibit cell proliferation, but combination treatment of these agents had a synergistic effect on SNU-475 cells. This result suggests that combined treatment with sorafenib and EKB-569 represents a promising strategy to overcome resistance against various EGFR-TKIs and sorafenib. EKB-569 alone and in combination with sorafenib requires further study to identify the exact signaling pathway. Nevertheless, these approaches might have important implications for the development of a tailored treatment strategy in patients with highly resistant hepatocellular carcinoma.
In this study, we found that EKB-569 had higher efficacy in HCC, compared to first generation, reversible EGFR inhibitors. Furthermore, the combination of sorafenib and EKB-569 might be able to overcome HCC resistance to EGFR inhibitors.
Footnotes
This study was supported by a Samsung Biomedical Research Institute grant (#SBRI C-A9-236).
AUTHOR SUMMARY
Novel EGFR-TK Inhibitor EKB-569 Inhibits Hepatocellular Carcinoma Cell Proliferation by AKT and MAPK Pathways
Heesue Kim and Ho Yeong Lim
We investigated drugs that can potentially overcome multi-drug resistance in hepatocellular carcinoma (HCC) cells. SK-Hep1 cells treated with EKB-569 showed the highest G0-G1 arrest and decreased the phosphorylation of AKT and ERK. The combination of sorafenib and EKB-569 showed a synergistic effect to inhibit proliferation of SNU-475, previously the most resistant HCC cell to EGFR-Tyrosin kinase inhibitors. These approaches might have important implications for the development of a tailored treatment strategy in patients.
References
- 1.Online analysis. GLOBOCAN 2008. [accessed on 26 Oct 2011]. Available at http://globocan.iarc.fr/
- 2.Related statistics. Korean National Cancer Center. [accessed on 1 April 2011]. Available at http://www.ncc.re.kr.
- 3.Strumberg D. Preclinical and clinical development of the oral multikinase inhibitor sorafenib in cancer treatment. Drugs Today (Barc) 2005;41:773–784. doi: 10.1358/dot.2005.41.12.937959. [DOI] [PubMed] [Google Scholar]
- 4.Sze KM, Wong KL, Chu GK, Lee JM, Yau TO, Oi-Lin Ng I. Loss of phosphatase and tensin homolog enhances cell invasion and migration through AKT/Sp-1 transcription factor/matrix metalloproteinase 2 activation in hepatocellular carcinoma and has clinicopathologic significance. Hepatology. 2011;53:1558–1569. doi: 10.1002/hep.24232. [DOI] [PubMed] [Google Scholar]
- 5.Minguez B, Tovar V, Chiang D, Villanueva A, Llovet JM. Pathogenesis of hepatocellular carcinoma and molecular therapies. Curr Opin Gastroenterol. 2009;25:186–194. doi: 10.1097/MOG.0b013e32832962a1. [DOI] [PubMed] [Google Scholar]
- 6.Chaparro M, González Moreno L, Trapero-Marugán M, Medina J, Moreno-Otero R. Pharmacological therapy for hepatocellular carcinoma with sorafenib and other oral agents. Aliment Pharmacol Ther. 2008;28:1269–1277. doi: 10.1111/j.1365-2036.2008.03857.x. [DOI] [PubMed] [Google Scholar]
- 7.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 8.Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, De Greve J, Douillard JY, Lathia C, Schwartz B, Taylor I, Moscovici M, Saltz LB. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293–4300. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 9.Bhide RS, Cai ZW, Zhang YZ, Qian L, Wei D, Barbosa S, Lombardo LJ, Borzilleri RM, Zheng X, Wu LI, Barrish JC, Kim SH, Leavitt K, Mathur A, Leith L, Chao S, Wautlet B, Mortillo S, Jeyaseelan R, Sr, Kukral D, Hunt JT, Kamath A, Fura A, Vyas V, Marathe P, D'Arienzo C, Derbin G, Fargnoli J. Discovery and preclinical studies of (R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[2,1-f][1,2,4] triazin-6-yloxy)propan-2-ol(BMS-540215), an in vivo active potent VEGFR-2 inhibitor. J Med Chem. 2006;49:2143–2146. doi: 10.1021/jm051106d. [DOI] [PubMed] [Google Scholar]
- 10.Huynh H, Ngo VC, Fargnoli J, Ayers M, Soo KC, Koong HN, Thng CH, Ong HS, Chung A, Chow P, Pollock P, Byron S, Tran E. Brivanib alaninate, a dual inhibitor of vascular endothelial growth factor receptor and fibroblast growth factor receptor tyrosine kinases, induces growth inhibition in mouse models of human hepatocellular carcinoma. Clin Cancer Res. 2008;14:6146–6153. doi: 10.1158/1078-0432.CCR-08-0509. [DOI] [PubMed] [Google Scholar]
- 11.Zhu XD, Zhang JB, Fan PL, Xiong YQ, Zhuang PY, Zhang W, Xu HX, Gao DM, Kong LQ, Wang L, Wu WZ, Tang ZY, Ding H, Sun HC. Antiangiogenic effects of pazopanib in xenograft hepatocellular carcinoma models: evaluation by quantitative contrast enhanced ultrasonography. BMC Cancer. 2011;11:28. doi: 10.1186/1471-2407-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendelsohn J. Targeting the epidermal growth factor receptor for cancer therapy. J Clin Oncol. 2002;20:1S–13S. [PubMed] [Google Scholar]
- 13.Kris MG, Natale RB, Herbst RS, Lynch TJ, Jr, Prager D, Belani CP, Schiller JH, Kelly K, Spiridonidis H, Sandler A, Albain KS, Cella D, Wolf MK, Averbuch SD, Ochs JJ, Kay AC. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290:2149–2158. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- 14.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabárbara P, Seymour L National Cancer Institute of Canada Clinical Trials Group. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 15.Thomas MB, Chadha R, Glover K, Wang X, Morris J, Brown T, Rashid A, Dancey J, Abbruzzese JL. Phase 2 study of erlotinib in patients with unresectable hepatocellular carcinoma. Cancer. 2007;110:1059–1067. doi: 10.1002/cncr.22886. [DOI] [PubMed] [Google Scholar]
- 16.Philip PA, Mahoney MR, Allmer C, Thomas J, Pitot HC, Kim G, Donehower RC, Fitch T, Picus J, Erlichman C. Phase II study of Erlotinib (OSI-774) in patients with advanced hepatocellular cancer. J Clin Oncol. 2005;23:6657–6663. doi: 10.1200/JCO.2005.14.696. [DOI] [PubMed] [Google Scholar]
- 17.Wissner A, Overbeek E, Reich MF, Floyd MB, Johnson BD, Mamuya N, Rosfjord EC, Discafani C, Davis R, Shi X, Rabindran SK, Gruber BC, Ye F, Hallett WA, Nilakantan R, Shen R, Wang YF, Greenberger LM, Tsou HR. Synthesis and structure-activity relationships of 6,7-disubstituted 4-anilinoquinoline-3-carbonitriles. The design of an orally active, irreversible inhibitor of the tyrosine kinase activity of the epidermal growth factor receptor (EGFR) and the human epidermal growth factor receptor-2 (HER-2) J Med Chem. 2003;46:49–63. doi: 10.1021/jm020241c. [DOI] [PubMed] [Google Scholar]
- 18.Ganne-Carrié N, Trinchet JC. Systemic treatment of hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2004;16:275–281. doi: 10.1097/00042737-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Herr I, Schemmer P, Buchler MW. On the TRAIL to therapeutic intervention in liver disease. Hepatology. 2007;46:266–274. doi: 10.1002/hep.21740. [DOI] [PubMed] [Google Scholar]
- 20.Ivanchuk SM, Rutka JT. The cell cycle: accelerators, brakes, and checkpoints. Neurosurgery. 2004;54:692–699. doi: 10.1227/01.neu.0000109534.28063.5d. [DOI] [PubMed] [Google Scholar]
- 21.Massagué J. G1 cell cycle control and cancer. Nature. 2004;432:298–306. doi: 10.1038/nature03094. [DOI] [PubMed] [Google Scholar]
- 22.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, Kosaka T, Holmes AJ, Rogers AM, Cappuzzo F, Mok T, Lee C, Johnson BE, Cantley LC, Jänne PA. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 23.Desbois-Mouthon C, Cacheux W, Blivet-Van Eggelpoël MJ, Barbu V, Fartoux L, Poupon R, Housset C, Rosmorduc O. Impact of IGF-1R/EGFR cross-talks on hepatoma cell sensitivity to gefitinib. Int J Cancer. 2006;119:2557–2566. doi: 10.1002/ijc.22221. [DOI] [PubMed] [Google Scholar]
- 24.García JM, Silva J, Peña C, Garcia V, Rodríguez R, Cruz MA, Cantos B, Provencio M, España P, Bonilla F. Promoter methylation of the PTEN gene is a common molecular change in breast cancer. Genes Chromosomes Cancer. 2004;41:117–124. doi: 10.1002/gcc.20062. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Trotman LC, Koppie T, Alimonti A, Chen Z, Gao Z, Wang J, Erdjument-Bromage H, Tempst P, Cordon-Cardo C, Pandolfi PP, Jiang X. NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell. 2007;128:129–139. doi: 10.1016/j.cell.2006.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, Willson JK, Markowitz S, Kinzler KW, Vogelstein B, Velculescu VE. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 27.Rouleau E, Spyratos F, Dieumegard B, Guinebretière JM, Lidereau R, Bièche I. KRAS mutation status in colorectal cancer to predict response to EGFR targeted therapies: the need for a more precise definition. Br J Cancer. 2008;99:2100. doi: 10.1038/sj.bjc.6604815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su MC, Lien HC, Jeng YM. Absence of epidermal growth factor receptor exon 18-21 mutation in hepatocellular carcinoma. Cancer Lett. 2005;224:117–121. doi: 10.1016/j.canlet.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Lee SC, Lim SG, Soo R, Hsieh WS, Guo JY, Putti T, Tao Q, Soong R, Goh BC. Lack of somatic mutations in EGFR tyrosine kinase domain in hepatocellular and nasopharyngeal carcinoma. Pharmacogenet Genomics. 2006;16:73–74. doi: 10.1097/01.fpc.0000184959.82903.02. [DOI] [PubMed] [Google Scholar]