Abstract
To date, clinico-physiologic indices have not been compared with quantitative CT imaging indices in determining the risk of chronic obstructive pulmonary disease (COPD) exacerbation. We therefore compared clinico-physiologic and CT imaging indices as risk factors for COPD exacerbation in patients with COPD. We retrospectively analyzed 260 COPD patients from pulmonary clinics at 11 hospitals in Korea from June 2005 to November 2009 and followed-up for at least one year. At the time of enrollment, none of these patients had COPD exacerbations for at least 2 months. All underwent clinico-physiologic and radiological evaluation for risk factors of COPD exacerbation. After 1 yr, 106 of the 260 patients had at least one exacerbation of COPD. Multiple logistic regression analysis showed that old age, high Charlson Index, and low FEV1 were significant in a clinico-physiologic model, with C-statistics of 0.69, and that increased age and emphysema index were significant in a radiologic model, with C-statistics of 0.64. The difference between the two models was statistically significant (P = 0.04 by bootstrap analysis). Combinations of clinico-physiologic risk factors may be better than those of imaging risk factors in predicting COPD exacerbation.
Keywords: Pulmonary Disease, Chronic Obstructive; Exacerbation; Risk Factors; Computed Tomography
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a major disease that is expected by 2020 to be the fifth most common cause of morbidity and the third most common cause of mortality worldwide (1, 2). COPD patients experience exacerbation of COPD an average of 1.3 times per year (3). Exacerbation aggravates lung function decline (4) and has a negative impact on morbidity, mortality (5), and quality of life (6). The costs of exacerbations are estimated to represent 35%-60% of the total direct costs associated with COPD (7-9). Hence, the prevention of COPD exacerbation is considered an important management goal. Among the risk factors associated with exacerbations are old age (10), low body mass index (11), gastroesophageal reflux disease (GERD) symptoms (12, 13), chronic productive cough (10, 14), severity of COPD and past history of exacerbations (15). Certain groups of patients are more susceptible to COPD exacerbations, irrespective of COPD severity (16) and a model has been developed to assess the risks of COPD exacerbations in patients with moderate-to-very severe COPD (10).
Advanced CT techniques were recently shown to have a potential application in assessing and monitoring COPD exacerbations in patients with COPD, with correlations of clinical and physiologic indices with CT-assessed pulmonary emphysema and airway dimension (17-19). Furthermore, CT-assessed emphysema was found to correlate with mortality in patients with COPD (20, 21). In contrast, the presence, severity, and distribution of emphysema on CT were found to have little impact on clinical indices, including dyspnea score, frequency of exacerbation, six minute walk distance, St. George Respiratory Questionnaires score and BODE index (a composite index of body mass index, airflow obstruction, dyspnea, and exercise capacity). In patients with mild-to-moderate COPD, it is unclear whether CT indices, such as emphysema, airway thickening and air trapping, are related to the development of COPD exacerbations (22). There is a report including clinico-physiologic and emphysema index, one of CT indices of COPD for evaluation of risk factors (16). However, other CT indices representing the bronchus and small airway as well as emphysema index have not been compared with clinico-physiologic indices. We have therefore compared clinico-physiologic and CT imaging indices as risk factors for COPD exacerbation in patients with COPD.
MATERIALS AND METHODS
Patients
This study is a part of the ongoing Korean Obstructive Lung Disease (KOLD) Cohort, in which a total of 800 patients with COPD or asthma will have been enrolled by 2012. For this study, we retrospectively analyzed 260 patients who had been recruited from pulmonary clinics in 11 hospitals throughout Korea from June 2005 to November 2009 and were followed up for at least one year. All 260 patients fulfilled the following three criteria: post-bronchodilator ratio of FEV1 to forced vital capacity (FEV1/FVC) < 0.7 after administration of 400 µg of inhaled albuterol, more than 10 pack-years of smoking history, and no or minimal abnormality on chest radiography. Moreover, none of these patients had exacerbations of COPD for at least 2 months at the time of enrollment.
Definition and confirmation of COPD exacerbation
Acute exacerbations were defined as worsening symptoms (dyspnea, cough, or sputum) requiring treatment with systemic steroids or antibiotics, a visit to the emergency room, and/or admission to a hospital, as decided by attending physicians.
Clinico-physiologic indices for COPD
Demographic and clinical data collected from all patients included age, sex, smoking status, cigarette smoking amount, GERD treated with medications such as proton pump inhibitors, and hospitalization due to exacerbation of COPD within 1 yr before enrollment. Comorbidities were assessed using the Charlson index, which has been shown to be a good predictor of prognosis in patients with COPD (23). Body mass index was calculated as the weight in kilograms divided by height in meters squared (kg/m2). Productive cough was defined as an affirmative answer to the question, "Did you cough up phlegm (in the morning or during the day) for at least 3 months each year during the past two years?" Dyspnea was assessed with the Modified Medical Research Council Dyspnea Scale (MMRC). The 6-min walking distance test was performed according to American Thoracic Society guidelines, except for the short distance of 20 meters. Health-related quality of life was assessed using the St. George Respiratory Questionnaire. Spirometry was performed using the Vmax 22 (SensorMedics, Yorba Linda, CA, USA; PFDX instrument; MedGraphics, St. Paul, MN, USA) (24).
CT indices for COPD assessment
CT indices for COPD assessment included the emphysema index, air-trapping index and airway dimensions. (17, 25-27). Patients underwent volumetric CT scans at full inspiration and expiration using a 16-MDCT scanner (CT machines of three manufacturers: the Somatom Sensation 16, Siemens Medical Solutions, Forchheim, Germany; the GE Lightspeed Ultra, General Electric Healthcare, Milwaukee, WI; and the Philips Brilliance 16, Philips Medical Systems, Best, Netherlands) (19). Scan parameters included 0.75-mm collimation, 100 effmAs, 140 kVP, and pitch 1.0. The scale of attenuation coefficients in this CT scanner ranges from -1,024 to 3,072 HU. Patients were scanned craniocaudally, with both scans performed in the supine position. Images were reconstructed using a soft kernel (B30f; Siemens Medical Systems) from the thoracic inlet to the lung base.
The emphysema index was determined by automatic calculation from CT data of the volume fraction of the lungs below -950 Hounsfield Units at full inspiration and the mean lung density (26). The CT air-trapping index was defined as the ratio of mean lung density on expiration and inspiration (19). Airway dimensions were measured near the origin of the four segmental bronchi (RB1, LB1+2, RB10, LB10) by a consensus of two radiologists using in-house software. Airway dimensions, including the wall area (WA), lumen area (LA), and wall area percent (WA %), defined as WA/(WA+LA) × 100, were measured in each segmental bronchus.
Statistical analysis
Continuous data and the frequencies and percentages of categorical data were expressed as mean ± standard deviation. A stepwise backward multiple logistic regression was used to construct models predictive of COPD exacerbation. Clinico-physiologic indices with a univariate P value < 0.2 were considered candidates in the backward stepwise multiple logistic regression. All three radiologic indices, irrespective of the result of univariate analysis, as well as age and gender, were included in the multiple logistic regression. The predictive accuracy of the models was quantified by calculating the C-statistics. The two models were compared by bootstrap analysis using R 2.12.0 (R Development Core Team, GNU General Public License, www.r-project.org). In addition, we performed a multiple logistic regression analysis with all of the variables including both clinico-physiologic indices and radiologic indices. All other statistical analyses were performed using a statistical package (SPSS version 12.1.1; SPSS, Chicago, IL, USA).
Ethics statement
This study was approved by the institutional review board of Asan Medical Center (Approval No. 2005-0345) and of other 10 hospitals. Written informed consent was obtained from all subjected patients.
RESULTS
Patient characteristics
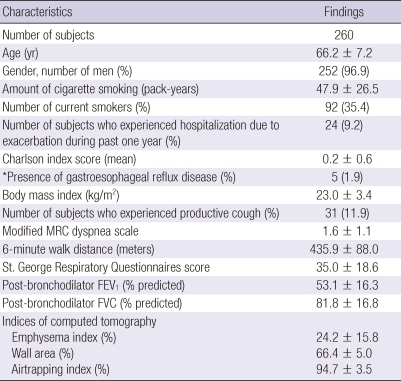
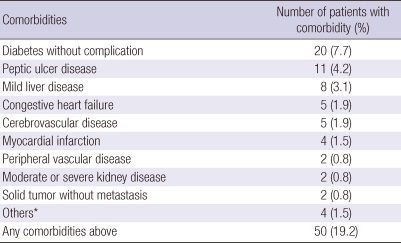
The clinical, physiologic, and radiologic characteristics of the 260 patients are shown in Table 1. According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, most patients had moderate-to-severe COPD. During the previous year, 24 (9.2%) patients had been hospitalized for COPD exacerbation. The mean Charlson index score in all 260 patients was 0.24. Fifty patients (19.2%) had at least one comorbidity, with diabetes mellitus being the most frequent (Table 2).
Table 1.
Baseline characteristics of the subjects
Data are presented as mean ± standard deviation. Numbers in parentheses are percentage of subjects. *Physician-diagnosed gastroesophageal reflux disease. Modified MRC, Modified Medical Research Council; FEV1, Forced expiratory volume in 1 second; FVC, Forced vital capacity; Emphysema index, the volume fraction of the lung below -950 Hounsfield Units (HU) at full inspiration. Wall area, % = wall area/(wall area + lumen area) × 100. Airtrapping index = ratio of mean lung density on expiration and inspiration × 100.
Table 2.
Comorbidities of the subjects
*Others include dementia, connective tissue disease, diabetes with organ disease, or hemiplegia.
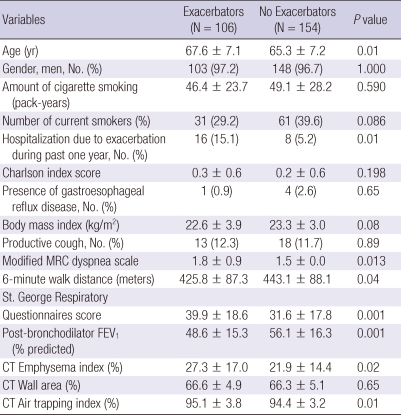
Comparison of characteristics between exacerbators and non-exacerbators
A total of 106 (40.8%) of 260 patients had experiences of acute exacerbation of COPD during 1 yr follow-up, who were designated as exacerbators. The characteristics of exacerbators and non-exacerbatiors were summarized in Table 3. Exacerbators were older and had more experiences of hospitalization due to COPD exacerbations, more dyspnea scale, worse quality of life and exercise capacity, lower FEV1, more emphysematous change and air-trapping on CT. Eighteen patients (17%) of 106 exacerbators were hospitalized for the treatment of COPD acute exacerbations during follow-up period.
Table 3.
Comparison of characteristics between exacerbators and no exacerbators
Data are presented as mean ± standard deviation. Numbers in parentheses are percentage of subjects. FEV1 = Forced expiratory volume in 1 second; FVC, Forced vital capacity; Emphysema index, the volume fraction of the lung below -950 Hounsfield Units (HU) at full inspiration; Wall area, % = wall area/(wall area + lumen area) × 100. Air trapping index, ratio of mean lung density on expiration and inspiration × 100.
Univariate analysis of risk factors for COPD exacerbation
Table 4 shows the univariate analysis for factors predictive of COPD exacerbation. Among clinico-physiologic indices, we found that old age, current smoking, hospitalization for exacerbation of COPD during the previous year, Charlson index score, BMI, MMRC dyspnea scale, six minute walk distance, the St. George Respiratory Questionnaire and FEV1 were related to COPD exacerbation. Among the CT imaging indices, emphysema and CT air-trapping index were related to exacerbation.
Table 4.
Univariate analysis for the risk factors of COPD exacerbation
COPD exacerbations were defined as worsening symptoms (dyspnea , cough, or sputum) requiring treatment with systemic steroids or antibiotics, a visit to the emergency room, and/or admission to a hospital which were decided by attending physicians.
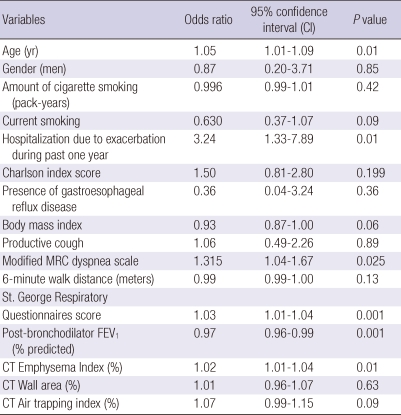
Multivariate models of risk factors for COPD exacerbation
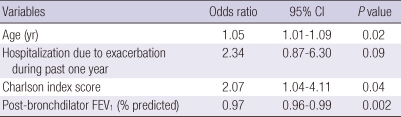
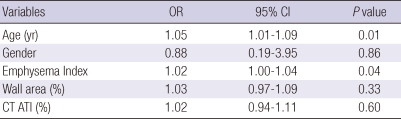
We utilized multiple logistic regression analysis to develop models of risk factors for COPD exacerbation (Table 5, 6). A model using clinico-physiologic indices showed that increased age, higher Charlson Index, and lower FEV1 were significantly associated with exacerbation of COPD, with C-statistics of 0.69. A CT imaging model showed that increased age and emphysema index were associated with COPD exacerbation, with C-statistics of 0.64. Bootstrap analysis showed that the clinico-physiologic model was significantly superior to the CT imaging indices model in predicting the occurrence of exacerbations of COPD (P = 0.04, bootstrap analysis).
Table 5.
A multivariate logistic regression model for the risk factors of COPD exacerbation using conventional clinical and physiological information
Hosmer-Lemeshow goodness-of-fit showed chi-square = 6.52 and P = 0.59, showing good fit of the models; C-statistics value was 0.694. Clinico-physiologic indices with a univariate P value < 0.2 were considered candidates in the backward stepwise multiple logistic regression. Initial variables for multiple logistic regression were age, current smoking, hospitalization due to exacerbation during past one year, Charlson index score, body mass index, modified MRC dyspnea scale, 6 minute walk distance, St. George Rspiratory Questionnaires score, FEV1, % predicted.
Table 6.
A multivariate logistic regression model for the risk factors of COPD exacerbation using computed tomography (CT) indices
Hosmer-Lemeshow goodness-of-fit showed chi-square = 10.41 and P = 0.24, showing good fit of the models; C-statistics value was 0.643. ATI, air trapping index. All radiologic indices irrespective of the result of univariate analysis were included in the multiple logistic regression.
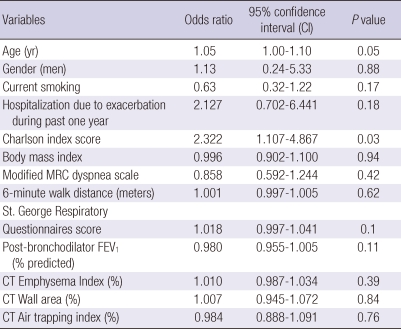
When a multiple logistic regression analysis was performed with all of the variables including both clinico-physiologic indices and radiologic indices, only Charlson Index was a significant risk factor of COPD exacerbation (Table 7). When the same analysis was done for hospitalizatioin due to COPD exacerbation as a dependent variable, the St. George Respiratory Questionnaires score and emphysema index were significant (data not shown).
Table 7.
Multivariate analysis for the risk factors of COPD exacerbation
Hosmer-Lemeshow goodness-of-fit showed chi-square = 3.48 and P = 0.90, showing good fit of the models; C-statistics value was 0.70. Clinico-physiologic indices with a univariate P value < 0.2 were considered candidates. Initial variables for multiple logistic regression were age, current smoking, hospitalization due to exacerbation during past one year, Charlson index score, body mass index, modified MRC dyspnea scale, 6 minute walk distance, St. George Rspiratory Questionnaires score, FEV1, % predicted. All radiologic indices irrespective of the result of univariate analysis were included in the multiple logistic regression.
DISCUSSION
Many clinical and physiological indices have been associated with COPD exacerbation (10-12, 16), with the phenotype of frequent exacerbations recently suggested to be an important risk factor (16, 28). To evaluate risk factors for COPD exacerbation we designed two models, a clinico-physiologic model and a CT imaging model, both using multivariate linear regression. A comparison of the two models showed that the model using clinicophysiologic indices was superior to the model using CT imaging indices. The clinico-physiologic measurements independently associated with COPD exacerbation over 1-yr follow-up were increased age, high Charlson index and low FEV1.
Several reports showed a relationship between quantitative assessment of COPD by CT and clinico-physiologic indices (17, 19), suggesting that emphysematous changes measured by CT may be predictive of mortality in COPD patients. Moreover, CTmeasured emphysematous changes have been found to predict respiratory mortality (21). However, little is known about the relationship between CT imaging indices and COPD exacerbations. Although studies have included CT-determined emphysematous changes as an index for prediction of COPD exacerbation, these associations were not clinically significant (16, 22). A recent study demonstrated that % wall area measuring airway thickness correlates with symptoms of chronic bronchitis and frequent exacerbation (29).
Although our study showed that emphysema index and CT air-trapping index were associated with exacerbations of COPD in univariate analysis, only emphysema index remained significant on multivariate analysis. However, when all of the clinical, physiologic and CT imaging indices were included in a multivariate logistic model, only the clinico-physiologic indices were statistically significant. Only hospitalization due to COPD exacerbation is limited to emphysema index, one of CT indices in our study, which is statistically significant by multivariate analysis. This result suggests that emphysema index may be useful for evaluation of the risk hospitalization due to COPD exacerbation. We did not find any relationship between air wall characteristics on CT and frequency of exacerbations, but this may be a consequence of the population studied, with only 11% of chronic bronchitis.
We found that increased age and higher Charlson index score were risk factors for COPD exacerbation. Charlson index score, which is used to assess comorbidities, has been shown to be a good predictor of prognosis in COPD patients (23) and has been associated with the number of hospitalizations of COPD patients for any cause during the previous year (30).
Studies of patients with COPD have shown a phenotype for frequent COPD exacerbation, with prior exacerbations of COPD being the strongest risk factor for subsequent COPD exacerbations (16). Of our 260 patients, 24 (9.2%) had been hospitalized at least once for COPD exacerbation during the previous year; however, they showed a marginally increased risk for COPD exacerbation during follow-up than patients not hospitalized for COPD exacerbation in clinico-physiologic multivariate analysis.
We found that other possible risk factors, such as productive cough and GERD, were not significant for exacerbation of COPD. However, few of our patients had productive cough (11.9%) or GERD (1.9%), which may have affected their statistical contribution.
This study had several limitations. First, considering a very low prevalence of comorbidities, our relatively small-sized population was very selective. So it is difficult to generalize our results to all patients with COPD. Second, we did not evaluate the number of previous exacerbations, one of the more relevant factors associated with current exacerbations, before enrollment. Third, we did not consider the impact of COPD medications on exacerbation, due to the heterogeneous use of medication during follow-up among our patients.
In conclusion, a combination of clinico-physiologic risk factors may be superior to a combination of CT imaging risk factors in predicting COPD exacerbation.
Footnotes
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health and Welfare, Republic of Korea (A102065).
AUTHOR SUMMARY
Comparison of Clinico-Physiologic and CT Imaging Risk Factors for COPD Exacerbation
Jung-Wan Yoo, Yoonki Hong, Joon Beom Seo, Eun Jin Chae, Seung Won Ra, Ji-Hyun Lee, Eun Kyung Kim, Seunghee Baek, Tae-Hyung Kim, Woo Jin Kim, Jin Hwa Lee, Sang-Min Lee, Sangyeub Lee, Seong Yong Lim, Tae Rim Shin, Ho Il Yoon, Seung Soo Sheen, Jae Seung Lee, Jin Won Huh, Yeon-Mok Oh and Sang-Do Lee
We compared clinico-physiologic and CT imaging indices as risk factors for COPD exacerbation in 260 patients with chronic obstructive pulmonary disease (COPD). Multivariate analysis showed that older age, higher Charlson Index, and lower FEV1 were significant in a clinico-physiologic model, and that increased age and emphysema index were significant in a radiologic model. Combinations of clinico-physiologic risk factors revealed to be better than combinations of radiological factors.
References
- 1.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 2.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriquez-Roisin R, van Weel C, Zielinski J Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 3.Singh JM, Palda VA, Stanbrook MB, Chapman KR. Corticosteroid therapy for patients with acute exacerbations of chronic obstructive pulmonary disease: a systematic review. Arch Intern Med. 2002;162:2527–2536. doi: 10.1001/archinte.162.22.2527. [DOI] [PubMed] [Google Scholar]
- 4.Kanner RE, Anthonisen NR, Connett JE Lung Health Study Research Group. Lower respiratory illnesses promote FEV(1) decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health study. Am J Respir Crit Care Med. 2001;164:358–364. doi: 10.1164/ajrccm.164.3.2010017. [DOI] [PubMed] [Google Scholar]
- 5.Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:1608–1613. doi: 10.1164/ajrccm.161.5.9908022. [DOI] [PubMed] [Google Scholar]
- 7.Andersson F, Borg S, Jansson SA, Jonsson AC, Ericsson A, Prütz C, Rönmark E, Lundbäck B. The costs of exacerbations in chronic obstructive pulmonary disease (COPD) Respir Med. 2002;96:700–708. doi: 10.1053/rmed.2002.1334. [DOI] [PubMed] [Google Scholar]
- 8.Miravitlles M, Murio C, Guerrero T, Gisbert R. Costs of chronic bronchitis and COPD: a 1-year follow-up study. Chest. 2003;123:784–791. doi: 10.1378/chest.123.3.784. [DOI] [PubMed] [Google Scholar]
- 9.Mittmann N, Kuramoto L, Seung SJ, Haddon JM, Bradley-Kennedy C, Fitzgerald JM. The cost of moderate and severe COPD exacerbations to the Canadian healthcare system. Respir Med. 2008;102:413–421. doi: 10.1016/j.rmed.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Niewoehner DE, Lokhnygina Y, Rice K, Kuschnner WG, Sharafkhaneh A, Sarosi GA, Krumpe P, Pieper K, Kesten S. Risk indexes for exacerbations and hospitalizations due to COPD. Chest. 2007;131:20–28. doi: 10.1378/chest.06-1316. [DOI] [PubMed] [Google Scholar]
- 11.Kessler R, Faller M, Fourgaut G, Mennecier B, Weitzenblum E. Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:158–164. doi: 10.1164/ajrccm.159.1.9803117. [DOI] [PubMed] [Google Scholar]
- 12.Rascon-Aguilar IE, Pamer M, Wludyka P, Cury J, Coultas D, Lambiase LR, Nahman NS, Vega KJ. Role of gastroesophageal reflux symptoms in exacerbations of COPD. Chest. 2006;130:1096–1101. doi: 10.1378/chest.130.4.1096. [DOI] [PubMed] [Google Scholar]
- 13.Rogha M, Behravesh B, Pourmoghaddas Z. Association of gastroesophageal reflux disease symptoms with exacerbations of chronic obstructive pulmonary disease. J Gastrointestin Liver Dis. 2010;19:253–256. [PubMed] [Google Scholar]
- 14.Burgel PR, Nesme-Meyer P, Chanez P, Caillaud D, Carré P, Perez T, Roche N Initiative Bronchipneumopathie Chronique Obstructive Scientific Committee. Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest. 2009;135:975–982. doi: 10.1378/chest.08-2062. [DOI] [PubMed] [Google Scholar]
- 15.Donaldson GC, Wedzicha JA. COPD exacerbations. 1: Epidemiology. Thorax. 2006;61:164–168. doi: 10.1136/thx.2005.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, Miller B, Lomas DA, Agusti A, Macnee W, Calverley P, Rennard S, Wouters EF, Wedzicha JA. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoint (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 17.Nakano Y, Muro S, Sakai H, Hirai T, Chin K, Tsukino M, Nishimura K, Itoh H, Paré PD, Hogg JC, Mishima M. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med. 2000;162:1102–1108. doi: 10.1164/ajrccm.162.3.9907120. [DOI] [PubMed] [Google Scholar]
- 18.Fujimoto K, Kitaguchi Y, Kubo K, Honda T. Clinical analysis of chronic obstructive pulmonary disease phenotypes classified using high-resolution computed tomography. Respirology. 2006;11:731–740. doi: 10.1111/j.1440-1843.2006.00930.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee YK, Oh YM, Lee JH, Kim EK, Lee JH, Kim N, Seo JB, Lee SD KOLD Study Group. Quantitative assessment of emphysema, air trapping, and airway thickening on computed tomography. Lung. 2008;186:157–165. doi: 10.1007/s00408-008-9071-0. [DOI] [PubMed] [Google Scholar]
- 20.Martinez FJ, Foster G, Curtis JL, Criner G, Weinmann G, Fishman A, DeCamp MM, Benditt J, Sciurba F, Make B, Mohsenifar Z, Diaz P, Hoffman E, Wise R NETT Research Group. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med. 2006;173:1326–1334. doi: 10.1164/rccm.200510-1677OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haruna A, Muro S, Nakano Y, Ohara T, Hoshino Y, Ogawa E, Hirai T, Niimi A, Nishimura K, Chin K, Mishima M. CT scan findings of emphysema predict mortality in COPD. Chest. 2010;138:635–640. doi: 10.1378/chest.09-2836. [DOI] [PubMed] [Google Scholar]
- 22.de Torres JP, Bastarrika G, Zagaceta J, Sáiz-Mendiguren R, Alcaide AB, Seijo LM, Montes U, Campo A, Zulueta JJ. Emphysema presence, severity, and distribution has little impact on the clinical presentation of a cohort of patients with mild to moderate COPD. Chest. 2011;139:36–42. doi: 10.1378/chest.10-0984. [DOI] [PubMed] [Google Scholar]
- 23.Almagro P, Calbo E, Ochoa de Echagüen A, Barreiro B, Quintana S, Heredia JL, Garau J. Mortality after hospitalization for COPD. Chest. 2002;121:1441–1448. doi: 10.1378/chest.121.5.1441. [DOI] [PubMed] [Google Scholar]
- 24.American Thoracic Society. Standardization of Spirometry, 1994 Update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 25.Madani A, Zanen J, de Maertelaer V, Gevenois PA. Pulmonary emphysema: objective quantification at multi-detector row CT--comparison with macroscopic and microscopic morphometry. Radiology. 2006;238:1036–1043. doi: 10.1148/radiol.2382042196. [DOI] [PubMed] [Google Scholar]
- 26.Gevenois PA, de Maertelaer V, De Vuyst P, Zanen J, Yernault JC. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1995;152:653–657. doi: 10.1164/ajrccm.152.2.7633722. [DOI] [PubMed] [Google Scholar]
- 27.Wood SA, Zerhouni EA, Hoford JD, Hoffman EA, Mitzner W. Measurement of three-dimensional lung tree structures by using computed tomography. J Appl Physiol. 1995;79:1687–1697. doi: 10.1152/jappl.1995.79.5.1687. [DOI] [PubMed] [Google Scholar]
- 28.Wan ES, DeMeo DL, Hersh CP, Shapiro SD, Rosiello RA, Sama SR, Fuhlbrigge AL, Foreman MG, Silverman EK. Clinical predictors of frequent exacerbations in subjects with severe chronic obstructive pulmonary disease (COPD) Respir Med. 2011;105:588–594. doi: 10.1016/j.rmed.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mair G, Maclay J, Miller JJ, McAllister D, Connell M, Murchison JT, Mac-Nee W. Airway dimensions in COPD: Relationship with clinical variables. Respir Med. 2010;104:1683–1690. doi: 10.1016/j.rmed.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 30.Almagro P, López Garciá F, Cabrera F, Montero L, Morchón D, Díez J, Soriano J Grupo Epoc De La Sociedad Española De Medicina Interna. Comorbidity and gender-related differences in patients hospitalized for COPD. The ECCO study. Respir Med. 2010;104:253–259. doi: 10.1016/j.rmed.2009.09.019. [DOI] [PubMed] [Google Scholar]