Abstract
Resurgent interest in antiviral drugs for the treatment of herpesvirus has led to the development of new compounds that are progressing through clinical trials. This is important because there are few therapeutic options for resistant infections and some viruses such as human cytomegalovirus remain underserved. New compounds include conventional DNA polymerase inhibitors such as valomaciclovir and cyclopropavir, as well as CMX001 that has a broad spectrum of antiviral activity that includes all the herpesviruses. It also includes compounds with new molecular targets such as maribavir, FV-100, AIC361, and AIC246. Recent advances with each of these compounds will be reviewed including their virus specificity, mechanism of action, and stage of development. The potential of these new compounds to improve clinical outcome will also be discussed.
Introduction
The herpesviruses are responsible for a wide variety of diseases in humans. Most of these viruses are endemic in the population, with the seroprevalence of some approaching 100% in the first years of life. These viruses establish lifelong latent or persistent infections that can initiate subsequent episodes of disease. While these infections are common, and with a few notable exceptions, they are typically mild and self limiting when held in check by the host’s immune system.
One characteristic shared by all the human herpesviruses is the progression to severe disease in immunocompromised hosts. Resistance to the therapies of choice also arises readily in such hosts as the viruses continue to replicate notwithstanding sustained treatment with first line drugs [1–2]. Treatment of resistant infections is problematic because secondary therapies are limited by a lack of oral bioavailability, modest potency, and are associated with significant toxicities [3–5]. The development of new therapies, particularly those with novel molecular targets, will be important not only to treat resistant infections, but also to prevent their occurrence with combination chemotherapy [6–7].
The development of acyclovir (ACV) for use as a therapy against the alphaherpesviruses represents the first example of a truly effective antiviral therapy [8]. Yet the potency of available therapies for herpesvirus infections pales in comparison to that of modern therapies for HIV infections. Indeed the modest antiviral activity of some approved therapies for the other herpesviruses coupled with dose-limiting toxicity limit their effectiveness and often result in the development of resistance in immunocompromised hosts [1]. New therapies are required that have improved efficacy as well as reduced toxicity to allow an extended course of therapy to suppress viral replication in the target population. The search for such therapies has identified several promising compounds that are progressing through various stages of clinical trials. Recent work with the most advanced compounds will be discussed and compared with existing therapies to provide perspective on their potential advantages.
New Developments in Antiviral Therapies for the Herpesviruses
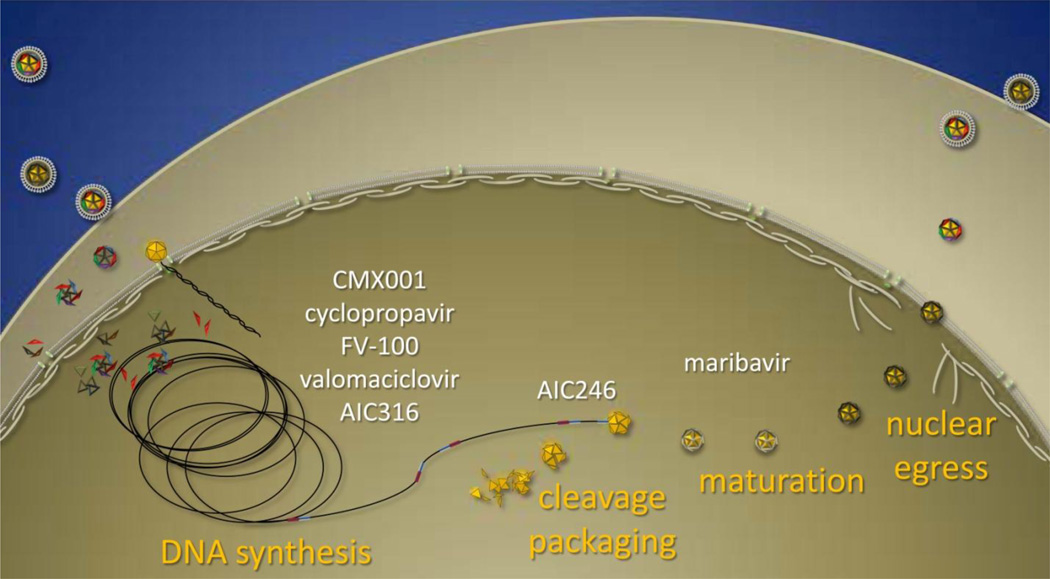
The search for novel inhibitors of the herpesviruses has not only taken advantage of conventional targets, such as the DNA polymerase, but has resulted in the discovery of new molecular targets and greatly improved our understanding of the biology of the herpesviruses (Figure 1). Novel inhibitors of viral infection will be reviewed with respect to their efficacy, spectrum of antiviral activity, and their molecular targets insofar as they have been described (Figure 2). Although many inhibitors of cellular targets have been identified that impact viral replication, this review will focus on inhibitors that specifically target viral gene products with an emphasis on recent developments in the field.
Figure 1.
Summary of compounds that inhibit various stages of herpesvirus replication. Infection is initiated by virions that bind to receptors at the cell surface and internalized capsids deliver the viral genome to the nucleus. The subsequent synthesis of genomic DNA is inhibited by nucleoside and nucleotide analogs inhibit the DNA polymerase and AIC316 that inhibits the helicase/primase. The coordinated steps of genome cleavage/packaging are inhibited by AIC246. Inhibitors of the viral UL97 kinase, such as maribavir, impact both early and late events in viral replication and inhibit the egress of mature capsids into the cytoplasm.
Figure 2.
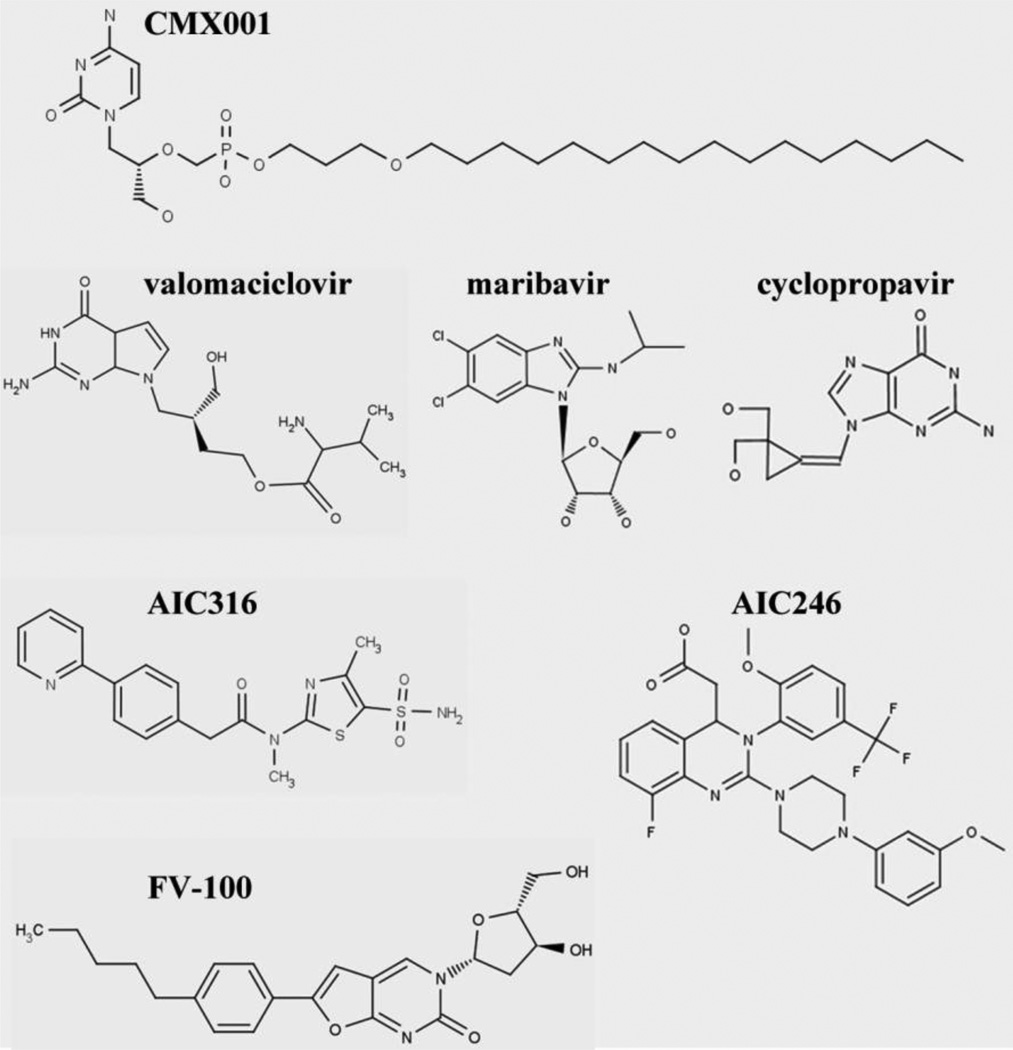
New inhibitors of herpesvirus replication. Many new inhibitors undergoing clinical development are not nucleoside analogs and belong to diverse chemical classes. The structure of each of the molecules discussed is shown.
FV-100
FV-100 is the prodrug form of cf-1743 (3-(2-Deoxy-β-D-ribofuanosyl)-6-(p-pentylphenyl)-2,3-dihydrofuro[2,3-d]pyrimidin-2-one), a bicyclic nucleoside analog (BCNA) that has highly specific antiviral activity against VZV [9–10]. The excellent potency of this compound makes it a good candidate to treat shingles that is underserved by current therapies. Although its precise mechanism of action is unclear, the VZV thymidine kinase appears to play a key role, as TK negative mutants in vitro are resistant to BCNAs [9]. The drug is phosphorylated by the VZV thymidine kinase and further phosphorylated by the VZV thymidylate kinase. Unlike ACV and other nucleoside analogs, a triphosphate form is not detected in cells making it uncertain if it directly inhibits DNA polymerase or acts via another mechanism [11]. The antiviral activity cf-1743 against VZV is 100 times more potent than that of ACV although FV-100 is about 4-fold less active than cf-1743 [10]. In a recent clinical trial with adults, FV-100 proved to be well tolerated [10,12]. A Phase 2 clinical trial evaluated the safety and efficacy of FV-100 compared to valacyclovir in the treatment of shingles but data have not yet been published (NCT00900783).
AIC316
AIC316, also known as Bay 57-1293 (N-[5-(aminosulfonyl)-4-methyl-1,3-thiazol-2-yl]-N-methyl-2-[4-(2-pyridinyl)phenyl]acetamide) is a helicase primase inhibitor that has good activity against HSV-1 and HSV-2 [13]. It inhibits the helicase primase complex encoded by the UL5, UL52 and UL8 genes of HSV and results in the inhibition of viral DNA synthesis [14]. The interaction of AIC316 with the complex is thought to be mediated by the region of UL5 close to helicase motif IV [15], and UL52 near the asparagine at position 242 that together are thought to form a binding pocket for the drug [16]. The in vitro potency of the compound is superior to ACV and is effective in mice infected with HSV [14]. Mutations in UL5 and UL52 confer resistance to AIC316 as well as other related compounds under development [17]. One mutation in UL5 that imparts resistance to AIC316 (K356N) is a naturally occurring polymorphism that is detectable in more than 10% of clinical isolates of HSV [13]. It is not clear how low levels of this polymorphism in patients would impact the clinical outcome, but it will be monitored closely as clinical trials proceed. This may not prove to be a problem since HSV isolates with this polymorphism represented at a frequency of 2% were successfully treated in mouse studies [18].
Because the mechanism of action of AIC316 is distinct from ACV it remains active against ACV-resistant isolates of HSV [19]. This indicates that it might be useful in the treatment of resistant infections, but more importantly might be used in combination to prevent the development of resistance in high risk populations. The compound has proven to be well tolerated in clinical studies and was shown to be effective in a recently completed phase 2 trial (NCT01047540).
Valomaciclovir
Valomaciclovir, the prodrug form of H2G (R-9[4-Hydroxy-2(hydroxymethy)butyl]guanine) is a guanosine analog that has activity against HSV-1, HSV-2, EBV, and VZV [20]. It does not have activity against HHV-6A, HHV-6B, HHV-7, HHV-8 or HCMV [21]. It is phosphorylated initially by viral thymidine kinase homologs encoded by susceptible viruses, and the triphosphate metabolite is thought to inhibit the viral DNA polymerase. It is not an obligate DNA chain terminator like ACV, but like GCV, is a competitive inhibitor of the viral DNA polymerase and interferes with the elongation of viral DNA. The compound also has some effect on the activity of human DNA polymerase alpha as well [20]. It is more potent than ACV in vitro against VZV [22], but mutations in the TK gene that confer resistance to ACV also confer resistance to H2G [23]. The compound appears to be well tolerated and Phase 2 studies have been undertaken to evaluate its effectiveness in infectious mononucleosis and shingles (ClinicalTrials.gov identifier: NCT00575185 and NCT00831103, respectively).
CMX001
CDV has good antiviral activity against all the human herpesviruses and has been licensed for the treatment of HCMV infection [24]. Recently, a series of lipid ester analogs of CDV was synthesized to help improve the oral bioavailability of the parent compound [25], and this modification unexpectedly enhanced the antiviral activity against all the human herpesviruses by 2 to 3 orders of magnitude compared to CDV [26]. One of these compounds, hexadecyloxypropyl CDV (HDP-CDV, CMX001) was also shown to be effective against clinical isolates of HCMV and HSV as well as GCV-resistant isolates of HCMV [26] and ACV-resistant isolates of HSV [27]. Following uptake into infected cells, the compound is metabolized to release CDV and is thought to inhibit viral replication by the inhibition of the viral DNA polymerases [25].
CMX001 exhibited good oral therapeutic activity against HCMV infections in SCID mice implanted with human retinal or thymus/liver tissue [28], against MCMV infections in mice[29], and guinea pig cytomegalovirus infections in guinea pigs [30]. It was also effective against HSV infections in murine models of infection [31], and potentiated the efficacy of ACV both in vitro and in vivo [32].
The in vitro and animal model data summarized above taken together with results from pharmacokinetic, metabolic, and toxicologic studies were used to select CMX001 as a candidate for clinical evaluation [33], and CMX001 has successfully completed Phase 1 clinical trials. This compound proved to be well tolerated and confirmed earlier studies in mice that showed significantly reduced accumulation of the compound in the kidney since unlike CDV, CMX001 is not concentrated by the organic anion transporter in renal proximal tubule cells [25]. Thus CMX001 avoids the nephrotoxicity that is observed frequently with CDV [31,33–34]. CMX001 is currently being evaluated in Phase 2 clinical studies for treatment of adenovirus and HCMV infections (ClinicalTrials.gov identifier: NCT01241344 and NCT00942305, respectively).
Maribavir
Maribavir (MBV) is a benzimidazole l riboside that has good antiviral activity against EBV and HCMV [35], and is a highly specific inhibitor of the HCMV UL97 kinase [36]. This compound is also effective in vivo against HCMV in SCID/hu mice [37]. Inhibition of UL97 kinase activity by MBV results in a complex set of replication defects that corresponds to the UL97 kinase null phenotype and has been recently reviewed [38]. Mutations in the UL97 kinase that confer resistance to MBV lie near the active site of the enzyme and are distinct from those of GCV-resistant mutants and some also lie outside the conserved kinase domains [39]. Consistent with these observations, the drug remains active against GCV-resistant strains and might be useful in the treatment of drug resistant infections [35]. However, the inhibition of UL97 kinase activity by MBV may interfere with the activation of GCV if administered concomitantly [27,40].
Interestingly, most resistant strains isolated in the laboratory do not have mutations in UL97, but rather in the UL27 open reading frame [41–42]. Compensatory mutations in UL27 arise when UL97 kinase activity is inhibited by MBV and impart low-level resistance to the drug [41]. The resistance conferred by mutations within UL27 is modest compared to those in the kinase, but they appear to occur much more frequently. A potential explanation for this effect was proposed recently in a report that indicated pUL27 promotes the proteasome-dependent degradation of Tip60 [43]. When UL27 is mutated, the presence of Tip60 leads to reduced levels of p21Waf/CIP1, which are insufficient to reduce the levels of cyclin-dependent kinases that can substitute for some of the activities of the UL97 kinase. Increased levels of cyclin-dependent kinases have the potential to compensate for decreased UL97 kinase activity since kinases such as cdc2 perform many of the same functions as UL97 kinase including the phosphorylation of retinoblastoma protein [44–45], and degradation of the nuclear lamina [46–47]. This is also consistent with the decreased efficacy of MBV in dividing cells that have increased levels of cdc2. MBV failed to meet its clinical endpoints in two Phase 3 clinical trials and was hypothesized to be related to the specific endpoint selected [48]. Despite these negative clinical results, UL97 kinase remains a good target for antiviral therapy.
Cyclopropavir
Methylenecyclopropane analogs have been shown to be effective against a number of the human herpesviruses. The lead compound, cyclopropavir (CPV, MBX 400), was evaluated against all the human herpesviruses and it was reported to be active in vitro against HCMV, HHV-6A, HHV-6B and HHV-8 [49–50]. It was also effective in vivo in two animal models of human HCMV infection [51]. This compound requires the UL97 kinase for its antiviral activity since recombinant viruses with large deletions in the UL97 open reading frame, or K355M mutations that abrogate the enzymatic activity of the kinase are resistant to the antiviral activity of CPV [50, data not presented]. This was confirmed in enzymatic studies that determined that CPV was a better substrate for the kinase than GCV [52]. The high affinity of CPV for UL97 kinase also appears to inhibit the normal function of the kinase in infected cells and likely contributes to the potency of the compound [27]. Once phosphorylated by the UL97 kinase, CPV monophosphate can be further phosphorylated by guanosine monophosphate kinase to the level of the triphosphate [53], which presumably inhibits the viral DNA polymerase and is consistent with the observed inhibition of viral DNA synthesis [50].
Resistance to CPV has been mapped to the UL97 kinase and M460I, H520Q, and C603R mutations confer resistance to this compound [54]. Although these mutations arise in the clinic in response to GCV therapy, they are rather uncommon mutations and most GCV-resistant clinical isolates remain susceptible to CPV. Deep sequencing of laboratory isolates resistant to CPV also identified a frameshift mutation in the UL27 open reading frame that deletes the critical carboxyl terminal domain important for the function of the protein [55], and is consistent with other experiments that suggest CPV inhibits UL97 kinase activity [27]. The selection of a frameshift mutation in the UL27 open reading frame in cells treated with CPV is a good indication that it is similar to MBV in that it compromises UL97 kinase activity. Mutations in UL27 might also have the potential to have a small impact on CPV susceptibility. This promising new compound is currently under late stages of preclinical development.
AIC246
AIC246 has been described recently as a potent inhibitor of HCMV replication that acts at a late stage of viral replication and is thought to be an inhibitor of the terminase complex [56]. The terminase complex is composed of pUL89 and pUL56 that interact with the UL104 virion portal protein to direct the sequence specific cleavage/packaging of viral genomes [57]. This process is essential for virus replication, and specific inhibitors of the complex have been identified and characterized including BDCRB and BAY 38-4766 [58–59]. Recently, specific mutations in the UL56 open reading frame were identified that were sufficient to confer resistance to the compound and thus confirmed that UL56 is a molecular target of AIC246 [60]. Since AIC246 is thought to target the terminase complex, it is predicted to be active against HCMV isolates resistant to nucleoside analogs and was effective in an immunocompromised patient that failed multiple therapies [61]. In clinical studies AIC246 has proven to be well tolerated and is currently being evaluated in phase 2 clinical studies (NCT01063829).
Conclusions
For more than a decade the development of new drugs for herpesvirus infections languished as industry focused on other priorities, and further research continued largely in the academic setting. But renewed interest in new therapies for herpesvirus infections is heartening and appropriate as an awareness of potential opportunities has grown. The tremendous successes in the therapy of HIV infections illustrate the power of highly effective therapies and illuminate the modest efficacy of ACV, which remains the best drug for the treatment of herpesvirus infections. The promising compounds summarized in this review have the potential to augment current therapies for HSV infections and greatly improve antiviral regimens for underserved herpesvirus infections.
Development of additional therapies with improved efficacy and reduced potential for toxicity is clearly required. As additional drugs are approved it will be important not only to understand the potential of each new molecule and new therapeutic target, but also to consider their potential use in multidrug regimens. Deep sequencing techniques have led to a new appreciation of the genetic diversity of herpesvirus genomes within an infected individual. Indeed the level of diversity suggests that mutations conferring resistance to antiviral drugs preexist and isolates that carry them can proliferate where the incomplete suppression of viral replication afforded by single agents cannot be augmented by the host immune system.
Combination therapy has proven to be highly effective in the management of HIV infections as it can suppress virus replication to undetectable levels and minimize the development of resistance [62]. A similar strategy will be required to manage herpesvirus infections in immunosuppressed hosts where extended therapeutic regimens are needed. A combination of agents has the potential to keep residual viral replication to a minimum and prevent the selection of resistant isolates that exist as subpopulations in infected individuals. Obvious candidate combinations would likely include DNA polymerase inhibitors, as well as other compounds with distinct molecular targets to minimize the development of resistance. Thus new agents that inhibit novel molecular targets will be critical. And since immunocompromised hosts are at risk for many herpesvirus infections, a broad spectrum agent such as CMX001 will also be important in prophylactic regimens. The rapid progression of compounds described here through clinical trials is crucial since they have the potential to successfully treat underserved diseases and improve clinical outcomes.
Highlights.
-
➢
We review recent advances in the development of new therapies for herpesvirus infections
-
➢
Spectrum of antiviral activity, mechanism of action, resistance and stage of development are discussed
-
➢
Opinions on future directions of the field are discussed
Acknowledgments
These studies were funded in whole or in part with Federal funds from the National Institutes of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract HHSN2722011000010C. NBP receives funding from The Dixon Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Emery VC, Griffiths PD. Prediction of cytomegalovirus load and resistance patterns after antiviral chemotherapy. Proc Natl Acad Sci U S A. 2000;97:8039–8044. doi: 10.1073/pnas.140123497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torres-Madriz G, Boucher HW. Immunocompromised hosts: perspectives in the treatment and prophylaxis of cytomegalovirus disease in solid-organ transplant recipients. Clin Infect Dis. 2008;47:702–711. doi: 10.1086/590934. [DOI] [PubMed] [Google Scholar]
- 3. Lurain NS, Chou S. Antiviral drug resistance of human cytomegalovirus. Clin Microbiol Rev. 2010;23:689–712. doi: 10.1128/CMR.00009-10.. This comprehensive review of resistance in human cytomegalovirus provides a complete listing of mutations that confer resistance to key drugs. It summarizes more than 20 years of research and clearly and concisely presents this important data.
- 4.Strasfeld L, Chou S. Antiviral drug resistance: mechanisms and clinical implications. Infect Dis Clin North Am. 2010;24:809–833. doi: 10.1016/j.idc.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Biron KK. Antiviral drugs for cytomegalovirus diseases. Antiviral Res. 2006;71:154–163. doi: 10.1016/j.antiviral.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 6. Andrei G, De Clercq E, Snoeck R. Drug targets in cytomegalovirus infection. Infect Disord Drug Targets. 2009;9:201–222. doi: 10.2174/187152609787847758.. This comprehensive review discusses a wide range of analogs effective against cytomegalovirus. It describes the biological function of the molecular targets of the compounds and provides a rich history of antiviral drug development.
- 7.Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother. 2011;55:459–472. doi: 10.1128/AAC.00615-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elion GB. Mechanism of action and selectivity of acyclovir. Am J Med. 1982;73:7–13. doi: 10.1016/0002-9343(82)90055-9. [DOI] [PubMed] [Google Scholar]
- 9.McGuigan C, Barucki H, Carangio A, Blewett S, Andrei G, Snoeck R, De Clercq E, Balzarini J, Erichsen JT. Highly potent and selective inhibition of varicella-zoster virus by bicyclic furopyrimidine nucleosides bearing an aryl side chain. J Med Chem. 2000;43:4993–4997. doi: 10.1021/jm000210m. [DOI] [PubMed] [Google Scholar]
- 10.McGuigan C, Pathirana RN, Migliore M, Adak R, Luoni G, Jones AT, Diez-Torrubia A, Camarasa MJ, Velazquez S, Henson G, et al. Preclinical development of bicyclic nucleoside analogues as potent and selective inhibitors of varicella zoster virus. J Antimicrob Chemother. 2007;60:1316–1330. doi: 10.1093/jac/dkm376. [DOI] [PubMed] [Google Scholar]
- 11.Sienaert R, Naesens L, Brancale A, De Clercq E, McGuigan C, Balzarini J. Specific recognition of the bicyclic pyrimidine nucleoside analogs, a new class of highly potent and selective inhibitors of varicella-zoster virus (VZV), by the VZV-encoded thymidine kinase. Mol Pharmacol. 2002;61:249–254. doi: 10.1124/mol.61.2.249. [DOI] [PubMed] [Google Scholar]
- 12. Pentikis HS, Matson M, Atiee G, Boehlecke B, Hutchins JT, Patti JM, Henson GW, Morris A. Pharmacokinetics and safety of FV-100, a novel oral anti-herpes zoster nucleoside analogue, administered in single and multiple doses to healthy young adult and elderly adult volunteers. Antimicrob Agents Chemother. 2011;55:2847–2854. doi: 10.1128/AAC.01446-10.. The authors show that FV-100 is well tolerated in adults, and that once daily dosing may be sufficient to achieve serum levels above the in vitro EC50 needed to treat VZV. This is the first published clinical trial using FV-100 in humans.
- 13. Biswas S, Field HJ, editors. Helicase–Primase Inhibitors: A New Approach to Combat Herpes Simplex Virus and Varicella Zoster Virus. Weinheim: WILEY-VCH Verlag GmbH & Co.; 2011. . This is an excellent and thorough review of AIC316. It reviews the antiviral activity and mechanism of action of the compoudn as well as a discussion of resistance associated with a polymorphism the occures in many clincal isolates.
- 14.Kleymann G, Fischer R, Betz UA, Hendrix M, Bender W, Schneider U, Handke G, Eckenberg P, Hewlett G, Pevzner V, et al. New helicase-primase inhibitors as drug candidates for the treatment of herpes simplex disease. Nat Med. 2002;8:392–398. doi: 10.1038/nm0402-392. [DOI] [PubMed] [Google Scholar]
- 15.Kleymann G. Agents and strategies in development for improved management of herpes simplex virus infection and disease. Expert Opin Investig Drugs. 2005;14:135–161. doi: 10.1517/13543784.14.2.135. [DOI] [PubMed] [Google Scholar]
- 16.Biswas S, Miguel RN, Sukla S, Field HJ. A mutation in helicase motif IV of herpes simplex virus type 1 UL5 that results in reduced growth in vitro and lower virulence in a murine infection model is related to the predicted helicase structure. J Gen Virol. 2009;90:1937–1942. doi: 10.1099/vir.0.011221-0. [DOI] [PubMed] [Google Scholar]
- 17.Biswas S, Swift M, Field HJ. High frequency of spontaneous helicase-primase inhibitor (BAY 57-1293) drug-resistant variants in certain laboratory isolates of HSV-1. Antivir Chem Chemother. 2007;18:13–23. doi: 10.1177/095632020701800102. [DOI] [PubMed] [Google Scholar]
- 18.Sukla S, Biswas S, Birkmann A, Lischka P, Ruebsamen-Schaeff H, Zimmermann H, Field HJ. Effects of therapy using a helicase-primase inhibitor (HPI) in mice infected with deliberate mixtures of wild-type HSV-1 and an HPI-resistant UL5 mutant. Antiviral Res. 2010;87:67–73. doi: 10.1016/j.antiviral.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Field HJ, Biswas S. Antiviral drug resistance and helicase-primase inhibitors of herpes simplex virus. Drug Resist Update. 2011;14:45–51. doi: 10.1016/j.drup.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Lowe DM, Alderton WK, Ellis MR, Parmar V, Miller WH, Roberts GB, Fyfe JA, Gaillard R, Ertl P, Snowden W, et al. Mode of action of (R)-9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine against herpesviruses. Antimicrob Agents Chemother. 1995;39:1802–1808. doi: 10.1128/aac.39.8.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Clercq E. Guanosine analogues as anti-herpesvirus agents. Nucleosides Nucleotides Nucleic Acids. 2000;19:1531–1541. doi: 10.1080/15257770008045444. [DOI] [PubMed] [Google Scholar]
- 22.Andrei G, Snoeck R, Reymen D, Liesnard C, Goubau P, Desmyter J, De Clercq E. Comparative activity of selected antiviral compounds against clinical isolates of varicella-zoster virus. Eur J Clin Microbiol Infect Dis. 1995;14:318–329. doi: 10.1007/BF02116525. [DOI] [PubMed] [Google Scholar]
- 23.Ng TI, Shi Y, Huffaker HJ, Kati W, Liu Y, Chen CM, Lin Z, Maring C, Kohlbrenner WE, Molla A. Selection and characterization of varicella-zoster virus variants resistant to (R)-9-[4-hydroxy-2-(hydroxymethy)butyl]guanine. Antimicrob Agents Chemother. 2001;45:1629–1636. doi: 10.1128/AAC.45.6.1629-1636.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hitchcock MJ, Jaffe HS, Martin JC, Stagg RJ. Cidofovir, a new agent with potent anti-herpesvirus activity. Antivir Chem Chemother. 1996;7:115–127. [Google Scholar]
- 25. Hostetler KY. Alkoxyalkyl prodrugs of acyclic nucleoside phosphonates enhance oral antiviral activity and reduce toxicity: current state of the art. Antiviral Res. 2009;82:A84–A98. doi: 10.1016/j.antiviral.2009.01.005.. This manuscript provides and excellent review of the development of alkoxyalkyl prodrugs including CMX001. It describes the biological properties and advantages of these analogs.
- 26.Williams-Aziz SL, Hartline CB, Harden EA, Daily SL, Prichard MN, Kushner NL, Beadle JR, Wan WB, Hostetler KY, Kern ER. Comparative activities of lipid esters of cidofovir and cyclic cidofovir against replication of herpesviruses in vitro. Antimicrob Agents Chemother. 2005;49:3724–3733. doi: 10.1128/AAC.49.9.3724-3733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James SH, Hartline CB, Harden EA, Driebe EM, Schupp JM, Engelthaler DM, Keim PS, Bowlin TL, Kern ER, Prichard MN. Cyclopropavir Inhibits the Normal Function of the Human Cytomegalovirus UL97 Kinase. Antimicrob Agents Chemother. 2011;55:4682–4691. doi: 10.1128/AAC.00571-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bidanset DJ, Beadle JR, Wan WB, Hostetler KY, Kern ER. Oral activity of ether lipid ester prodrugs of cidofovir against experimental human cytomegalovirus infection. J Infect Dis. 2004;190:499–503. doi: 10.1086/421912. [DOI] [PubMed] [Google Scholar]
- 29.Kern ER, Collins DJ, Wan WB, Beadle JR, Hostetler KY, Quenelle DC. Oral treatment of murine cytomegalovirus infections with ether lipid esters of cidofovir. Antimicrob Agents Chemother. 2004;48:3516–3522. doi: 10.1128/AAC.48.9.3516-3522.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bravo FJ, Bernstein DI, Beadle JR, Hostetler KY, Cardin RD. Oral hexadecyloxypropyl-cidofovir therapy in pregnant guinea pigs improves outcome in the congenital model of cytomegalovirus infection. Antimicrob Agents Chemother. 2011;55:35–41. doi: 10.1128/AAC.00971-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quenelle DC, Lampert B, Collins DJ, Rice TL, Painter GR, Kern ER. Efficacy of CMX001 against herpes simplex virus infections in mice and correlations with drug distribution studies. J Infect Dis. 2010;202:1492–1499. doi: 10.1086/656717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prichard MN, Kern ER, Hartline CB, Lanier ER, Quenelle DC. CMX001 Potentiates the Efficacy of Acyclovir in Herpes Simplex Virus Infections. Antimicrob Agents Chemother. 2011;55:4728–4734. doi: 10.1128/AAC.00545-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Painter GR, Trost LC, Lambert BL, Almond MR, Buller M, Kern ER, Painter GP, Robertson AT, O’Mahony R. CMX001. Drugs of the future. 2008;33:1–7. [Google Scholar]
- 34.Ciesla SL, Trahan J, Wan WB, Beadle JR, Aldern KA, Painter GR, Hostetler KY. Esterification of cidofovir with alkoxyalkanols increases oral bioavailability and diminishes drug accumulation in kidney. Antiviral Res. 2003;59:163–171. doi: 10.1016/s0166-3542(03)00110-4. [DOI] [PubMed] [Google Scholar]
- 35.Williams SL, Hartline CB, Kushner NL, Harden EA, Bidanset DJ, Drach JC, Townsend LB, Underwood MR, Biron KK, Kern ER. In vitro activities of benzimidazole D- and L-ribonucleosides against herpesviruses. Antimicrob Agents Chemother. 2003;47:2186–2192. doi: 10.1128/AAC.47.7.2186-2192.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biron K. In: Maribavir: a promising new antiherpes therapeutic agent. Bogner E, Holzenburg A, editors. Dordrecht, the Netherlands: Springer; 2006. [Google Scholar]
- 37.Kern ER, Hartline CB, Rybak RJ, Drach JC, Townsend LB, Biron KK, Bidanset DJ. Activities of benzimidazole D- and L-ribonucleosides in animal models of cytomegalovirus infections. Antimicrob Agents Chemother. 2004;48:1749–1755. doi: 10.1128/AAC.48.5.1749-1755.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Prichard MN. Function of human cytomegalovirus UL97 kinase in viral infection and its inhibition by maribavir. Rev Med Virol. 2009;19:215–229. doi: 10.1002/rmv.615.. This review describes the biology of UL97 kinase and its potential as a target for antiviral therapy. It integrates results from pharmacologic and genetic approaches used to understand the function of this viral kinase.
- 39.Chou S. Cytomegalovirus UL97 mutations in the era of ganciclovir and maribavir. Rev Med Virol. 2008;18:233–246. doi: 10.1002/rmv.574. [DOI] [PubMed] [Google Scholar]
- 40.Chou S, Marousek GI. Maribavir antagonizes the antiviral action of ganciclovir on human cytomegalovirus. Antimicrob Agents Chemother. 2006;50:3470–3472. doi: 10.1128/AAC.00577-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chou S. Diverse cytomegalovirus UL27 mutations adapt to loss of viral UL97 kinase activity under maribavir. Antimicrob Agents Chemother. 2009;53:81–85. doi: 10.1128/AAC.01177-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Komazin G, Townsend LB, Drach JC. Role of a mutation in human cytomegalovirus gene UL104 in resistance to benzimidazole ribonucleosides. J Virol. 2004;78:710–715. doi: 10.1128/JVI.78.2.710-715.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Reitsma JM, Savaryn JP, Faust K, Sato H, Halligan BD, Terhune SS. Antiviral inhibition targeting the HCMV kinase pUL97 requires pUL27-dependent degradation of Tip60 acetyltransferase and cell-cycle arrest. Cell Host Microbe. 2011;9:103–114. doi: 10.1016/j.chom.2011.01.006.. This recent article was the first to describe how mutations in the UL27 open reading frame migh impact susceptibility to maribavir and other inhibitors of UL97 kinase.
- 44.Hume AJ, Finkel JS, Kamil JP, Coen DM, Culbertson MR, Kalejta RF. Phosphorylation of retinoblastoma protein by viral protein with cyclin-dependent kinase function. Science. 2008;320:797–799. doi: 10.1126/science.1152095. [DOI] [PubMed] [Google Scholar]
- 45.Prichard MN, Sztul E, Daily SL, Perry AL, Frederick SL, Gill RB, Hartline CB, Streblow DN, Varnum SM, Smith RD, et al. Human cytomegalovirus UL97 kinase activity is required for the hyperphosphorylation of retinoblastoma protein and inhibits the formation of nuclear aggresomes. J Virol. 2008;82:5054–5067. doi: 10.1128/JVI.02174-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamirally S, Kamil JP, Ndassa-Colday YM, Lin AJ, Jahng WJ, Baek MC, Noton S, Silva LA, Simpson-Holley M, Knipe DM, et al. Viral mimicry of Cdc2/cyclin-dependent kinase 1 mediates disruption of nuclear lamina during human cytomegalovirus nuclear egress. PLoS Pathog. 2009;5:e1000275. doi: 10.1371/journal.ppat.1000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marschall M, Marzi A, aus dem Siepen P, Jochmann R, Kalmer M, Auerochs S, Lischka P, Leis M, Stamminger T. Cellular p32 recruits cytomegalovirus kinase pUL97 to redistribute the nuclear lamina. J Biol Chem. 2005;280:33357–33367. doi: 10.1074/jbc.M502672200. [DOI] [PubMed] [Google Scholar]
- 48.Marty FM, Ljungman P, Papanicolaou GA, Winston DJ, Chemaly RF, Strasfeld L, Young JA, Rodriguez T, Maertens J, Schmitt M, et al. Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: a phase 3, double-blind, placebo-controlled, randomised trial. Lancet Infect Dis. 2011;11:284–292. doi: 10.1016/S1473-3099(11)70024-X. [DOI] [PubMed] [Google Scholar]
- 49.Zhou S, Breitenbach JM, Borysko KZ, Drach JC, Kern ER, Gullen E, Cheng YC, Zemlicka J. Synthesis and antiviral activity of (Z)- and (E)-2,2-[bis(hydroxymethyl)cyclopropylidene]methylpurines and -pyrimidines: second-generation methylenecyclopropane analogues of nucleosides. J Med Chem. 2004;47:566–575. doi: 10.1021/jm030316s. [DOI] [PubMed] [Google Scholar]
- 50.Kern ER, Kushner NL, Hartline CB, Williams-Aziz SL, Harden EA, Zhou S, Zemlicka J, Prichard MN. In vitro activity and mechanism of action of methylenecyclopropane analogs of nucleosides against herpesvirus replication. Antimicrob Agents Chemother. 2005;49:1039–1045. doi: 10.1128/AAC.49.3.1039-1045.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kern ER, Bidanset DJ, Hartline CB, Yan Z, Zemlicka J, Quenelle DC. Oral activity of a methylenecyclopropane analog, cyclopropavir, in animal models for cytomegalovirus infections. Antimicrob Agents Chemother. 2004;48:4745–4753. doi: 10.1128/AAC.48.12.4745-4753.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gentry BG, Kamil JP, Coen DM, Zemlicka J, Drach JC. Stereoselective phosphorylation of cyclopropavir by pUL97 and competitive inhibition by maribavir. Antimicrob Agents Chemother. 2010;54:3093–3098. doi: 10.1128/AAC.00468-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gentry BG, Gentry SN, Jackson TL, Zemlicka J, Drach JC. Phosphorylation of antiviral and endogenous nucleotides to di- and triphosphates by guanosine monophosphate kinase. Biochem Pharmacol. 2011;81:43–49. doi: 10.1016/j.bcp.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 54.Chou S, Bowlin TL. Cytomegalovirus UL97 mutations affecting cyclopropavir and ganciclovir susceptibility. Antimicrob Agents Chemother. 2011;55:382–384. doi: 10.1128/AAC.01259-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hakki M, Drummond C, Houser B, Marousek G, Chou S. Resistance to maribavir is associated with the exclusion of pUL27 from nucleoli during human cytomegalovirus infection. Antiviral Res. 2011 doi: 10.1016/j.antiviral.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lischka P, Hewlett G, Wunberg T, Baumeister J, Paulsen D, Goldner T, Ruebsamen-Schaeff H, Zimmermann H. In vitro and in vivo activities of the novel anticytomegalovirus compound AIC246. Antimicrob Agents Chemother. 2010;54:1290–1297. doi: 10.1128/AAC.01596-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bogner E. Human cytomegalovirus terminase as a target for antiviral chemotherapy. Rev Med Virol. 2002;12:115–127. doi: 10.1002/rmv.344. [DOI] [PubMed] [Google Scholar]
- 58.Krosky PM, Underwood MR, Turk SR, Feng KW, Jain RK, Ptak RG, Westerman AC, Biron KK, Townsend LB, Drach JC. Resistance of human cytomegalovirus to benzimidazole ribonucleosides maps to two open reading frames: UL89 and UL56. J Virol. 1998;72:4721–4728. doi: 10.1128/jvi.72.6.4721-4728.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reefschlaeger J, Bender W, Hallenberger S, Weber O, Eckenberg P, Goldmann S, Haerter M, Buerger I, Trappe J, Herrington JA, et al. Novel non-nucleoside inhibitors of cytomegaloviruses (BAY 38-4766): in vitro and in vivo antiviral activity and mechanism of action. J Antimicrob Chemother. 2001;48:757–767. doi: 10.1093/jac/48.6.757. [DOI] [PubMed] [Google Scholar]
- 60.Goldner T, Hewlett G, Ettischer N, Ruebsamen-Schaeff H, Zimmermann H, Lischka P. The Novel Anticytomegalovirus Compound AIC246 (Letermovir) Inhibits Human Cytomegalovirus Replication through a Specific Antiviral Mechanism That Involves the Viral Terminase. J Virol. 2011;85:10884–10893. doi: 10.1128/JVI.05265-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaul DR, Stoelben S, Cober E, Ojo T, Sandusky E, Lischka P, Zimmermann H, Rubsamen-Schaeff H. First report of successful treatment of multidrug-resistant cytomegalovirus disease with the novel anti-CMV compound AIC246. Am J Transplant. 2011;11:1079–1084. doi: 10.1111/j.1600-6143.2011.03530.x. [DOI] [PubMed] [Google Scholar]
- 62.Este JA, Cihlar T. Current status and challenges of antiretroviral research and therapy. Antiviral Res. 2010;85:25–33. doi: 10.1016/j.antiviral.2009.10.007. [DOI] [PubMed] [Google Scholar]