Abstract
Rationale
The interoceptive and reinforcing effects of 3,4-methylenedioxymethamphetamine (MDMA) are similar to those of psychostimulants, but the role of dopamine in the behavioral effects of MDMA is not well documented, especially in primates.
Objective
The aim of this study was to assess the role of dopamine in the behavioral effects of MDMA in two nonhuman primate species.
Methods
The behavioral effects of MDMA, with and without serotonergic or dopaminergic pretreatments, were studied in squirrel monkeys trained to respond under a fixed-interval schedule of stimulus termination; effects on caudate dopamine levels were studied in a separate group of squirrel monkeys using in vivo microdialysis. Positron emission tomography neuroimaging with the dopamine transporter (DAT) ligand [18F]FECNT was used to determine DAT occupancy by MDMA in rhesus monkeys.
Results
MDMA (0.5–1.5 mg/kg) did not induce behavioral stimulant effects, but the highest dose of MDMA suppressed responding. Pretreatment with fluoxetine (3.0 mg/kg) or the selective 5HT2A antagonist M100907 (0.03–0.3 mg/kg) attenuated the rate suppressing effects of MDMA. In contrast, pretreatment with the selective dopamine transporter inhibitor RTI-177 (0.1 mg/kg) did not alter the rate suppressing effects of MDMA. Administration of MDMA at a dose that suppressed operant behavior had negligible effects on extracellular dopamine. The percent DAT occupancy of MDMA at a dose that suppressed operant behavior also was marginal and reflected low in vivo potency for DAT binding.
Conclusions
Collectively, these results indicate that behaviorally relevant doses of MDMA do not induce behavioral stimulant or dopamine transporter-mediated effects in nonhuman primates.
Keywords: MDMA, PET neuroimaging, Microdialysis, Dopamine, Serotonin, Operant behavior Nonhuman primates
Introduction
3,4-Methylenedioxymethamphetamine (MDMA) is a substituted phenethylamine with chemical similarities to mescaline-type hallucinogens and amphetamine-type psychostimulants. Studies investigating the behavioral pharmacology of MDMA have typically focused on serotonergic mechanisms (Bankson and Cunningham 2001; Bankson and Cunningham 2002), but more recently, dopaminergic mechanisms have been investigated. In rodents, MDMA has been shown to increase synaptic dopamine via interactions with the dopamine transporter (Iravani et al. 2000; White et al. 1996; Nash and Brodkin 1991; Yamamoto and Spanos 1988; Baumann et al. 2004). In behavioral studies, MDMA self-administration and MDMA-induced locomotor stimulation were attenuated in rats pretreated with the D1-like antagonist SCH 23390 (Daniela et al. 2004). Similarly, MDMA-elicited hyper-locomotion was strongly correlated with elevations in extracellular DA in nucleus accumbens, striatum, and prefrontal cortex in the rat (Baumann et al. 2008). The pharmacological basis for this correlation may involve interactions of DA released following MDMA administration, as S(+)-MDMA-stimulated locomotor activity is dependent on D1 and D2 receptor activation, and D1 receptors also appear to play a role in the discriminative stimulus properties of (+)-MDMA (Bubar et al. 2004). On a more molecular level, MDMA-induced ERK phosphorylation within the nucleus accumbens was blocked by the D1-like antagonist SCH 39166, but not by the D2-like antagonist raclopride (Acquas et al. 2007). A more complex role for dopaminergic systems in the locomotor stimulant effects of MDMA has been described in the mouse (Risbrough et al. 2006).
In primates, however, the role of dopaminergic mechanisms in the behavioral effects of MDMA has not been intensively investigated. Nevertheless, in rhesus monkeys trained to discriminate amphetamine from saline, MDMA dose-dependently and fully substituted for the amphetamine cue (Kamien et al. 1986). Similarly, MDMA exhibits amphetamine-like discriminative stimulus and subjective effects in humans (Peroutka et al. 1988; Tancer and Johanson 2001, 2007; Johanson et al. 2006). No previous studies have used selective antagonists to attempt to ascertain the neuropharmacological underpinnings of the discriminative stimulus effects of MDMA in human or nonhuman primates. Furthermore, while MDMA is self-administered by rhesus monkeys (Beardsley et al. 1986; Fantegrossi et al. 2002; Lamb and Griffiths 1987; Lile et al. 2005; Wang and Woolverton 2007), it is usually done so to a lesser extent than traditional psychostimulants such as cocaine or methamphetamine. Importantly, administration of 5-HT2 antagonists (selective and nonselective) suggests that the reinforcing effects of MDMA are mediated through central serotonergic systems in rhesus monkeys (Fantegrossi et al. 2002). Analogous studies using dopamine antagonists have not yet been undertaken in primates. Thus, the interoceptive and reinforcing effects of MDMA in primates are consistent with those of other dopaminergic drugs, although serotonergic mechanisms of action are much more pronounced. The behavioral profile of MDMA is thus not easily defined as stimulant-like in nonhuman primates, and the effects of MDMA on motor activity in nonhuman primates do not conform to those demonstrated in rodents. For example, MDMA did not alter spontaneous home cage activity in rhesus monkeys, although administration of methamphetamine to these same animals reliably increased motor behavior (Crean et al. 2006). Taffe and colleagues have reported that MDMA-treated rhesus monkeys appeared drug intoxicated but otherwise were normally responsive without evidence of repetitive stereotyped movement (Taffe et al. 2006). This description does not contradict our own observations of reduced motor activity, closed eyes, and a blunted responsiveness to environmental stimuli in squirrel monkeys following MDMA administration. The contrast between what is observed in rodents and nonhuman primates is indeed striking.
Accordingly, the present studies were designed to assess directly the role of dopaminergic systems in the behavioral effects of MDMA in two nonhuman primate species. The behavioral effects of MDMA were studied in squirrel monkeys (Saimiri sciureus) trained to respond in an operant procedure that is especially sensitive to increases in response rates induced by various dopaminergics drugs (Kimmel et al. 2007). The effects on caudate dopamine levels of doses at least as high as those found to be active in the behavioral procedure were studied in a separate group of squirrel monkeys using in vivo microdialysis. Lastly, positron emission tomography (PET) neuroimaging with the dopamine transporter (DAT) ligand [18F]FECNT was used to determine DAT occupancy by a challenge injection of MDMA in rhesus monkeys (Macaca mulatta). Collectively, the results indicate that behaviorally relevant doses of MDMA do not induce behavioral stimulant or dopamine transporter-mediated effects in nonhuman primates.
Materials and methods
Subjects
Eight adult male squirrel monkeys weighing 800–1,150 g served as subjects in behavioral (N=6) and neurochemical (N=2) studies. Three adult rhesus monkeys (two female, one male) weighing between 7.8 and 13.4 kg served as subjects in PET neuroimaging experiments. All animals lived in individual stainless steel home cages and were provided daily access to food (Harlan Teklad monkey chow; Harlan Teklad, Madision, WI, USA; fresh fruit and vegetables) and unlimited water. Environmental enrichment devices were provided on a regular rotating basis. All monkeys had prior exposure to cocaine and other drugs with selective dopaminergic or serotonergic activity in various behavioral studies. Animal use procedures were in strict accordance with the National Institutes of Health “Guide for Care and Use of Laboratory Animals” (Publication No. 85-23, revised 1985) and were approved by the Institutional Animal Care and Use Committee of Emory University.
Procedures
Effects of MDMA on schedule-controlled behavior in squirrel monkeys
During daily test sessions, squirrel monkeys (s-170, s-171, s-177, s-187, s-189, s-195) used in behavioral studies were seated in Plexiglas chairs equipped with stimulus lights, a response lever, and equipment for delivering a mild electrical stimulus to the tail. Behavioral test sessions lasted 90-min each day and occurred within ventilated, sound-attenuating chambers (MED Associates, Georgia, VT, USA). All animals were trained under a fixed-interval (FI) 300-s schedule of stimulus termination. At the beginning of each testing session, the behavioral chamber was illuminated with a red light for 300 s. At the end of this interval, monkeys were required to press the lever once within 3 s to terminate the red light, which was associated with an impending electrical stimulus. Upon termination of the red light, a white light was illuminated for 15 s, followed by a 60-s timeout. If the lever was not pressed during the 3-s limited hold period, the animal received a 200-ms 3-mA stimulus to the tail, followed by a 60-s timeout. Responding during timeout periods had no scheduled consequences. Animals were tested each day, 5 days/week, and each daily session consisted of 15 consecutive FI components.
Drug experiments began when rates and patterns of responding stabilized (less than 20% variability in response rates for five consecutive test sessions). Drugs were administered on Tuesdays and Fridays and saline was administered on Thursdays as a control. A single dose of drug (MDMA or methamphetamine) or saline was administered i.m. 5 s before the beginning of the experimental session. In drug interaction experiments, a dose of the selective serotonin transporter (SERT) inhibitor, fluoxetine, the 5HT2A receptor antagonist, M100907, or the selective DAT inhibitor, RTI-177, was administered i.m. 15 min pre-session. Subjects s-170, s-171, s-177, and s-187 received methamphetamine, MDMA, fluoxetine and M100907. Subjects s-170, s-187, s-189 and s-195 received MDMA and RTI-177. Drug interaction experiments were conducted first with fluoxetine followed by M100907 and RTI-177. Drug doses were administered in a quasi-random order and each dose or drug combination was determined twice. Response rate maintained by the FI 300-s schedule of stimulus termination was computed separately for each FI component by dividing the total number of responses in a component by the total time the red light was present. Mean control rate was determined for each monkey by averaging response rate for all saline (control) sessions. Data are presented for the group of monkeys as the mean response rate ± SEM during all sessions for a particular drug condition and are expressed as a percent of control rate obtained when saline was administered.
Guide cannula implantation
A stereotaxic apparatus was used to implant CMA/11 guide cannulae (CMA/Microdialysis, Acton, MA, USA) bilaterally to target both caudate nuclei in two squirrel monkeys (s-165, s-175) as described previously (Czoty et al. 2000). Anesthesia was initiated with Telazol (tiletamine hydrochloride and zolazepam hydrochloride, 3.0 mg) and atropine. Inhaled isoflurane (1.0–2.0%) was administered to maintain depth of anesthesia during the procedure. A stainless steel stylet was placed in the guide cannula when not in use. Analgesics [Banamine (flunixin meglumine)] and antibiotics [Rocephin (ceftriaxone)] were prescribed as necessary by veterinary staff. Animals were closely monitored during recovery from anesthesia, and a minimum of 2 weeks was allowed before microdialysis experiments were performed. The guide cannulae provided a very specific path for the insertion of the probe such that the correct placement of the guide ensured correct placement of the probe.
In vivo microdialysis
Microdialysis experiments were conducted in squirrel monkeys seated in a Plexiglas chair and fitted with an adjustable Lexan neckplate that was positioned perpendicular to the medial plane of the body just above the shoulder. All squirrel monkeys had been acclimated to the chairs over several months. CMA/11 dialysis probes with a shaft length of 14 mm and active dialysis membrane measuring 4 mm long and 0.24 mm diameter were flushed with artificial cerebrospinal fluid (1.0 mM Na2HPO4, 150 mM NaCl, 3 mM KCl, 1.3 mM Ca Cl2, 1.0 mM mg Cl2, and 0.15 mM ascorbic acid, final pH=7.4–7.56) for at least 20 min. Probes were inserted into the guide cannulae and connected to a Harvard PicoPlus microinfusion pump via FEP Teflon tubing. Probes were perfused with artificial cerebrospinal fluid at a rate of 2.0µl/min for the duration of the experiment. Continuous samples were collected every 10 min in microcentrifuge tubes and immediately refrigerated. Following a 60-min equilibration, three consecutive 10-min samples were collected for determination of baseline dopamine concentration. Following collection of baseline samples, a dose of MDMA was administered IM and 10-min samples were collected for an additional 120 min. Both subjects received 1.5 mg/kg first followed by 3.0 mg/kg at least 2 weeks later. Finally, to assess voltage dependency of dopamine release and tissue integrity, aCSF with a high concentration of KCl (75 mM) was perfused through the probe and dialysate was collected for a 10-min period. A robust increase in extracellular dopamine in response to high KCl verified tissue integrity. Animals were tested a maximum of one time per week, and each site was accessed no more often than once every 2 weeks. This regimen of repeated access of the caudate has produced consistent responses to drug treatment without significant gliosis (Czoty et al. 2000).
High-performance liquid chromatography (HPLC) and electrochemical detection were used to quantify levels of dopamine. The HPLC system consisted of a small bore (3.2 mm × 150 mm, 3µm) column (ESA, Inc., Chelmsford, MA, USA) with a commercially available mobile phase (MD-TM, ESA, Inc.) delivered by an ESA 582 solvent delivery pump at a flow rate of 0.6 ml/min. After loading onto the refrigerated sample tray, samples (20µl) were automatically mixed with 3µl of ascorbate oxidase, and 18µl of the mixture was injected into the HPLC system by an ESA Model 542 autosampler. Samples were analyzed within 12 h of collection, remaining either in a refrigerator or in the refrigerated autosampler tray during this time. Electrochemical analyses were performed using an ESA dual-channel analytical cell (model 5040) and guard cell (model 5020, potential = 350 mV) and an ESA Coulochem II detector. The potential of channel 1 was set to −150 mV for oxidation, while the potential of channel 2 was set to 275 mV for reduction. A full range of dopamine standards (1–50 nM) was analyzed both before and after each set of samples to evaluate possible degradation of dopamine. Levels of dopamine below 0.5 nM were considered below the limit of detection. A desktop computer collected data and chromatograms were generated by EZChrom Elite software (version 3.1, Scientific Software, Pleasanton, CA, USA). The chromatograms were analyzed using the EZChrom software, comparing the experimental samples with the standards. Mean baseline dopamine concentrations for an individual subject were defined as the mean of the three samples preceding MDMA administration. Samples were collected from both the left and right caudate in separate experiments. For each animal, dopamine levels from the two studies were averaged, resulting in a single value for that animal for each experimental condition. Data are presented for individual subjects, and the effects of MDMA are expressed as a percent of baseline dopamine concentrations.
PET neuroimaging
PET neuroimaging was performed in rhesus monkeys (RLm-1, RDn-2, RBp-3) at the Emory University PET Center on a Concorde microPET P4 scanner (Concorde MicroSystems, Knoxville, TN, USA) using [18F]FECNT. During image acquisition, vital signs were continuously monitored, and heart rate and rectal temperature were noted every 10 min by a trained technician. On study days, subjects were immobilized in their home cage with 4.0 mg/kg Telazol and transported to the Emory University PET Center. Subsequently, animals were intubated and anesthesia was maintained with 1.0% to 2.0% isoflurane. Subjects were positioned in the scanner and connected to the physiological monitoring equipment.
A 15-min transmission scan was obtained for attenuation correction, then a slow bolus of approximately 5.0 mCi of [18F]FECNT (specific activity 1.5 Ci/µmol) was injected over 5 to 6 min at a rate of 1.0 ml/min. The PET ligand FECNT is an N-fluoroethyl nortropane derivative that is selective for the DAT (Goodman et al. 2000), and displacement studies with cocaine previously determined that FECNT and cocaine bind to the same site in the striatum and that corresponding DAT occupancy measures for cocaine are dose-dependent (Votaw et al. 2002). Scanning began coincident with the start of radiotracer injection. The initial acquisition was a 28-frame dynamic sequence starting with 30-s scans and ending with 20-min scans for a total duration of 90 min. At 90 min, a single bolus dose (1.5 mg/kg) of MDMA was injected, and a second dynamic sequence was acquired starting with 2-min frames and ending with 5-min frames for a total duration of 3 h for both sequences.
A generalized reference tissue method was used to analyze the resulting occupancy data (Votaw et al. 2002). All images were reconstructed with measured attenuation correction, zoom factor 8, and Shepp-Logan reconstruction filter cut-off at 1 cycle/cm. The axial slice thickness was 3.375 mm. All images were decay-corrected to the time of injection. Regions of interest were manually drawn on the late images over the putamen, caudate, and cerebellum. The regions of interest were then overlaid on all images to obtain time–activity curves. Data were collected in two sections with the assumption that drug infusion changed only the k3 (Bmaxkon) rate constant (i.e., MDMA competes with FECNT for binding at the DAT and thus decreases the apparent Bmax) from ka/3 (predrug infusion) to kb/3 (postdrug infusion). Five rate constants (R, k2, ka/3, kb/3, and k4) and the time–activity curve from the reference region (cerebellum) were used to model the putamen and caudate time–activity curve. In the modeling, it was assumed that the competing ligand did not affect the blood–brain barrier parameters (flow or extraction; R or k2) or the transporter-FECNT kinetic properties (kon, koff= k4). Thus, these parameters were kept constant over the entire experiment. After optimization, the covariance matrix was numerically determined and used to estimate the uncertainty in the fit values. From this, it was found that the variance in the k3/k4 ratio is much less than the variance in either parameter alone due to their nonvanishing covariance. Accordingly, the k3/k4 ratio was taken as a measure of DAT density. The fraction of transporters occupied by the competing ligand was then estimated as 1–(kb/3/k4)(ka/3/k4).
Physiological measures
During PET neuroimaging experiments, subjects were fitted with electronic thermistor and pulse oximetry probes coupled to a computerized medical monitor in order to continuously observe rectal temperature and heart rate, respectively. Baseline measures were obtained by averaging three observations collected at 5 min increments over 15 min immediately preceding injection of MDMA, while experimental observations were determined every 5 min following MDMA infusion. For the three rhesus monkeys studied under deep anesthesia for PET experiments, baseline rectal temperature was 92.08±0.42°C, and baseline heart rate was 91.22±3.47 beats per min prior to MDMA administration.
Drugs
Racemic MDMA and methamphetamine HCl and RTI-177 were supplied by the National Institute on Drug Abuse (Research Technology Branch, Research Triangle Park, NC, USA). Fluoxetine HCl was supplied by Eli Lilly and Company (Indianapolis, IN, USA). M100907 was supplied as a generous gift from Kenner C. Rice, Ph.D. at the Laboratory of Medicinal Chemistry at the NIH/NIDDK. All drugs were dissolved in physiological saline prior to injection and doses are expressed as the salt. The purity of MDMA was independently verified by two separate labs (the University of Utah College of Pharmacy, Department of Pharmacology and Toxicology, Center for Human Toxicology, and Research Triangle Institute (RTI) International) using liquid chromatography/mass spectrometry with positive ion electrospray ionization and selected-ion monitoring (Utah) and nuclear magnetic resonance spectroscopy (RTI). Surgical and anesthetic drugs were purchased from commercial sources. [18F]FECNT was synthesized and radiolabeled at the Emory University PET Center core facilities. All HPLC reagents were purchased from commercial sources.
Data analysis
In behavioral experiments, the effects of methamphetamine (0.3 mg/kg) and fluoxetine (3.0 mg/kg) were compared to saline using a repeated-measures t test. The effects of MDMA (0.5–1.5 mg/kg), M100907 (0.01–0.3 mg/kg), and MDMA in combination with M100907 were analyzed with repeated-measures one-way ANOVA. Drug time course data comparing the effects of MDMA (1.5 mg/kg) alone to MDMA in combination with fluoxetine, M100907, and RTI-177 were analyzed with repeated-measures two-way ANOVA. In microdialysis experiments, the equal variance test failed so the effects of MDMA were analyzed with Friedman repeated-measures ANOVA on ranks. In physiological experiments, the effects of MDMA were analyzed with repeatedmeasures one-way ANOVA. Significant (p<0.05) main effects were followed with Dunnett’s post hoc analyses corrected for multiple comparisons.
Results
Schedule-controlled behavior in squirrel monkeys
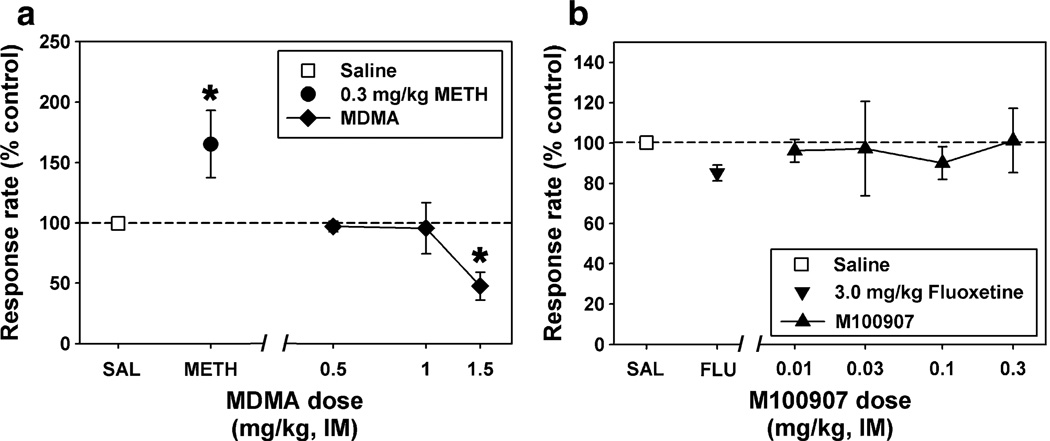
Responding during nondrug sessions or following saline administration was characteristic of typical performance maintained by FI schedules of reinforcement in squirrel monkeys. Response rate was low early in the interval and increased as the interval elapsed. All subjects terminated most stimuli, and electric stimulus presentation was infrequent. Mean response rate for the group of six subjects during saline control sessions (n>10) was 0.74±0.32 responses/s. Methamphetamine (0.3 mg/kg) produced a significant (p=0.024) stimulant effect, increasing mean session response rate to over 200% of control rate (Fig. 1a). In contrast, MDMA (0.5–1.5 mg/kg) did not produce a stimulant effect over a range of drug doses. Rather, MDMA produced a significant (p=0.009) decrease in responding, with the highest dose decreasing mean session response rate to below 50% of control rates. Injection of 0.5 mg/kg MDMA resulted in a pattern of responding which was virtually identical to that observed following saline administration.
Fig. 1.
a Effects of saline (square), 0.3 mg/kg METH (circle), or various doses of MDMA (diamonds) on behavior maintained by a fixed-interval schedule of stimulus termination. b Effects of saline (square), 3.0 mg/kg fluoxetine (inverted triangle), or various doses of M100907 (triangles) on behavior maintained by a fixed-interval schedule of stimulus termination. Each point represents the mean ± SEM (n=4 squirrel monkeys). Abscissae: injection condition. Ordinates: response rate (per second) converted to percent of control. Asterisks indicate significant differences from saline control
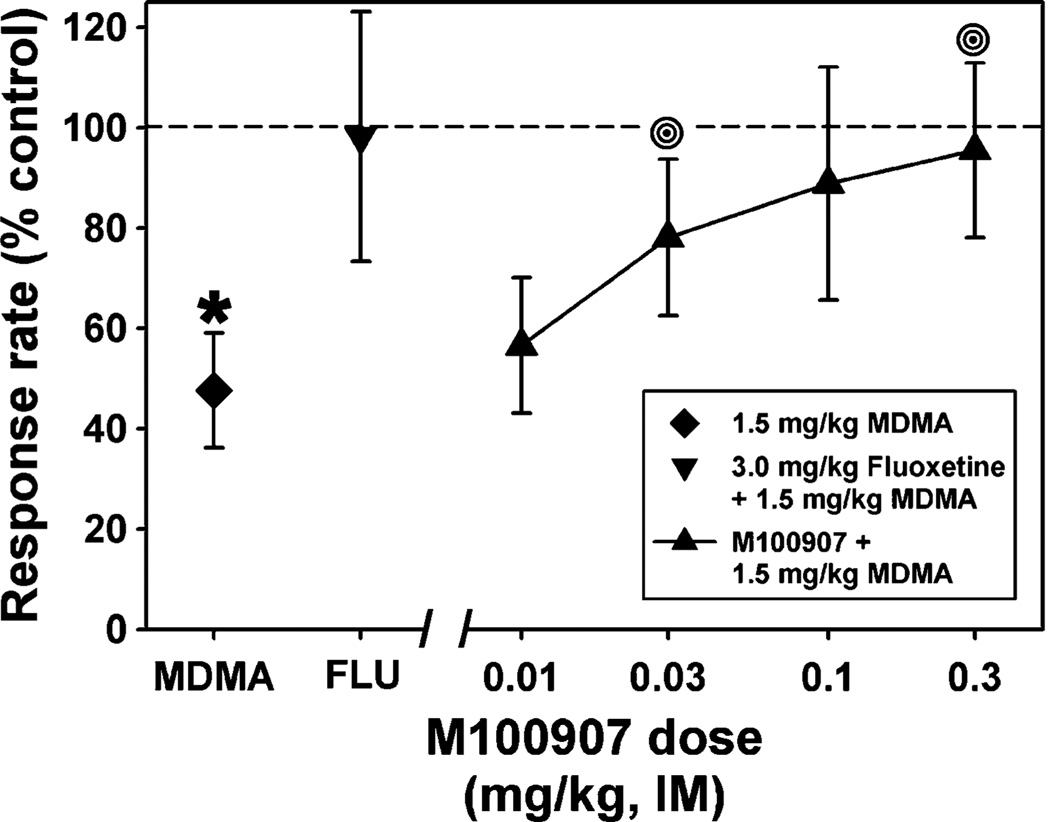
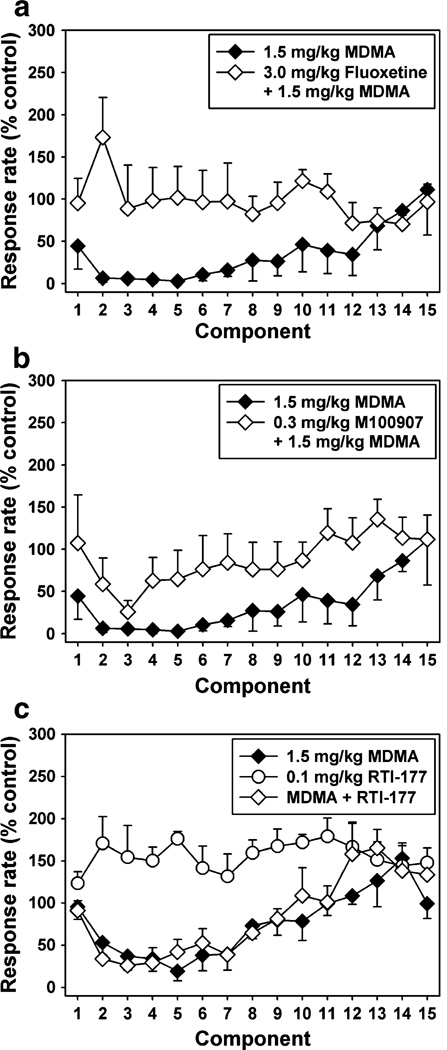
Administration of the selective SERT inhibitor, fluoxetine (3.0 mg/kg), or the selective 5HT2A antagonist, M100907 (0.03–0.3 mg/kg), alone had no significant effect on responding (Fig. 1b). However, pretreatment with fluoxetine (3.0 mg/kg) completely blocked the rate suppressing effects of the highest dose (1.5 mg/kg) of MDMA (Fig. 2). Moreover, a twofold higher dose (3.0 mg/kg) of MDMA was ineffective in suppressing response rate when administered following pretreatment with fluoxetine (data not shown). Similarly, pretreatment with the selective 5HT2A antagonist, M100907 (0.03–0.3 mg/kg), significantly (p=0.007) attenuated the rate suppressing effects of MDMA in a dose-dependent manner (Fig. 2). In drug time course experiments (Fig. 3), there was a significant main effect of fluoxetine treatment (p=0.028) and time (p= 0.029) and a significant interaction (p=0.005, Fig. 3a). Likewise, the effect of M100907 treatment approached significance (p=0.062) and there was a main effect of time (p=0.001, Fig. 3b). Lastly, administration of the selective DAT inhibitor, RTI-177 (0.1 mg/kg) had a significant (p=0.02) stimulant effect (Fig. 3c). However, pretreatment with RTI-177 had no significant effect on MDMA-induced decreases in response rate.
Fig. 2.
Effects of 1.5 mg/kg MDMA alone (diamond), or following pretreatment with 3.0 mg/kg fluoxetine (inverted triangle) or various doses of M100907 (triangles) on behavior maintained by a fixed-interval schedule of stimulus termination. Each point represents the mean ± SEM (n=4 squirrel monkeys). Abscissa and ordinate as described in Fig. 1. Asterisks indicate significant differences from saline control, while bull’s eyes represent significant differences from MDMA alone
Fig. 3.
a Within-session analysis of behavior maintained by a fixed-interval schedule of stimulus termination following injection with 1.5 mg/kg MDMA alone (filled diamonds) or the combination of 3.0 mg/kg fluoxetine and MDMA (open diamonds). b Within-session analysis of behavior maintained by a fixed-interval schedule of stimulus termination following injection with 1.5 mg/kg MDMA alone (filled diamonds) or the combination of 0.3 mg/kg M100907 and MDMA (open diamonds). c Within-session analysis of behavior maintained by a fixed-interval schedule of stimulus termination following injection with 1.5 mg/kg MDMA alone (filled diamonds), 0.1 mg/kg RTI-177 alone (open circles) or the combination of 0.1 mg/kg RTI-177 and MDMA (open diamonds). Each point represents the mean ± SEM (n=4 squirrel monkeys). Abscissae: successive 5-min components of the stimulus termination session. Ordinates: response rate (per second) converted to percent of control
In vivo microdialysis
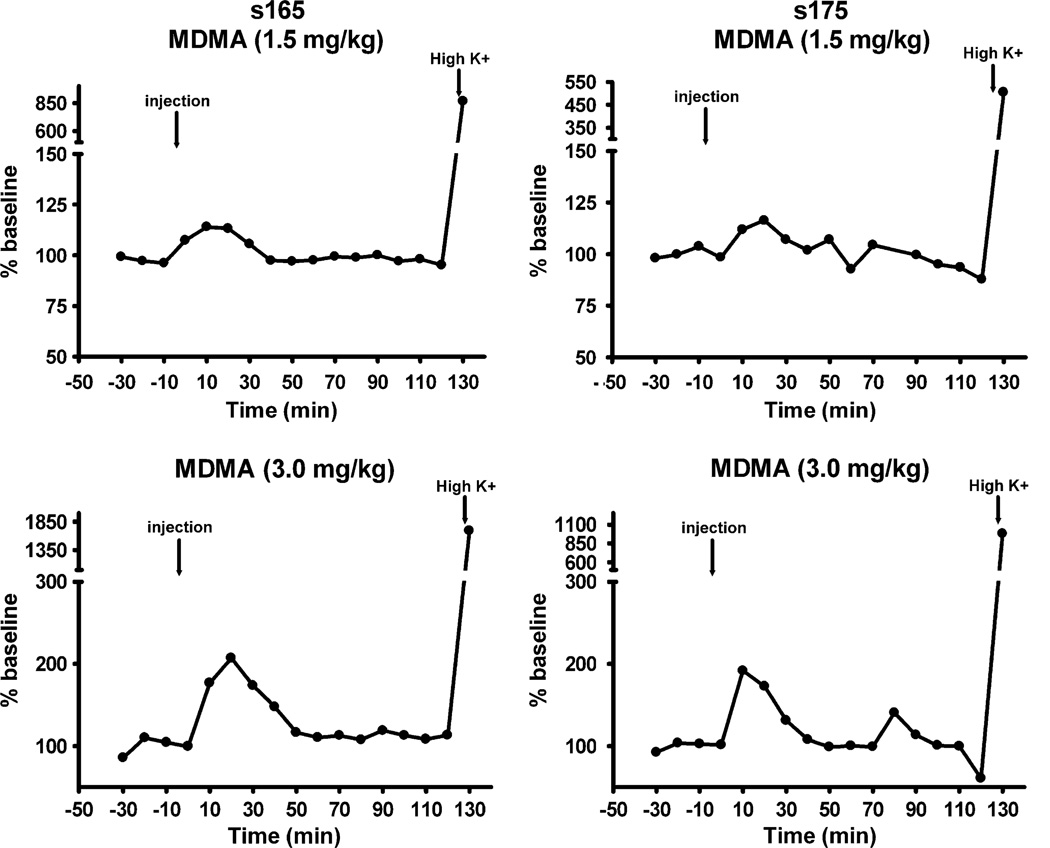
In vivo microdialysis experiments were conducted in conscious squirrel monkeys in order to examine the neurochemical effects of behaviorally active doses of MDMA (1.5 and 3.0 mg/kg). In all experiments, extracellular dopamine levels stabilized within 60 min of probe insertion. Mean basal dopamine levels unadjusted for probe recovery were 3–6 nM, as reported previously (Czoty et al 2000, 2002). Administration of MDMA at a dose (1.5 mg/kg) that markedly suppressed operant behavior had negligible effects on dopamine in both subjects (Fig. 4). A twofold higher dose (3.0 mg/kg) did effectively increase dopamine to approximately 200% of baseline and the effect approached significance (p=0.091). However, it is important to note that both subjects were in a semi-cataleptic state and largely unresponsive to environmental stimuli following this highest dose. These effects were not observed in behavioral experiments when 3.0 mg/kg MDMA was administered following fluoxetine pretreatment. A robust increase in dopamine was always obtained during perfusion with high KCl, verifying integrity of caudate tissue.
Fig. 4.
Effects of 1.5 mg/kg (top) or 3.0 mg/kg (bottom) MDMA on extracellular dopamine levels within the caudate nucleus of squirrel monkeys s-165 (left) and s-175 (right). Abscissae: time (minutes) relative to injection of MDMA. Ordinates: extracellular dopamine (nanomolar), converted to percent of baseline
PET neuroimaging
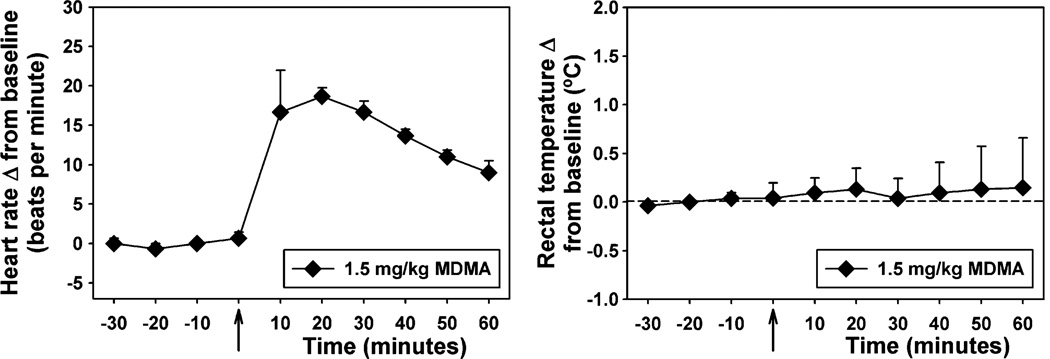
Drug-induced displacement of [18F]FECNT binding in rhesus monkeys was used to determine the percent dopamine transporter occupancy of MDMA at a dose (1.5 mg/kg) that significantly suppressed operant behavior in squirrel monkeys. Mean percent occupancy in a group of three subjects was 20±13% in the caudate and 12±10% in the putamen (Table 1). Note that percent occupancy was below the limit of detection in both regions of interest in one subject. The results reflect low in vivo potency for DAT binding. Physiological monitoring during acquisition of PET data revealed a significant (p=0.007) increase in heart rate with no significant effect on rectal temperature following MDMA administration (Fig. 5).
Table 1.
Individual and mean (±SEM) DAT occupancy in caudate and putamen by 1.5 mg/kg MDMA, administered IV to anesthetized rhesus monkeys 90 min after injection of [18F]FECNT
| DAT occupancy | ||
|---|---|---|
| Monkey | Caudate | Putamen |
| RBp | 23.55 | 6.90 |
| RDn | 0.00 | 0.00 |
| RLm | 37.28 | 28.65 |
| Mean±SEM | 20.28±13.33 | 11.85±10.57 |
Occupancy was calculated as 1−([k3b/k4]/[k3a/k4])
Fig. 5.
Effects of 1.5 mg/kg MDMA on heart rate (left) and rectal temperature (right). Each point represents the mean ± SEM (n=3 rhesus monkeys). Abscissae: time (minutes) relative to injection of MDMA. Ordinates: heart rate (beats per minute, left) or rectal temperature (°C, right), reported as a change from baseline
Discussion
Previous research on the persistent neurochemical effects of MDMA in rodents and nonhuman primates generally indicates that rodents are less sensitive to monoamine depletion following repeated MDMA administration (e.g., Slikker et al. 1988; Slikker et al. 1989; Fischer et al. 1995), but few studies have directly compared the behavioral effects of MDMA in rodents and primates. Nevertheless, interesting differences between the in vivo effects of MDMA in rodents and primates have been previously reported. For example, while MDMA decreased prepulse inhibition of the startle response in rats, MDMA increased prepulse inhibition in human subjects (Vollenweider et al. 1999). Similarly, although human subjects rate the positive subjective effects of MDMA as higher than those of amphetamine (Tancer and Johanson 2003), self-administration studies in nonhuman subjects suggest that the reinforcing effects of MDMA are less than those of amphetamine-like stimulants (Beardsley et al. 1986; Fantegrossi et al. 2002; Lamb and Griffiths 1987; Lile et al. 2005; Wang and Woolverton 2007). This pattern of data may represent species differences in MDMA effects or may simply be due to methodological differences in assays designed for rodents, nonhuman primates, and human subjects.
The studies presently reported detail the effects of MDMA on behavior and dopamine neurochemistry in squirrel monkeys and rhesus monkeys. The major conclusion to be drawn from the preponderance of these data is that, at the doses tested, MDMA does not function as a traditional psychostimulant in nonhuman primates. In contrast to cocaine and its tropane analogs (Kimmel et al. 2007), as well as being distinct from the effects of amphetamine (Katz et al. 1995), MDMA failed to increase response rate in an assay of schedule-controlled behavior in squirrel monkeys. Indeed, across the dose range tested, MDMA had no effect on, or suppressed, responding. Dose–effect curves with traditional psychostimulants using this assay are biphasic (Howell and Byrd 1995; Howell et al. 1997; Kimmel et al. 2007), with high doses decreasing response rates via behavioral disruption, but it is important to note that MDMA never increased responding in the present studies. The lowest dose of MDMA tested (0.5 mg/kg) did not alter baseline response rates, and the increasing doses resulted in a monotonic and dose-dependent reduction in schedule-controlled responding. These results are similar to those previously reported by Frederick et al. (1995), where 1.0 mg/kg MDMA decreased rates of operant responding maintained by food presentation in rhesus monkeys. Importantly, the behavioral effects of MDMA herein reported appeared to be mediated via central 5-HT systems, as pretreatment with both the SSRI, fluoxetine, and selective 5-HT2A antagonist, M100907, completely reversed the disruptive effects of MDMA on response rate. In contrast, pretreatment with the selective DAT inhibitor, RTI-177, was ineffective in attenuating MDMA-induced suppression of behavior. However, additional studies with more extensive parametric manipulation of drug dose are clearly warranted. Moreover, under this behavioral paradigm, the role of central 5-HT in the effects of psychostimulants such as cocaine and GBR12909 is more complex; SSRIs insurmountably attenuate the behavioral stimulant effects of cocaine, whereas nonselective 5-HT2 antagonists potentiate the behavioral stimulant effects of low to intermediate doses (Howell and Byrd 1995; Howell et al. 1997).
Doses of MDMA which were at least as high as those which effectively suppressed response rates had only minor effects on extracellular DA in the squirrel monkey caudate. Again, contrary to the effects of cocaine and its tropane analogs (Ginsburg et al. 2005), MDMA administration elicited a much smaller caudate DA response than would be expected for a psychostimulant. In fact, doses of methamphetamine and RTI-177 that induced significant stimulant effects in the present study also induced robust increases in extracellular dopamine in squirrel monkeys (Czoty et al. 2004; Kimmel et al. 2007). However, no more than a doubling of caudate DA was quantified following MDMA administration, and this effect returned to baseline levels in under an hour. Moreover, the high dose of MDMA required to induce modest increases in dopamine caused pronounced behavioral effects resembling a catatonic state that lasted for several hours. In the rat, the effects of MDMA on extracellular DA are more robust than we have measured in the monkey (Baumann et al. 2008; Gudelsky and Yamamoto 2008). Although doses of MDMA and amphetamine can be balanced to induce equivalent DA overflow in the rat, the influence of pharmacological manipulation of central serotonin systems on these two drugs differs markedly. For example, pretreatment with the SSRI fluoxetine blunts the effects of MDMA on extracellular DA, but enhances amphetamine-induced dopamine release in rat striatum (Gudelsky and Yamamoto 2008). It seems plausible to speculate that the interaction of MDMA with SERT (Iravani et al. 2000) in the squirrel monkey may inhibit its effects on extracellular DA.
The rhesus monkey PET experiments presently reported demonstrate a relative lack of MDMA occupancy of the DAT, a common site of action among both the amphetamine-type and cocaine-type psychostimulants in nonhuman primates (Ritz and Kuhar 1989). In one previous study (Frederick et al. 1995), a chronic MDMA dosing regimen involving both constant and escalating doses administered to rhesus monkeys resulted in both the typical serotonergic depletions commonly observed, as well as a focused increase in dopamine concentrations within the caudate nucleus. The subjects undergoing PET procedures in the present studies had only minimal exposure to MDMA, and thus are not likely to have undergone either of these neurochemical adaptations. It also seems unlikely that these present PET results can be explained as a failure to test a sufficiently high dose of MDMA, because the dose of MDMA here tested (1.5 mg/kg, delivered as an IV bolus) is within the range of MDMA self-administered by rhesus monkeys over an entire session (Fantegrossi et al. 2004; Banks et al. 2008) and had significant effects on heart rate during PET experiments. In contrast, doses of cocaine that maintain maximal self-administration behavior in rhesus monkeys occupied at least 65% of DAT (Wilcox et al. 2002) and the dose of RTI-177 that induced significant stimulant effects in the present study resulted in over 70% DAT occupancy (Lindsey et al. 2004) when measured with PET procedures similar to those in the present study (Wilcox et al., 2002). Finally, the in vitro affinity of MDMA for the DAT is low in both competition binding assays at human cloned transporters (Ki>10,000 nM, NIMH Psychoactive Drug Screening Program—Ki determinations were generously provided by the National Institute of Mental Health's Psychoactive Drug Screening Program, Contract # NO1MH32004 (NIMH PDSP). The NIMH PDSP is Directed by Bryan L. Roth MD, PhD at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscol at NIMH, Bethesda MD, USA. For experimental details please refer to the PDSP web site http://pdsp.med.unc.edu/ and click on "Binding Assay" or "Functional Assay" on the menu bar.) and functional assays in rat caudate (EC50~278 nM, Setola et al. 2003). To our knowledge, analogous studies have not been performed using rhesus monkey tissue.
In summary, the chemical structure of MDMA is psychostimulant-like, and MDMA bears numerous behavioral and neurochemical similarities to amphetamine-type and cocaine-type stimulants in rodents. Nevertheless, MDMA failed to induce a behavioral stimulant effect in squirrel monkeys in an assay of schedule-controlled behavior, had only minor effects on caudate DA neurochemistry in squirrel monkeys, and exhibited very low occupancy at DAT in the rhesus monkey. Importantly, the disruptive effects of MDMA on response rates in squirrel monkeys were completely abolished by pretreatment with fluoxetine and M100907, suggesting that these behavioral effects are dependent on central 5-HT in general, and 5-HT2A receptors in particular. More mechanistic studies regarding the apparent lack of stimulant-like effects of MDMA in nonhuman primates are warranted.
Acknowledgments
Expert technical assistance with all nonhuman primate procedures was provided by Tango Howard, Carol Nichols, Jodi Godfrey, and Lisa Neidert at the Yerkes National Primate Research Center. The authors express their gratitude to Larry Williams at the Emory University PET Center for his skilled aid in the conduct of the imaging procedures. These studies were supported by USPHS Grants DA10344 (LLH), DA12514 (LLH), DA00517 (LLH), DA20645 (WEF), RR00165 (LLH and WEF), and RR020146 (WEF).
Contributor Information
William E. Fantegrossi, Department of Pharmacology and Toxicology, University of Arkansas for Medical Sciences, Little Rock, AR, USA Division of Neuroscience, Yerkes National Primate Research Center, Emory University, 954 Gatewood Road NE, Atlanta, GA 30329, USA.
Rayna M. Bauzo, Division of Neuroscience, Yerkes National Primate Research Center, Emory University, 954 Gatewood Road NE, Atlanta, GA 30329, USA
Daniel M. Manvich, Division of Neuroscience, Yerkes National Primate Research Center, Emory University, 954 Gatewood Road NE, Atlanta, GA 30329, USA
Jose C. Morales, Division of Neuroscience, Yerkes National Primate Research Center, Emory University, 954 Gatewood Road NE, Atlanta, GA 30329, USA
John R. Votaw, Department of Radiology, Emory University School of Medicine, Atlanta, GA, USA
Mark M. Goodman, Department of Radiology, Emory University School of Medicine, Atlanta, GA, USA
Leonard L. Howell, Email: lhowell@emory.edu, Division of Neuroscience, Yerkes National Primate Research Center, Emory University, 954 Gatewood Road NE, Atlanta, GA 30329, USA.
References
- Acquas E, Pisanu A, Spiga S, Plumitallo A, Zernig G, Di Chiara G. Differential effects of intravenous R,S-(+/−)-3,4-methylenedioxymethamphetamine (MDMA, Ecstasy) and its S(+)- and R(−)-enantiomers on dopamine transmission and extracellular signal regulated kinase phosphorylation (pERK) in the rat nucleus accumbens shell and core. J Neurochem. 2007;102(1):121–132. doi: 10.1111/j.1471-4159.2007.04451.x. [DOI] [PubMed] [Google Scholar]
- Banks ML, Sprague JE, Czoty PW, Nader MA. Effects of ambient temperature on the relative reinforcing strength of MDMA using a choice procedure in monkeys. Psychopharmacology. 2008;196(1):63–70. doi: 10.1007/s00213-007-0932-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankson MG, Cunningham KA. 3, 4-Methylenedioxyme thamphetamine (MDMA) as a unique model of serotonin receptor function and serotonin–dopamine interaction. J Pharmacol Exp Ther. 2001;297:846–852. [PubMed] [Google Scholar]
- Bankson MG, Cunningham KA. Pharmacological studies of the acute effects of (+)-3, 4-methylenedioxymethamphetamine on locomotor activity: role of 5-HT1b/1d and 5-HT2 receptors. Neuropsychopharmacology. 2002;26:40–52. doi: 10.1016/S0893-133X(01)00345-1. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Budzynski AG, Partilla JS, Blough BE, Rothman RB. Effects of “Legal X” piperazine analogs on dopamine and serotonin release in rat brain. Ann N Y Acad Sci. 2004;1025:189–197. doi: 10.1196/annals.1316.024. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Rothman RB. Locomotor stimulation produced by 3, 4-methylenedioxymethamphetamine (MDMA) is correlated with dialysate levels of serotonin and dopamine in rat brain. Pharmacol Biochem Behav. 2008;90(2):208–217. doi: 10.1016/j.pbb.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Balster RL, Harris LS. Self-administration of methylenedioxymethamphetamine (MDMA) by rhesus monkeys. Drug Alcohol Depend. 1986;18(2):149–157. doi: 10.1016/0376-8716(86)90047-5. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Pack KM, Frankel PS, Cunningham KA. Effects of dopamine D1- or D2-like receptor antagonists on the hyper-motive and discriminative stimulus effects of (+)-MDMA. Psychopharmacology. 2004;173(3–4):326–336. doi: 10.1007/s00213-004-1790-1. [DOI] [PubMed] [Google Scholar]
- Crean RD, Davis SA, Von Huben SN, Lay CC, Katner SN, Taffe MA. Effects of (+/−) 3, 4-methylenedioxymethamphetamine, (+/−) 3, 4-methylenedioxyamphetamine and methamphetamine on temperature and activity in rhesus macaques. Neuroscience. 2006;142(2):515–525. doi: 10.1016/j.neuroscience.2006.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Justice JB, Jr, Howell LL. Cocaine-induced changes in extracellular dopamine determined by microdialysis in awake squirrel monkeys. Psychopharmacology. 2000;148(3):299–306. doi: 10.1007/s002130050054. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Ginsburg BC, Howell LL. Serotonergic attenuation of the reinforcing and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 2002;300(3):831–837. doi: 10.1124/jpet.300.3.831. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Makriyannis A, Bergman J. Methamphetamine discrimination and in vivo microdialysis in squirrel monkeys. Psychopharmacology. 2004;175(2):170–178. doi: 10.1007/s00213-004-1798-6. [DOI] [PubMed] [Google Scholar]
- Daniela E, Brennan K, Gittings D, Hely L, Schenk S. Effect of SCH 23390 on (+/−)-3, 4-methylenedioxymethamphetamine hyperactivity and self-administration in rats. Pharmacol Biochem Behav. 2004;77(4):745–750. doi: 10.1016/j.pbb.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Ullrich T, Rice KC, Woods JH, Winger G. 3, 4-Methylenedioxy-methamphetamine (MDMA, “Ecstasy”) and its stereoisomers as reinforcers in rhesus monkeys: serotonergic involvement. Psychopharmacology. 2002;161:356–364. doi: 10.1007/s00213-002-1021-6. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Woolverton WL, Kilbourn M, Sherman P, Yuan J, Hatzidimitriou G, Ricaurte GA, Woods JH, Winger G. Behavioral and neurochemical consequences of long-term intravenous self-administration of MDMA and its enantiomers by rhesus monkeys. Neuropsychopharmacology. 2004;29(7):1270–1281. doi: 10.1038/sj.npp.1300442. [DOI] [PubMed] [Google Scholar]
- Fischer C, Hatzidimitriou G, Wlos J, Katz J, Ricaurte G. Reorganization of ascending 5-HT axon projections in animals previously exposed to the recreational drug (+/−) 3, 4-methylenedioxymethamphetamine (MDMA, “ecstasy”) J Neurosci. 1995;15(8):5476–5485. doi: 10.1523/JNEUROSCI.15-08-05476.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick DL, Ali SF, Slikker W, Jr, Gillam MP, Allen RR, Paule MG. Behavioral and neurochemical effects of chronic methylenedioxymethamphetamine (MDMA) treatment in rhesus monkeys. Neurotoxicol Teratol. 1995;17(5):531–543. doi: 10.1016/0892-0362(95)00013-h. [DOI] [PubMed] [Google Scholar]
- Ginsburg BC, Kimmel HL, Carroll FI, Goodman MM, Howell LL. Interaction of cocaine and dopamine transporter inhibitors on behavior and neurochemistry in monkeys. Pharmacol Biochem Behav. 2005;80(3):481–491. doi: 10.1016/j.pbb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Goodman MM, Kilts CD, Keil R, Shi B, Martarello L, Xing D, Votaw J, Ely TD, Lambert P, Owens MJ, Camp VM, Malveaux E, Hoffman JM. 18F-labeled FECNT: a selective radioligand for PET imaging of brain dopamine transporters. Nucl Med Biol. 2000;27(1):1–12. doi: 10.1016/s0969-8051(99)00080-3. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA, Yamamoto BK. Actions of 3, 4-methylenedioxymethamphetamine (MDMA) on cerebral dopaminergic, serotonergic and cholinergic neurons. Pharmacol Biochem Behav. 2008;90(2):198–207. doi: 10.1016/j.pbb.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Byrd LD. Serotonergic modulation of the behavioral effects of cocaine in the squirrel monkey. J Pharmacol Exp Ther. 1995;275(3):1551–1559. [PubMed] [Google Scholar]
- Howell LL, Czoty PW, Byrd LD. Pharmacological interactions between serotonin and dopamine on behavior in the squirrel monkey. Psychopharmacology. 1997;131(1):40–48. doi: 10.1007/s002130050263. [DOI] [PubMed] [Google Scholar]
- Iravani MM, Asari D, Patel J, Wieczorek WJ, Kruk ZL. Direct effects of 3, 4-methylenedioxymethamphetamine (MDMA) on serotonin or dopamine release and uptake in the caudate putamen, nucleus accumbens, substantia nigra pars reticulata, and the dorsal raphe nucleus slices. Synapse. 2000;36:275–285. doi: 10.1002/(SICI)1098-2396(20000615)36:4<275::AID-SYN4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Kilbey M, Gatchalian K, Tancer M. Discriminative stimulus effects of 3, 4-methylenedioxymethamphetamine (MDMA) in humans trained to discriminate among d-amphetamine, meta-chlorophenylpiperazine and placebo. Drug Alcohol Depend. 2006;81(1):27–36. doi: 10.1016/j.drugalcdep.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Kamien JB, Johanson CE, Schuster CR, Woolverton WL. The effects of (+/−)-methylenedioxymethamphetamine and (+/−)-methylenedioxyamphetamine in monkeys trained to discriminate (+)-amphetamine from saline. Drug Alcohol Depend. 1986;18(2):139–147. doi: 10.1016/0376-8716(86)90046-3. [DOI] [PubMed] [Google Scholar]
- Katz JL, Alling K, Shores E, Witkin JM. Effects of D1 dopamine agonists on schedule-controlled behavior in the squirrel monkey. Behav Pharmacol. 1995;6(2):143–148. [PubMed] [Google Scholar]
- Kimmel HL, O'Connor JA, Carroll FI, Howell LL. Faster onset and dopamine transporter selectivity predict stimulant and reinforcing effects of cocaine analogs in squirrel monkeys. Pharmacol Biochem Behav. 2007;86(1):45–54. doi: 10.1016/j.pbb.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RJ, Griffiths RR. Self-injection of d, 1–3, 4-methylenedioxymethamphetamine (MDMA) in the baboon. Psychopharmacology. 1987;91(3):268–272. doi: 10.1007/BF00518175. [DOI] [PubMed] [Google Scholar]
- Lile JA, Ross JT, Nader MA. A comparison of the reinforcing efficacy of 3, 4-methylenedioxymethamphetamine (MDMA, “ecstasy”) with cocaine in rhesus monkeys. Drug Alcohol Depend. 2005;78(2):135–140. doi: 10.1016/j.drugalcdep.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Lindsey KP, Wilcox KM, Votaw JR, Goodman MM, Plisson C, Carroll FI, Rice KC, Howell LL. Effects of dopamine transporter inhibitors on cocaine self-administration in rhesus monkeys: relationship to transporter occupancy determined by positron emission tomography neuroimaging. J Pharmacol Exp Ther. 2004;309(3):959–969. doi: 10.1124/jpet.103.060293. [DOI] [PubMed] [Google Scholar]
- Nash JF, Brodkin J. Microdialysis studies on 3, 4-methylenedioxymehtamphetmine-induced dopamine release: effect of dopamine uptake inhibitors. J Pharmacol Exp Ther. 1991;259:820–825. [PubMed] [Google Scholar]
- Peroutka SJ, Newman H, Harris H. Subjective effects of 3,4-methylenedioxymethamphetamine in recreational users. Neuropsychopharmacology. 1988;1(4):273–277. [PubMed] [Google Scholar]
- Risbrough VB, Masten VL, Caldwell S, Paulus MP, Low MJ, Geyer MA. Differential contributions of dopamine D1, D2, and D3 receptors to MDMA-induced effects on locomotor behavior patterns in mice. Neuropsychopharmacology. 2006;31(11):2349–2358. doi: 10.1038/sj.npp.1301161. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Kuhar MJ. Relationship between self-administration of amphetamine and monoamine receptors in brain: comparison with cocaine. J Pharmacol Exp Ther. 1989;248(3):1010–1017. [PubMed] [Google Scholar]
- Setola V, Hufeisen SJ, Grande-Allen KJ, Vesely I, Glennon RA, Blough B, Rothman RB, Roth BL. 3, 4-Methylenedioxy methamphetamine (MDMA, “Ecstasy”) induces fenfluramine-like proliferative actions on human cardiac valvular interstitial cells in vitro. Mol Pharmacol. 2003;63(6):1223–1229. doi: 10.1124/mol.63.6.1223. [DOI] [PubMed] [Google Scholar]
- Slikker W, Jr, Ali SF, Scallet AC, Frith CH, Newport GD, Bailey JR. Neurochemical and neurohistological alterations in the rat and monkey produced by orally administered methylenedioxymethamphetamine (MDMA) Toxicol Appl Pharmacol. 1988;94(3):448–457. doi: 10.1016/0041-008x(88)90285-2. [DOI] [PubMed] [Google Scholar]
- Slikker W, Jr, Holson RR, Ali SF, Kolta MG, Paule MG, Scallet AC, McMillan DE, Bailey JR, Hong JS, Scalzo FM. Behavioral and neurochemical effects of orally administered MDMA in the rodent and nonhuman primate. Neurotoxicology. 1989;10:529–542. [PubMed] [Google Scholar]
- Taffe MA, Lay CC, Von Huben SN, Davis SA, Crean RD, Katner SN. Hyperthermia induced by 3, 4-methylenedioxyme thamphetamine in unrestrained rhesus monkeys. Drug Alcohol Depend. 2006;82(3):276–281. doi: 10.1016/j.drugalcdep.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tancer ME, Johanson CE. The subjective effects of MDMA and mCPP in moderate users. Drug Alcohol Depend. 2001;65(1):97–101. doi: 10.1016/s0376-8716(01)00146-6. [DOI] [PubMed] [Google Scholar]
- Tancer M, Johanson CE. Reinforcing, subjective, and physiological effects of MDMA in humans: a comparison with d-amphetamine and mCPP. Drug Alcohol Depend. 2003;72(1):33–44. doi: 10.1016/s0376-8716(03)00172-8. [DOI] [PubMed] [Google Scholar]
- Tancer M, Johanson CE. The effects of fluoxetine on the subjective and physiological effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology. 2007;189(4):565–573. doi: 10.1007/s00213-006-0576-z. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Remensberger S, Hell D, Geyer MA. Opposite effects of 3, 4-methylenedioxymethamphetamine (MDMA) on sensorimotor gating in rats versus healthy humans. Psychopharmacology. 1999;143(4):365–372. doi: 10.1007/s002130050960. [DOI] [PubMed] [Google Scholar]
- Votaw JR, Howell LL, Martarello L, Hoffman JM, Kilts CD, Lindsey KP, Goodman MM. Measurement of dopamine transporter occupancy for multiple injections of cocaine using a single injection of [F-18]FECNT. Synapse. 2002;44(4):203–210. doi: 10.1002/syn.10068. [DOI] [PubMed] [Google Scholar]
- Wang Z, Woolverton WL. Estimating the relative reinforcing strength of (±)-3, 4-methylenedioxymethamphetamine (MDMA) and its isomers in rhesus monkeys: comparison to (+)-methamphetamine. Psychopharmacology. 2007;189:483–488. doi: 10.1007/s00213-006-0599-5. [DOI] [PubMed] [Google Scholar]
- White SR, Obradovic T, Imel KM, Wheaton MJ. The effects of methylenedioxymethamphetamine (MDMA, ‘ecstasy’) on monoaminergic neurotransmission in the central nervous system. Prog Neurobiol. 1996;49:455–479. doi: 10.1016/0301-0082(96)00027-5. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Lindsey KP, Votaw JR, Goodman MM, Martarello L, Carroll FI, Howell LL. Self-administration of cocaine and the cocaine analog RTI-113: relationship to dopamine transporter occupancy determined by PET neuroimaging in rhesus monkeys. Synapse. 2002;43(1):78–85. doi: 10.1002/syn.10018. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Spanos LJ. The acute effects of methylenedioxymethamphetamine on dopamine release in the awake-behaving rat. Eur J Pharmacol. 1988;148:195–203. doi: 10.1016/0014-2999(88)90564-x. [DOI] [PubMed] [Google Scholar]