Abstract
NKT cells are a heterogeneous subset of specialized, self-reactive T cells, with innate and adaptive immune properties, which allow them to bridge innate and adaptive immunity and profoundly influence autoimmune and malignant disease outcomes. NKT cells mediate these activities through their ability to rapidly express pro- and anti-inflammatory cytokines that influence the type and magnitude of the immune response. Not only do NKT cells regulate the functions of other cell types, but experimental evidence has found NKT cell subsets can modulate the functions of other NKT subsets. Depending on underlying mechanisms, NKT cells can inhibit or exacerbate autoimmunity and malignancy, making them potential targets for disease intervention. NKT cells can respond to foreign and endogenous antigenic glycolipid signals that are expressed during pathogenic invasion or ongoing inflammation, respectively, allowing them to rapidly react to and influence a broad array of diseases. In this article we review the unique development and activation pathways of NKT cells and focus on how these attributes augment or exacerbate autoimmune disorders and malignancy. We also examine the growing evidence that NKT cells are involved in liver inflammatory conditions that can contribute to the development of malignancy.
Keywords: autoimmunity, cancer, inflammation, NAFLD, type I NKT cells, type II NKT cells
NKT cells are unconventional T cells that have been implicated in the regulation of a broad range of diseases, including those associated with inflammation [1]. Unlike conventional T cells, NKT cells are capable of orienting the immune system toward either inflammation or tolerance, by rapidly producing Th1, Th2, Th3 and/or Th17 cytokines, without the need for prior priming or polarization [2–6]. In addition, NKT cells can modify early inflammatory mediators, such as macrophages and neutrophils, as well as late stage T cell responses [7–10], thereby affecting all stages of the inflammatory response. Collectively, these results define NKT cells as critical cells in regulatory events that direct the magnitude and type of inflammatory responses. In this article we will briefly discuss the functional attributes of NKT cells, the underlying mechanisms by which they naturally regulate cancer and autoimmunity, and the inflammatory conditions that contribute to these disease processes.
NKT cell phenotype & distribution
Two subsets of NKT cells have been described, type I and type II. Unlike conventional T cells that recognize peptide antigens bound to MHC molecules, both subsets of NKT cells recognize lipid antigens bound to CD1d molecules [2,11,12]. CD1d is MHC-related molecule that has a deep hydrophobic groove capable of binding lipids with long alkyl chains [2,11,12]. NKT cells can recognize lipid antigens from pathogens, as well as endogenous antigens expressed during inflammatory responses [13–18]. The divergent recognition of distinct classes of antigens by T cells and NKT cells increases the ability of the host immune system to recognize a wide variety of pathogens and mediate immunosurveillance of diverse inflammatory conditions.
Type I NKT cells express an invariant T cell receptor (TCR; Vα14Jα18 in mice and Vα24Jα18 in humans), while type II NKT cells express variable TCRs [2,19–21]. The restricted TCR of type I NKT cells allows the recognition of foreign [15,16,22,23] and endogenous [14,24–27] glycolipid antigens that have structural similarities [28]. Taking advantage of the conserved TCR of type I NKT cells, antigen-specific tetramers were developed that allow for a more accurate phenotypic and functional characterization of these cells [29]. α-galactosyl ceramide (αGalCer) was the first antigen discovered that could bind to the CD1d molecule and activate NKT cells [30], and was also the first antigen used to load CD1d tetramers for the detection of NKT cells [29]. αGalCer was isolated from a marine sponge as part of a screen for antimetastatic compounds [29].
In both mice and humans, type I NKT cells can be CD4+CD8− or CD4−CD8−, and humans have an additional CD4−CD8+ subset [31]. The distribution of type I NKT cells between humans and mice appears to be similar. Type I NKT cells are found in tissues where lymphocytes normally reside, including the spleen, blood, bone marrow, lymph node, thymus and liver. However, the frequency of type I NKT cells is much lower in humans compared with mice. The frequency of type I NKT cells in humans is variable among individuals, ranging from 0.001 to 3% of total leukocytes in the blood, bone marrow and spleen [32], with the highest concentration of approximately 0.5% [33] found in the liver. By contrast, the frequency of these cells among leukocytes in mice ranges between 0.2 and 0.5% in the blood, bone marrow and spleen, and between 15 and 35% in the liver.
In contrast to type I NKT cells, type II NKT cells are less studied because reagents that specifically detect these cells are not currently available [19–21]. Type II NKT cells are either CD4+CD8− or CD4−CD8+, with no report of a CD4−CD8− population [34]. While a majority of NKT cells in mice are type I, in humans the majority of NKT cells are type II [34]. Type II NKT cells reside in the same tissues as type I NKT cells.
In this review we will denote NKT cells as NKT cells when describing studies that did not differentiate between type I and type II NKT cells and type I invariant NKT cells will be annotated as invariant NKT (iNKT) cells and type II variant NKT cells will be annotated as variant NKT (vNKT) cells.
NKT-cell development
The diverse immune attributes between T-cell lineages are acquired during development. NKT cells and conventional T cells develop in the thymus from a common precursor [35,36]. However, distinct intrathymic TCR interactions occuring during development activate cell-specific transcriptional programs that instruct the unique functions of each cell type. NKT cells are selected by TCR interactions with CD4/CD8 double positive thymocytes expressing endogenous glycolipids in the context of CD1 molecules [37–39]. The interaction of NKT cell precursors with double positive thymocytes triggers Src kinase Fyn and Sap signaling, mediated through Slamf1 and Slamf6 receptors, which is necessary for iNKT cell differentiation and proliferation [40–44]. By contrast, conventional T cells are selected by TCR interactions with thymic epithelial cells expressing peptides in the context of MHC molecules [45], and do not require Fyn or Sap for development. The difference in the nature of selecting cells and selecting antigens between NKT and conventional T cells allows for diverse cell programming, which broadens peripheral immune coverage and allows more complex cell–cell interactions.
Autoimmune reactions are controlled in part by the depletion of T cells bearing a high affinity TCR for endogenous self-antigens, a process termed negative selection [46]. Experiments examining iNKT cells found the processing and presentation of endogenous lipid antigens was required for iNKT cell development [47–49], and that they receive stronger TCR signals during development than conventional T cells [50], demonstrating that iNKT cells are positively selected by self-lipid antigens. However, it remains unclear if iNKT cells avoid negative selection, or if they have a greater capacity to survive negative selection compared with conventional T cells. Immediately after positive selection iNKT cells, but not conventional T cells, express high levels of promyelocytic leukemia zinc finger transcription factor (PLZF) transcription factor [51,52] and become functionally responsive to TCR ligation by expressing high levels of IL-4 but low levels of IFN-γ. As iNKT cells move through the selection process and mature, the PLZF levels decrease, but remain high compared with conventional T cells, and a shift in cytokine expression profile occurs in which iNKT cells become high expressors of IFN-γ and reduced expressors of IL-4.
During the postselection stage of development, conventional T cells egress from the thymus as naive cells, while thymic iNKT cells immediately upregulate IL-2/IL-15R β-chain (CD122) [53] and undergo multiple rounds of cell division, whereupon they begin to express innate markers of the NK cell lineage, including NK1.1, Ly49s and CD94. PLZF-deficient iNKT cells do not undergo multiple rounds of cell division or express NK cell lineage markers and lose the ability to coexpress IL-4 and IFN-γ [51,52]. The thymic iNKT cell expansion and innate marker acquisition phase requires IL-15 signaling and the transcription factors T-box expressed in T-cells (T-bet) [54] and GATA-binding protein-3 (GATA-3) [55]. iNKT cell developmental dependence on T-bet and IL-15 may be related because T-bet promotes the expression of CD122 necessary for IL-15 signaling [56,57]. Intriguingly, T-bet and GATA-3 have been shown to induce chromatin remodeling of the IFN-γ and IL-4 gene loci, respectively, in conventional T cells [58,59], which could be the mechanism allowing early iNKT cell progenitors to rapidly express IFN-γ and IL-4. Consistent with this hypothesis is the discovery that iNKT cells undergo chromatin remodeling of the IFN-γ and IL-4 gene loci and acquire constitutive expression of IL-4 and IFN-γ transcripts, a hallmark of innate cell function [60]. By contrast to conventional T cells that need to acquire an effector phenotype for chromatin remodeling, iNKT cells undergo chromatin remodeling early in development [60]. Furthermore, induction and maintenance of antigen-educated effector CD8+ T cells requires T-bet [61] and IL-15 [62,63]. Taken together these findings suggest that iNKT cells develop as antigen-instructed effector cells. Consistent with an effector phenotype, mature iNKT cells upregulate CD44, a marker of antigen experience, and the early activation marker CD69. Unlike T cells, iNKT cells naturally acquire their effector phenotype even in germ-free mice, demonstrating that iNKT cell function is shaped by endogenous antigens [64]. Taken together these findings demonstrating that iNKT cells develop a unique functional phenotype with attributes of both innate and effector T cells.
Owing to a lack of specific phenotypic markers, the development of vNKT cells has not been well characterized and it is not clear how closely these cells follow the development of iNKT cells. Both types of NKT cells are selected by CD1d molecules expressing endogenous antigens. However, it was recently reported that mice deficient in suppressor of cytokine signaling (SOCS1) have fewer iNKT cells in the periphery and do not respond to the iNKT cell antigen, αGalCer [65]. By contrast, the authors found SOCS1-deficient mice responded normally to the vNKT cell antigen, sulfatide, suggesting that peripheral numbers of vNKT cells were unaffected by SOCS1 deficiency. This difference in the requirements for the selection of NKT cell subsets suggests that distinct functional directives for iNKT versus vNKT cells.
NKT-cell activation
NKT cells occupy a niche bridging innate and adaptive immune components. Although iNKT cells have been shown to express perforin, granzyme B and FasL; the major immune role of NKT cells is the coordination of adaptive and innate cells, through the expression of cell surface costimulatory molecules and secretion of cytokines, rather than acting directly as effector cells [66–69]. NKT cells sense changes in their local microenvironment through multiple means that function individually or in conjunction with each other, to influence the type and magnitude of response. These pathways of activation are mediated primarily by cytokines, pathogen-associated molecular patterns and glycolipid antigens.
Invariant NKT cells express receptors to a variety of chemokines and cytokines, which regulate their movement and activation [70,71]. iNKT cells express chemokine receptors that direct them to sites of inflammation [72]. Once at the inflammatory site, iNKT cells are primarily activated by antigens and cytokines such as IL-12 and IL-18, which are expressed early by macrophages in response to microbes. These attributes taken together enable NKT cells to dramatically affect the early orientation of an inflammatory response.
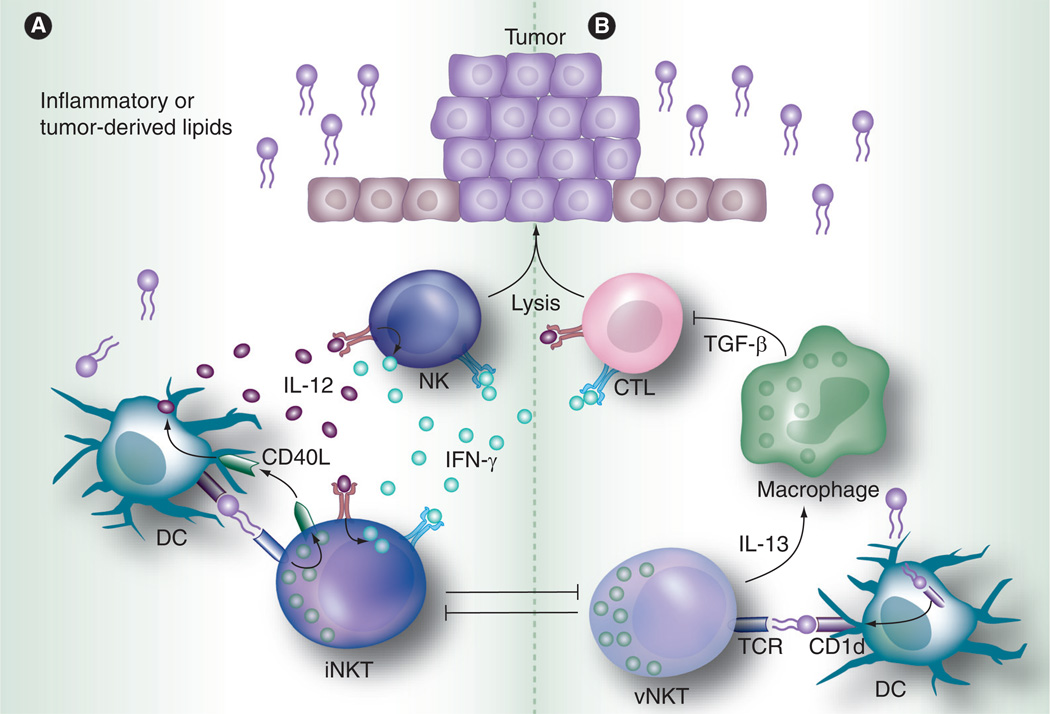
Antigenic activation of NKT cells can occur directly or indirectly. Direct activation involves the endocytosis of glycolipid antigens such as αGalCer (iNKT) or sulfatide (vNKT) by antigen-presenting cells (APCs) and their presentation to NKT cells in CD1d-antigen complexes. This pathway results in the rapid expression of regulatory/Th2 (e.g., IL-13, IL-10 and IL-4) and proinflammatory (IFN-γ and TNF) cytokines by iNKT cells [2,54]. The reciprocal cross-talk between dendritic cells (DCs) and iNKT cells involves the upregulation of CD154 (CD40L) that, in turn, triggers CD40 signaling in DCs, inducing their maturation and secretion of IL-12 [73]. DC-derived IL-12 then activates NK and NKT cells for IFN-γ production that in turn modulates bystander activity of innate NK cells and adaptive CD8+ T cells (Figure 1A) [66,68]. During this cross-talk process, mature DCs can antigen prime T helper cells and they, along with activated NKT cells, can enhance antibody responses by providing B-cell help [74]. iNKT cells can also directly provide B-cell help that is mediated through cognate antigenic interactions between CD1d-expressing B cells and iNKT cells [75–77]. Thus, antigenic activation of iNKT cells can help mediate both cell mediated and humoral responses.
Figure 1. NKT cell immunoregulatory axis.
(A) The reciprocal cross-talk between DCs and iNKT cells involves the upregulation of CD154 (CD40L) that, in turn, triggers CD40 signaling in DCs, inducing their maturation and secretion of IL-12 [61]. IL-12 expressed by DCs then activates NK and NKT cells for IFN-γ production that in turn modulates bystander activity of innate NK cells and adaptive CD8+ T cells [51,53]. (B) IL-13 released by vNKT cells induces TGF-β expression by myeloid cells that inhibits CD8+ T cell effector function. Antigen-activated vNKT cells can suppress the activation of iNKT cells [143].
DC: Dendritic cells; iNKT: Invariant NKT; vNKT: Variant NKT.
Indirect NKT-cell activation by antigens occurs when APCs are activated through Toll-like receptors that trigger the loading of CD1d molecules with endogenous glycolipid antigens that are then presented to NKT cells [13,78]. Whether other inflammatory signals that activate APCs can also induce the indirect antigen activation of NKT cells remains to be determined. Furthermore, pharmacokinetic studies of endogenous antigen(s) need to be determined, because the quality of iNKT cell activation is influenced by the binding affinity, concentration and stability of glycolipid –CD1d complexes [79–82]. Low antigen concentration or binding affinity of human iNKT cells induces granulocyte-macrophage colony stimulating (GM-CSF) and IL-13, while a higher antigen concentration or binding affinity induces IL-4 and IFN-γ, along with increased expression of GM-CSF and IL-13, demonstrating a hierarchy in cytokine expression [82]. However, antigen concentration and binding affinity are only part of the properties that determine the type of cytokines NKT cells express. Antigen–CD1d complex stability also determines the type of cytokines NKT cells produce and the subsequent quality of the bystander activity. Localization of CD1d in lipid rafts help to stabilize the CD1d–antigen complex [83–85]. The lipid, C-glycoside, an analog of αGalCer, has a weak binding affinity to the iNKT cell TCR but is still able to induce IFN-γ production because it was found to form a stable complex with CD1d and thus survives longer in vivo [81].
How antigens are loaded onto CD1d molecules determines whether the CD1d–antigen complex forms in the presence of lipid rafts [86]. CD1d rapidly loads less hydrophobic antigens onto the cell surface, to the exclusion of lipid rafts, resulting in iNKT cell cytokine expression with a Th2 bias. By contrast, hydrophobic antigens are intracellularly loaded onto CD1d molecules, leading to organized transport of the CD1d–antigen complex into lipid raft regions on the cell surface, resulting in iNKT cells expressing IFN-γ. Thus, NKT-cell responses are fine-tuned by the pharmacokinetics of both endogenous and pathogen-derived antigens. This layer of complexity, along with the other activation pathways, allows NKT cells to proactively regulate a broad array of inflammatory responses.
Protective/pathogenic roles of NKT cells in autoimmune & allergic disorders
Autoimmune diseases derive from protracted immune response(s) targeting self-tissues, causing prolonged inflammation and subsequent tissue destruction. The aberrant frequency and/or function of NKT cells in the peripheral blood of patients with autoimmune and allergic inflammation diseases suggest the involvement of these cells in disease pathology [87,88]. Clinical and animal studies found NKT cells have a profound and diverse role in this subset of diseases, with the remarkable capacity for both protective and pathogenic activities (Figure 2).
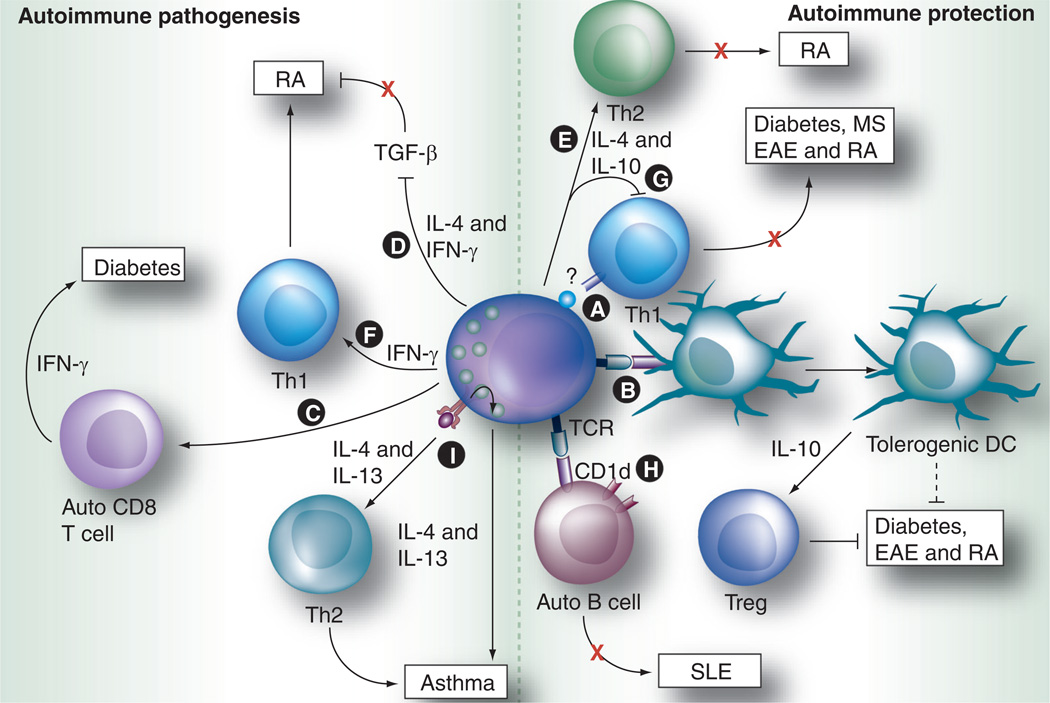
Figure 2. Dual role of NKT cells in autoimmunity and allergic inflammation.
The autoimmunity and allergic disease-promoting roles of NKT cells are mainly related to the balance in their production of Th1 versus Th2 cytokines, as well as their interactions with DCs, CD4+ and CD8+ T cells ([A-I] are described in detail in the ‘Protective/pathogenic roles of NKT cells in autoimmune & allergic disorders’ section).
DC: Dendritic cell; EAE: Experimental autoimmune encephalomyelitis; MS: Multiple sclerosis; RA: Rheumatoid arthritis; SLE: Systemic lupus erythematosus; TCR: T cell receptor.
The immunological role for NKT cells in the pathogenesis of Type 1 diabetes and rheumatoid arthritis is complicated by the fact that seemingly conflicting results have been observed in animal studies, dependent upon the genetic background of the host, animal model used or, in some cases, the stage of the disease studied. Thus, in the discussion below we will highlight the principal mechanisms that underlie the contradictory roles of NKT cells in autoimmune diseases.
Type 1 diabetes
Clinical studies examining human NKT-cell frequencies and function from Type 1 diabetes patients have revealed conflicting results. Wilson et al. reported that Type 1 diabetes patients had a lower frequency of peripheral blood iNKT cells that were anergic for the production of cytokines [89], while other studies found normal levels of peripheral blood iNKT cells in Type 1 diabetes patients compared with normal subjects [90]. In nonobese diabetic (NOD) mice that are classically used for diabetes studies, iNKT cells exhibit remarkable defects in both their frequency and cytokine production [90]. Adoptive transfer of iNKT cells from wild-type mice or, alternatively, the restoration of their function by αGalCer treatment or TCR or CD1d overexpression can prevent the development of diabetes in NOD mice [90–92]. From these studies, it was concluded that iNKT cells have a protective role during diabetes development. The studies in the past decade showed the protective mechanism of NKT cells during the development of Type 1 diabetes can be complex. First, NKT cells can impair the differentiation of anti-islet reactive T cells into Th1 effector cells in a cell–cell contact dependent manner, which did not require Th2 cytokine production or CD1d recognition (Figure 2A) [93–95]. Second, NKT cells accumulating in the pancreas can indirectly suppress diabetogenic CD4+ T cells via IFN-γ production [96]. Last, anergic iNKT cells induced by protracted αGalCer stimulation can induce the production of noninflammatory DCs, which inhibit diabetes development in an Ag-specific fashion (Figure 2B) [97]. These findings point to an important protective role for NKT cells during autoimmune pathogenesis in the pancreas.
On the other hand, a considerable amount of evidence has also highlighted the pathogenic role of NKT cells in Type 1 diabetes. Using a mouse diabetes model established by the adoptive transfer of diabetogenic CD8 T cells, Griseri et al. demonstrated that iNKT cells could exacerbate diabetes by enhancing the activation, expansion and IFN-γ production of these CD8 T cells (Figure 2C) [98]. These contrasting results suggest that NKT cells might have a dual role in the modulation of diabetogenic CD8+ and CD4+ T cells. It also implies that the protective roles of NKT cells in diabetes might vary depending on stage of disease development. specifically, NKT cells may inhibit the development and progression of diabetes, but once CD8+ T cells become diabetogenic, NKT cells may promote their pathogenesis. However, results from experimental diabetes studies need to be carefully analyzed. specifically, a recent study showed that the transfer of iNKT-conditioned DCs that were of a C57BL/6 genetic background resulted in an immune response that enhances rather than suppresses Type 1 diabetes development, whereas the transfer of iNKT-conditioned DCs from NOD mice prevents Type 1 diabetes [99]. This finding suggests that the genetic background could influence the roles of iNKT cells in the development of Type 1 diabetes and may also explain why diabetic patients exhibit high variability in their iNKT frequency and function in peripheral blood compared with normal human subjects [89,100–102].
Rheumatoid arthritis
A reduced frequency and a Th1 bias in iNKT cells have been reported in the peripheral blood of rheumatoid arthritis patients [103–105]. TGF-β, which suppresses pathogenic Th1 cells in arthritis, was elevated in CD1d–knockout mice [106]. Adoptive transfer experiments demonstrated that IL-4 and IFN-γ produced by iNKT cells within the joints suppressed TGF-β production, allowing pathogenic Th1 cells to cause joint injury (Figure 2D) [106]. Using IL-12p35−/− mice, Park et al. recently demonstrated that the IL-12p35/IFN-γ axis promotes antibody-induced joint inflammation, by activating iNKT cells through the IL-12β2 receptor and suppressing TGF-β production [107]. Stimulation of iNKT cells with αGalCer or OCH (an αGalCer analog with a truncated sphingosine chain) resulted in the protection of certain mouse strains against arthritis in the collagen-induced arthritis model [108], which correlated with the induction of IL-4 and IL-10 expression and a Th2 shift in collagen-specific T-cell responses (Figure 2e). However, in the antibody-induced arthritis model, αGalCer treatment moderately increased joint inflammation [106],indicating that iNKT cells are capable of functioning as suppressors of pathogenic T cells during the induction phase of disease, as well as inflammation promoters during the effector phase, when antibody-mediated destruction is prominent. Additional studies revealed that iNKT cells in the joint can be activated by engagement of IgG antibodies via FcγRIII receptors expressed on NKT cells [109]. Using CD1d- and Jα18-knockout mice, which lack all NKT cells, or only iNKT cells, respectively, several studies demonstrated that iNKT cells had a pathogenic role in both collagen-induced arthritis and antibody-mediated arthritis mouse models that was mediated by IFN-γ (Figure 2F) [106,108,110,111].
By contrast, Teige et al. found that NKT cells suppressed arthritis development [112]. The authors found induction of acute and chronic arthritis, along with inflammation, was more severe in CD1d-knockout mice, when compared with wild-type mice, with the proposed mechanism in both models being the inhibition of pathogenic Th1 cells (Figure 2g) [112]. This contradiction in the role of iNKT cells in arthritis is most likely due to functional differences between iNKT and vNKT cells, since the study that showed a protective role used CD1d-knockout mice that lack both types of NKT cells, while the other studies that found a pathogenic role for NKT cells focused only on the iNKT-cell subset. Taken together these studies suggest that vNKT cells suppress arthritis while iNK cells have a pathogenic role in these disease models.
Systemic lupus erythematosus
A classic hallmark of systemic lupus erythematosus (SLE) is the activation of autoreactive B cells, which produce autoantibodies against nuclear antigens, a process controlled by autoreactive T helper cells and Tregs. Many patients with SLE have a reduced frequency of CD1d-restricted NKT cells [113]. Similar to their roles in diabetes and rheumatoid arthritis, NKT cells can be pathogenic and/or protective in SLE animal studies [114–116]. The mechanisms underlying the protective versus promoting roles for NKT cells in the pathogenesis of SLE is likely to be related to their interactions with disease promoting, autoreactive B cells. Takahashi et al. reported that iNKT cells from Lupus-prone NZB/W F1 mice increased the spontaneous secretion of IgM and IgG anti-dsDNA by B cells that was dependent on CD1d antigen presentation and CD40 ligation [115]. However, two recent reports found that iNKT cells could also suppress SLE. The first report found that iNKT cells could limit activation of autoreactive CD1d-positive B cells in a contact- and CD1d-dependent manner (Figure 2H) [117]. However, another study examining SLE triggered by an increased load of circulating apoptotic cells, found that the absence or reduction of iNKT cells and the absence of CD1d expression on B cells led to increased autoreactive B-cell activation and disease severity (Figure 2i) [118]. All three studies required CD1d interactions for disease modulation, suggesting that the regulatory effects of NKT cells are dependent on antigenic signals. Given the contradictory results in these animal studies, it is proposed that iNKT cells may play distinct roles in the different stages of SLE progression [88]. Alternatively, it is possible that NKT-cell subsets may have disparate effects in SLE.
Primary biliary cirrhosis
Primary biliary cirrhosis (PBC) is an autoimmune cholangitis characterized by the destruction of intrahepatic bile ducts and the appearance of highly specific autoantibodies against the mitochondrial enzyme, pyruvate dehydrogenase complex-E2 component (PDC-E2). In the liver, iNKT cells comprise a major cell leukocyte population that patrols the sinusoids in a random pattern [119]. At present, the pathogenic role for hepatic NKT cells has been more readily apparent in patients with PBC than in the other autoimmune disorders previously described. PBC patients exhibit an increased frequency of hepatic iNKT cells compared with normal donors [120], and a pathogenic role for iNKT cells has been supported by studies in different experimental models of PBC [121]. Recently, Mattner et al. demonstrated that infection of mice with the gram-negative bacterium, Novosphingobium aromaticivorans, produces glycolipids that are recognized by NKT cells in the sinusoids, causing the production of PDC-E2-specific antibodies that trigger chronic T-cell-mediated autoimmunity against small bile ducts [122]. The livers from these mice were enlarged owing to inflammation and had bile duct damage, biliary lesions and granuloma formation. Infection of CD1d-knockout mice, that lack NKT cells, did not result in enlargement of the liver or development of biliary lesions. Conversely, infection of B6.Vα14 transgenic mice, which overexpress iNKT cells resulted in more severe portal inflammation, bile duct damage and granuloma formation. Subsequently, in another model of PBC, induced by immunizing mice with 2-octynoic acid coupled with bovine serum albumin, exposure to αGalCer resulted in a dramatic exacerbation of autoimmune cholangitis [123]. Collectively, these findings point to disease-promoting potential for hepatic iNKT cells for autoimmune biliary diseases, however, the precise mechanisms underlying this process remain to be determined.
Allergic asthma
Asthma is a complicated and heterogeneous disease characterized by airway hyper-reactivity (AHR) and inflammation. Allergic asthma is the most common form of asthma. Since Th2 cells play crucial roles in the development of allergic asthma, it has been hypothesized that NKT cells can promote AHR [124]. Evidence that iNKT cells are required for the development of allergic asthma in humans was first hypothesized when a study found that iNKT cells were the predominant T cell in the lungs and bronchoalveolar lavage fluid of patients suffering from this disease [125]. In that study, iNKT cells were found to exclusively produce Th2 cytokines. Several recent studies confirmed that the number of iNKT cells is increased in patients with asthma [87,126]. However, two competing studies found iNKT cells were not the predominant T cell in patients suffering from asthma and suggested iNKT cells modulate instead of cause asthma [127,128]. As asthma is a multifactorial disease with varying severity, it is possible that the causative agent and disease severity determine the role, if any, of iNKT cells. Indeed, one study found that iNKT cells were increased in patients with severe asthma compared with controls and those with mild asthma [129].
Studies using animal models have begun to elucidate the complex mechanisms used by NKT cells to develop and regulate asthma. Challenging mice with ovalbumin (OVA) antigen induces Th2 cytokines IL-13 and IL-4 and severe AHR. OVA challenged Jα18(−/−) mice, that lack iNKT cells, did not have elevated IL-13 or IL-4 expression, nor did they develop AHR [130]. Adoptive transfer of iNKT cells from wild-type, but not IL-4- or IL-13-deficient mice, into Jα18(−/−) mice reconstituted AHR. A pathogenic Th2 cytokine storm in AHR is known to be mediated by IL-25 (IL-17E) [131]. A recent study identified a subset of iNKT cells that express the IL-25 receptor IL-17Rb as the target for IL-25-induced Th2 cytokine storm in AHR-initiated mice (Figure 2H) [132]. In addition, NKT cells can be activated directly through TIM-1, an apoptotic sensor pathogen-associated molecular pattern [133]. Furthermore, AHR induced by apoptotic cells can be prevented by blocking TIM-1 or in CD1d-knockout mice.
Manipulations that skew NKT cells towards a Th1 bias have efficiently prevented asthma in many animal studies. For example, the activation of iNKT cells with αGalCer during the sensitization phase suppressed allergic airway inflammation and Th2 responses, via the production of IFN-γ [134]. Moreover, a single dose of the CD1d-binding lipid antagonist, di-palmitoyl-phosphatidyl-ethanolamine polyethylene glycol (DPPE-PEG), could prevent the development of AHR and pulmonary infiltration of lymphocytes upon OVA challenge [135]. In addition, the transfer of αGalCer-loaded bone marrow-derived DCs prevented the development of lung allergic responses in a manner that was dependent on IFN-γ production by host iNKT cells [136]. Th1 skewing was further verified in a study where infection of suckling mice with influenza A virus could protect adult mice from AHR, through preferential expansion of CD4−CD8− iNKT cells that had skewed IFN-γ production, and required T-bet, TLR7 and CD1d ligation [137]. These findings suggest that novel therapeutic strategies that eliminate or skew iNKT cells toward Th1 functions may hold promise for the therapy of asthma.
Multiple sclerosis
Multiple sclerosis (MS) patients exhibit a reduction in the frequency of iNKT cells in the peripheral blood and their ability to produce IL-4 [105,138]. Myelin oligodendrocyte glycoprotein (MOG35–55)-immunized NOD mice develop experimental autoimmune encephalomyelitis (EAE) and serve as an animal model of MS. MOG35–55-immunized NOD mice that overexpress iNKT cells (Va14- Jα18 transgenic NOD) developed less severe EAE that depended on iNKT cell derived IL-4 inhibition of IFN-γ-producing splenocytes (Figure 2g) [139]. This Th2 skewing was independent of CD1d antigenic signaling; suggesting alternative cognate and/or soluble factors are involved [140]. OCH, a sphingosine-truncated analog of αGalCer, was shown to induce Th2 cytokines in iNKT cells [141]. Treating MOG35–55 mice with OCH attenuated EAE development in an IL-4-dependent manner [142]. Thus, one mechanism used by iNKT cells for alleviating EAE may be by inhibition of pathogenic Th1 cells via Th2 skewing of the immune response.
NKT cells can also inhibit EAE severity through interactions of semi-matured DCs and CD1d antigenic signaling. NKT cells activated by TNF-treated semi-mature DCs induce high serum levels of IL-4 and IL-13, and protection from EAE [143]. This protection was dependent on endogenous glycolipid antigen presentation to NKT cells and CD4+ T-cell activation by the same DC. A subsequent study found semi-mature-DC-induced protection from EAE was mediated through interactions with vNKT cells and independent of iNKT cells [144]. Deficiencies in B7-H1 (PDL-1) enhance EAE protection induced by semi-mature DCs and vNKT cell interactions. Together, these studies suggest both iNKT and vNKT cell subsets can protect from EAE, but while vNKT cells require antigenic signals for protection, iNKT protection is antigen independent. A recent addition to the NKT cell family is NKT cells expressing the regulatory T-cell-associated transcriptional factor (FoxP3), termed ‘NKTreg’ cells. NKT regulatory cells develop in a TGF-β rich environment, and are found in the cervical lymph nodes of mice that are protected from EAE following αGalCer treatment [145]. These authors also found human iNKT cells can be induced to express FoxP3 with TGF-β in vitro. However, their role in autoimmunity is still unclear and these observations are ripe for further investigation.
Role of NKT cells in inflammation & cancer
Cancer therapies targeting NKT cells
Numerous studies have examined the antitumor effects of iNKT cells following exogenous IL-12 or αGalCer activation (exceptionally covered in these reviews [1,2,146]). Antitumor activity following iNKT-cell activation is dependent on secondary responses that trigger IL-12 expression by myeloid cells causing bystander IFN-γ production and the downstream activation of NK- and CD8+ T-cell effector functions [66–68]. The iNKT-cell antigen αGalCer was first discovered as part of a screen for antimetastatic compounds [29]. Clinical trials studying the efficacy of αGalCer monotherapy in patients with solid tumors, hepatitis B virus and hepatitis C virus infection have had limited success [147–149]. Peripheral levels of iNKT cells dropped precipitously in all patients treated with αGalCer, which is consistent with studies in mice [150]. The clinical response to αGalCer therapy was directly attributed to the patient’s baseline frequency of iNKT cells. Only patients with high baseline levels of iNKT had immune activation of NK and CD8+ T cells and an increase in serum IFN-γ, TNFα and IL-6. This is a problem for broad applicability of this therapeutic approach, since iNKT-cell frequencies vary widely between individuals. Furthermore, studies found peripheral frequencies of iNKT cells are decreased in patients with melanoma [151,152], primary lung cancer [153], breast cancer [151] and prostate cancer [154]. These findings may limit the value of using αGalCer as a monotherapy in treating cancer, since the target cell population of the therapy may be absent or limiting in number. By contrast to αGalCer given alone, αGalCer-pulsed DCs induced significant resistance to B16 melanoma with prolonged expansion of IFN-γ-producing iNKT cells [155]. Subsequent clinical trials evaluating the effectiveness of αGalCer-pulsed DCs in treating patients with solid tumors in the liver or lung, as well as unresectable head and neck cancers, have shown some promise [156–158]. Cancer patients that received αGalCer-pulsed DCs had enhanced NK and CD8+ T-cell responses with the expansion of iNKT cells even in those patients with initial low baseline frequency. In addition, partial responders were reported in patients who received the vaccine, even in the challenging Phase I trial setting.
Next generation preclinical studies found αGalCer-pulsed DCs loaded with tumor antigen or tumor tissue had even better antitumor activity against established B16 melanoma (subcutaneous) or CMS4 sarcomas (intra-hepatic) compared with αGalCer-pulsed DCs alone [159,160]. These results suggest the nature of APCs that activate iNKT cells is important for the therapeutic outcome. Free αGalCer can be endocytosed and presented to iNKT cells by a number of APCs, including DCs, monocytes, macrophages, B cells and even some epithelial and mesenchymal cells, such as hepatocytes [161]. Each type of presenting cell can express different levels of CD1d and costimulatory molecules that influence NKT cell activation status [69,162]. Consistent with the scope and bias of NKT-cell activation being determined by the functional phenotype of APCs, one study found mature DCs were superior to immature DCs for the activation of iNKT cells [163] and mature DCs pulsed with αGalCer showed long-term expansion of iNKT cells and an increase in memory CD8+ T cells in patients with cancer [164].
Physiological role of NKT cells in tumor immunity
A paradox exists in the natural ability of NKT cells to both promote and suppress immune responses. An early study that laid the ground-work for examining the endogenous role of iNKT cells in tumor immunity found methycholanthrene (MCA)-induced fibrosarcomas grew faster in mice deficient in iNKT cells, suggesting iNKT cells provide protection through immunosurveillance [165]. The authors subsequently found that iNKT-cell mediated protection against MCA-induced sarcomas required NK cells, CD8+ T cells and perforin (Figure 1A) [166]. However, protection was maintained even when perforin-deficient NKT cells were transferred into MCA-initiated mice, suggesting that iNKT-cell protection was not mediated directly through perforin but rather through the downstream induction of other perforin expressing cells.
In apparent contrast to the MCA-initiated tumor model, two separate studies found NKT cells inhibited tumor surveillance. Our laboratory, using a metastatic model of renal cell carcinoma, found that CD1d-deficient mice (which lack both iNKT and vNKT cells) had significantly reduced numbers of hepatic tumor nodules, compared with NKT-cell competent mice [167]. Furthermore, we demonstrated that IL-18 plus IL-12 therapeutic intervention against liver tumor nodules was enhanced in CD1d-deficient mice [167]. Another study showed that subcutaneous 15–12RM fbrosarcoma, which naturally regresses and relapses, failed to relapse in CD1d-deficient mice [168]. These authors found IL-13 released by NKT cells induced TGF-β expression by myeloid cells that inhibited CD8+ T-cell effector function (Figure 1B). Two subsequent studies by this group partially resolved the apparent contradictory role that NKT cells play in immunosurveillance. The first study demonstrated that vNKT cells were sufficient for the downregulation of tumor immunosurveillance and relapse growth of 15–12RM fibrosarcoma in an antigen-dependent manner [169], while the second study found that activation of vNKT cells with sulfatide antigen could suppress the activation of iNKT cells [170]. These two studies collectively demonstrate vNKT cells can inhibit iNKT-cells functions, establishing a regulatory axis between tumor immunosurveillance and escape (Figure 1B).
However, this immunregulatory axis is not universally active in all tumor settings. iNKT cells were found to slow the onset of sarcomas and hematopoietic cancers that develop in p53+/− mice, while vNKT cells played no role in this model [171]. It remains unclear whether iNKT cells always mediate antitumor immunity, since in transplantation and autoimmunity, iNKT cells suppress rather than promote cell-mediated immunity. Consistent with these immunoregulatory functions, iNKT cells are able to produce IL-13 that can drive the TGF-β-mediated suppressive cascade used by vNKT cells (Figure 1B). Furthermore, iNKT cells can promote suppression through the expression of IL-2, which is essential for Treg maintenance, as well as TNF-α that activates and expands functionally suppressive Tregs [172,173]. More work is needed to determine the mechanisms that promote NKT cells to suppress or promote cell-mediated immunity to more accurately determine their immune contributions to the outcomes of different tumor types. These studies, along with the findings that antitumor activity varied among iNKT-cell subsets from different tissues [31,174,175] and tumor-shed glycolipids could inhibit iNKT-cell responses [176,177], suggest that tumor etiology and location may determine the natural role of each NKT-cell subset in tumor surveillance.
Role of NKT cells in inflammatory conditions that lead to cancer
In the 19th century, Rudolf Virchow found that tumors frequently developed at sites of chronic inflammation and that they often contained inflammatory infiltrates, implicating inflammation as a key underlying mechanism for the development of malignancy [178]. Recent studies have provided cellular and molecular evidence for this link between inflammation and cancer [179]. Better understanding of the inflammatory milieu involved in the promotion and regulation of inflammatory conditions could reveal important intervention points for the improved therapy of inflammation-related cancers. In this regard, recent studies reveal NKT cells as important mediators of inflammatory responses. NKT cells express chemokine receptors that focus their homing to inflammatory sites [72] and during inflammatory responses, activated APCs upregulate CD1d molecules, which sensitize them to antigen activation [162]. Lysophosphatidylcholine, a phospholipid associated with inflammation [180], was found to compete with endogenous glycolipids for the binding of CD1d molecules, causing human iNKT cell activation [14]. Furthermore, inflammatory conditions are often associated with increased levels of acute phase protein serum amyloid (SA)-A1. SAA-1 promotes CD40- and CD1d-dependent iNKT-cell interaction with neutrophils [7]. This interaction reverses the suppressive phenotype of neutrophils by decreasing their ability to produce IL-10. These data taken together suggest that NKT cells can monitor and react to host inflammatory conditions. A central question then is whether NKT cells play a pathogenic or beneficial role in the outcome of inflammation-associated disease conditions, including development of cancer.
Multiple myeloma, a malignancy associated with inflammation [181,182], is associated with an increased frequency of vNKT cells and elevated levels of several lysophosphatidylcholine species bound to CD1d molecules [181,183]. A pathogenic role has been suggested for vNKT cells in myeloma patients since vNKT cells can exhibit a skewed IL-13 cytokine secretion profile, and IL-13 is a growth factor for myeloma, as well as a promoter of some inflammatory conditions [184,185]. In addition, as previously described in mice, IL-13 expressed by vNKT cells can modulate the immunoregulatory axis and contribute to the suppression of iNKT responses (Figure 1B) [146,184]. Indeed, myeloma disease progression has been associated with dysfunction in iNKT-cell activation [186].
The liver represents a potentially important organ site for NKT-cell-mediated modulation of inflammatory diseases that lead to cancer. It is noteworthy that NKT cells are enriched in the liver. NKT cells respond to lipid antigens, and most endogenous lipids and lipoproteins are synthesized by the liver, with their production and metabolism altered during liver inflammation or cancer progression [187–189]. Nonalcoholic fatty liver disease (NAFLD) is the most common inflammatory liver disease in western countries [190]. NAFLD is initiated with fatty liver deposits (steatosis) and, with the help of chronic inflammation, can progress to steatohepatitis (NASH) followed by cirrhosis and finally hepatocellular carcinoma [191,192]. An overall increase in hepatocellular carcinoma, initiated by NAFLD, is expected, based on the epidemic of obesity now seen in many nations [193].
Monounsaturated fatty acid-induced NAFLD reduces hepatic iNKT-cell numbers and impairs the ability of hepatocytes to present endogenous antigens to iNKT cells [194]. Diet-induced and leptin-deficient ob/ob murine models of NAFLD also have decreased NK1.1+ CD3+ or NK1.1+ CD4+ NKT cells [195–197], further implicating a role for these cells in the pathogenesis of this disease. Factors that might contribute to changes in iNKT cells relevant to these effects could include IL-12, which causes the loss of iNKT cells, and IL-15, which is a prosurvival factor for iNKT cells [53,167,198]. Specifically, the loss of iNKT and NK1.1+ CD4+ NKT cells in diet-induced and leptin-deficient ob/ob mice were found to be dependent on Kupffer cell-induced IL-12 expression [199,200]. Furthermore, leptin-deficient mice had increased IL-12 expression by Kupffer cells, concurrent with a loss in IL-15 expression, which might further contribute to iNKT cell loss in this model. Further analysis found decreased NK1.1+ CD3+ cell numbers correlated with increased Th1 proinflammatory cytokines in mouse models of NAFLD and, because Th1 proinflammatory cytokines are frequently found in obese mice with steatotic livers [196,197], this suggests that iNKT cells may play an important regulatory role in NAFLD. Indeed, the adoptive transfer of NK1.1+ CD3+ cells into ob/ob mice decreased hepatic fat content and ameliorated NAFLD pathology [201]. However, because NK1.1+ CD3+ or NK1.1+ CD4+ cells encompass both iNKT cells and vNKT cells further studies using CD1d−/− and Ja18−/− knockout mice are needed to determine the beneficial as well as pathological roles of each subset in NAFLD. By contrast to the liver, examination of adipose tissue from mice with diet-induced NAFLD had an increase in iNKT cells [202]. Using β2-micro-globulin knockout mice that lack NKT cells, the authors found a reduced frequency in infiltrating macrophages and an amelioration of glucose intolerance, suggesting that iNKT cells can exacerbate the inflammatory response in NAFLD via macrophage recruitment. However, β2-microglobulin knockout mice also lack CD8+ T cells and vNKT cells, which could be playing a pathogenic role, suggesting additional studies are needed to d etermine the implications of this finding.
In humans, the role for NKT cells in NAFLD is less clear. Two reports found NKT cell levels decreased in patients with NAFLD [199,203], while two other reports demonstrated an increase in these cells [204,205]. The first report found that iNKT cells were decreased in the peripheral blood of patients with NAFLD [203]. However, peripheral changes in immune cells do not always correlate with disease status [206] and this study did not report hepatic iNKT cell levels. Moreover, we found activated iNKT cells are recruited to the liver, suggesting a loss of peripheral iNKT cells in patients with NAFLD could be the result of iNKT cells migrating from the peripheral blood to the inflamed liver [207]. Further studies examining the hepatic NKT cell levels in NAFLD patients could help reveal the role of NKT cells in this disease and determine the relative contributions at various stages of the disease process. specifically, NAFLD severity ranges from steatosis to NASH and cirrhosis, and thus, NKT cell levels could change in relation to, or influence, disease severity at different points in the inflammatory progression. In fact, disease severity is generally reflected by an accumulation of hepatic inflammatory cells. Kremer et al. found a decrease in hepatic iNKT cells in patients with NAFLD. However, interpretation of these results is complicated by the exclusion of samples from those patients who presented with fibrosis, cirrhosis and significant inflammation, which are the more severe cases of NAFLD [199]. Tajiri et al. found levels of hepatic iNKT cells increased as NAFLD severity score increased [205]. By examining the more severe cases of NAFLD, Syn et al. found accumulation of hepatic iNKT cells in patients with NASH cirrhosis [204]. Using a mouse NASH model (patch-deficient mice fed a methionine choline-deficient diet), this group found an increase in hepatic iNKT cells directly correlated to fibrosis and increased IL-15 expression. Knocking out NKT cells in these mice protected them from fibrosis. Interestingly, the NAFLD mouse models previously described more closely resemble steatosis and had a loss in hepatic iNKT cells, whereas the NASH mouse model described here had increased hepatic iNKT cells. Consistent with an increase in iNKT cells proportional to disease severity, patients with liver cancer, which can be considered the culmination of inflammatory liver disease, have an enrichment of iNKT cells [208]. Overall, these results suggest that the roles of iNKT cells may vary as a f unction of increasing severity of NAFLD.
Specifically, iNKT cells appear to inhibit early disease progression with their loss facilitating disease progression, while at later inflammatory stages, NKT cells promote disease progression. Clearly, more work needs to be carried out to determine the underlying mechanisms NKT cells use to switch from protecting the host from NAFLD to promoting the disease.
Future perspective
Phase I clinical trials, using αGalCer-loaded DCs to target iNKT cells, have shown promise in treating some patients with cancer. As humans have a low frequency of iNKT cells, therapies that combine αGalCer-loaded DCs with agents that boost iNKT cells (e.g., IL-15 or IL-18) might be a useful strategy to enhance therapeutic efficacy. While most of the therapeutic approaches focus on iNKT cell activation, blocking vNKT cells that modulate the immuneregulatory axis toward suppression may play an equally important role in some malignancies. Continuing research needs to be carried out to determine which human malignancies may be regulated by vNKT cells. Based on findings that IL-13 produced by vNKT cells inhibits iNKT cells and CD8+ T-cell responses in some mouse cancer models and in myeloma patients, therapies designed to use a push–pull method of blocking IL-13 expressed by vNKT cells in combination with activation of iNKT cells with αGalCer-loaded DCs may also have merit.
Accumulating evidence demonstrates that NKT cells can play important roles in the development of autoimmune and allergic diseases in mouse models. Although alterations in function and frequency suggest the involvement of NKT cells in patients with autoimmune and allergic inflammation, more research is needed to determine if the observed function of NKT cells in mouse models translate to humans with autoimmune disorders. Strategies that specifically manipulate NKT cells, such as the use of αGalCer or its synthetic analogs [209], the infusion of ex vivo expanded NKT or NKT-induced tolerant DCs, and the use of antibodies that inhibit activated pathogenic iNKT cells in vivo [210] may also hold promise for the management of autoimmune diseases. In addition, chronic activation with αGalCer causes iNKT cell anergy [211] and then long-term loss [207], which could be a useful strategy for inhibiting iNKT cells, in order to alleviate autoimmune diseases where iNKT cells are pathogenic. However, owing to the complexity and sometimes inconsistent findings relating to the roles of NKT cells reported in preclinical studies, additional investigations are warranted for a more thorough understanding of the intrinsic biology of different NKT-cell subsets and the roles of NKT cells in different disease stages. The findings from such studies may allow predictable harnessing and tailoring of NKT functions to appropriate stages of various autoimmune or neoplastic diseases through rationally designed clinical trials.
Executive summary.
NKT cells in autoimmunity
-
▪
The aberrant frequency and/or function of NKT cells in the peripheral blood of patients with autoimmune and allergic inflammation diseases suggest that they are involved in the disease pathology.
-
▪
The diverse functional roles of NKT cells in autoimmune disease probably depend on the particular autoimmune disease, the NKT-cell subset being studied, the stage of disease and the genetic background of the host or animal model used for study.
-
▪
Invariant NKT (iNKT) cells have a dual role in mouse models of diabetes. They appear to inhibit the development of diabetes, but can also promote pathogenic CD8+ diabetogenic T cells once they emerge.
-
▪
Th2 cytokine production by iNKT cells protects mice from rheumatoid arthritis and multiple sclerosis.
-
▪
Both iNKT and variant NKT (vNKT) cell subsets can protect mice from autoimmune encephalomyelitis, however, while vNKT cells require antigenic signals for protection, iNKT-mediated protection is antigen independent.
-
▪
Th1 skewing of iNKT cells prevented allergic asthma in mouse models.
NKT cells in cancer
-
▪
Phase I clinical trials using α-galactosyl ceramide-loaded dendritic cells expanded iNKT cells and caused bystander activation of CD8+ T cells and NK cells, and is associated with some positive clinical outcomes.
-
▪
vNKT cells can inhibit the antitumor activity of iNKT cells and CD8+ T cells, establishing a regulatory axis between tumor immunosurveillance and escape. The inhibition of CD8+ T cells by vNKT cells was mediated through the expression of IL-13 and the downstream induction of TGF-β. This immunoregulatory axis may be operative in myeloma patients, where vNKT cells express IL-13 and iNKT cells are dysfunctional for activation.
-
▪
Depending on disease stage, human iNKT cells appear to differentially regulate nonalcoholic fatty liver disease. iNKT-cell frequency is reduced in humans with steatosis, suggesting that these cells inhibit early disease progression, while at a later inflammatory NASH stage, NKT cell frequency is increased. This suggests that they may promote disease progression. Similar findings have been reported in mouse models, and these results are strikingly similar to the differential roles that iNKT cells appear to play in development versus promotion of nonobese diabetic-induced diabetes in mice.
Acknowledgments
This work was supported by the Intramural Research Program of the National Cancer Institute (NCI)/NIH.
Footnotes
Author disclosure
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the US government.
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
- 1.van der Vliet HJ, Molling JW, von Blomberg BM, et al. The immunoregulatory role of CD1d-restricted natural killer T cells in disease. Clin. Immunol. 2004;112:8–23. doi: 10.1016/j.clim.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu. Rev. Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 3.Coquet JM, Chakravarti S, Kyparissoudis K, et al. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc. Natl Acad. Sci. USA. 2008;105:11287–11292. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michel ML, Keller AC, Paget C, et al. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J. Exp. Med. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang SH, Jin JZ, Lee SH, et al. Role of NKT cells in allogeneic islet graft survival. Clin. Immunol. 2007;124:258–266. doi: 10.1016/j.clim.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Godfrey DI, Hammond KJ, Poulton LD, Smyth MJ, Baxter AG. NKT cells: facts, functions and fallacies. Immunol. Today. 2000;21:573–583. doi: 10.1016/s0167-5699(00)01735-7. [DOI] [PubMed] [Google Scholar]
- 7.De Santo C, Arscott R, Booth S, et al. Invariant NKT cells modulate the suppressive activity of IL-10-secreting neutrophils differentiated with serum amyloid A. Nat. Immunol. 2010;11:1039–1046. doi: 10.1038/ni.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hermans IF, Silk JD, Gileadi U, et al. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J. Immunol. 2003;171:5140–5147. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 9.Kim JH, Chung DH. CD1d-restricted IFN-γ-secreting NKT cells promote immune complex-induced acute lung injury by regulating macrophage-inflammatory protein-1α production and activation of macrophages and dendritic cells. J. Immunol. 2011;186:1432–1441. doi: 10.4049/jimmunol.1003140. [DOI] [PubMed] [Google Scholar]
- 10.Ley K, Smith E, Stark MA. IL-17a-producing neutrophil-regulatory Tn lymphocytes. Immunol. Res. 2006;34:229–242. doi: 10.1385/IR:34:3:229. [DOI] [PubMed] [Google Scholar]
- 11.Cohen NR, Garg S, Brenner MB. Antigen presentation by CD1 lipids, T cells, and NKT cells in microbial immunity. Adv. Immunol. 2009;102:1–94. doi: 10.1016/S0065-2776(09)01201-2. [DOI] [PubMed] [Google Scholar]
- 12.Zeng Z, Castano AR, Segelke BW, Stura EA, Peterson PA, Wilson IA. Crystal structure of mouse CD1: an MHC-like fold with a large hydrophobic binding groove. Science. 1997;277:339–345. doi: 10.1126/science.277.5324.339. [DOI] [PubMed] [Google Scholar]
- 13.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat. Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 14. Fox LM, Cox DG, Lockridge JL, et al. Recognition of lyso-phospholipids by human natural killer T lymphocytes. PLoS Biol. 2009;7:E1000228. doi: 10.1371/journal.pbio.1000228. ▪ Demonstrates that invariant NKT (iNKT) cells can be activated by inflammation-associated lipids, linking iNKT cells to inflammation.
- 15.Kinjo Y, Wu D, Kim G, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 16.Kinjo Y, Tupin E, Wu D, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat. Immunol. 2006;7:978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 17.Mattner J, Debord KL, Ismail N, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 18.Salio M, Cerundolo V. Linking inflammation to natural killer T cell activation. PLoS Biol. 2009;7:E1000226. doi: 10.1371/journal.pbio.1000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behar SM, Podrebarac TA, Roy CJ, Wang CR, Brenner MB. Diverse TCRs recognize murine CD1. J. Immunol. 1999;162:161–167. [PubMed] [Google Scholar]
- 20.Cardell S, Tangri S, Chan S, Kronenberg M, Benoist C, Mathis D. CD1-restricted CD4+ T cells in major histocompatibility complex class II-deficient mice. J. Exp. Med. 1995;182:993–1004. doi: 10.1084/jem.182.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiu YH, Jayawardena J, Weiss A, et al. Distinct subsets of CD1d-restricted T cells recognize self-antigens loaded in different cellular compartments. J. Exp. Med. 1999;189:103–110. doi: 10.1084/jem.189.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer K, Scotet E, Niemeyer M, et al. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc. Natl Acad. Sci. USA. 2004;101:10685–10690. doi: 10.1073/pnas.0403787101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park SH, Bendelac A. CD1-restricted T-cell responses and microbial infection. Nature. 2000;406:788–792. doi: 10.1038/35021233. [DOI] [PubMed] [Google Scholar]
- 24.Cox D, Fox L, Tian R, et al. Determination of cellular lipids bound to human CD1d molecules. PLoS ONE. 2009;4:E5325. doi: 10.1371/journal.pone.0005325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pei B, Speak AO, Shepherd D, et al. Diverse endogenous antigens for mouse NKT cells: self-antigens that are not glycosphingolipids. J. Immunol. 2011;186:1348–1360. doi: 10.4049/jimmunol.1001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan W, Kang SJ, Evans JE, Cresswell P. Natural lipid ligands associated with human CD1d targeted to different subcellular compartments. J. Immunol. 2009;182:4784–4791. doi: 10.4049/jimmunol.0803981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou D, Mattner J, Cantu C, III, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 28.Stronge VS, Salio M, Jones EY, Cerundolo V. A closer look at CD1d molecules: new horizons in studying NKT cells. Trends Immunol. 2007;28:455–462. doi: 10.1016/j.it.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Sidobre S, Kronenberg M. CD1 tetramers: a powerful tool for the analysis of glycolipid-reactive T cells. J. Immunol. Methods. 2002;268:107–121. doi: 10.1016/s0022-1759(02)00204-1. [DOI] [PubMed] [Google Scholar]
- 30.Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 31.Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J. Exp. Med. 2002;195:625–636. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berzins SP, Smyth MJ, Baxter AG. Presumed guilty: natural killer T cell defects and human disease. Nat. Rev. Immunol. 2011;11:131–142. doi: 10.1038/nri2904. [DOI] [PubMed] [Google Scholar]
- 33.Kenna T, Golden-Mason L, Porcelli SA, et al. NKT cells from normal and tumor-bearing human livers are phenotypically and functionally distinct from murine NKT cells. J. Immunol. 2003;171:1775–1779. doi: 10.4049/jimmunol.171.4.1775. [DOI] [PubMed] [Google Scholar]
- 34.Exley MA, Tahir SM, Cheng O, et al. A major fraction of human bone marrow lymphocytes are Th2-like CD1d-reactive T cells that can suppress mixed lymphocyte responses. J. Immunol. 2001;167:5531–5534. doi: 10.4049/jimmunol.167.10.5531. [DOI] [PubMed] [Google Scholar]
- 35.Aliahmad P, Kaye J. Development of all CD4 T lineages requires nuclear factor TOX. J. Exp. Med. 2008;205:245–256. doi: 10.1084/jem.20071944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benlagha K, Wei DG, Veiga J, Teyton L, Bendelac A. Characterization of the early stages of thymic NKT cell development. J. Exp. Med. 2005;202:485–492. doi: 10.1084/jem.20050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Coles MC, Raulet DH. NK1.1+ T cells in the liver arise in the thymus and are selected by interactions with class I molecules on CD4+CD8+ cells. J. Immunol. 2000;164:2412–2418. doi: 10.4049/jimmunol.164.5.2412. ▪ Along with [38] this is the first paper to demonstrate that NKT cells are self-reactive T cells that undergo a unique T-cell development pathway in the thymus.
- 38. Gapin L, Matsuda JL, Surh CD, Kronenberg M. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat. Immunol. 2001;2:971–978. doi: 10.1038/ni710. ▪ Along with [37] this is the first paper to demonstrate that NKT cells are self-reactive T cells that undergo a unique T-cell development pathway in the thymus.
- 39.Sandberg JK, Stoddart CA, Brilot F, Jordan KA, Nixon DF. Development of innate CD4+ α-chain variable gene segment 24 (Vα24) natural killer T cells in the early human fetal thymus is regulated by IL-7. Proc. Natl Acad. Sci. USA. 2004;101:7058–7063. doi: 10.1073/pnas.0305986101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eberl G, Lowin-Kropf B, MacDonald HR. Cutting edge: NKT cell development is selectively impaired in Fyn-deficient mice. J. Immunol. 1999;163:4091–4094. [PubMed] [Google Scholar]
- 41.Gadue P, Morton N, Stein PL. The Src family tyrosine kinase Fyn regulates natural killer T cell development. J. Exp. Med. 1999;190:1189–1196. doi: 10.1084/jem.190.8.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griewank K, Borowski C, Rietdijk S, et al. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 2007;27:751–762. doi: 10.1016/j.immuni.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nichols KE, Hom J, Gong SY, et al. Regulation of NKT cell development by SAP, the protein defective in XLP. Nat. Med. 2005;11:340–345. doi: 10.1038/nm1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pasquier B, Yin L, Fondaneche MC, et al. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J. Exp. Med. 2005;201:695–701. doi: 10.1084/jem.20042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bousso P, Bhakta NR, Lewis RS, Robey E. Dynamics of thymocyte-stromal cell interactions visualized by two-photon microscopy. Science. 2002;296:1876–1880. doi: 10.1126/science.1070945. [DOI] [PubMed] [Google Scholar]
- 46.Kishimoto H, Sprent J. Negative selection in the thymus includes semimature T cells. J. Exp. Med. 1997;185:263–271. doi: 10.1084/jem.185.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiu YH, Park SH, Benlagha K, et al. Multiple defects in antigen presentation and T cell development by mice expressing cytoplasmic tail-truncated CD1d 11. Nat. Immunol. 2002;3:55–60. doi: 10.1038/ni740. [DOI] [PubMed] [Google Scholar]
- 48.Honey K, Benlagha K, Beers C, et al. Thymocyte expression of cathepsin l is essential for NKT cell development. Nat. Immunol. 2002;3:1069–1074. doi: 10.1038/ni844. [DOI] [PubMed] [Google Scholar]
- 49.Jayawardena-Wolf J, Benlagha K, Chiu YH, Mehr R, Bendelac A. CD1d endosomal trafficking is independently regulated by an intrinsic CD1d-encoded tyrosine motif and by the invariant chain. Immunity. 2001;15:897–908. doi: 10.1016/s1074-7613(01)00240-0. [DOI] [PubMed] [Google Scholar]
- 50.Moran AE, Holzapfel KL, Xing Y, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J. Exp. Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kovalovsky D, Uche OU, Eladad S, et al. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat. Immunol. 2008;9:1055–1064. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Savage AK, Constantinides MG, Han J, et al. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsuda JL, Gapin L, Sidobre S, et al. Homeostasis of V α 14i NKT cells. Nat. Immunol. 2002;3:966–974. doi: 10.1038/ni837. [DOI] [PubMed] [Google Scholar]
- 54.Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat. Immunol. 2010;11:197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- 55.Kim PJ, Pai SY, Brigl M, et al. GATA-3 regulates the development and function of invariant NKT cells. J. Immunol. 2006;177:6650–6659. doi: 10.4049/jimmunol.177.10.6650. [DOI] [PubMed] [Google Scholar]
- 56.Matsuda JL, Zhang Q, Ndonye R, Richardson SK, Howell AR, Gapin L. T-bet concomitantly controls migration, survival, and effector functions during the development of Vα14i NKT cells. Blood. 2006;107:2797–2805. doi: 10.1182/blood-2005-08-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Townsend MJ, Weinmann AS, Matsuda JL, et al. T-bet regulates the terminal maturation and homeostasis of NK and Vα14i NKT cells. Immunity. 2004;20:477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 58.Lee HJ, Takemoto N, Kurata H, et al. GATA-3 induces T helper cell type 2 (Th2) cytokine expression and chromatin remodeling in committed Th1 cells. J. Exp. Med. 2000;192:105–115. doi: 10.1084/jem.192.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mullen AC, High FA, Hutchins AS, et al. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 60. Stetson DB, Mohrs M, Reinhardt RL, et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J. Exp. Med. 2003;198:1069–1076. doi: 10.1084/jem.20030630. ▪ Reveals the mechanism behind the ability of NKT cells to rapidly produce cytokines, and provided further evidence that these cells have innate cell function.
- 61.Sullivan BM, Juedes A, Szabo SJ, von HM, Glimcher LH. Antigen-driven effector CD8 T cell function regulated by T-bet. Proc. Natl Acad. Sci. USA. 2003;100:15818–15823. doi: 10.1073/pnas.2636938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 63.Liu K, Catalfamo M, Li Y, Henkart PA, Weng NP. IL-15 mimics T cell receptor crosslinking in the induction of cellular proliferation, gene expression, and cytotoxicity in CD8+ memory T cells. Proc. Natl Acad. Sci. USA. 2002;99:6192–6197. doi: 10.1073/pnas.092675799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park SH, Benlagha K, Lee D, Balish E, Bendelac A. Unaltered phenotype, tissue distribution and function of Vα14(+) NKT cells in germ-free mice. Eur. J. Immunol. 2000;30:620–625. doi: 10.1002/1521-4141(200002)30:2<620::AID-IMMU620>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 65.Hashimoto M, Hiwatashi K, Ichiyama K, et al. SOCS1 regulates type I/type II NKT cell balance by regulating IFN{γ} signaling. Int. Immunol. 2011;23:165–176. doi: 10.1093/intimm/dxq469. [DOI] [PubMed] [Google Scholar]
- 66. Carnaud C, Lee D, Donnars O, et al. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J. Immunol. 1999;163:4647–4650. ▪ Identifies NKT cells as potent inducers of bystander activation of NK cells
- 67.Chamoto K, Takeshima T, Kosaka A, et al. NKT cells act as regulatory cells rather than killer cells during activation of NK cell-mediated cytotoxicity by α-galactosylceramide in vivo. Immunol Lett. 2004;95:5–11. doi: 10.1016/j.imlet.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 68.Nakagawa R, Nagafune I, Tazunoki Y, et al. Mechanisms of the antimetastatic effect in the liver and of the hepatocyte injury induced by α-galactosylceramide in mice. J. Immunol. 2001;166:6578–6584. doi: 10.4049/jimmunol.166.11.6578. [DOI] [PubMed] [Google Scholar]
- 69.van den Heuvel MJ, Garg N, van Kaer L, Haeryfar SM. NKT cell costimulation: experimental progress and therapeutic promise. Trends Mol. Med. 2011;17:65–77. doi: 10.1016/j.molmed.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johnston B, Kim CH, Soler D, Emoto M, Butcher EC. Differential chemokine responses and homing patterns of murine TCR α β NKT cell subsets. J. Immunol. 2003;171:2960–2969. doi: 10.4049/jimmunol.171.6.2960. [DOI] [PubMed] [Google Scholar]
- 71.Lin H, Nieda M, Hutton JF, Rozenkov V, Nicol AJ. Comparative gene expression analysis of NKT cell subpopulations. J. Leukoc. Biol. 2006;80:164–173. doi: 10.1189/jlb.0705421. [DOI] [PubMed] [Google Scholar]
- 72.Thomas SY, Hou R, Boyson JE, et al. CD1d-restricted NKT cells express a chemokine receptor profile indicative of Th1-type inflammatory homing cells. J. Immunol. 2003;171:2571–2580. doi: 10.4049/jimmunol.171.5.2571. [DOI] [PubMed] [Google Scholar]
- 73.Kitamura H, Iwakabe K, Yahata T, et al. The natural killer T (NKT) cell ligand α-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J. Exp. Med. 1999;189:1121–1128. doi: 10.1084/jem.189.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tonti E, Galli G, Malzone C, Abrignani S, Casorati G, Dellabona P. NKT-cell help to B lymphocytes can occur independently of cognate interaction. Blood. 2009;113:70–376. doi: 10.1182/blood-2008-06-166249. [DOI] [PubMed] [Google Scholar]
- 75.Barral P, Eckl-Dorna J, Harwood NE, et al. B cell receptor-mediated uptake of CD1d-restricted antigen augments antibody responses by recruiting invariant NKT cell help in vivo. Proc. Natl Acad. Sci. USA. 2008;105:8345–8350. doi: 10.1073/pnas.0802968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lang GA, Devera TS, Lang ML. Requirement for CD1d expression by B cells to stimulate NKT cell-enhanced antibody production. Blood. 2008;111:2158–2162. doi: 10.1182/blood-2007-10-117309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leadbetter EA, Brigl M, Illarionov P, et al. NK T cells provide lipid antigen-specific cognate help for B cells. Proc. Natl Acad. Sci. USA. 2008;105:8339–8344. doi: 10.1073/pnas.0801375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paget C, Mallevaey T, Speak AO, et al. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 2007;27:597–609. doi: 10.1016/j.immuni.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 79.Oki S, Chiba A, Yamamura T, Miyake S. The clinical implication and molecular mechanism of preferential IL-4 production by modified glycolipid-stimulated NKT cells. J. Clin. Invest. 2004;113:1631–1640. doi: 10.1172/JCI20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stanic AK, Shashidharamurthy R, Bezbradica JS, et al. Another view of T cell antigen recognition: cooperative engagement of glycolipid antigens by Vα14Ja18 natural T(iNKT) cell receptor [corrected] J. Immunol. 2003;171:4539–4551. doi: 10.4049/jimmunol.171.9.4539. [DOI] [PubMed] [Google Scholar]
- 81.Sullivan BA, Nagarajan NA, Wingender G, et al. Mechanisms for glycolipid antigen-driven cytokine polarization by Vα14i NKT cells. J. Immunol. 2010;184:141–153. doi: 10.4049/jimmunol.0902880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang X, Chen X, Rodenkirch L, et al. Natural killer T-cell autoreactivity leads to a specialized activation state. Blood. 2008;112:4128–4138. doi: 10.1182/blood-2008-05-157529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lang GA, Maltsev SD, Besra GS, Lang ML. Presentation of a-galactosylceramide by murine CD1d to natural killer T cells is facilitated by plasma membrane glycolipid rafts. Immunology. 2004;112:386–396. doi: 10.1111/j.1365-2567.2004.01896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park YK, Lee JW, Ko YG, Hong S, Park SH. Lipid rafts are required for efficient signal transduction by CD1d. Biochem. Biophys. Res. Commun. 2005;327:1143–1154. doi: 10.1016/j.bbrc.2004.12.121. [DOI] [PubMed] [Google Scholar]
- 85.Peng W, Martaresche C, Escande-Beillard N, Cedile O, Reynier-Vigouroux A, Boucraut J. influence of lipid rafts on CD1d presentation by dendritic cells. Mol. Membr. Biol. 2007;24:475–484. doi: 10.1080/09687680701359408. [DOI] [PubMed] [Google Scholar]
- 86.Im JS, Arora P, Bricard G, et al. Kinetics and cellular site of glycolipid loading control the outcome of natural killer T cell activation. Immunity. 2009;30:888–898. doi: 10.1016/j.immuni.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Matangkasombut P, Marigowda G, Ervine A, et al. Natural killer T cells in the lungs of patients with asthma. J. Allergy Clin. Immunol. 2009;123:1181–1185. doi: 10.1016/j.jaci.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu L, van Kaer L. Natural killer T cells and autoimmune disease. Curr. Mol. Med. 2009;9:4–14. doi: 10.2174/156652409787314534. [DOI] [PubMed] [Google Scholar]
- 89.Wilson SB, Kent SC, Patton KT, et al. Extreme Th1 bias of invariant Vα24JαQ T cells in type 1 diabetes. Nature. 1998;391:177–181. doi: 10.1038/34419. [DOI] [PubMed] [Google Scholar]
- 90.Fletcher MT, Baxter AG. Clinical application of NKT cell biology in Type I (autoimmune) diabetes mellitus. Immunol. Cell Biol. 2009;87:315–323. doi: 10.1038/icb.2009.5. [DOI] [PubMed] [Google Scholar]
- 91.Lehuen A, Lantz O, Beaudoin L, et al. Overexpression of natural killer T cells protects Va14- Jα281 transgenic nonobese diabetic mice against diabetes. J. Exp. Med. 1998;188:1831–1839. doi: 10.1084/jem.188.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang B, Geng YB, Wang CR. CD1-restricted NK T cells protect nonobese diabetic mice from developing diabetes. J. Exp. Med. 2001;194:313–320. doi: 10.1084/jem.194.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Beaudoin L, Laloux V, Novak J, Lucas B, Lehuen A. NKT cells inhibit the onset of diabetes by impairing the development of pathogenic T cells specific for pancreatic β cells. Immunity. 2002;17:725–736. doi: 10.1016/s1074-7613(02)00473-9. ▪ Reveals iNKT cells are able to inhibit the conventional diabetogenic αβ CD4+ T cell differentiation in vivo.
- 94.Novak J, Beaudoin L, Griseri T, Lehuen A. Inhibition of T cell differentiation into effectors by NKT cells requires cell contacts. J. Immunol. 2005;174:1954–1961. doi: 10.4049/jimmunol.174.4.1954. [DOI] [PubMed] [Google Scholar]
- 95.Novak J, Beaudoin L, Park S, et al. Prevention of Type 1 diabetes by invariant NKT cells is independent of peripheral CD1d expression. J. Immunol. 2007;178:1332–1340. doi: 10.4049/jimmunol.178.3.1332. [DOI] [PubMed] [Google Scholar]
- 96.Cain JA, Smith JA, Ondr JK, Wang B, Katz JD. NKT cells and IFN-γ establish the regulatory environment for the control of diabetogenic T cells in the nonobese diabetic mouse. J. Immunol. 2006;176:1645–1654. doi: 10.4049/jimmunol.176.3.1645. [DOI] [PubMed] [Google Scholar]
- 97.Wang J, Cho S, Ueno A, et al. Ligand-dependent induction of noninflammatory dendritic cells by anergic invariant NKT cells minimizes autoimmune inflammation. J. Immunol. 2008;181:2438–2445. doi: 10.4049/jimmunol.181.4.2438. [DOI] [PubMed] [Google Scholar]
- 98.Griseri T, Beaudoin L, Novak J, et al. Invariant NKT cells exacerbate Type 1 diabetes induced by CD8 T cells. J. Immunol. 2005;175:2091–2101. doi: 10.4049/jimmunol.175.4.2091. [DOI] [PubMed] [Google Scholar]
- 99.Driver JP, Scheuplein F, Chen YG, Grier AE, Wilson SB, Serreze DV. Invariant natural killer T-cell control of Type 1 diabetes: a dendritic cell genetic decision of a silver bullet or Russian roulette. Diabetes. 2010;59:423–432. doi: 10.2337/db09-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee PT, Putnam A, Benlagha K, Teyton L, Gottlieb PA, Bendelac A. Testing the NKT cell hypothesis of human IDDM pathogenesis. J. Clin. Invest. 2002;110:793–800. doi: 10.1172/JCI15832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Oikawa Y, Shimada A, Yamada S, et al. High frequency of vα24(+) vβ11(+) T-cells observed in Type 1 diabetes. Diabetes Care. 2002;25:1818–1823. doi: 10.2337/diacare.25.10.1818. [DOI] [PubMed] [Google Scholar]
- 102.Tsutsumi Y, Jie X, Ihara K, et al. Phenotypic and genetic analyses of T-cell-mediated immunoregulation in patients with Type 1 diabetes. Diabet. Med. 2006;23:1145–1150. doi: 10.1111/j.1464-5491.2006.01951.x. [DOI] [PubMed] [Google Scholar]
- 103.Kojo S, Adachi Y, Keino H, Taniguchi M, Sumida T. Dysfunction of T cell receptor AV24AJ18+, BV11+ double-negative regulatory natural killer T cells in autoimmune diseases. Arthritis Rheum. 2001;44:1127–1138. doi: 10.1002/1529-0131(200105)44:5<1127::AID-ANR194>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 104.Linsen L, Thewissen M, Baeten K, et al. Peripheral blood but not synovial fuid natural killer T cells are biased towards a Th1-like phenotype in rheumatoid arthritis. Arthritis Res. Ther. 2005;7:R493–R502. doi: 10.1186/ar1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van der Vliet HJ, von Blomberg BM, Nishi N, et al. Circulating V(α24+) Vβ11+ NKT cell numbers are decreased in a wide variety of diseases that are characterized by autoreactive tissue damage. Clin. Immunol. 2001;100:144–148. doi: 10.1006/clim.2001.5060. [DOI] [PubMed] [Google Scholar]
- 106.Kim HY, Kim HJ, Min HS, et al. NKT cells promote antibody-induced joint inflammation by suppressing transforming growth factor β1 production. J. Exp. Med. 2005;201:41–47. doi: 10.1084/jem.20041400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Park Y, Kim HS, Ahn JY, et al. IL-12p35 promotes antibody-induced joint inflammation by activating NKT cells and suppressing TGF-β. J. Immunol. 2010;185:1476–1484. doi: 10.4049/jimmunol.1000425. [DOI] [PubMed] [Google Scholar]
- 108.Chiba A, Kaieda S, Oki S, Yamamura T, Miyake S. The involvement of V(α)14 natural killer T cells in the pathogenesis of arthritis in murine models. Arthritis Rheum. 2005;52:1941–1948. doi: 10.1002/art.21056. [DOI] [PubMed] [Google Scholar]
- 109.Kim HY, Kim S, Chung DH. FcγRIII engagement provides activating signals to NKT cells in antibody-induced joint inflammation. J. Clin. Invest. 2006;116:2484–2492. doi: 10.1172/JCI27219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Coppieters K, Dewint P, van Beneden K, et al. NKT cells: manipulable managers of joint inflammation. Rheumatology (Oxford) 2007;46:565–571. doi: 10.1093/rheumatology/kel437. [DOI] [PubMed] [Google Scholar]
- 111.Ohnishi Y, Tsutsumi A, Goto D, et al. TCR Vα14 natural killer T cells function as effector T cells in mice with collagen-induced arthritis. Clin. Exp. Immunol. 2005;141:47–53. doi: 10.1111/j.1365-2249.2005.02817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Teige A, Bockermann R, Hasan M, Olofsson KE, Liu Y, Issazadeh-Navikas S. CD1d-dependent NKT cells play a protective role in acute and chronic arthritis models by ameliorating antigen-specific Th1 responses. J. Immunol. 2010;185:345–356. doi: 10.4049/jimmunol.0901693. [DOI] [PubMed] [Google Scholar]
- 113.Wither J, Cai YC, Lim S, et al. Reduced proportions of natural killer T cells are present in the relatives of lupus patients and are associated with autoimmunity. Arthritis Res. Ther. 2008;10:R108. doi: 10.1186/ar2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Forestier C, Molano A, Im JS, et al. Expansion and hyperactivity of CD1d-restricted NKT cells during the progression of systemic lupus erythematosus (New Zealand Black × New Zealand White) F1 mice. J. Immunol. 2005;175:763–770. doi: 10.4049/jimmunol.175.2.763. [DOI] [PubMed] [Google Scholar]
- 115.Takahashi T, Strober S. Natural killer T cells and innate immune B cells from lupus-prone NZB/W mice interact to generate IgM and IgG autoantibodies. Eur. J. Immunol. 2008;38:156–165. doi: 10.1002/eji.200737656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang JQ, Wen X, Liu H, et al. Examining the role of CD1d and natural killer T cells in the development of nephritis in a genetically susceptible lupus model. Arthritis Rheum. 2007;56:1219–1233. doi: 10.1002/art.22490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Yang JQ, Wen X, Kim PJ, Singh RR. Invariant NKT cells inhibit autoreactive B cells in a contact- and CD1d-dependent manner. J. Immunol. 2011;186:1512–1520. doi: 10.4049/jimmunol.1002373. ▪ Along with [118] this is one of first reports to identify the direct interaction between iNKT cells and autoreactive B cells in autoimmune disease.
- 118. Wermeling F, Lind SM, Jordo ED, Cardell SL, Karlsson MC. Invariant NKT cells limit activation of autoreactive CD1d-positive B cells. J. Exp. Med. 2010;207:943–952. doi: 10.1084/jem.20091314. ▪ Along with [117] this is one of first reports to identify the direct interaction between iNKT cells and autoreactive B cells in autoimmune disease.
- 119.Lee WY, Moriarty TJ, Wong CH, et al. An intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nat. Immunol. 2010;11:295–302. doi: 10.1038/ni.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kita H, Naidenko OV, Kronenberg M, et al. Quantitation and phenotypic analysis of natural killer T cells in primary biliary cirrhosis using a human CD1d tetramer. Gastroenterology. 2002;123:1031–1043. doi: 10.1053/gast.2002.36020. [DOI] [PubMed] [Google Scholar]
- 121.Joyce S, van Kaer L. Invariant natural killer T cells trigger adaptive lymphocytes to churn up bile. Cell Host Microbe. 2008;3:275–277. doi: 10.1016/j.chom.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 122.Mattner J, Savage PB, Leung P, et al. Liver autoimmunity triggered by microbial activation of natural killer T cells. Cell Host Microbe. 2008;3:304–315. doi: 10.1016/j.chom.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu SJ, Yang YH, Tsuneyama K, et al. Innate immunity and primary biliary cirrhosis: activated invariant natural killer T cells exacerbate murine autoimmune cholangitis and fibrosis. Hepatology. 2011;53:915–925. doi: 10.1002/hep.24113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Iwamura C, Nakayama T. Role of NKT cells in allergic asthma. Curr. Opin Immunol. 2010;22:807–813. doi: 10.1016/j.coi.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 125.Akbari O, Faul JL, Hoyte EG, et al. CD4+ invariant T-cell-receptor+ natural killer T cells in bronchial asthma. N. Engl. J. Med. 2006;354:1117–1129. doi: 10.1056/NEJMoa053614. [DOI] [PubMed] [Google Scholar]
- 126.Reynolds C, Barkans J, Clark P, et al. Natural killer T cells in bronchial biopsies from human allergen challenge model of allergic asthma. J. Allergy Clin. Immunol. 2009;124:860–862. doi: 10.1016/j.jaci.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 127.Thomas SY, Chyung YH, Luster AD. Natural killer T cells are not the predominant T cell in asthma and likely modulate, not cause, asthma. J. Allergy Clin. Immunol. 2010;125:980–984. doi: 10.1016/j.jaci.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vijayanand P, Seumois G, Pickard C, et al. Invariant natural killer T cells in asthma and chronic obstructive pulmonary disease. N. Engl. J. Med. 2007;356:1410–1422. doi: 10.1056/NEJMoa064691. [DOI] [PubMed] [Google Scholar]
- 129.Hamzaoui A, Cheik RS, Grairi H, et al. NKT cells in the induced sputum of severe asthmatics. Mediators Infamm. 2006;2006(2):71214. doi: 10.1155/MI/2006/71214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Akbari O, Stock P, Meyer E, et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyper-reactivity. Nat. Med. 2003;9:582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 131.Tamachi T, Maezawa Y, Ikeda K, et al. IL-25 enhances allergic airway inflammation by amplifying a TH2 cell-dependent pathway in mice. J. Allergy Clin. Immunol. 2006;118:606–614. doi: 10.1016/j.jaci.2006.04.051. [DOI] [PubMed] [Google Scholar]
- 132.Stock P, Lombardi V, Kohlrautz V, Akbari O. Induction of airway hyper-reactivity by IL-25 is dependent on a subset of invariant NKT cells expressing IL-17RB. J. Immunol. 2009;182:5116–5122. doi: 10.4049/jimmunol.0804213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee HH, Meyer EH, Goya S, et al. Apoptotic cells activate NKT cells through T cell Ig-like mucin-like-1 resulting in airway hyper-reactivity. J. Immunol. 2010;185:5225–5235. doi: 10.4049/jimmunol.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cui J, Watanabe N, Kawano T, et al. Inhibition of T helper cell type 2 cell differentiation and immunoglobulin E response by ligand-activated Vα14 natural killer T cells. J. Exp. Med. 1999;190:783–792. doi: 10.1084/jem.190.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lombardi V, Stock P, Singh AK, et al. A CD1d-dependent antagonist inhibits the activation of invariant NKT cells and prevents development of allergen-induced airway hyper-reactivity. J. Immunol. 2010;184:2107–2115. doi: 10.4049/jimmunol.0901208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Matsuda H, Takeda K, Koya T, et al. Plasticity of invariant NKT cell regulation of allergic airway disease is dependent on IFN-γ production. J. Immunol. 2010;185:253–262. doi: 10.4049/jimmunol.0902301. [DOI] [PubMed] [Google Scholar]
- 137. Chang YJ, Kim HY, Albacker LA, et al. influenza infection in suckling mice expands an NKT cell subset that protects against airway hyper-reactivity. J. Clin. Invest. 2011;121:57–69. doi: 10.1172/JCI44845. ▪ This study found that influenza infection of suckling mice provided long-term protection from airway hyper-reactivity by Th1 polarization of iNKT cells.
- 138.Gausling R, Trollmo C, Hafer DA. Decreases in interleukin-4 secretion by invariant CD4(−) CD8(−)V α 24J α Q T cells in peripheral blood of patientswith relapsing-remitting multiple sclerosis. Clin. Immunol. 2001;98:11–17. doi: 10.1006/clim.2000.4942. [DOI] [PubMed] [Google Scholar]
- 139.Mars LT, Laloux V, Goude K, et al. Cutting edge: V α 14-J α 281 NKT cells naturally regulate experimental autoimmune encephalomyelitis in nonobese diabetic mice. J. Immunol. 2002;168:6007–6011. doi: 10.4049/jimmunol.168.12.6007. [DOI] [PubMed] [Google Scholar]
- 140.Mars LT, Gautron AS, Novak J, et al. Invariant NKT cells regulate experimental autoimmune encephalomyelitis and infiltrate the central nervous system in a CD1d-independent manner. J. Immunol. 2008;181:2321–2329. doi: 10.4049/jimmunol.181.4.2321. [DOI] [PubMed] [Google Scholar]
- 141.Oki S, Tomi C, Yamamura T, Miyake S. Preferential T(h)2 polarization by OCH is supported by incompetent NKT cell induction of CD40L and following production of inflammatory cytokines by bystander cells in vivo. Int. Immunol. 2005;17:1619–1629. doi: 10.1093/intimm/dxh342. [DOI] [PubMed] [Google Scholar]
- 142.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 143.Wiethe C, Schiemann M, Busch D, et al. Interdependency of MHC class II/self-peptide and CD1d/self-glycolipid presentation by TNF-matured dendritic cells for protection from autoimmunity. J. Immunol. 2007;178:4908–4916. doi: 10.4049/jimmunol.178.8.4908. [DOI] [PubMed] [Google Scholar]
- 144.Brandl C, Ortler S, Herrmann T, Cardell S, Lutz MB, Wiendl H. B7-H1-deficiency enhances the potential of tolerogenic dendritic cells by activating CD1d-restricted Type II NKT cells. PLoS ONE. 2010;5:E10800. doi: 10.1371/journal.pone.0010800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Monteiro M, Almeida CF, Caridade M, et al. Identification of regulatory Foxp3+ invariant NKT cells induced by TGF-β. J. Immunol. 2010;185:2157–2163. doi: 10.4049/jimmunol.1000359. [DOI] [PubMed] [Google Scholar]
- 146.Terabe M, Berzofsky JA. NKT cells in immunoregulation of tumor immunity: a new immunoregulatory axis. Trends Immunol. 2007;28:491–496. doi: 10.1016/j.it.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 147.Giaccone G, Punt CJ, Ando Y, et al. A Phase I study of the natural killer T-cell ligand α-galactosylceramide (KRN7000) in patients with solid tumors. Clin. Cancer Res. 2002;8:3702–3709. [PubMed] [Google Scholar]
- 148.Veldt BJ, van der Vliet HJ, von Blomberg BM, et al. Randomized placebo controlled phase I/II trial of α-galactosylceramide for the treatment of chronic hepatitis. C. J. Hepatol. 2007;47:356–365. doi: 10.1016/j.jhep.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 149.Woltman AM, Ter Borg MJ, Binda RS, et al. α-galactosylceramide in chronic hepatitis B infection: results from a randomized placebo-controlled Phase I/II trial. Antivir. Ther. 2009;14:809–818. doi: 10.3851/IMP1295. [DOI] [PubMed] [Google Scholar]
- 150.Matsuda JL, Naidenko OV, Gapin L, et al. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 2000;192:741–754. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Crough T, Purdie DM, Okai M, Maksoud A, Nieda M, Nicol AJ. Modulation of human Vα24(+)Vβ11(+) NKT cells by age, malignancy and conventional anticancer therapies. Br. J. Cancer. 2004;91:1880–1886. doi: 10.1038/sj.bjc.6602218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kawano T, Nakayama T, Kamada N, et al. Antitumor cytotoxicity mediated by ligand-activated human V α24 NKT cells. Cancer Res. 1999;59:5102–5105. [PubMed] [Google Scholar]
- 153.Motohashi S, Kobayashi S, Ito T, et al. Preserved IFN-α production of circulating Vα24 NKT cells in primary lung cancer patients. Int. J. Cancer. 2002;102:159–165. doi: 10.1002/ijc.10678. [DOI] [PubMed] [Google Scholar]
- 154.Tahir SM, Cheng O, Shaulov A, et al. Loss of IFN-γ production by invariant NK T cells in advanced cancer. J. Immunol. 2001;167:4046–4050. doi: 10.4049/jimmunol.167.7.4046. [DOI] [PubMed] [Google Scholar]
- 155.Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-γ-producing NKT response induced with α-galactosylceramide-loaded DCs. Nat. Immunol. 2002;3:867–874. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- 156.Ishikawa A, Motohashi S, Ishikawa E, et al. A Phase I study of α-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin. Cancer Res. 2005;11:1910–1917. doi: 10.1158/1078-0432.CCR-04-1453. [DOI] [PubMed] [Google Scholar]
- 157.Nieda M, Okai M, Tazbirkova A, et al. Therapeutic activation of Vα24+Vβ11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood. 2004;103:383–389. doi: 10.1182/blood-2003-04-1155. [DOI] [PubMed] [Google Scholar]
- 158.Uchida T, Horiguchi S, Tanaka Y, et al. Phase I study of α-galactosylceramide-pulsed antigen presenting cells administration to the nasal submucosa in unresectable or recurrent head and neck cancer. Cancer Immunol. Immunother. 2008;57:337–345. doi: 10.1007/s00262-007-0373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Petersen TR, Sika-Paotonu D, Knight DA, et al. Potent anti-tumor responses to immunization with dendritic cells loaded with tumor tissue and an NKT cell ligand. Immunol. Cell Biol. 2010;88:596–604. doi: 10.1038/icb.2010.9. [DOI] [PubMed] [Google Scholar]
- 160.Toura I, Kawano T, Akutsu Y, Nakayama T, Ochiai T, Taniguchi M. Cutting edge: inhibition of experimental tumor metastasis by dendritic cells pulsed with α-galactosylceramide. J. Immunol. 1999;163:2387–2391. [PubMed] [Google Scholar]
- 161.Dougan SK, Kaser A, Blumberg RS. CD1 expression on antigen-presenting cells. Curr. Top. Microbiol. Immunol. 2007;314:113–141. doi: 10.1007/978-3-540-69511-0_5. [DOI] [PubMed] [Google Scholar]
- 162.Skold M, Xiong X, Illarionov PA, Besra GS, Behar SM. Interplay of cytokines and microbial signals in regulation of CD1d expression and NKT cell activation. J. Immunol. 2005;175:3584–3593. doi: 10.4049/jimmunol.175.6.3584. [DOI] [PubMed] [Google Scholar]
- 163.Fujii S, Shimizu K, Steinman RM, Dhodapkar MV. Detection and activation of human Vα24+ natural killer T cells using α-galactosyl ceramide-pulsed dendritic cells. J. Immunol. Methods. 2003;272:147–159. doi: 10.1016/s0022-1759(02)00497-0. [DOI] [PubMed] [Google Scholar]
- 164.Chang DH, Osman K, Connolly J, et al. Sustained expansion of NKT cells and antigen-specific T cells after injection of α-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J. Exp. Med. 2005;201:1503–1517. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Smyth MJ, Thia KY, Street SE, et al. Differential tumor surveillance by natural killer (NK) and NKT cells. J. Exp. Med. 2000;191:661–668. doi: 10.1084/jem.191.4.661. ▪ This study was the first to indentify the natural ability of NKT cells to provide immunosurveillance of tumors.
- 166.Crowe NY, Smyth MJ, Godfrey DI. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J. Exp. Med. 2002;196:119–127. doi: 10.1084/jem.20020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Subleski JJ, Hall VL, Back TC, Ortaldo JR, Wiltrout RH. Enhanced antitumor response by divergent modulation of natural killer and natural killer T cells in the liver. Cancer Res. 2006;66:11005–11012. doi: 10.1158/0008-5472.CAN-06-0811. [DOI] [PubMed] [Google Scholar]
- 168.Terabe M, Matsui S, Park JM, et al. Transforming growth factor-β production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J. Exp. Med. 2003;198:1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Terabe M, Swann J, Ambrosino E, et al. A nonclassical non-Vα14Jα18 CD1d-restricted (type II) NKT cell is sufficient for downregulation of tumor immunosurveillance. J. Exp. Med. 2005;202:1627–1633. doi: 10.1084/jem.20051381. ▪ Along with reference [170] uncovered a new immunoregulatory axis where variant NKT (vNKT) cells can inhibit the antitumor activity of iNKT cells and CD8+ T cells.
- 170. Ambrosino E, Terabe M, Halder RC, et al. Cross-regulation between type I and type II NKT cells in regulating tumor immunity: a new immunoregulatory axis. J. Immunol. 2007;179:5126–5136. doi: 10.4049/jimmunol.179.8.5126. ▪ Along with reference [169] uncovered a new immunoregulatory axis where vNKT cells can inhibit the antitumor activity of iNKT cells and CD8+ T cells
- 171.Swann JB, Uldrich AP, van Dommelen S, et al. type I natural killer T cells suppress tumors caused by p53 loss in mice. Blood. 2009;113:6382–6385. doi: 10.1182/blood-2009-01-198564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chen X, Oppenheim JJ. TNF-α: an activator of CD4+FoxP3+TNFR2+ regulatory T cells. Curr. Dir. Autoimmun. 2010;11:119–134. doi: 10.1159/000289201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Maloy KJ, Powrie F. Fueling regulation: IL-2 keeps CD4+ Treg cells fit. Nat. Immunol. 2005;6:1071–1072. doi: 10.1038/ni1105-1071. [DOI] [PubMed] [Google Scholar]
- 174.Crowe NY, Coquet JM, Berzins SP, et al. Differential antitumor immunity mediated by NKT cell subsets in vivo. J. Exp. Med. 2005;202:1279–1288. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu TY, Uemura Y, Suzuki M, et al. Distinct subsets of human invariant NKT cells differentially regulate T helper responses via dendritic cells. Eur. J. Immunol. 2008;38:1012–1023. doi: 10.1002/eji.200737838. [DOI] [PubMed] [Google Scholar]
- 176.Park JE, Wu DY, Prendes M, et al. Fine specificity of natural killer T cells against GD3 ganglioside and identification of GM3 as an inhibitory natural killer T-cell ligand. Immunology. 2008;123:145–155. doi: 10.1111/j.1365-2567.2007.02760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sriram V, Cho S, Li P, et al. Inhibition of glycolipid shedding rescues recognition of a CD1+ T cell lymphoma by natural killer T (NKT) cells. Proc. Natl Acad. Sci. USA. 2002;99:8197–8202. doi: 10.1073/pnas.122636199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 179.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 180.Jackson SK, Abate W, Tonks AJ. Lysophospholipid acyltransferases: novel potential regulators of the inflammatory response and target for new drug discovery. Pharmacol. Ther. 2008;119:104–114. doi: 10.1016/j.pharmthera.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 181.Bell JK. The TOLL of inflammation in multiple myeloma. Cancer Biol. Ther. 2011;11:68–70. doi: 10.4161/cbt.11.1.14153. [DOI] [PubMed] [Google Scholar]
- 182.Mantovani A, Garlanda C. Inflammation and multiple myeloma: the Toll connection. Leukemia. 2006;20:937–938. doi: 10.1038/sj.leu.2404229. [DOI] [PubMed] [Google Scholar]
- 183. Chang DH, Deng H, Matthews P, et al. Inflammation-associated lysophospholipids as ligands for CD1d-restricted T cells in human cancer. Blood. 2008;112:1308–1316. doi: 10.1182/blood-2008-04-149831. ▪ This report provided the first evidence that lipids are increased in a pathological cancer setting, and that NKT cells can respond to inflammatory lipids.
- 184.Bataille R, Harousseau JL. Multiple myeloma. N. Engl. J. Med. 1997;336:1657–1664. doi: 10.1056/NEJM199706053362307. [DOI] [PubMed] [Google Scholar]
- 185.Wynn TA. IL-13 effector functions. Annu. Rev. Immunol. 2003;21:425–456. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 186. Dhodapkar MV, Geller MD, Chang DH, et al. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J. Exp. Med. 2003;197:1667–1676. doi: 10.1084/jem.20021650. ▪ This report offers evidence that the vNKT cells can inhibit the activation of iNKT cells, and that this immunoregulatory axis might also exist in cancer patients.
- 187.Bradbury MW. Lipid metabolism liver inflammation. I Hepatic fatty acid uptake: possible role in steatosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G194–G198. doi: 10.1152/ajpgi.00413.2005. [DOI] [PubMed] [Google Scholar]
- 188.Jiang JT, Xu N, Zhang XY, Wu CP. Lipids changes in liver cancer. J. Zhejiang Univ. Sci. B. 2007;8:398–409. doi: 10.1631/jzus.2007.B0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Reddy JK, Rao MS. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G852–G858. doi: 10.1152/ajpgi.00521.2005. [DOI] [PubMed] [Google Scholar]
- 190.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 191.Abrams GA, Kunde SS, Lazenby AJ, Clements RH. Portal fibrosis and hepatic steatosis in morbidly obese subjects: a spectrum of nonalcoholic fatty liver disease. Hepatology. 2004;40:475–483. doi: 10.1002/hep.20323. [DOI] [PubMed] [Google Scholar]
- 192.Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36:1349–1354. doi: 10.1053/jhep.2002.36939. [DOI] [PubMed] [Google Scholar]
- 193.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 194.Hua J, Ma X, Webb T, Potter JJ, Oelke M, Li Z. Dietary fatty acids modulate antigen presentation to hepatic NKT cells in nonalcoholic fatty liver disease. J. Lipid Res. 2010;51:1696–1703. doi: 10.1194/jlr.M003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Guebre-Xabier M, Yang S, Lin HZ, Lin HZ, Schwenk R, Krzych U, Diehl AM. Altered hepatic lymphocyte subpopulations in obesity-related murine fatty livers: potential mechanism for sensitization to liver damage. Hepatology. 2000;31:633–640. doi: 10.1002/hep.510310313. [DOI] [PubMed] [Google Scholar]
- 196.Kremer M, Hines IN, Milton RJ, Wheeler MD. Favored T helper 1 response in a mouse model of hepatosteatosis is associated with enhanced T cell-mediated hepatitis. Hepatology. 2006;44:216–227. doi: 10.1002/hep.21221. [DOI] [PubMed] [Google Scholar]
- 197.Li Z, Soloski MJ, Diehl AM. Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology. 2005;42:880–885. doi: 10.1002/hep.20826. [DOI] [PubMed] [Google Scholar]
- 198.Eberl G, MacDonald HR. Rapid death and regeneration of NKT cells in anti-CD3ε- or IL-12-treated mice: a major role for bone marrow in NKT cell homeostasis. Immunity. 1998;9:345–353. doi: 10.1016/s1074-7613(00)80617-2. [DOI] [PubMed] [Google Scholar]
- 199.Kremer M, Thomas E, Milton RJ, et al. Kupffer cell and interleukin-12-dependent loss of natural killer T cells in hepatosteatosis. Hepatology. 2010;51:130–141. doi: 10.1002/hep.23292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li Z, Lin H, Yang S, Diehl AM. Murine leptin deficiency alters Kupffer cell production of cytokines that regulate the innate immune system. Gastroenterology. 2002;123:1304–1310. doi: 10.1053/gast.2002.35997. [DOI] [PubMed] [Google Scholar]
- 201.Elinav E, Pappo O, Sklair-Levy M, et al. Adoptive transfer of regulatory NKT lymphocytes ameliorates non-alcoholic steatohepatitis and glucose intolerance in ob/ob mice and is associated with intrahepatic CD8 trapping. J. Pathol. 2006;209:121–128. doi: 10.1002/path.1950. [DOI] [PubMed] [Google Scholar]
- 202.Ohmura K, Ishimori N, Ohmura Y, et al. Natural killer T cells are involved in adipose tissues inflammation and glucose intolerance in diet-induced obese mice. Arterioscler. Thromb. Vasc. Biol. 2010;30:193–199. doi: 10.1161/ATVBAHA.109.198614. [DOI] [PubMed] [Google Scholar]
- 203.Xu CF, Yu CH, Li YM, et al. Association of the frequency of peripheral natural killer T cells with nonalcoholic fatty liver disease. World J. Gastroenterol. 2007;13:4504–4508. doi: 10.3748/wjg.v13.i33.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Syn WK, Oo YH, Pereira TA, Xu L, Du J, Shen Z. Accumulation of natural killer T cells in progressive nonalcoholic fatty liver disease. Hepatology. 2010;51:1998–2007. doi: 10.1002/hep.23599. ▪ This important study determined the role of NKT cells in patients with nonalcoholic fatty liver disease.
- 205.Tajiri K, Shimizu Y, Tsuneyama K, Sugiyama T. Role of liver-infiltrating CD3+CD56+ natural killer T cells in the pathogenesis of nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2009;21:673–680. doi: 10.1097/MEG.0b013e32831bc3d6. [DOI] [PubMed] [Google Scholar]
- 206.Weiss JM, Back TC, Scarzello AJ, et al. Successful immunotherapy with IL-2/anti-CD40 induces the chemokine-mediated mitigation of an immunosuppressive tumor microenvironment. Proc. Natl Acad. Sci. USA. 2009;106:19455–19460. doi: 10.1073/pnas.0909474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Subleski JJ, Hall VL, Wolfe TB, et al. TCR-dependent and -independent activation underlie liver-specific regulation of NKT cells. J. Immunol. 2011;186:838–847. doi: 10.4049/jimmunol.1001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bricard G, Cesson V, Devevre E, et al. Enrichment of human CD4+ V(α)24/Vβ11 invariant NKT cells in intrahepatic malignant tumors. J. Immunol. 2009;182:5140–5151. doi: 10.4049/jimmunol.0711086. [DOI] [PubMed] [Google Scholar]
- 209.Liu Y, Teige A, Mondoc E, Ibrahim S, Holmdahl R, Issazadeh-Navikas S. Endogenous collagen peptide activation of CD1d-restricted NKT cells ameliorates tissue-specific inflammation in. mice. J. Clin. Invest. 2011;121:249–264. doi: 10.1172/JCI43964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Araujo LM, Chauvineau A, Zhu R, et al. Cutting edge: intravenous Ig inhibits invariant NKT cell-mediated allergic airway inflammation through Fc{γ}RIIIA-dependent mechanisms. J. Immunol. 2011;186:3289–3293. doi: 10.4049/jimmunol.1003076. [DOI] [PubMed] [Google Scholar]
- 211.Parekh VV, Wilson MT, Olivares-Villagomez D, et al. Glycolipidantigen induces long-term natural killer T cell anergy in mice. J. Clin. Invest. 2005;115:2572–2583. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]