Abstract
Secondary pulmonary hypertension (PH) is emerging as one of the leading causes of mortality and morbidity in patients with hemolytic anemias such as sickle cell disease (SCD) and thalassemia. Impaired nitric oxide (NO) bioavailability represents the central feature of endothelial dysfunction, and is a major factor in the pathophysiology of PH. Inactivation of NO correlates with hemolytic rate and is associated with the erythrocyte release of cell-free hemoglobin, which consumes NO directly, and the simultaneous release of the arginine-metabolizing enzyme arginase, which limits bioavailability of the NO synthase substrate arginine during the process of intravascular hemolysis. Rapid consumption of NO is accelerated by oxygen radicals that exists in both SCD and thalassemia. A dysregulation of arginine metabolism contributes to endothelial dysfunction and PH in SCD, and is strongly associated with prospective patient mortality. The central mechanism responsible for this metabolic disorder is enhanced arginine turnover, occurring secondary to enhanced plasma arginase activity. This is consistent with a growing appreciation of the role of excessive arginase activity in human diseases, including asthma and pulmonary arterial hypertension. New treatments aimed at improving arginine and NO bioavailability through arginase inhibition, suppression of hemolytic rate, oral arginine supplementation, or use of NO donors represent potential therapeutic strategies for this common pulmonary complication of hemolytic disorders.
Keywords: Pulmonary hypertension, sickle cell disease, thalassemia, hemoglobinopathies, arginine, nitric oxide, arginase, hemolysis
Introduction
Sickle cell disease (SCD) is an inherited hemoglobin disorder that affects more than 70,000 primarily African Americans in the US, and millions more worldwide. The clinical phenotype varies widely, and is characterized by anemia, severe pain, and potentially life-threatening complications such as bacterial sepsis, splenic sequestration, acute chest syndrome, stroke and chronic organ damage. These and other manifestations result from chronic hemolysis and intermittent episodes of vascular occlusion that cause tissue injury and organ dysfunction [1, 2]. Genetically, SCD is caused by an amino acid substitution of valine for glutamic acid in the sixth position of the beta subunits of hemoglobin. This structural change results in intracellular polymerization under hypoxic conditions that injures and ultimately distorts the erythrocyte membrane, causing hemolytic anemia. Intracellular hemoglobin S polymerization also creates a rigid “sickled” red cell that blocks the microcirculation and gives rise to the vaso-occlusive nature of the disease [1].
Although polymerization is the primary event in its pathophysiology, SCD also affects vascular function [3]. In SCD, increased expression of adhesion molecules on erythrocytes and endothelial cells occurs, which causes young sickle red cells to adhere to the vascular endothelial cells. Interactions with leukocytes, increased levels of circulating inflammatory cytokines, reduced blood flow from increased viscosity and vascoconstriction, hemostatic activation, and endothelial damage are all thought to contribute to obstruction of the microvasculature by sickled erythrocytes [4, 5]. Ultimately these red cell changes initiate a cascade of events that results in episodic vaso-occlusion and subsequent ischemia-reperfusion injury, leading to clinical sequelae.
The thalassemia syndromes are a heterogeneous group of inherited hemoglobin disorders resulting from unbalanced production of the alpha and beta globin subunits of the hemoglobin tetramer. Thalassemia is most common in individuals whose ancestors originated from the Mediterranean region, Africa, southern China, Southeast Asia, and India. An estimated 900,000 births of clinically significant thalassemia disorders are expected in the next 20 years [6]. The clinical spectrum is a consequence of chronic hemolytic anemia and imbalanced globin chain accumulation [7]. The two clinically significant categories of beta-thalassemia have been designated thalassemia major and thalassemia intermedia. Thalassemia major is characterized by severe anemia starting during the first year of life and requiring life-long transfusion therapy for survival, while thalassemia intermedia has a later clinical onset with a milder anemia, does not typically require chronic transfusions, and offers a longer life expectancy [8].
Intravascular or intramedullary hemolysis, chronic anemia, and a high frequency of pulmonary hypertension (PH) are common features of both SCD and thalassemia. There is growing support for a new model of hemolysis-associated endothelial dysfunction and PH [9–16] that has important implications for these specific hemoglobinopathies. Hemolytic rate is also associated with a growing list of clinical complications of SCD, including multi-organ failure [17], priapism [13, 18], leg ulcers [13, 18, 19], and stroke [20], in addition to PH and mortality and constitute what is now considered the hemolytic subphenotypes of SCD [16]. Rapid consumption of NO by cell-free hemoglobin [21] coupled with the simultaneous release of erythrocyte arginase during hemolysis [22] is a fundamental aspect of this model in PH, and is the focus of this review.
Pulmonary Hypertension
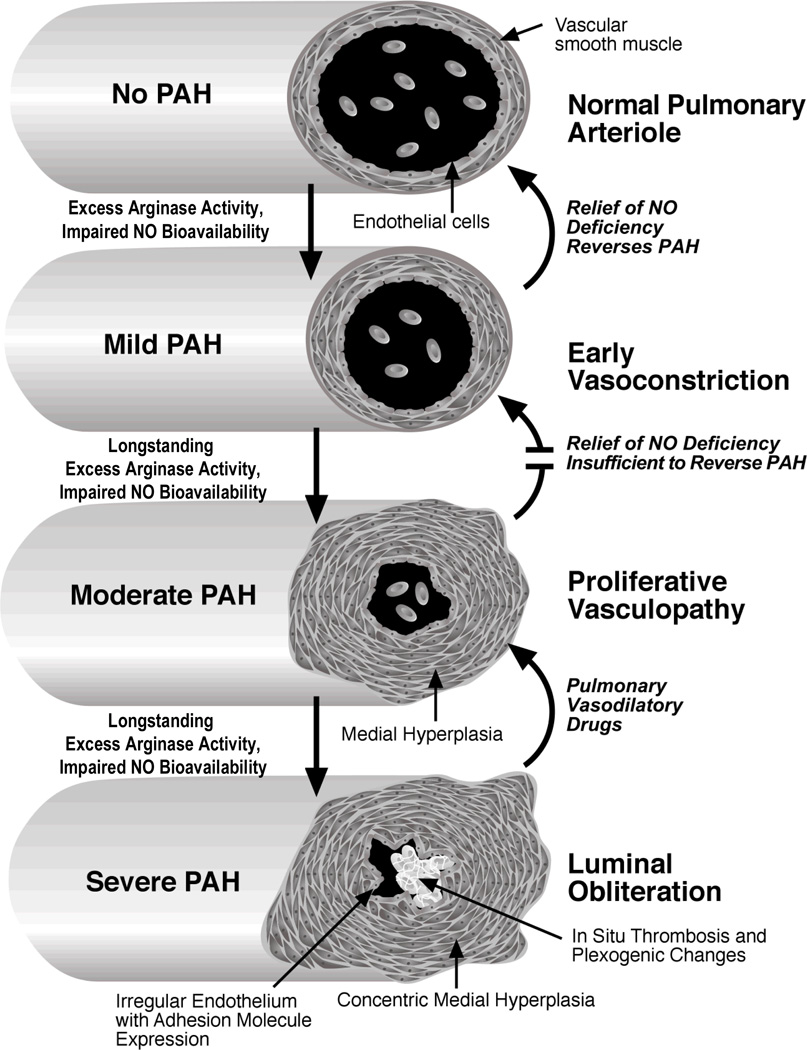
PH is a vascular disorder of the lung in which the pulmonary artery pressure rises above normal levels, compromises oxygenation and right-heart function, and can ultimately become life-threatening [23]. PH is defined as a mean pulmonary artery pressure of ≥ 25 mmHg at rest or ≥ 30 mmHg during exercise, and can result from a wide range of conditions [24]. PH is a poorly understood, complex syndrome with many clinical phenotypes. The initial injury leading to pulmonary artery hypertension in different disease states may vary; however, they share a common pathway of vascular remodeling that results in a similar clinical and histopathologic condition. Histopathological findings of autopsy studies in both SCD and thalassemia include plexiform and concentric medial hyperplastic pulmonary vascular lesions, and in situ pulmonary artery thrombosis [25–30], which are common to all forms of PH. PH in SCD and thalassemia is associated with vasoconstriction, vascular smooth muscle proliferation, and irregular endothelium in pulmonary arteries with associated thrombosis. These conditions all contribute to luminal narrowing, and eventual right ventricular failure [31] “Fig (1)”.
Figure 1. Progression of pulmonary hypertension in sickle cell disease and thalassemia.
In this hypothetical model, impaired NO bioavailability that results from chronic hemolysis and oxidative stress triggers chronic pulmonary vasoconstriction, mildly elevating pulmonary vascular resistance and pulmonary artery pressures. Excess arginase liberated from the erythrocyte during hemolysis consumes arginine, the obligate substrate for NO production, and creates a shift towards ornithine production that contributes to collagen deposition and vascular smooth muscle proliferation. Overabundant thrombin generation contributes to a chronic hypercoagulable state, increases arginase activity, and stimulates polyamine synthesis in vascular smooth muscle cells. As this becomes more long-standing, vascular smooth muscle hyperplasia begins to create a relatively fixed lesion, compounded in later stages by irregular, activated endothelium with expression of adhesion molecules. In situ thrombosis further occludes the vessel lumen, and results in plexogenic changes, further accelerating the progression of the pulmonary artery hypertension. (Modified Figure reproduced with permission [31]).
The responsiveness of PH to therapy will likely depend in large part upon the severity of vascular remodeling, hence early identification and treatment of PH before irreversible changes have occurred is crucial. Recent investigations suggest that endothelial dysfunction is key to the pathogenesis of pulmonary artery hypertension [32]. Because impaired nitric oxide (NO) bioavailability is a central feature of endothelial dysfunction [33], NO bioavailability represents an important target for therapy.
NO is one of the most potent vasodilators known [34] and is essential to vascular homeostasis. It plays an important role in the maintenance of vasomotor tone, limits platelet aggregation and ischemia-reperfusion injury, modulates endothelial proliferation, and possesses anti-inflammatory properties. Arginine is the precursor to NO, which is catalyzed by a family of enzymes, the NO synthases. NO causes vasodilation by activating soluble guanylate cyclase to produce the intracellular messenger, cyclic guanylate monophosphate (cGMP) [35]. Increased consumption and decreased production of both NO and arginine appear to contribute to the complications associated with PH.
Pulmonary Hypertension in Sickle Cell Disease
Pulmonary complications, which are common in SCD [36], compromise oxygenation and contribute to a vicious cycle of erythrocyte sickling. PH occurs frequently in both adults [9, 22, 37–40] and children [41, 42] with SCD. In the adult SCD population, PH occurs in >30% of patients [9], and it has been identified as a common cause of death on autopsy [26, 27, 43–45]. A Doppler echocardiogram measured tricuspid regurgitant jet velocity (TRV) of 2.5 m/sec or greater suggesting PH is currently the strongest predictor for early death in SCD, with approximately 10-fold increased risk of early mortality [9, 46, 47], and a 40% mortality risk within 3 years of diagnosis [9]. A TRV ≥ 2.5 m/sec is 2 standard deviations greater than normal.Ten percent of adult patients with SCD have a TRV ≥ 3 m/sec and of these most have mean pulmonary artery pressures greater than 25 mm Hg on right heart catheterization [48].
It remains to be determined whether this association reflects a causal relationship between PH per se and mortality, or whether PH represents a surrogate for disease severity. It has been speculated that the high risk of sudden death associated with SCD may be linked to PH. Surprisingly, despite a high risk of mortality associated with PH in SCD, it is estimated that only 10 percent of patients are monitored for pulmonary disease according to the preliminary recommendations of investigators at the National Institute of Health [40]. Even when there is monitoring, the diagnosis is often unrecognized, even with Doppler echocardiography evidence of PH [49]. This clinical misinterpretation is likely related to pulmonary pressures provoking symptoms at lower values in patients with SCD, compared to levels typically associated with PH in non-hemolytic conditions. In addition, acute elevations in pulmonary pressures occur during cardiopulmonary stressors such as pain crisis, exercise [50], and acute chest syndrome [51] and may be contributing to morbidity and mortality. Thus, universal echo screening of adult patients with SCD to identify a high-risk group who, despite the apparently mild elevation of pulmonary artery pressures [52], may be responsive to intervention has been recommended [9].
Because patients with SCD and PH are frequently asymptomatic with mildly elevated TRV [9], cases of PH are often identified only late in the course of the disease, after symptoms have developed that arouse clinical suspicion. Elevated TRV is also a common finding in children. Early studies demonstrated a prevalence similar to adults [31, 41, 42, 49, 53], although the long-term implications of this finding in the younger patients are unknown. The average age of pediatric patients with SCD with PH receiving care at the Northern California Comprehensive Sickle Cell Center is 12.7 years, but children as young as 7 years of age exhibited echocardiogram abnormalities suggestive of PH [49]. Therefore, screening recommendations should target children as well as adults.
In one study at Children’s Hospital & Research Center Oakland, children with PH had a different clinical profile of complications than adults with PH [49] (Table 1), indicating that other correlates in children may be contributing to the cause of their PH. For children, a history of sepsis, acute chest syndrome (ACS), or asthma was associated with PH, possibly reflecting distinct aspects of endothelial dysfunction. These factors are quite distinct from the high-risk profile of end-organ damage and chronic transfusion in adults associated with PH [49].
Table 1.
Clinical Variables Associated with Pulmonary Hypertension in SCD [49]
| Adult Variables |
| Age |
| Renal Abnormality |
| Hepatitis C |
| Oxygen Use |
| Chronic transfusions |
| History of Acute Chest |
| Syndrome* |
| Pediatric Variables |
| Asthma or Obstructive Disease |
| History of Sepsis/Bacteremia |
| History of Acute Chest Syndrome |
protective odds ratio in Adults only
Asthma [54–57], sepsis [58–60] and ACS [61] are all conditions associated with arginine dysregulation and low NO bioavailability, a shared mechanism with hemolysis-associated PH [22, 62, 63]. Sepsis remained independently associated with PH in logistic regression analysis of these 3 significant univariate variables, with the history of asthma approaching significance [49]. Recently, sepsis has also been identified as a significant risk factor for death within five years in a neural network exploration of severity of illness in SCD [64]. It is interesting to note that in this population, a history of ACS was a risk factor for PH in children, while it was protective in adults [49], possibly reflecting the consequences of therapy intensification, including hydroxyurea use and chronic transfusion that often occurs in patients with a history of chest syndrome at our institution. A better understanding of these differing phenotypes may provide insight into mechanisms contributing to the development of PH.
Although the pathogenesis of PH in SCD is multifactorial, it is strongly linked to the intensity of hemolysis [9, 13, 16]. Increased platelet activation, implicated as another important mechanism in PH [65], likely contributes to the hypercoagulable state commonly found in patients with hemoglobinopathies [66–68], and correlates strongly with both PH severity and biomarkers of hemolysis in SCD [69]. We have found that soluble VCAM-1 is a marker of endothelial dysfunction linked to hemolytic rate [17], and that lactate dehydrogenase levels reflect hemolysis-associated endothelial dysfunction and severity of PH [13]. Plasma arginine:ornithine ratio, a reflection of arginase activity [22, 63], and brain natriuretic factor [70] are additional biomarkers of PH associated with mortality in SCD that could provide clinicians with important prognostic and diagnostic information [22, 70]. It is likely that biomarkers of hemolytic rate, either independently or in combination, may help identify a high risk sub-population in need for more aggressive interventions [9].
Pulmonary Hypertension in Thalassemia
A growing body of data suggests that thalassemia has many biologic and epidemiologic factors in common with SCD, including chronic hypoxia, long term effect of splenectomy, red cell membrane pathology [71–75], coagulation abnormalities [67, 68, 74], platelet activation [67, 68, 74], oxidative stress [76], iron overload [77–82] and chronic hemolysis [40].
Earlier studies in both thalassemia intermediate and major demonstrate that adults frequently have undetected PH, with a prevalence of 60–75% reported [79, 83–86], and a growing body of literature suggests that asymptomatic PH is a leading factor in heart failure and death in thalassemia [83]. In a study of thalassemia major, pulmonary systolic pressure above 30 mm/Hg was found in all patients over 22 years [84]. Recent PH studies in more uniformly treated thalassemia major and intermedia patients have shown a lower frequency of increased pulmonary pressure in chronically transfused thalassemia major patients [86], while in thalassemia intermedia patients, increased pulmonary pressure was more frequently detected. In one study 60% of thalassemia intermedia patients had a pulmonary systolic pressure greater than 30 mm/Hg with preserved left ventricular systolic function [83] and another study showed a pulmonary systolic pressure greater than 35 mm/Hg in 23% of thalassemia patients [86] .
Analogous to SCD, hemolysis-associated PH is emerging as an important entity in thalassemia. [10, 62, 87]. Red cell destruction, elevated free plasma hemoglobin, anemia, and abnormal NO metabolism exist in both non-transfused and transfused patients [88]. A study of 202 TM patients concluded that strict compliance with chronic transfusion and chelation therapy to prevent iron overload reduces the occurrence of heart failure, and prevents PH [79, 86, 89]. Although more aggressive transfusion programs may provide greater protection from the development of PH, the occurrence of intramedullary hemolysis, platelet activation, iron deposits and a resultant vasculopathy may still be able to induce PH which likely progresses at a slower rate. Nevertheless, these findings strongly suggest a beneficial effect of regular transfusion in either prevention or slowing down the progression of PH in thalassemia [90].
Nitric Oxide Scavenging in Sickle Cell Disease
NO is a potent vasodilator [34], with properties that can impact many aspects of SCD: decreasing platelet activation [91, 92] and adhesion receptor expression on the vascular endothelium, decreasing vascular smooth muscle proliferation [34, 35, 93–95], limiting ischemia-reperfusion injury [96], modulating endothelial proliferation [3], and regulating inflammation [97]. Given the crucial role of NO depletion in endothelial dysfunction and PH due to other etiologies [98, 99], it is not surprising that NO depletion is associated with PH in SCD.
NO depletion has not been well studied in thalassemia [100], however given the overlap of hemolysis and oxidative stress in these hemoglobinopathies, alterations in NO bioavailability is inevitable. NO participates in the compensatory response to chronic vascular injury that occurs in SCD [61, 101, 102]. Although NO synthase expression and activity is increased [101], SCD is characterized by a state of NO resistance and increased NO inactivation [21, 102, 103]. Impaired NO bioavailability in this disorder is demonstrated by the blunted response to endothelium-dependent vasodilators in sickle cell mouse models [104, 105], as well as by a reduced flow-mediated vasodilation in human patients with SCD [102, 106, 107]. NO metabolites are elevated in patients with SCD at steady-state compared to normal controls [108], while peripheral vascular resistance [109] and resting blood pressures are low [110, 111]. Adding to this paradox, a “relative hypertension” occurs in SCD as blood pressures, while lower than in normal subjects, are higher in some patients [112, 113]. Mortality in SCD is associated with relative systolic hypertension in both male patients [113] and in patients with PH [9, 114].
Under conditions of increased hemolysis, inflammation and/or oxidative stress, the compensatory upregulation of NO likely is inadequate. Indeed, levels of NO metabolites are low during vaso-occlusive crisis [61, 115] and ACS [61], varying inversely with the degree of pain [116]. The rate of NO inactivation correlates with the hemolytic rate, and is associated with the release of cell-free hemoglobin [21]. Superoxide, already increased in SCD [117] and augmented further by circulating xanthine oxidase [104], consumes NO to form the potent oxidant, peroxynitrite [103, 118], further limiting NO bioavailability and possibly inducing cellular injury. This, in turn, could lead to increased arginine metabolism in a compensatory attempt to produce more NO, thereby depleting arginine stores further during periods of heightened oxidative stress or hemolysis.
Finally, NO bioavailability is limited by the hemolytic process itself. As hemoglobin is liberated into plasma during hemolysis, it reacts with and destroys NO, resulting in abnormally high NO consumption and the formation of reactive oxygen species [10, 21]. Consequently, smooth muscle guanylate cyclase is not activated and vasodilation is inhibited. Reduced NO bioavailability can also cause further impairment in vascular endothelial function via transcription depression of adhesion molecules, including VCAM-1 and E-selectin, and vasoconstrictor/growth factors such as endothelin-1 [10]. The simultaneous release of erythrocyte arginase during hemolysis [22] will limit the availability of arginine to the NO synthases, further enhancing the deficiency of NO.
Novel Role of Arginase in Pulmonary Disease
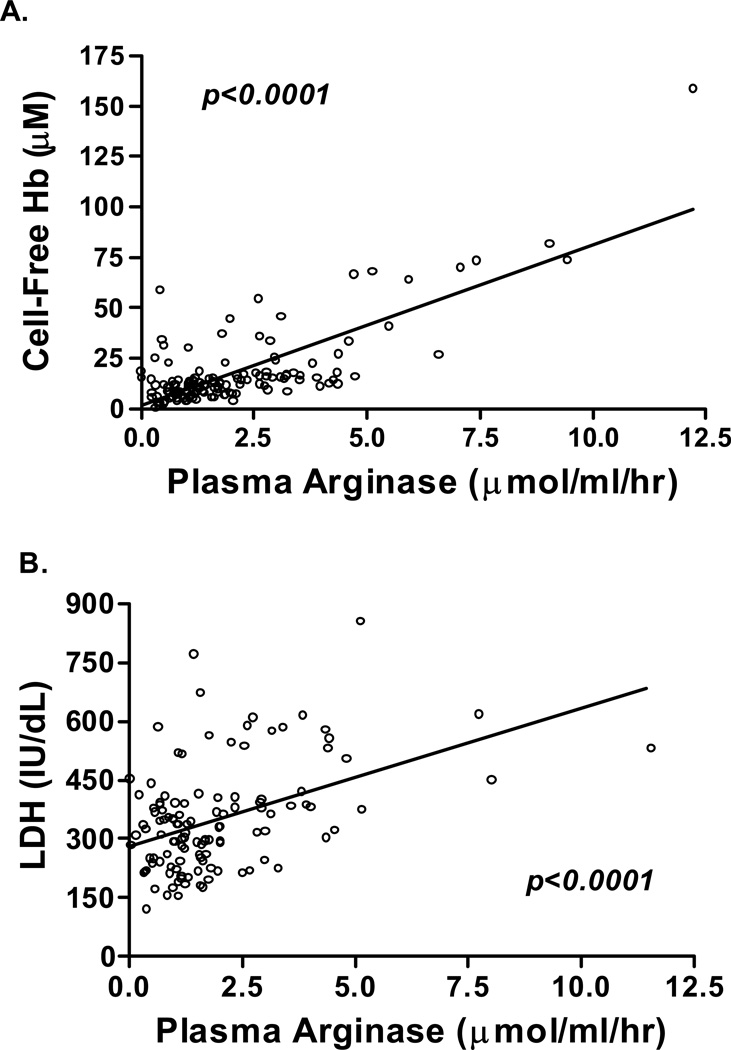
Although NO has long been implicated in endothelial dysfunction, the role of elevated arginase activity in the pathogenesis of PH and other pulmonary conditions has only been recently discovered [22, 55, 62, 63, 119–123]. Arginase is an essential enzyme in the urea cycle, responsible for the conversion of arginine to ornithine and urea [124]. Found predominantly in liver and kidneys [125], arginase is also found in the red blood cells of humans and other primates [126, 127], making it an intriguing enzyme to study in hemolytic disorders. Plasma arginase activity is elevated in SCD as a consequence of inflammation, liver dysfunction and, most significantly, by the release of erythrocyte arginase during intravascular hemolysis, which has been demonstrated by the strong correlation between plasma arginase leves and cell-free hemoglobin levels and other markers of increased hemolytic rate “Fig (2)” [22].
Figure 2. Association of arginase activity with hemolytic rate.
Correlation of plasma arginase activity (µmol/ml/hr) to A.cell-free hemoglobin (Cell-Free Hb, n=138, p< 0 .001) (Reproduced from Morris et al. [22], with permission from the American Medical Association) and B. serum lactate dehydrogenase levels (LDH, n=121, p<0.001) in patients with sickle cell disease.
Erythrocyte arginase release during hemolysis will alter normal arginine metabolism and is associated with the development of pulmonary complications [22]. Once released into circulation, arginase will convert arginine to ornithine, which is the precursor to proline. Proline levels are elevated in thalassemia [62] and SCD [128, 129]. Proline is an amino acid involved in collagen formation [130], lung fibrosis, airway remodeling and vascular smooth muscle proliferation [22, 121, 131], all of which are common features of pulmonary dysfunction in thalassemia [81, 132] and SCD [36, 49]. A low arginine:ornithine ratio is associated with increase mortality in SCD [22], and severity of PH of various etiologies [122]. By creating a shift towards ornithine metabolism, arginase triggers a proliferative pathway that may contribute to the clinical scenario of PH, asthma and lung fibrosis. Analogous to its proposed roles in asthma [133, 134], elevated proline levels in thalassemia [62] and SCD [128, 129] may play a role in this pulmonary pathology. Like PH, asthma is another condition that occurs more frequently in SCD than the general population [135–138] that is associated with elevated arginase activity [55, 133, 134, 139] and an acute arginine [55] and NO deficiency [54]. Although asthma is often unrecognized by clinicians, it is treatable co-morbidity associated with PH in children with SCD [49], that is also commonly identified in primary pulmonary hypertension [140]. Recently, abnormal findings on pulmonary function testing that typically occur in asthma were found to be associated with severity of PH and markers of hemolysis in patients with SCD [53]. Asthma is known to cause hypoxemia that is associated with stroke [141], ACS [136, 141, 142], and mortality [143] in SCD. Therefore, asthma in SCD should be aggressively treated based on published NIH guidelines for the diagnosis and management of asthma [144, 145], and early consultation with a pulmonary specialist is advisable. It remains to be determined whether airway hyperresponsiveness is a contributing factor or a result of PH, however dysregulated arginine metabolism and excess production of proline and polyamines likely contribute to many forms of abnormal pulmonary function in SCD, with implications in thalassemia that warrant further evaluation.
Increased ornithine levels also may promote synthesis of polyamines, which are required for cell proliferation that occurs in vascular remodeling [133, 134]. Furthermore, since arginine and ornithine compete for the same transport system for cellular uptake (cationic amino acid transporter, CAT) [146, 147], a decrease in the arginine:ornithine ratio resulting from increased arginase activity [22, 63] could also impair arginine bioavailability for NO production, even when plasma arginine concentration appears sufficient . Altered arginase activity within the platelets of patients with SCD likely contributes to pathological platelet activation, and the hypercoagulopathy and vasculopathy in this disorder [148]. High thrombin generation is known to occur in both SCD [66, 149, 150] and thalassemia syndromes [68, 73], and contributes to a chronic hypercoaguable state. Thrombin itself increases arginase activity in human endothelial cells [151–153], and will stimulate vascular smooth muscle cell polyamine synthesis by increasing CAT and ornithine decarboxylase gene expression [154], thus playing a role in endothelial dysfunction. Additionally, Villagra and colleagues have recently demonstrated that increased platelet activation in SCD correlates strongly with both PH severity and biomarkers of hemolysis [69], while Ataga and colleagues found that measures of hemolytic rate correlated with indices of hypercoagulability [155]. A mechanistic model is emerging that links coagulation abnormalities to dysregulation of the arginine-NO pathway in PH that has important implications for hemolytic disorders.
Arginase activity is higher in the erythrocytes of patients with SCD compared to normal controls, and strongly correlates to plasma arginase activity [22]. Erythrocyte arginase activity is also elevated in thalassemia patients [156, 157]. In addition, arginase activity is higher in immature red blood cells and reticulocytes [127], compared to older cells. When these early cells are destroyed in the bone marrow, a high concentration of arginase will be released, contributing to arginine dysregulation. It is therefore likely that erythrocyte release of arginase during hemolysis will limit the availability of arginine to NO synthase, resulting in a deficiency of NO and dysregulation of arginine metabolism in thalassemia patients through a similar mechanism identified in SCD.
PH also develops in most hereditary and chronic hemolytic anemias in addition to SCD [10] and thalassemia [68, 79, 83–86, 88]; thus, erythrocyte arginase release during hemolysis may contribute to endothelial dysfunction and the pathogenesis of PH through impaired production of NO [9, 13, 22, 62, 121] and stimulation of vascular smooth muscle proliferation. [151–153] These observations support a novel mechanism of disease that links oxidative stress, chronic organ damage, hypercoagulopathy, and hemolytic rate to endothelial dysfunction and PH [9, 10, 13, 21, 62]. Arginase activity and alterations in arginine metabolic pathways have also recently been implicated in the pathophysiology of primary pulmonary hypertension and pulmonary artery hypertension associated with collagen vascular disease [122, 123], suggesting a common mechanism in the pathogenesis of otherwise distinct forms of PH.
Dysregulation of Arginine Metabolism in Sickle Cell Disease and Thalassemia
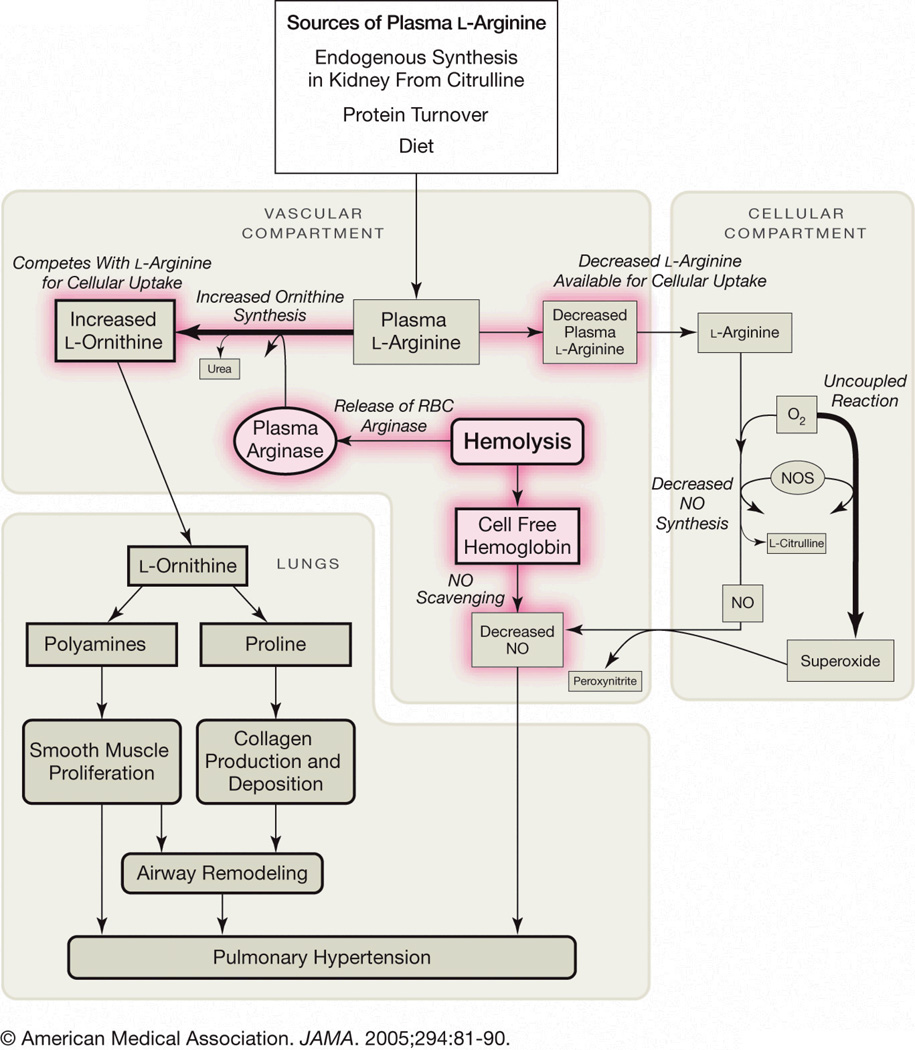
There is growing evidence that PH is a disease process that involves altered arginine metabolism or decreased arginine bioavailability [121]. Arginine metabolism is impaired in SCD “Fig.(3)” and contributes to endothelial dysfunction, PH and increased risk of mortality [22, 121]. Adults with SCD are arginine deficient at steady-state [61, 158–160], while children have plasma levels that are similar to normal controls [61]. An arginine deficiency develops with age and is influenced by acute events [61]. There is an associated increase in erythrocyte arginine transport in SCD compared to normal controls that may be a compensatory reaction to low plasma levels [161]. Plasma arginine concentration decreases significantly in both adults and children during vaso-occlusive crisis and ACS, and is associated with low NO metabolite levels [61, 162, 163]. Ongoing intermittent vaso-occlusion may lead to a chronic depletion of arginine stores that are worsened by acute events. Of interest, acute events and exercise are also associated with worsening PH [50].
Figure 3. Altered arginine metabolism in hemolytic disorders.
Arginine is synthesized endogenously from citrulline primarily via the intestinal-renal axis. Arginase and nitric oxide synthase (NOS) compete for arginine, their common substrate. In sickle cell disease (SCD), bioavailability of arginine and nitric oxide (NO) are decreased by several mechanisms linked to hemolysis, and similar mechanisms are postulated for thalassemia and other hemolytic disorders. The release of erythrocyte arginase during hemolysis increases plasma arginase levels and shifts arginine metabolism towards ornithine production, decreasing the amount available for NO production. The bioavailability of arginine is further decreased by increased ornithine levels because ornithine and arginine compete for the same transporter system for cellular uptake. Endogenous synthesis of arginine from citrulline may be compromised by renal dysfunction, commonly associated with sickle cell disease. Despite an increase in NOS in SCD, NO bioavailability is low due to low substrate availability, NOS dysfunction, NO scavenging by cell-free hemoglobin released during hemolysis, and through reactions with free radicals such as superoxide. Superoxide is elevated in SCD due to low superoxide dismutase activity, high xanthine oxidase activity and potentially as a result of uncoupled NOS in an environment of low arginine and/or tetrahydrobiopterin concentration or potentially as a result of altered redox potential of NADPH, a critical NOS cofactor. Endothelial dysfunction resulting from NO depletion and increased levels of the downstream products of ornithine metabolism (polyamines and proline) likely contribute to the pathogenesis of lung injury, fibrosis and pulmonary hypertension. (Reproduced from Morris et al. [22], with permission from the American Medical Association).
Low arginine bioavailability itself may contribute to increased consumption and decreased production of NO. Under conditions of low arginine or tetrahydrobiopterin (an essential NO synthase cofactor) availability [164, 165], NO synthase is uncoupled, producing reactive oxygen species in lieu of NO [166, 167], potentially further reducing NO bioavailability. An imbalance between eNO synthase-derived NO and superoxide generation has been established in SCD by Wood et al [168]. These authors were also the first to suggest that abnormal tetrahydrobiopterin function or availability may be yet another mechanism contributing to dysregulation of the arginine-NO pathway in SCD. This is a mechanism now well described in systemic hypertension [168–172] that has only recently been addressed in PH [173]. Upregulation of NO synthase would therefore enhance oxidative stress when arginine, tetrahydrobiopterin, or other NO synthase cofactors are deficient and NO synthase becomes uncoupled. Indeed, studies in transgenic sickle cell mice demonstrate that NO synthase activity is paradoxically increased [103, 174, 175] and uncoupled [15] in a disease state involving a marked decrease in NO bioavailability.
Arginine is an endogenously synthesized nonessential amino acid [176] that becomes essential under conditions involving an increased catabolic state such as sepsis, burn injury, and trauma, when the capacity of endogenous arginine synthesis is exceeded [177]. It is likely that SCD represents a catabolic state whereby the body’s ability to maintain an arginine balance is disrupted. The majority of whole-body arginine synthesis in adults is performed in a metabolic collaboration by the small intestines and kidneys in what has been termed the “intestinal-renal axis” [130]. Intestine-derived citrulline is released into the circulation and taken up primarily by the kidneys for arginine synthesis [178]. Renal dysfunction is a common occurrence in SCD [9, 46], and is associated with PH in adults with this hemoglobinopathy [46, 49]. Kidney dysfunction will impair the major route for endogenous arginine biosynthesis, thereby contributing to a global reduction in arginine bioavailability, and likely contributes to the pathogenesis of PH. The presence of proteinuria in SCD suggests kidney dysfunction and may identify patients at increased risk for the development of PH, a finding that warrants screening for PH by echocardiography [46].
Low arginine bioavailability may be exacerbated further by the presence of elevated methylarginines, which are competitive inhibitors of arginine transport [179] and all NO synthase isozymes [180, 181]. Circulating methylarginine levels are elevated in several conditions of endothelial dysfunction, including SCD [182], and have also been implicated in the pathophysiology of systemic [182–189] and pulmonary hypertension [188, 189]. Elevated asymmetric dimethylarginine may also induce NO synthase uncoupling [190], thereby contributing to oxidative stress and dysregulated arginine metabolism in SCD.
As in SCD [22], dysregulated arginine metabolism also occurs in patients with thalassemia [62, 87]. The implications of elevated arginase activity in thalassemia remain to be determined, however, given the association of low arginine bioavailability in SCD with increased hemolytic rate, the severity of PH and mortality, this relationship in patients with thalassemia is of significant clinical interest.
Oxidative Stress and Pulmonary Hypertension
Chronic hemolysis may play an important role in the pathogenesis of PH in SCD and thalassemia. A growing body of evidence suggests additional mechanisms are also involved including a complex interaction of platelets, coagulation system, erythrocytes and endothelial cells along with inflammatory and vascular mediators. Oxidative stress also plays a pivotal role in the pathophysiology of PH [191–193]; the erythrocyte may be a major determinant of the global redox environment in hemolytic disorders such as SCD and thalassemia. The sickle and thalassemia erythrocyte have increased concentrations of reactive oxygen species compared with normal erythrocytes [194–197]. Given the presence of alterations in the glutathione buffering system that occurs in these hemoglobinopathies [196–204], it is likely that these erythrocytes are incapable of handling the increased oxidant burden, which might predispose them to hemolysis.
Recently, a depletion of erythrocyte glutamine concentration as well as aberrations in erythrocyte glutathione metabolism have been linked to PH in SCD [204]. Because alterations in the erythrocyte redox environment can contribute to both increased oxidative stress and hemolysis, the erythrocyte redox environment itself represents a mechanistic model bridging two critical pathways in the pathogenesis of PH [9, 10, 13, 16, 17, 22, 62, 191, 192, 205] in hemolytic disorders. Glutamine bioavailability may influence the redox potential of the sickle erythrocyte via the loss of nicotinamide adenine dinucleotide phosphate (NADPH) biosynthesis capacity.
NADPH is an essential co-factor required for glutathione recycling, and low bioavailability would negatively impact the conversion of oxidized glutathione (GSSG) to reduced glutathione, resulting in an overall increase in intracellular GSSG and potential efflux [206]. The downstream effects of reduced NADPH synthesis would be a loss in the overall erythrocyte pool of glutathione and a shift in the redox potential rendering them more susceptible to hemolysis. Clinical trials have demonstrated that altered erythrocyte NAD redox potential is improved by oral L-glutamine therapy [207, 208], suggesting that glutamine limitation is playing a role in oxidative stress. Dysfunctional NADPH biosynthesis has global implications due to its role as a critical co-factor for a number of enzymes, including the NO synthases and may contribute to NO synthase dysfunction [15] and further dysregulation of the arginine-NO pathway.
During NADPH biosynthesis, intracellular glutamine is metabolized to glutamate. The erythrocyte glutamine:glutamate ratio inversely correlates with both cell free hemoglobin and plasma arginase concentration as well as tricuspid regurgitant jet velocity in patients with SCD [204], suggesting a novel link between altered glutamine metabolism and hemolysis. The glutamine:glutamate ratio may therefore represent a novel biomarker of hemolysis and oxidative stress as well as PH. The value of erythrocyte amino acid analysis in SCD should be validated in future investigations, as it is more reflective of the total amino acid pools than plasma alone. In addition, selective derangement of erythrocytes amino acid pools has systemic consequences that are poorly understood. A better understanding of metabolic processes and oxidative stress occurring within the sickle erythrocyte may shed light on disease pathogenesis.
Further oxidative stress is caused by hemolysis itself and by the presence of iron overload and free-radical formation common in both SCD and thalassemia. Iron overload also affects PH by other mechanisms. It induces interstitial pulmonary fibrosis as well as left and right cardiac hemosiderosis, which subsequently results in cardiac dysfunction and affects pulmonary vascular resistance [81]. Ferriten level, a reflection of iron overload and inadequate chelation, is an independent predictor of mortality in patients with SCD [70]. Transgenic mouse models of SCD exhibit evidence of ischemia-reperfusion injury in hypoxia suggesting a role for the vascular endothelium in the modulation of the redox balance [209–212] possibly via its interactions with the sickle erythrocyte, while alteration in endothelial adhesion molecules have been linked to both PH and mortality in patients with SCD [17, 22].
Conclusion
PH is a complication observed in several hereditary and acquired hemolytic anemias [9–12, 213]. The release of hemoglobin from erythrocytes to the plasma rapidly consumes endothelial NO and increases the body’s metabolic requirement for arginine. Plasma arginine bioavailability, however, is itself decreased due to the simultaneous release of erythrocyte arginase that results from hemolysis. Treatments aimed at reversing endothelial dysfunction and oxidative stress, re-coupling NO synthase, minimizing hemolysis, and enhancing arginine and NO bioavailability in hemolytic conditions including SCD and thalassemia may prove efficacious. Controlled clinical trials for the treatment of PH in these populations are needed to guide future management.
Though several drugs have been approved by the FDA for the treatment of PH, there are only limited studies of the efficacy or toxicities of these therapies in patients with hemoglobinopathies. There is currently no standard therapy recommended for hemolysis-associated PH. To date, only two studies have been published for the treatment of PH in SCD, and only case report in thalassemia. Arginine supplementation improved pulmonary artery pressures estimated by Doppler echocardiography by more than 15% after only 5 days of therapy [63], and treatment with sildenafil improved exercise tolerance in patients with SCD and PH [214]. Plans to investigate sildenafil, arginine, and therapies already approved for use in PH are in development or underway for both SCD and thalassemia. More aggressive screening and a comprehensive pulmonary exam that includes periodic Doppler echocardiography and pulmonary function testing may help identify patients at risk for PH, while early intervention may decrease mortality and morbidity for these patients and improve quality of life.
ACKNOWLEDGEMENTS
This study was supported in part by the NIH CTSI grant UL1 RR024131 (to CRM). GJK and MTG receive intramural support from the National Institutes of Health (NIH). MTG holds a cooperative research and development agreement (CRADA) through the NIH with INO Therapeutics, Inc.
List of Abbreviations (in order of appearance)
- PH
Pulmonary hypertension
- SCD
Sickle Cell Disease
- NO
Nitric Oxide
- ACS
Acute Chest Syndrome
- CAT
Cationic Amino Acid Transporter
- NADPH
Nicotinamide Adenine Dinucleotide Phosphate;
Footnotes
Authors declare no conflicts of interest.
References
- 1.Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997;337:762–769. doi: 10.1056/NEJM199709113371107. [DOI] [PubMed] [Google Scholar]
- 2.Stuart MJ, Nagel RL. Sickle-cell disease. Lancet. 2004;364:1343–1360. doi: 10.1016/S0140-6736(04)17192-4. [DOI] [PubMed] [Google Scholar]
- 3.Hebbel RP, Osarogiagbon KD. The endothelial biology of sickle cell disease: Inflammation and a chronic vasculopathy. Microcirculation. 2004;11:129–151. [PubMed] [Google Scholar]
- 4.Frenette PS. Sickle cell vaso-occlusion: multistep and multicellular paradigm. Curr Opin Hematol. 2002;9:101–106. doi: 10.1097/00062752-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Parise LV, Telen MJ. Erythrocyte adhesion in sickle cell disease. Curr Hematol Rep. 2003;2:102–108. [PubMed] [Google Scholar]
- 6.Vichinsky EP. Changing patterns of thalassemia worldwide. Ann N Y Acad Sci. 2005;1054:18–24. doi: 10.1196/annals.1345.003. [DOI] [PubMed] [Google Scholar]
- 7.Olivieri NF. The beta-thalassemias. N Engl J Med. 1999;341:99–109. doi: 10.1056/NEJM199907083410207. [DOI] [PubMed] [Google Scholar]
- 8.Giardina PJ, Hilgartner MW. Update on thalassemia. Pediatr Rev. 1992;13:55–62. doi: 10.1542/pir.13-2-55. [DOI] [PubMed] [Google Scholar]
- 9.Gladwin M, Sachdev V, Jison M, Shizukuda Y, Plehn J, Minter K, Brown B, Coles W, Nichols J, Ernst I, Hunter L, Blackwelder W, Schechter A, Rodgers G, Castro O, Ognibene F. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:22–31. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 10.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: A novel mechanism of human disease. JAMA. 2005;293:1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 11.Lin EE, Rodgers GP, Gladwin MT. Hemolytic anemia-associated pulmonary hypertension in sickle cell disease. Curr Hematol Rep. 2005;4:117–125. [PubMed] [Google Scholar]
- 12.Gladwin MT, Kato GJ. Cardiopulmonary complications of sickle cell disease: role of nitric oxide and hemolytic anemia. Hematology (Am Soc Hematol Educ Program) 2005:51–57. doi: 10.1182/asheducation-2005.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato GJ, McGowan V, Machado RF, Little JA, Taylor Jt, Morris CR, Nichols JS, Wang X, Poljakovic M, Morris SM, Jr, Gladwin MT. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006;107:2279–2285. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato GJ, Hsieh M, Machado R, Taylor Jt, Little J, Butman JA, Lehky T, Tisdale J, Gladwin MT. Cerebrovascular disease associated with sickle cell pulmonary hypertension. Am J Hematol. 2006;81:503–510. doi: 10.1002/ajh.20642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu LL, Champion HC, Campbell-Lee SA, Bivalacqua TJ, Manci EA, Diwan BA, Schimel DM, Cochard AE, Wang X, Schechter AN, Noguchi CT, Gladwin MT. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood. 2007;109:3088–3098. doi: 10.1182/blood-2006-08-039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: Reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 2007;21:37–47. doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato GJ, Martyr S, Blackwelder WC, Nichols JS, Coles WA, Hunter LA, Brennan ML, Hazen SL, Gladwin MT. Levels of soluble endothelium-derived adhesion molecules in patients with sickle cell disease are associated with pulmonary hypertension, organ dysfunction, and mortality. Br J Haematol. 2005;130:943–953. doi: 10.1111/j.1365-2141.2005.05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nolan VG, Wyszynski DF, Farrer LA, Steinberg MH. Hemolysis-associated priapism in sickle cell disease. Blood. 2005;106:3264–3267. doi: 10.1182/blood-2005-04-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nolan VG, Adewoye A, Baldwin C, Wang L, Ma Q, Wyszynski DF, Farrell JJ, Sebastiani P, Farrer LA, Steinberg MH. Sickle cell leg ulcers: associations with haemolysis and SNPs in Klotho, TEK and genes of the TGF-beta/BMP pathway. Br J Haematol. 2006;133:570–578. doi: 10.1111/j.1365-2141.2006.06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lezcano NE, Odo N, Kutlar A, Brambilla D, Adams RJ. Regular Transfusion Lowers Plasma Free Hemoglobin in Children With Sickle-Cell Disease at Risk for Stroke. Stroke. 2006;37:1424–1426. doi: 10.1161/01.STR.0000221173.97108.01. [DOI] [PubMed] [Google Scholar]
- 21.Reiter C, Wang X, Tanus-Santos J, Hogg N, Cannon R, Schechter A, Gladwin M. Cell-free hemoglobin limits nitric oxide bioavailability in sickle cell disease. Nat Med. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 22.Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, V S, Hazen SL, Vichinsky EP, Morris SM, Jr, Gladwin MT. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension and mortality in sickle cell disease. JAMA. 2005;294:81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351:1655–1665. doi: 10.1056/NEJMra035488. [DOI] [PubMed] [Google Scholar]
- 24.Rubin LJ. Primary pulmonary hypertension. N Engl J Med. 1997;336:111–117. doi: 10.1056/NEJM199701093360207. [DOI] [PubMed] [Google Scholar]
- 25.Adedeji MO, Cespedes J, Allen K, Subramony C, Hughson MD. Pulmonary thrombotic arteriopathy in patients with sickle cell disease. Arch Pathol Lab Med. 2001;125:1436–1441. doi: 10.5858/2001-125-1436-PTAIPW. [DOI] [PubMed] [Google Scholar]
- 26.Haque AK, Grinberg AR, Lencioni M, Molina MM, Roncoroni AJ. Pulmonary hypertention in sickle cell hemoglobinopathy: A clinicopathologic study of 20 cases. Hum Pathol. 2002;33:1037–1043. doi: 10.1053/hupa.2002.128059. [DOI] [PubMed] [Google Scholar]
- 27.Manci EA, Culberson DE, Yang YM, Gardner TM, Powell R, Haynes J, Jr, Shah AK, Mankad VN. Causes of death in sickle cell disease: an autopsy study. Br J Haematol. 2003;123:359–365. doi: 10.1046/j.1365-2141.2003.04594.x. [DOI] [PubMed] [Google Scholar]
- 28.Sonakul D, Fucharoen S. Pulmonary thromboembolism in thalassemic patients. Southeast Asian J Trop Med Public Health. 1992;23 Suppl 2:25–28. [PubMed] [Google Scholar]
- 29.Sonakul D, Pacharee P, Thakerngpol K. Pathologic findings in 76 autopsy cases of thalassemia. Birth Defects Orig Artic Ser. 1988;23:157–176. [PubMed] [Google Scholar]
- 30.Sonakul D, Suwanagool P, Sirivaidyapong P, Fucharoen S. Distribution of pulmonary thromboembolic lesions in thalassemic patients. Birth Defects Orig Artic Ser. 1987;23:375–384. [PubMed] [Google Scholar]
- 31.Kato GJ, Onyekwere OC, Gladwin MT. Pulmonary Hypertension In Sickle Cell Disease: Relevance To Children. Pediatr Hematol Oncol. 2007;24:159–170. doi: 10.1080/08880010601185892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004;109:159–165. doi: 10.1161/01.CIR.0000102381.57477.50. [DOI] [PubMed] [Google Scholar]
- 33.Voetsch B, Jin RC, Loscalzo J. Nitric oxide insufficiency and atherothrombosis. Histochem Cell Biol. 2004;122:353–367. doi: 10.1007/s00418-004-0675-z. [DOI] [PubMed] [Google Scholar]
- 34.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 35.Ignarro LJ. Heme-dependent activation of soluble guanylate cyclase by nitric oxide: Regulation of enzyme activity by porphyrins and metalloporphyrins. Sem Hematol. 1989;26:63–76. [PubMed] [Google Scholar]
- 36.Klings ES, Wyszynski DF, Nolan VG, Steinberg MH. Abnormal Pulmonary Function in Adults with Sickle Cell Anemia. Am J Respir Crit Care Med. 2006;173:1264–1269. doi: 10.1164/rccm.200601-125OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ataga KI, Sood N, De Gent G, Kelly E, Henderson AG, Jones S, Strayhorn D, Lail A, Lieff S, Orringer EP. Pulmonary hypertension in sickle cell disease. Am J Med. 2004;117:665–669. doi: 10.1016/j.amjmed.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 38.Castro O, Hoque M, Brown M. Pulmonary hypertension in sickle cell disease: cardiac catheterization results and survival. Blood. 2003;101:1257–1261. doi: 10.1182/blood-2002-03-0948. [DOI] [PubMed] [Google Scholar]
- 39.Sutton LL, Castro O, Cross DJ, Spencer JE, Lewis LF. Pulmonary hypertension in sickle cell disease. Am J Cardiol. 1994;74:626–628. doi: 10.1016/0002-9149(94)90760-9. [DOI] [PubMed] [Google Scholar]
- 40.Vichinsky EP. Pulmonary hypertension in sickle cell disease: A time for intervention. N Engl J Med. 2004;350:857–859. doi: 10.1056/NEJMp038250. [DOI] [PubMed] [Google Scholar]
- 41.Suell MN, Bezold LI, Okcu MF, Mahoney DH, Jr, Shardonofsky F, Mueller BU. Increased pulmonary artery pressures among adolescents with sickle cell disease. J Pediatr Hematol Oncol. 2005;27:654–658. doi: 10.1097/01.mph.0000194022.17968.bf. [DOI] [PubMed] [Google Scholar]
- 42.Ambrusko SJ, Gunawardena S, Sakara A, Windsor B, Lanford L, Michelson P, Krishnamurti L. Elevation of tricuspid regurgitant jet velocity, a marker for pulmonary hypertension in children with sickle cell disease. Pediatr Blood Cancer. 2006 doi: 10.1002/pbc.20791. [DOI] [PubMed] [Google Scholar]
- 43.Darbari DS, Kple-Faget P, Kwagyan J, Rana S, Gordeuk VR, Castro O. Circumstances of death in adult sickle cell disease patients. Am J Hematol. 2006;81:858–863. doi: 10.1002/ajh.20685. [DOI] [PubMed] [Google Scholar]
- 44.Graham JK, Mosunjac M, Hanzlick RL, Mosunjac M. Sickle cell lung disease and sudden death: a retrospective/prospective study of 21 autopsy cases and literature review. Am J Forensic Med Pathol. 2007;28:168–172. doi: 10.1097/01.paf.0000257397.92466.50. [DOI] [PubMed] [Google Scholar]
- 45.Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005;84:363–376. doi: 10.1097/01.md.0000189089.45003.52. [DOI] [PubMed] [Google Scholar]
- 46.De Castro LM, Jonassaint JC, Graham FL, Ashley-Koch A, Telen MJ. Pulmonary hypertension associated with sickle cell disease: Clinical and laboratory endpoints and disease outcomes. Am J Hematol. 2007 doi: 10.1002/ajh.21058. [DOI] [PubMed] [Google Scholar]
- 47.Ataga KI, Moore CG, Jones S, Olajide O, Strayhorn D, Hinderliter A, Orringer EP. Pulmonary hypertension in patients with sickle cell disease: a longitudinal study. Br J Haematol. 2006;134:109–1015. doi: 10.1111/j.1365-2141.2006.06110.x. [DOI] [PubMed] [Google Scholar]
- 48.Anthi A, Machado RF, Jison ML, Taveira-Dasilva AM, Rubin LJ, Hunter L, Hunter CJ, Coles W, Nichols J, Avila NA, Sachdev V, Chen CC, Gladwin MT. Hemodynamic and functional assessment of patients with sickle cell disease and pulmonary hypertension. Am J Respir Crit Care Med. 2007;175:1272–1279. doi: 10.1164/rccm.200610-1498OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hagar W, Michlitsch J, Gardner J, Vichinsky EP, Morris CR. Differences in Disease Patterns between Adults and Children with Pulmonary Hypertension and Sickle Cell Disease. Brit J Haematol. 2007 doi: 10.1111/j.1365-2141.2007.06822.x. in press. [DOI] [PubMed] [Google Scholar]
- 50.Machado RF, Kyle Mack A, Martyr S, Barnett C, Macarthur P, Sachdev V, Ernst I, Hunter LA, Coles WA, Nichols JP, Kato GJ, Gladwin MT. Severity of pulmonary hypertension during vaso-occlusive pain crisis and exercise in patients with sickle cell disease. Br J Haematol. 2007;136:319–325. doi: 10.1111/j.1365-2141.2006.06417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dessap AM, Leon R, Habibi A, Nzouakou R, Roudot-Thoraval F, Adnot S, Godeau B, Galacteros F, Brun-Buisson C, Brochard L, Maitre B. Pulmonary Hypertension and Cor Pulmonale during Severe Acute Chest Syndrome in Sickle Cell Disease. Am J Respir Crit Care Med. 2008 doi: 10.1164/rccm.200710-1606OC. [DOI] [PubMed] [Google Scholar]
- 52.Machado RF, Gladwin MT. Chronic sickle cell lung disease: new insights into the diagnosis, pathogenesis and treatment of pulmonary hypertension. Br J Haematol. 2005;129:449–464. doi: 10.1111/j.1365-2141.2005.05432.x. [DOI] [PubMed] [Google Scholar]
- 53.Onyekwere OC, Campbell A, Teshome M, Onyeagoro S, Sylvan C, Akintilo A, Hutchinson S, Ensing G, Gaskin P, Kato G, Rana S, Kwagyan J, Gordeuk V, Williams J, Castro O. Pulmonary Hypertension in Children and Adolescents with Sickle Cell Disease. Pediatr Cardiol. 2007 doi: 10.1007/s00246-007-9018-x. [DOI] [PubMed] [Google Scholar]
- 54.de Boer J, Duyvendak M, Schuurman FE, Pouw FM, Zaagsma J, Meurs H. Role of L-arginine in the deficiency of nitric oxide and airway hyperreactivity after the allergen-induced early asthmatic reaction in guinea-pigs. Br J Pharmacol. 1999;128:1114–11120. doi: 10.1038/sj.bjp.0702882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morris CR, Poljakovic M, Lavisha L, Machado L, Kuypers F, Morris SM., Jr Decreased arginine bioavailability and increased arginase activity in asthma. Am J Respir Crit Care Med. 2004;170:148–153. doi: 10.1164/rccm.200309-1304OC. [DOI] [PubMed] [Google Scholar]
- 56.King NE, Rothenberg ME, Zimmermann N. Arginine in asthma and lung inflammation. J Nutr. 2004;134:2830S–2836S. doi: 10.1093/jn/134.10.2830S. discussion 2853S. [DOI] [PubMed] [Google Scholar]
- 57.Maarsingh H, Leusink J, Bos IS, Zaagsma J, Meurs H. Arginase strongly impairs neuronal nitric oxide-mediated airway smooth muscle relaxation in allergic asthma. Respir Res. 2006;7:6. doi: 10.1186/1465-9921-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carraway MS, Piantadosi CA, Jenkinson CP, Huang YC. Differential expression of arginase and iNOS in the lung in sepsis. Exp Lung Res. 1998;24:253–268. doi: 10.3109/01902149809041533. [DOI] [PubMed] [Google Scholar]
- 59.Luiking YC, Poeze M, Dejong CH, Ramsay G, Deutz NE. Sepsis: an arginine deficiency state? Crit Care Med. 2004;32:2135–2145. doi: 10.1097/01.ccm.0000142939.81045.a0. [DOI] [PubMed] [Google Scholar]
- 60.Luiking YC, Poeze M, Ramsay G, Deutz NE. The role of arginine in infection and sepsis. JPEN J Parenter Enteral Nutr. 2005;29:S70–S74. doi: 10.1177/01486071050290S1S70. [DOI] [PubMed] [Google Scholar]
- 61.Morris CR, Kuypers FA, Larkin S, Vichinsky E, Styles L. Patterns of arginine and nitric oxide in sickle cell disease patients with vaso-occlusive crisis and acute chest syndrome. J Pediatr Hematol Oncol. 2000;22:515–520. doi: 10.1097/00043426-200011000-00009. [DOI] [PubMed] [Google Scholar]
- 62.Morris C, Kuypers F, Kato G, Lavrisha L, Larkin S, Singer T, Vichinsky E. Hemolysis-associated pulmonary hypertension in thalassemia. An NY Acad Sci. 2005;1054:481–485. doi: 10.1196/annals.1345.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morris CR, Morris SM, Jr, Hagar W, van Warmerdam J, Claster S, Kepka-Lenhart K, Machado L, Kuypers FA, Vichinsky EP. Arginine Therapy: A new treatment for pulmonary hypertension in sickle cell disease? Am J Respir Crit Care Med. 2003;168:63–69. doi: 10.1164/rccm.200208-967OC. [DOI] [PubMed] [Google Scholar]
- 64.Sebastiani P, Nolan VG, Baldwin CT, Abad-Grau MM, Wang L, Adewoye AH, McMahon LC, Farrer LA, Taylor JGt, Kato GJ, Gladwin MT, Steinberg MH. A network model to predict the risk of death in sickle cell disease. Blood. 2007 doi: 10.1182/blood-2007-04-084921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herve P, Humbert M, Sitbon O, Parent F, Nunes H, Legal C, Garcia G, Simonneau G. Pathobiology of pulmonary hypertension. The role of platelets and thrombosis. Clin Chest Med. 2001;22:451–458. doi: 10.1016/s0272-5231(05)70283-5. [DOI] [PubMed] [Google Scholar]
- 66.Ataga KI, Orringer EP. Hypercoagulability in sickle cell disease: a curious paradox. Am J Med. 2003;115:721–728. doi: 10.1016/j.amjmed.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 67.Eldor A, Rachmilewitz EA. The hypercoagulable state in thalassemia. Blood. 2002;99:36–43. doi: 10.1182/blood.v99.1.36. [DOI] [PubMed] [Google Scholar]
- 68.Singer ST, Kuypers FA, Styles L, Vichinsky EP, Foote D, Rosenfeld H. Pulmonary hypertension in thalassemia: association with platelet activation and hypercoagulable state. Am J Hematol. 2006;81:670–675. doi: 10.1002/ajh.20640. [DOI] [PubMed] [Google Scholar]
- 69.Villagra J, Shiva S, Hunter LA, Machado RF, Gladwin MT, Kato GJ. Platelet activation in patients with sickle disease, hemolysis-associated pulmonary hypertension and nitric oxide scavenging by cell-free hemoglobin. Blood. 2007;110:2166–2172. doi: 10.1182/blood-2006-12-061697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Machado RF, Anthi A, Steinberg MH, Bonds D, Sachdev V, Kato GJ, Taveira-DaSilva AM, Ballas SK, Blackwelder W, Xu X, Hunter L, Barton B, Waclawiw M, Castro O, Gladwin MT. N-terminal pro-brain natriuretic peptide levels and risk of death in sickle cell disease. Jama. 2006;296:310–318. doi: 10.1001/jama.296.3.310. [DOI] [PubMed] [Google Scholar]
- 71.Phrommintikul A, Sukonthasarn A, Kanjanavanit R, Nawarawong W. Splenectomy: a strong risk factor for pulmonary hypertension in patients with thalassaemia. Heart. 2006;92:1467–1472. doi: 10.1136/hrt.2005.079970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Atichartakarn V, Likittanasombat K, Chuncharunee S, Chandanamattha P, Worapongpaiboon S, Angchaisuksiri P, Aryurachai K. Pulmonary arterial hypertension in previously splenectomized patients with beta-thalassemic disorders. Int J Hematol. 2003;78:139–145. doi: 10.1007/BF02983382. [DOI] [PubMed] [Google Scholar]
- 73.Eldor A, Durst R, Hy-Am E, Goldfarb A, Gillis S, Rachmilewitz EA, Abramov A, MacLouf J, Godefray YC, De Raucourt E, Guillin MC. A chronic hypercoagulable state in patients with beta-thalassaemia major is already present in childhood. Br J Haematol. 1999;107:739–746. doi: 10.1046/j.1365-2141.1999.01758.x. [DOI] [PubMed] [Google Scholar]
- 74.Borenstain-Ben Yashar V, Barenholz Y, Hy-Am E, Rachmilewitz EA, Eldor A. Phosphatidylserine in the outer leaflet of red blood cells from beta-thalassemia patients may explain the chronic hypercoagulable state and thrombotic episodes. Am J Hematol. 1993;44:63–65. doi: 10.1002/ajh.2830440114. [DOI] [PubMed] [Google Scholar]
- 75.Borgna Pignatti C, Carnelli V, Caruso V, Dore F, De Mattia D, Di Palma A, Di Gregorio F, Romeo MA, Longhi R, Mangiagli A, Melevendi C, Pizzarelli G, Musumeci S. Thromboembolic events in beta thalassemia major: an Italian multicenter study. Acta Haematol. 1998;99:76–79. doi: 10.1159/000040814. [DOI] [PubMed] [Google Scholar]
- 76.Walter PB, Fung EB, Killilea DW, Jiang Q, Hudes M, Madden J, Porter J, Evans P, Vichinsky E, Harmatz P. Oxidative stress and inflammation in iron-overloaded patients with beta-thalassaemia or sickle cell disease. Br J Haematol. 2006;135:254–263. doi: 10.1111/j.1365-2141.2006.06277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fung EB, Harmatz P, Milet M, Ballas SK, De Castro L, Hagar W, Owen W, Olivieri N, Smith-Whitley K, Darbari D, Wang W, Vichinsky E. Morbidity and mortality in chronically transfused subjects with thalassemia and sickle cell disease: A report from the multi-center study of iron overload. Am J Hematol. 2006 doi: 10.1002/ajh.20809. [DOI] [PubMed] [Google Scholar]
- 78.Fung EB, Harmatz PR, Lee PD, Milet M, Bellevue R, Jeng MR, Kalinyak KA, Hudes M, Bhatia S, Vichinsky EP. Increased prevalence of iron-overload associated endocrinopathy in thalassaemia versus sickle-cell disease. Br J Haematol. 2006;135:574–582. doi: 10.1111/j.1365-2141.2006.06332.x. [DOI] [PubMed] [Google Scholar]
- 79.Aessopos A, Farmakis D. Pulmonary hypertension in beta-thalassemia. Ann N Y Acad Sci. 2005;1054:342–349. doi: 10.1196/annals.1345.041. [DOI] [PubMed] [Google Scholar]
- 80.Hahalis G, Alexopoulos D, Kremastinos DT, Zoumbos NC. Heart failure in beta-thalassemia syndromes: a decade of progress. Am J Med. 2005;118:957–967. doi: 10.1016/j.amjmed.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 81.Zakynthinos E, Vassilakopoulos T, Kaltsas P, Malagari E, Daniil Z, Roussos C, Zakynthinos SG. Pulmonary hypertension, interstitial lung fibrosis, and lung iron deposition in thalassaemia major. Thorax. 2001;56:737–739. doi: 10.1136/thorax.56.9.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aessopos A, Farmakis D, Karagiorga M, Voskaridou E, Loutradi A, Hatziliami A, Joussef J, Rombos J, Loukopoulos D. Cardiac involvement in thalassemia intermedia: a multicenter study. Blood. 2001;97:3411–6. doi: 10.1182/blood.v97.11.3411. [DOI] [PubMed] [Google Scholar]
- 83.Aessopos A, Stamatelos G, Skoumas V, Vassilopoulos G, Mantzourani M, Loukopoulos D. Pulmonary hypertension and right heart failure in patients with beta-thalassemia intermedia. Chest. 1995;107:50–53. doi: 10.1378/chest.107.1.50. [DOI] [PubMed] [Google Scholar]
- 84.Du ZD, Roguin N, Milgram E, Saab K, Koren A. Pulmonary hypertension in patients with thalassemia major. Am Heart J. 1997;134:532–537. doi: 10.1016/s0002-8703(97)70091-7. [DOI] [PubMed] [Google Scholar]
- 85.Grisaru D, Rachmilewitz EA, Mosseri M, Gotsman M, Lafair JS, Okon E, Goldfarb A, Hasin Y. Cardiopulmonary assessment in beta-thalassemia major. Chest. 1990;98:1138–1142. doi: 10.1378/chest.98.5.1138. [DOI] [PubMed] [Google Scholar]
- 86.Aessopos A, Farmakis D, Deftereos S, Tsironi M, Tassiopoulos S, Moyssakis I, Karagiorga M. Thalassemia heart disease: a comparative evaluation of thalassemia major and thalassemia intermedia. Chest. 2005;127:1523–1530. doi: 10.1378/chest.127.5.1523. [DOI] [PubMed] [Google Scholar]
- 87.Morris CR, Vichinsky E, Singer ST. Pulmonary hypertension in thalassemia: Association with hemolysis, arginine metabolism dysregulation and a hypercoaguable state. Advances in Pulmonary Hypertension. 2007;5:31–38. [Google Scholar]
- 88.Hagar RW, Morris CR, Vichinsky EP. Pulmonary hypertension in thalassaemia major patients with normal left ventricular systolic function. Br J Haematol. 2006;133:433–435. doi: 10.1111/j.1365-2141.2006.06053.x. [DOI] [PubMed] [Google Scholar]
- 89.Aessopos A, Farmakis D, Hatziliami A, Fragodimitri C, Karabatsos F, Joussef J, Mitilineou E, Diamanti-Kandaraki E, Meletis J, Karagiorga M. Cardiac status in well-treated patients with thalassemia major. Eur J Haematol. 2004;73:359–366. doi: 10.1111/j.1600-0609.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- 90.Atichartakarn V, Chuncharunee S, Chandanamattha P, Likittanasombat K, Aryurachai K. Correction of hypercoagulability and amelioration of pulmonary arterial hypertension by chronic blood transfusion in an asplenic hemoglobin E/beta-thalassemia patient. Blood. 2004;103:2844–2846. doi: 10.1182/blood-2003-09-3094. [DOI] [PubMed] [Google Scholar]
- 91.Cheung P, Salas E, Schulz R, Radomski MW. Nitric oxide and platelet function: Implications for neonatology. Semin Perinatol. 1997;21:409–417. doi: 10.1016/s0146-0005(97)80006-7. [DOI] [PubMed] [Google Scholar]
- 92.Adams MR, Forsyth CJ, Jessup W, Robinson J, Celermajer DS. Oral L-arginine inhibits platelet aggregation but does not enhance endothelium-dependent dilation in healthy young men. J Am Coll Cardiol. 1995;26:1054–1061. doi: 10.1016/0735-1097(95)00257-9. [DOI] [PubMed] [Google Scholar]
- 93.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nat Med. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 94.Kam PC, Govender G. Nitric oxide: Basic science and clinincal applications. Anaesthesia. 1994;49:515–521. doi: 10.1111/j.1365-2044.1994.tb03525.x. [DOI] [PubMed] [Google Scholar]
- 95.Hobbs AJ, Ignarro LJ. The Nitric Oxide-Cyclic GMP Signal Transduction System. In: Zapol WM, Bloch KD, editors. Nitric Oxide and the Lung. Marcel Dekker, Inc; 1997. p. 469. 1. [Google Scholar]
- 96.Kaul DK, Hebbel RP. Hypoxia/reoxygenation causes inflammatory response in transgenic sickle mice but not in normal mice. J Clin Invest. 2000;106:411–420. doi: 10.1172/JCI9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peng H-B, Spiecker M, Liao J. Inducible nitric oxide: An autoregulatory feedback inhibitor of vascular inflammation. J Immunol. 1998;161:1970–1976. [PubMed] [Google Scholar]
- 98.Hampl V, Herget J. Role of nitric oxide in the pathogenesis of chronic pulmonary hypertension. Physiol Rev. 2000;80:1337–1372. doi: 10.1152/physrev.2000.80.4.1337. [DOI] [PubMed] [Google Scholar]
- 99.Michelakis ED. The role of the NO axis and its therapeutic implications in pulmonary arterial hypertension. Heart Fail Rev. 2003;8:5–21. doi: 10.1023/a:1022150819223. [DOI] [PubMed] [Google Scholar]
- 100.Afanas’ev IB. Interplay between superoxide and nitric oxide in thalassemia and Fanconi’s anemia. Hemoglobin. 2006;30:113–118. doi: 10.1080/03630260500455557. [DOI] [PubMed] [Google Scholar]
- 101.Reiter CD, Gladwin MT. An emerging role for nitric oxide in sickle cell disease vascular homeostatis and therapy. Curr Opin Hematol. 2003;10:99–107. doi: 10.1097/00062752-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 102.Gladwin M, Schechter A, Ognibene F, Coles W, Reiter C, Schenke W, Csako G, Waclawiw M, Panza J, Cannon R. Divergent nitric oxide bioavailability in men and women with sickle cell disease. Circulation. 2003;107:271–278. doi: 10.1161/01.cir.0000044943.12533.a8. [DOI] [PubMed] [Google Scholar]
- 103.Kaul DK, Liu X, Chang H, Nagel RL, Fabry ME. Effect of fetal hemoglobin on microvascular regulation in sickle transgenic-knockout mice. J Clin Invest. 2004;114:1136–1145. doi: 10.1172/JCI21633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aslan M, Ryan TM, Adler B, Townes TM, Parks DA, Thompson JA, Tousson A, Gladwin MT, Patel RP, Tarpey MM, Batinic-Haberle I, White CR, Freeman BA. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc Natl Acad Sci U S A. 2001;98:15215–15220. doi: 10.1073/pnas.221292098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kaul DK, Liu XD, Fabry ME, Nagel RL. Impaired nitric oxide-mediated vasodilation in transgenic sickle mouse. Am J Physiol Heart Circ Physiol. 2000;278:H1799–H1806. doi: 10.1152/ajpheart.2000.278.6.H1799. [DOI] [PubMed] [Google Scholar]
- 106.Eberhardt RT, McMahon L, Duffy SJ, Steinberg MH, Perrine SP, Loscalzo J, Coffman JD, Vita JA. Sickle cell anemia is associated with reduced nitric oxide bioactivity in peripheral conduit and resistance vessels. Am J Hematol. 2003;74:104–111. doi: 10.1002/ajh.10387. [DOI] [PubMed] [Google Scholar]
- 107.Belhassen L, Pelle G, Sediame S, Bachir D, Carville C, Bucherer C, Lacombe C, Galacteros F, Adnot S. Endothelial dysfunction in patients with sickle cell disease is related to selective impairment of shear stress-mediated vasodilation. Blood. 2001;97:1584–1589. doi: 10.1182/blood.v97.6.1584. [DOI] [PubMed] [Google Scholar]
- 108.Rees DC, Cervi P, Grimwade D, O’Driscoll A, Hamilton M, Parker NE, Porter JB. The metabolites of nitric oxide in sickle-cell disease. Br J Haematol. 1995;91:834–837. doi: 10.1111/j.1365-2141.1995.tb05397.x. [DOI] [PubMed] [Google Scholar]
- 109.Lonsdorfer J, Bogui P, Otayeck A, Bursaux E, Poyart C, Cabannes R. Cardiorespiratory adjustments in chronic sickle cell anemia. Bull Eur Physiopathol Respir. 1983;19:339–344. [PubMed] [Google Scholar]
- 110.Johnson CS, Giorgio AJ. Arterial blood pressure in adults with sickle cell disease. Arch Intern Med. 1981;141:891–893. [PubMed] [Google Scholar]
- 111.Karayaylali I, Onal M, Yildizer K, Seyrek N, Paydas S, Akoglu E, Gurcay AA, Birand A, Sagliker Y. Low blood pressure, decreased incidence of hypertension, and renal cardiac, and autonomic nervous system functions in patients with sickle cell syndromes. Nephron. 91(202):535–537. doi: 10.1159/000064306. [DOI] [PubMed] [Google Scholar]
- 112.Rodgers GP, Walker EC, Podgor MJ. Is “relative” hypertension a risk factor for vaso-occlusive complications in sickle cell disease? Am J Med Sci. 1993;305:150–156. doi: 10.1097/00000441-199303000-00004. [DOI] [PubMed] [Google Scholar]
- 113.Pegelow CH, Colangelo L, Steinberg M, Wright EC, Smith J, Phillips G, Vichinsky E. Natural history of blood pressure in sickle cell disease: risks for stroke and death associated with relative hypertension in sickle cell anemia. Am J Med. 1997;102:171–177. doi: 10.1016/s0002-9343(96)00407-x. [DOI] [PubMed] [Google Scholar]
- 114.Gordeuk VR, Sachdev V, Taylor JG, Gladwin MT, Kato G, Castro OL. Relative systemic hypertension in patients with sickle cell disease is associated with risk of pulmonary hypertension and renal insufficiency. Am J Hematol. 2007 doi: 10.1002/ajh.21016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lopez BL, Barnett J, Ballas SK, Christopher TA, Davis-Moon L, Ma X. Nitric oxide metabolite levels in acute vaso-occlusive sickle-cell crisis. Acad Emerg Med. 1996;3:1098–1103. doi: 10.1111/j.1553-2712.1996.tb03367.x. [DOI] [PubMed] [Google Scholar]
- 116.Lopez B, Davis-Moon L, Ballas S. Sequential nitric oxide measurements during the emergency department treatment of acute vasoocclusive sickle cell crisis. Am J Hematol. 2000;64:15–19. doi: 10.1002/(sici)1096-8652(200005)64:1<15::aid-ajh3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 117.Dias-Da-Motta P, Arruda V, Muscara M, Saad S. The release of nitric oxide and superoxide anion by neutrophils and mononuclear cells from patients with sickle cell anaemia. Brit J Haematol. 1996;93:333–340. doi: 10.1046/j.1365-2141.1996.4951036.x. [DOI] [PubMed] [Google Scholar]
- 118.Demiryurek A, Dakici I, Danzik I. Peroxynitrite: A putative cytotoxin. Pharm Toxicology. 1998;82:113–117. doi: 10.1111/j.1600-0773.1998.tb01408.x. [DOI] [PubMed] [Google Scholar]
- 119.Endo M, Oyadomari S, Terasaki Y, Takeya M, Suga M, Mori M, Gotoh T. Induction of arginase I and II in bleomycin-induced fibrosis of mouse lung. Am J Physiol Lung Cell Mol Physiol. 2003;285:L313–L321. doi: 10.1152/ajplung.00434.2002. [DOI] [PubMed] [Google Scholar]
- 120.Grasemann H, Schwiertz R, Matthiesen S, Racke K, Ratjen F. Increased arginase activity in cystic fibrosis airways. Am J Respir Crit Care Med. 2005;172:1523–1528. doi: 10.1164/rccm.200502-253OC. [DOI] [PubMed] [Google Scholar]
- 121.Morris CR. New Strategies for the Treatment of Pulmonary Hypertension in Sickle Cell Disease : The Rationale for Arginine Therapy. Treat Respir Med. 2006;5:31–45. doi: 10.2165/00151829-200605010-00003. [DOI] [PubMed] [Google Scholar]
- 122.Morris CR, Teehankee C, Kato G, Gardner J, McGlothlin D, Malloy M, DeMarco T. Decreased arginine bioavailability contributes to the pathogenesis of pulmonary artery hypertension; Orlando, Florida. American College of Cardiology Annual Meeting.2005. [Google Scholar]
- 123.Xu W, Kaneko TF, Zheng S, Comhair SA, Janocha AJ, Goggans T, Thunnissen FB, Farver C, Hazen SL, Jennings C, Dweik RA, Arroliga AC, Erzurum SC. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB. 2004;18:1746–1748. doi: 10.1096/fj.04-2317fje. [DOI] [PubMed] [Google Scholar]
- 124.Morris SM., Jr Regulation of enzymes of the urea cycle and arginine metabolism. Annu Rev Nutr. 2002;22:87–105. doi: 10.1146/annurev.nutr.22.110801.140547. [DOI] [PubMed] [Google Scholar]
- 125.Morris SM, Jr, Bhamidipati D, Kepka-Lenhart D. Human type II arginase: Sequence analysis and tissue-specific expression. Gene. 1997;193:157–161. doi: 10.1016/s0378-1119(97)00099-1. [DOI] [PubMed] [Google Scholar]
- 126.Kim P, Iyer R, Lu K, Yu H, Karimi A, Kern R, Tai D, Cederbaum S, Grody W. Expression of the liver form of arginase in erythrocytes. Mol Genet Metab. 2002;76:100–110. doi: 10.1016/s1096-7192(02)00034-3. [DOI] [PubMed] [Google Scholar]
- 127.Azizi E, Dror Y, Wallis K. Arginase activity in erythrocytes of healthy and ill children. Clin Chim Acta. 1970;28:391–396. doi: 10.1016/0009-8981(70)90063-x. [DOI] [PubMed] [Google Scholar]
- 128.Schnog JB, Jager EH, van der Dijs FP, Duits AJ, Moshage H, Muskiet FD, Muskiet FA. Evidence for a metabolic shift of arginine metabolism in sickle cell disease. Ann Hematol. 2004;83:371–375. doi: 10.1007/s00277-004-0856-9. [DOI] [PubMed] [Google Scholar]
- 129.VanderJagt DJ, Kanellis GJ, Isichei C, Pastuszyn A, Glew RH. Serum and urinary amino acid levels in sickle cell disease. J Trop Pediatr. 1997;43:220–225. doi: 10.1093/tropej/43.4.220. [DOI] [PubMed] [Google Scholar]
- 130.Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Morris CR, Singer ST, Walters MC. Clinical hemoglobinopathies: iron, lungs and new blood. Curr Opin Hematol. 2006;13:407–418. doi: 10.1097/01.moh.0000245685.24462.4e. [DOI] [PubMed] [Google Scholar]
- 132.Piatti G, Allegra L, Fasano V, Gambardella C, Bisaccia M, Cappellini MD. Lung function in beta-thalassemia patients: a longitudinal study. Acta Haematol. 2006;116:25–29. doi: 10.1159/000092344. [DOI] [PubMed] [Google Scholar]
- 133.Meurs H, Maarsingh H, Zaagsma J. Arginase and asthma: novel insights into nitric oxide homeostatis and airway hyperresponsiveness. Trends Pharmacol Sci. 2003;24:450–455. doi: 10.1016/S0165-6147(03)00227-X. [DOI] [PubMed] [Google Scholar]
- 134.Zimmermann N, King NE, Laporte J, Yoan M, Mishra A, Pope SM, Muntel EE, Witte DP, Pegg AA, Foster PS, Hamid Q, Rothenberg ME. Dissection of experimental asthma with DNA microarray analysis identifies arginase in asthma pathogenesis. J Clin Invest. 2003;111:1863–1874. doi: 10.1172/JCI17912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bryant R. Asthma in the pediatric sickle cell patient with acute chest syndrome. J Pediatr Health Care. 2005;19:157–162. doi: 10.1016/j.pedhc.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 136.Knight-Madden JM, Forrester TS, Lewis NA, Greenough A. Asthma in children with sickle cell disease and its association with acute chest syndrome. Thorax. 2005;60:206–210. doi: 10.1136/thx.2004.029165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Boyd JH, Moinuddin A, Strunk RC, DeBaun MR. Asthma and acute chest in sickle-cell disease. Pediatr Pulmonol. 2004;38:229–232. doi: 10.1002/ppul.20066. [DOI] [PubMed] [Google Scholar]
- 138.Koumbourlis AC, Zar HJ, Hurlet-Jensen A, Goldberg MR. Prevalence and reversibility of lower airway obstruction in children with sickle cell disease. J Pediatr. 2001;138:188–192. doi: 10.1067/mpd.2001.111824. [DOI] [PubMed] [Google Scholar]
- 139.Ricciardolo FL, Zaagsma J, Meurs H. The therapeutic potential of drugs targeting the arginase pathway in asthma. Expert Opin Investig Drugs. 2005;14:1221–1231. doi: 10.1517/13543784.14.10.1221. [DOI] [PubMed] [Google Scholar]
- 140.Rastogi D, Ngai P, Barst RJ, Koumbourlis AC. Lower airway obstruction, bronchial hyperresponsiveness, and primary pulmonary hypertension in children. Pediatr Pulmonol. 2004;37:50–55. doi: 10.1002/ppul.10363. [DOI] [PubMed] [Google Scholar]
- 141.Nordness ME, Lynn J, Zacharisen MC, Scott PJ, Kelly KJ. Asthma is a risk factor for acute chest syndrome and cerebral vascular accidents in children with sickle cell disease. Clin Mol Allergy. 2005;3:2. doi: 10.1186/1476-7961-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sylvester KP, Patey RA, Broughton S, Rafferty GF, Rees D, Thein SL, Greenough A. Temporal relationship of asthma to acute chest syndrome in sickle cell disease. Pediatr Pulmonol. 2007;42:103–106. doi: 10.1002/ppul.20430. [DOI] [PubMed] [Google Scholar]
- 143.Boyd JH, Macklin EA, Strunk RC, Debaun MR. Asthma is associated with increased mortality in patients with sickle cell anemia. Haematologica. 2007;92:1115–1118. doi: 10.3324/haematol.11213. [DOI] [PubMed] [Google Scholar]
- 144.New NHLBI guidelines for the diagnosis and management of asthma. National Heart, Lung and Blood Institute. Lippincott Health Promot Lett. 1997;2:8–9. 1. [PubMed] [Google Scholar]
- 145.Rastogi D, Shetty A, Neugebauer R, Harijith A. National Heart, Lung, and Blood Institute guidelines and asthma management practices among inner-city pediatric primary care providers. Chest. 2006;129:619–623. doi: 10.1378/chest.129.3.619. [DOI] [PubMed] [Google Scholar]
- 146.Zharikov S, Block E. Association of L-arginine transporters with fodrin: implications for hypoxic inhibition of arginine uptake. Am J Physiol Lung Cell Mol Physiol. 2000;278:L111–L117. doi: 10.1152/ajplung.2000.278.1.L111. [DOI] [PubMed] [Google Scholar]
- 147.Graf P, Forstermann U, Closs E. The transport activity of the human cationic amino acid transporter hCAT-1 is downregulated by activation of protein kinase C. Br J Pharmacol. 2001;132:1193–1200. doi: 10.1038/sj.bjp.0703921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Raghavachari N, Xu X, Harris A, Villagra J, Logun C, Barb J, Solomon MA, Suffredini AF, Danner RL, Kato G, Munson PJ, Morris SM, Jr, Gladwin MT. Amplified Expression Profiling of Platelet Transcriptome Reveals Changes in Arginine Metabolic Pathways in Patients With Sickle Cell Disease. Circulation. 2007 doi: 10.1161/CIRCULATIONAHA.106.658641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Helley D, Eldor A, Girot R, Ducrocq R, Guillin MC, Bezeaud A. Increased procoagulant activity of red blood cells from patients with homozygous sickle cell disease and beta-thalassemia. Thromb Haemost. 1996;76:322–327. [PubMed] [Google Scholar]
- 150.Peters M, Plaat BE, ten Cate H, Wolters HJ, Weening RS, Brandjes DP. Enhanced thrombin generation in children with sickle cell disease. Thromb Haemost. 1994;71:169–172. [PubMed] [Google Scholar]
- 151.Ming XF, Barandier C, Viswambharan H, Kwak BR, Mach F, Mazzolai L, Hayoz D, Ruffieux J, Rusconi S, Montani JP, Yang Z. Thrombin stimulates human endothelial arginase enzymatic activity via RhoA/ROCK pathway: implications for atherosclerotic endothelial dysfunction. Circulation. 2004;110:3708–3714. doi: 10.1161/01.CIR.0000142867.26182.32. [DOI] [PubMed] [Google Scholar]
- 152.Topal G, Brunet A, Walch L, Boucher JL, David-Dufilho M. Mitochondrial arginase II modulates nitric-oxide synthesis through nonfreely exchangeable L-arginine pools in human endothelial cells. J Pharmacol Exp Ther. 2006;318:1368–1374. doi: 10.1124/jpet.106.103747. [DOI] [PubMed] [Google Scholar]
- 153.Yang L, Lewis CM, Chandrasekharan UM, Kinney CM, Dicorleto PE, Kashyap VS. Arginase activity is increased by thrombin: a mechanism for endothelial dysfunction in arterial thrombosis. J Am Coll Surg. 2006;203:817–826. doi: 10.1016/j.jamcollsurg.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 154.Durante W, Liao L, Peyton KJ, Schafer AI. Thrombin stimulates vascular smooth muscle cell polyamine synthesis by inducing cationic amino acid transporter and ornithine decarboxylase gene expression. Circ Res. 1998;83:217–223. doi: 10.1161/01.res.83.2.217. [DOI] [PubMed] [Google Scholar]
- 155.Ataga KI, Moore CG, Hillery CA, Jones S, Whinna HC, Strayhorn D, Sohier C, Hinderliter A, Parise LV, Orringer EP. Coagulation activation and inflammation in sickle cell disease-associated pulmonary hypertension. Haematologica. 2008;93:20–26. doi: 10.3324/haematol.11763. [DOI] [PubMed] [Google Scholar]
- 156.Belfiore F. Enzymatic activities of the blood serum in thalassemia and in thalassodrepanocytosis. Riforma Med. 1964;78:1052–1055. [PubMed] [Google Scholar]
- 157.Reynolds J, Follette JH, Valentine WH. The arginase activity of erythrocytes and leukocytes with particular reference to pernicious anemia and thalassemia major. J Lab Clin Med. 1957;50:78–92. [PubMed] [Google Scholar]
- 158.Enwonwu CO. Increased metabolic demand for arginine in sickle cell anaemia. Med Sci Res. 1989;17:997–998. [Google Scholar]
- 159.Enwonwu CO, Xu X, Turner E. Nitrogen metabolism in sickle cell anemia: Free amino acids in plasma and urine. Am J Med Sci. 1990;300:366–371. doi: 10.1097/00000441-199012000-00005. [DOI] [PubMed] [Google Scholar]
- 160.Waugh W, Daeschner C, Files B, Gordon D. Evidence that L-arginine is a key amino acid in sickle cell anemia - a preliminary report. Nutritional Research. 1999;19:501–518. [Google Scholar]
- 161.Mendes Ribeiro AC, Brunini TM. L-arginine transport in disease. Curr Med Chem Cardiovasc Hematol Agents. 2004;2:123–131. doi: 10.2174/1568016043477288. [DOI] [PubMed] [Google Scholar]
- 162.Morris CR, Kuypers FA, Larkin S, Sweeter N, Simon J, Vichinsky EP, Styles L. Arginine therapy: A novel strategy to increase nitric oxide production in sickle cell disease. Brit J Haematol. 2000;111:498–500. doi: 10.1046/j.1365-2141.2000.02403.x. [DOI] [PubMed] [Google Scholar]
- 163.Lopez B, Kreshak A, Morris CR, Davis-Moon L, Ballas S, Ma X. L-arginine levels are diminished in adult acute vaso-occlusive sickle cell crisis in the emergency department. Br J Haematol. 2003;120:532–534. doi: 10.1046/j.1365-2141.2003.04109.x. [DOI] [PubMed] [Google Scholar]
- 164.Berka V, Yeh HC, Gao D, Kiran F, Tsai AL. Redox function of tetrahydrobiopterin and effect of L-arginine on oxygen binding in endothelial nitric oxide synthase. Biochemistry. 2004;43:13137–13148. doi: 10.1021/bi049026j. [DOI] [PubMed] [Google Scholar]
- 165.Berka V, Wu G, Yeh HC, Palmer G, Tsai AL. Three different oxygen-induced radical species in endothelial nitric-oxide synthase oxygenase domain under regulation by L-arginine and tetrahydrobiopterin. J Biol Chem. 2004;279:32243–32251. doi: 10.1074/jbc.M404044200. [DOI] [PubMed] [Google Scholar]
- 166.Xia Y, Dawson V, Dawson T, Snyder S, Zweier J. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc Natl Acad Sci. 1996;93:6770–6774. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Heinzel B, John M, Klatt P, Bohme E, Mayer B. Ca2+/calmodulin-dependent formation of hydrogen peroxide by brain nitric oxide synthase. Biochem J. 1992;281:627–630. doi: 10.1042/bj2810627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wood KC, Hebbel RP, Lefer DJ, Granger DN. Critical role of endothelial cell-derived nitric oxide synthase in sickle cell disease-induced microvascular dysfunction. Free Radic Biol Med. 2006;40:1443–1453. doi: 10.1016/j.freeradbiomed.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 169.Li H, Witte K, August M, Brausch I, Godtel-Armbrust U, Habermeier A, Closs EI, Oelze M, Munzel T, Forstermann U. Reversal of endothelial nitric oxide synthase uncoupling and up-regulation of endothelial nitric oxide synthase expression lowers blood pressure in hypertensive rats. J Am Coll Cardiol. 2006;47:2536–2544. doi: 10.1016/j.jacc.2006.01.071. [DOI] [PubMed] [Google Scholar]
- 170.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 171.Moens AL, Kass DA. Tetrahydrobiopterin and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2006;26:2439–2444. doi: 10.1161/01.ATV.0000243924.00970.cb. [DOI] [PubMed] [Google Scholar]
- 172.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Khoo JP, Zhao L, Alp NJ, Bendall JK, Nicoli T, Rockett K, Wilkins MR, Channon KM. Pivotal role for endothelial tetrahydrobiopterin in pulmonary hypertension. Circulation. 2005;111:2126–2133. doi: 10.1161/01.CIR.0000162470.26840.89. [DOI] [PubMed] [Google Scholar]
- 174.Osei S, Ahima j, Fabry M, Nagel R, Bank N. Immunohistochemical localization of hempatic nitric oxide synthase in normal and transgenic sickle cell mice: The effect of hypoxia. Blood. 1996;88:3583–3588. [PubMed] [Google Scholar]
- 175.Bank N, Aynedjian H, Qiu J, Osei S, Ahima R, Fabry M, Nagel R. Renal nitric oxide synthases in transgenic sickle cell mice. Kidney Int. 1996;50:184–189. doi: 10.1038/ki.1996.301. [DOI] [PubMed] [Google Scholar]
- 176.Wu G, Meininger CJ, Knabe DA, Bazer FW, Rhoads JM. Arginine nutrition in development, health and disease. Curr Opin Clin Nutr Metab Care. 2000;3:59–66. doi: 10.1097/00075197-200001000-00010. [DOI] [PubMed] [Google Scholar]
- 177.Hallemeesch MM, Lamers WH, Deutz NE. Reduced arginine availability and nitric oxide production. Clin Nutr. 2002;21:273–279. doi: 10.1054/clnu.2002.0571. [DOI] [PubMed] [Google Scholar]
- 178.Wu G. Intestinal mucosal amino acid catabolism. J Nutr. 1998;128:1249–1252. doi: 10.1093/jn/128.8.1249. [DOI] [PubMed] [Google Scholar]
- 179.Closs EI, Mann GE. Membrane transport of L-arginine and cationic amino acid analogs. San Diego: Academic Press; 2000. pp. 225–241. [Google Scholar]
- 180.Vallance P. The asymmetrical dimethylarginine/dimethylarginine dimethylaminohydrolase pathway in the regulation of nitric oxide generation. Clin Sci. 2001;100:159–60. [PubMed] [Google Scholar]
- 181.Cooke JP, Mont-Reynaud R, Tsao PS, Maxwell AJ. Nitric oxide and vascular disease. In: Ignarro LJ, editor. Nitric Oxide: Biology and Pathology. Academic Press; 2000. pp. 759–783. New Your. [Google Scholar]
- 182.Schnog JB, Teerlink T, van der Dijs FP, Duits AJ, Muskiet FA. Plasma levels of asymmetric dimethylarginine (ADMA), an endogenous nitric oxide synthase inhibitor, are elevated in sickle cell disease. Ann Hematol. 2005;84:282–286. doi: 10.1007/s00277-004-0983-3. [DOI] [PubMed] [Google Scholar]
- 183.Leiper J, Nandi M, Torondel B, Murray-Rust J, Malaki M, O’Hara B, Rossiter S, Anthony S, Madhani M, Selwood D, Smith C, Wojciak-Stothard B, Rudiger A, Stidwill R, McDonald NQ, Vallance P. Disruption of methylarginine metabolism impairs vascular homeostasis. Nat Med. 2007;13:198–203. doi: 10.1038/nm1543. [DOI] [PubMed] [Google Scholar]
- 184.Toth J, Racz A, Kaminski PM, Wolin MS, Bagi Z, Koller A. Asymmetrical Dimethylarginine Inhibits Shear Stress-Induced Nitric Oxide Release and Dilation and Elicits Superoxide-Mediated Increase in Arteriolar Tone. Hypertension. 2007;49:563–568. doi: 10.1161/01.HYP.0000256764.86208.3d. [DOI] [PubMed] [Google Scholar]
- 185.Meinitzer A, Seelhorst U, Wellnitz B, Halwachs-Baumann G, Boehm BO, Winkelmann BR, Marz W. Asymmetrical dimethylarginine independently predicts total and cardiovascular mortality in individuals with angiographic coronary artery disease (the ludwigshafen risk and cardiovascular health study) Clin Chem. 2007;53:273–283. doi: 10.1373/clinchem.2006.076711. [DOI] [PubMed] [Google Scholar]
- 186.Matsuguma K, Ueda S, Yamagishi S, Matsumoto Y, Kaneyuki U, Shibata R, Fujimura T, Matsuoka H, Kimoto M, Kato S, Imaizumi T, Okuda S. Molecular mechanism for elevation of asymmetric dimethylarginine and its role for hypertension in chronic kidney disease. J Am Soc Nephrol. 2006;17:2176–2183. doi: 10.1681/ASN.2005121379. [DOI] [PubMed] [Google Scholar]
- 187.Schulze F, Lenzen H, Hanefeld C, Bartling A, Osterziel KJ, Goudeva L, Schmidt-Lucke C, Kusus M, Maas R, Schwedhelm E, Strodter D, Simon BC, Mugge A, Daniel WG, Tillmanns H, Maisch B, Streichert T, Boger RH. Asymmetric dimethylarginine is an independent risk factor for coronary heart disease: results from the multicenter Coronary Artery Risk Determination investigating the Influence of ADMA Concentration (CARDIAC) study. Am Heart J. 2006;152:e1–e8. doi: 10.1016/j.ahj.2006.06.005. 493. [DOI] [PubMed] [Google Scholar]
- 188.Kielstein JT, Bode-Boger SM, Hesse G, Martens-Lobenhoffer J, Takacs A, Fliser D, Hoeper MM. Asymmetrical dimethylarginine in idiopathic pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol. 2005;25:1414–1418. doi: 10.1161/01.ATV.0000168414.06853.f0. [DOI] [PubMed] [Google Scholar]
- 189.Pullamsetti S, Kiss L, Ghofrani HA, Voswinckel R, Haredza P, Klepetko W, Aigner C, Fink L, Muyal JP, Weissmann N, Grimminger F, Seeger W, Schermuly RT. Increased levels and reduced catabolism of asymmetric and symmetric dimethylarginines in pulmonary hypertension. Faseb J. 2005;19:1175–1177. doi: 10.1096/fj.04-3223fje. [DOI] [PubMed] [Google Scholar]
- 190.Sydow K, Munzel T. ADMA and oxidative stress. Atheroscler Suppl. 2003;4:41–51. doi: 10.1016/s1567-5688(03)00033-3. [DOI] [PubMed] [Google Scholar]
- 191.Black SM, Fineman JR. Oxidative and nitrosative stress in pediatric pulmonary hypertension: roles of endothelin-1 and nitric oxide. Vascul Pharmacol. 2006;45:308–316. doi: 10.1016/j.vph.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 192.Bowers R, Cool C, Murphy RC, Tuder RM, Hopken MW, Flores SC, Voelkel NF. Oxidative stress in severe pulmonary hypertension. Am J Respir Crit Care Med. 2004;169:764–769. doi: 10.1164/rccm.200301-147OC. [DOI] [PubMed] [Google Scholar]
- 193.Klings ES, Safaya S, Adewoye AH, Odhiambo A, Frampton G, Lenburg M, Gerry N, Sebastiani P, Steinberg MH, Farber HW. Differential gene expression in pulmonary artery endothelial cells exposed to sickle cell plasma. Physiol Genomics. 2005;21:293–298. doi: 10.1152/physiolgenomics.00246.2004. [DOI] [PubMed] [Google Scholar]
- 194.Aslan M, Freeman BA. Oxidant-mediated impairment of nitric oxide signaling in sickle cell disease--mechanisms and consequences. Cell Mol Biol (Noisy-le-grand) 2004;50:95–105. [PubMed] [Google Scholar]
- 195.Hebbel RP, Eaton JW, Balasingam M, Steinberg MH. Spontaneous oxygen radical generation by sickle erythrocytes. J Clin Invest. 1982;70:1253–1259. doi: 10.1172/JCI110724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chakraborty D, Bhattacharyya M. Antioxidant defense status of red blood cells of patients with beta-thalassemia and Ebeta-thalassemia. Clin Chim Acta. 2001;305:123–129. doi: 10.1016/s0009-8981(00)00428-9. [DOI] [PubMed] [Google Scholar]
- 197.Cheng ML, Ho HY, Tseng HC, Lee CH, Shih LY, Chiu DT. Antioxidant deficit and enhanced susceptibility to oxidative damage in individuals with different forms of alpha-thalassaemia. Br J Haematol. 2005;128:119–127. doi: 10.1111/j.1365-2141.2004.05257.x. [DOI] [PubMed] [Google Scholar]
- 198.Reid ME, Badaloo A, Forrester T, Jahoor F. In vivo rates of erythrocyte glutathione synthesis in adults with Sickle Cell Disease. Am J Physiol Endocrinol Metab. 2006;291:E73–9. doi: 10.1152/ajpendo.00287.2005. [DOI] [PubMed] [Google Scholar]
- 199.Fujii S, Kaneko T. Selenium, glutathione peroxidase and oxidative hemolysis in sickle cell disease. Acta Haematol. 1992;87:105–106. doi: 10.1159/000204730. [DOI] [PubMed] [Google Scholar]
- 200.Saad S, Salles SI, Velho PE. Decreased reduced glutathione and glutathione reductase activity in subjects with hemoglobin C. Nouv Rev Fr Hematol. 1991;33:11–14. [PubMed] [Google Scholar]
- 201.Adelekan DA, Thurnham DI, Adekile AD. Reduced antioxidant capacity in paediatric patients with homozygous sickle cell disease. Eur J Clin Nutr. 1989;43:609–614. [PubMed] [Google Scholar]
- 202.Varma RN, Mankad VN, Phelps DD, Jenkins LD, Suskind RM. Depressed erythrocyte glutathione reductase activity in sickle cell disease. Am J Clin Nutr. 1983;38:884–887. doi: 10.1093/ajcn/38.6.884. [DOI] [PubMed] [Google Scholar]
- 203.Wetterstroem N, Brewer GJ, Warth JA, Mitchinson A, Near K. Relationship of glutathione levels and Heinz body formation to irreversibly sickled cells in sickle cell anemia. J Lab Clin Med. 1984;103:589–596. [PubMed] [Google Scholar]
- 204.Morris CR, Suh JH, Hagar W, Larkin S, Bland DA, Steinberg MH, Vichinsky EP, Shigenaga M, Ames B, Kuypers FA, Klings ES. Erythrocyte Glutamine Depletion, Altered Redox Environment, and Pulmonary Hypertension in Sickle Cell Disease. Blood. 2007 doi: 10.1182/blood-2007-04-081703. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Grobe AC, Wells SM, Benavidez E, Oishi P, Azakie A, Fineman JR, Black SM. Increased oxidative stress in lambs with increased pulmonary blood flow and pulmonary hypertension: role of NADPH oxidase and endothelial NO synthase. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1069–L1077. doi: 10.1152/ajplung.00408.2005. [DOI] [PubMed] [Google Scholar]
- 206.Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med. 1999;27:922–935. doi: 10.1016/s0891-5849(99)00176-8. [DOI] [PubMed] [Google Scholar]
- 207.Niihara Y, Matsui NM, Shen YM, Akiyama DA, Johnson CS, Sunga MA, Magpayo J, Embury SH, Kalra VK, Cho SH, Tanaka KR. L-glutamine therapy reduces endothelial adhesion of sickle red blood cells to human umbilical vein endothelial cells. BMC Blood Disord. 2005;5:4. doi: 10.1186/1471-2326-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Niihara Y, Zerez CR, Akiyama DS, Tanaka KR. Oral L-glutamine therapy for sickle cell anemia: I. Subjective clinical improvement and favorable change in red cell NAD redox potential. Am J Hematol. 1998;58:117–121. doi: 10.1002/(sici)1096-8652(199806)58:2<117::aid-ajh5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 209.Osarogiagbon UR, Choong S, Belcher JD, Vercellotti GM, Paller MS, Hebbel RP. Reperfusion injury pathophysiology in sickle transgenic mice. Blood. 2000;96:314–320. [PubMed] [Google Scholar]
- 210.Belcher JD, Marker PH, Weber JP, Hebbel RP, Vercellotti GM. Activated monocytes in sickle cell disease: potential role in the activation of vascular endothelium and vaso-occlusion. Blood. 2000;96:2451–2459. [PubMed] [Google Scholar]
- 211.Belcher JD, Mahaseth H, Welch TE, Vilback AE, Sonbol KM, Kalambur VS, Bowlin PR, Bischof JC, Hebbel RP, Vercellotti GM. Critical role of endothelial cell activation in hypoxia-induced vasoocclusion in transgenic sickle mice. Am J Physiol Heart Circ Physiol. 2005;288:H2715–H2725. doi: 10.1152/ajpheart.00986.2004. [DOI] [PubMed] [Google Scholar]
- 212.Nath KA, Grande JP, Haggard JJ, Croatt AJ, Katusic ZS, Solovey A, Hebbel RP. Oxidative stress and induction of heme oxygenase-1 in the kidney in sickle cell disease. Am J Pathol. 2001;158:893–903. doi: 10.1016/S0002-9440(10)64037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Jison ML, Gladwin MT. Hemolytic anemia-associated pulmonary hypertension of sickle cell disease and the nitric oxide/arginine pathway. Am J Respir Crit Care Med. 2003;168:3–4. doi: 10.1164/rccm.2304002. [DOI] [PubMed] [Google Scholar]
- 214.Machado RF, Martyr S, Kato GJ, Barst RJ, Anthi A, Robinson MR, Hunter L, Coles W, Nichols J, Hunter C, Sachdev V, Castro O, Gladwin MT. Sildenafil therapy in patients with sickle cell disease and pulmonary hypertension. Br J Haematol. 2005;130:445–453. doi: 10.1111/j.1365-2141.2005.05625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]