Abstract
Estradiol (E2) is an important modifier of the activity of the fetal hypothalamus-pituitary-adrenal axis. We have reported that estradiol-3-sulfate (E2SO4) circulates in fetal blood in far higher concentrations than E2 and that the fetal brain expresses steroid sulfatase, required for local deconjugation of E2SO4. We performed the present study to test the hypothesis that chronic infusion of E2SO4 chronically increases ACTH and cortisol secretion and that it shortens gestation. Chronically catheterized fetal sheep were treated with E2SO4 intracerebroventricular (n = 5), E2SO4 iv (n = 4), or no steroid infusion (control group, n = 5). Fetuses were subjected to arterial blood sampling every other day until spontaneous birth for plasma hormone analysis. Treatment with E2SO4 attenuated preparturient increases in ACTH secretion near term without affecting the ontogenetic rise in plasma cortisol. Infusion of E2SO4 intracerebroventricularly significantly increased plasma E2, plasma E2SO4, and plasma progesterone and shortened gestation compared with all other groups. These results are consistent with the conclusion that E2SO4: 1) interacts with the hypothalamus-pituitary-adrenal axis primarily by stimulating cortisol secretion and inhibiting ACTH and pro-ACTH secretion by negative feedback; and 2) stimulates the secretion of E2 and E2SO4. We conclude that the endocrine response to E2SO4 in the fetus is not identical with the response to E2.
In fetal sheep, the hypothalamus-pituitary-adrenal (HPA) axis is a central component of the fetal stress response and plays a central role in the initiation of parturition (1–4). The activity of the fetal HPA axis is influenced by both physiological (blood gases, blood pressure, etc.) and endocrine variables. We have previously reported that estradiol (E2) is a potent stimulator of the fetal HPA axis, increasing neuronal activity in brain regions known to control HPA axis function and increasing the expression of genes within the fetal brain that are known to be important for HPA axis activity (5–8). The effect of E2 on HPA axis activity in the fetus mirrors the effect of E2 and other estrogens in adult animals. Estrogen has been shown to augment HPA activity in adult rats. Female rats have higher basal and stimulated corticosterone than males (9) and exert maximum responses to stress during proestrus, when plasma estrogen is elevated (10–14). Ovariectomy attenuates adrenal steroid production in response to stress; however, activity of the HPA axis is ameliorated after hormone replacement with E2 (15, 16).
The major forms of estrogen circulating in fetal plasma are estrone-3-sulfate (E1SO4) and estradiol-3-sulfate (E2SO4) (17, 18). These sulfoconjugated forms of estrogen are not known to be directly biologically active. Sulfoconjugated steroids are abundant in fetal plasma (18, 19) and are able to bind estrogen receptors within target tissues only after deconjugation by the enzyme steroid sulfatase (STS) (20, 21). Accordingly, we hypothesized and demonstrated the presence of both STS and estrogen sulfotransferase (STF) in the fetal brain, allowing for bidirectional conversion of the conjugated and unconjugated forms of the steroids within an important target tissue (22, 23). We have also demonstrated that short-term infusion of E2SO4 into fetal plasma increases fetal HPA axis activity and stimulates cellular responses in the fetal brain that are consistent with increased neuronal activity (18, 24).
Although we have demonstrated that relatively short infusions of E2SO4 augment fetal HPA axis activity, we could not conclude that long-term exposure to elevated levels of E2SO4 produced persistent endocrine responses. We therefore hypothesized that infusion of E2SO4 into either the vascular compartment or the central nervous system of the fetal sheep produce persistent increases in fetal ACTH and cortisol concentrations. The present study was designed to test this hypothesis.
Materials and Methods
Fourteen pregnant ewes with time-dated singleton fetuses (120–125 d gestation) were used in this study. They were divided into three experimental groups: control (n = 5), E2SO4 infusion intracerebroventricular (icv; 1 mg/d, n = 5), and E2SO4 infusion iv (1 mg/d, n = 4). Plasma hormone data for the fetuses in the control group have been published previously (25). The three groups reported in this study were performed at the same time as a test of estrogen receptor blockade (25). The control group is therefore a time- and conditions-matched control for both studies except for the position of the infusion (into the lateral cerebral ventricle). All experiments were approved by the University of Florida Institutional Animal Care and Use Committee and were performed in accordance with the American Physiological Society's Guiding Principles of the Care and Use of Animals (26). Animals were housed in individual pens and were given access to food and water ad libitum.
Surgical preparation
Ewes were fasted for 24 h before surgery. Ewes were intubated and anesthetized with halothane (0.5–2%) in oxygen during surgery. Before the start of surgery, 750 mg ampicillin (Polyflex; Fort Dodge Laboratories, Fort Dodge, IA) was administered im. The uterus was exposed through a midline incision and the fetal hindlimbs were located by palpation. The hindlimbs were delivered through a small incision in the uterus. Vascular catheters were inserted into fetal tibial arteries and saphenous veins, and the catheter tips were advanced to the femoral arteries and veins, as described previously (25, 27). The catheters were filled with heparin to prevent clotting and closed at the end using a sterile brass nail. An additional catheter (outside diameter 0.09 in.; inside diameter, 0.05 in.) was sewn to the skin of one fetal hindlimb for access to amniotic fluid. Fetuses in the E2SO4 iv group received an infusion of E2SO4 into one of the venous catheters using an osmotic minipump (size 2mL4; Alza Corp., Palo Alto, CA). Fetuses receiving icv infusions were instrumented with arterial catheters and an extra catheter placed in the lateral cerebral ventricle as previously described (28). E2SO4 was infused using an osmotic minipump (size 2mL4; Alza). Antibiotics (750 mg ampicillin) were administered into the amniotic cavity via direct injection.
Postoperatively, ewes were given 1 mg/kg flunixin meglumine (Webster Veterinary, Sterling, MA) for analgesia and returned to their pens in which they were monitored until they could stand on their own. Twice daily during a 5-d recovery period, ewes were given antibiotic (ampicillin, 750 mg, im) and rectal temperatures were monitored for indication of postoperative infection.
Blood collection
After the recovery period, fetal blood samples were drawn from the arterial catheter every other morning (between 0800 and 1000 h) for use in hormone assays. Samples were kept on ice until centrifuged at 3000 × g for 15 min at 4 C to separate red blood cells and plasma. Plasma was stored at −20 C until analysis. Blood gases were measured at the time of blood sampling using an ABL 77 radiometer (Radiometer America Inc., Cleveland, OH) blood gas analyzer.
Plasma hormone assays
ACTH1–39 was measured using a two-site immunoradiometric assay purchased from DiaSorin (Stillwater, MN; catalog no. 27130). Proopiomelanocortin (POMC) and pro-ACTH (reported herein as POMC) was measured using an ELISA kit from Immunodiagnostic Systems Ltd. (Fountain Hills, AZ; catalog no. AC-71F1) according to the manufacturer's instructions. Cross-reactivity with POMC and pro-ACTH is 100%, as reported by the manufacturer.
Plasma E2 and E2SO4 were measured using the estradiol ELISA kit from Oxford Biomedical Research (Oxford, MI; catalog no. EA70) as previously reported (18). E2 was extracted from plasma using hexane and ethyl acetate (3:2), and E2SO4 was extracted from plasma using ethanol (18). Cross-reactivity with 17β-estradiol, estriol, and estrone in this kit is 100, 0.41, and 0.10%, respectively, as reported by the manufacturer. Cross-reactivity with E2SO4 is 100%, as we have reported previously (18). Plasma estrone (E1) was measured using the estrone EIA kit from Cayman Chemical (Ann Arbor, MI; catalog no. 582301). E1 was extracted from plasma using ethanol, as described for the estradiol assay. Cross-reactivity with estrone, E1SO4, estrone-3-glucuronide, cortisol, and 17β-estradiol are 100, 100, 100, less than 0.01, and less than 0.01%, respectively. Values reported from this assay represent total E1 (unconjugated plus conjugated E1).
Plasma cortisol was measured using a cortisol ELISA kit from Oxford Biomedical Research (catalog no. EA65). Cortisol was extracted using ethanol, as described previously (29). Cross-reactivity with cortisol, cortisone, 11-deoxycortisol, and corticosterone in this kit is 100, 15.77, 15, and 4.81%, respectively, as reported by the manufacturer. Dehydroepiandrosterone sulfate (DHAS) was measured with a 125I-DHEA-SO4 Coat-A-Count kit from Diagnostic Products Corp. (Los Angeles, CA; catalog no. TKDS5). Percent cross-reactivity with DHAS, dehydroepiandrosterone, and E1SO4 was 100, 0.57 and 0.25%, respectively, as reported by the manufacturer. Progesterone was measured with a 125I-Progesterone Coat-A-Count kit from Diagnostic Products (catalog no. TKPG5) according to the manufacturer's instructions.
Statistics and estimation of secretion rates
Two-way ANOVA was used to analyze differences among treatment groups in this study. One-way ANOVA was used to test the effect of time within each treatment group. Pairwise multiple comparisons were performed using the Student-Newman-Keuls multiple-range test. Effect of treatment on day of parturition was analyzed using the modified Wilcoxon method for survival analysis (30). SPSS version 17 (SPSS Corp., Chicago, IL) was used for analyses. A significance level of P < 0.05 was used to reject the null hypothesis. Values are reported as mean ± sem. To estimate endogenous secretion rates for E2 and E2SO4, metabolic clearance rates (MCR) were calculated for each hormone using infusion rates and changes in plasma concentration from this (for E2SO4) and one previous (for E2) study from this laboratory (27) using the following formula: MCR = (infusion rate)/(change in plasma concentration at steady state).
Results
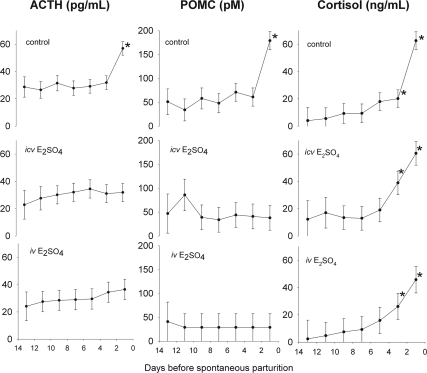
In the control group, we measured an increase in fetal ACTH1-39, POMC, and cortisol (Fig. 1) before parturition. ACTH1-39 concentrations were not different among groups (P = NS by ANOVA), whereas POMC was significantly lower in the iv and icv groups compared with the control group (P < 0.001 for main effect of group in ANOVA and P < 0.05 by Student Newman-Keuls test for comparison of groups). Despite the lack of overall significant differences among groups for ACTH1-39, there was a statistically significant (P < 0.05 by simple effects contrast) increase in plasma ACTH1-39 concentration in the control group but not in the other experimental groups. Although there was no preparturient increase in plasma concentrations of ACTH1-39 and POMC in the iv and icv groups near term and although the increase in ACTH1-39 concentration was limited to the last measurement in utero, fetal plasma cortisol concentrations increased markedly in all groups before parturition (Fig. 1). Fetal plasma cortisol was constant until d −7 when it began to increase until the end of gestation in all experimental groups. There were no significant differences in plasma cortisol among groups. The trend for increased cortisol over time was statistically significant by two-way ANOVA (P < 0.001 for main effect of gestational age), although there was no statistically significant difference between groups (P = NS for main effect of group in ANOVA).
Fig. 1.
Plasma ACTH1-39 (left panels), POMC (middle panels), and cortisol (right panels) concentrations in chronically catheterized singleton ovine fetuses throughout the last 13 d of gestation. Fetal sheep were treated with no steroid infusion (control, top panels), estradiol sulfate icv (middle panels), estradiol sulfate iv (bottom panels). Values are represented as mean ± sem. Asterisks denote statistically significant change from −14 d.
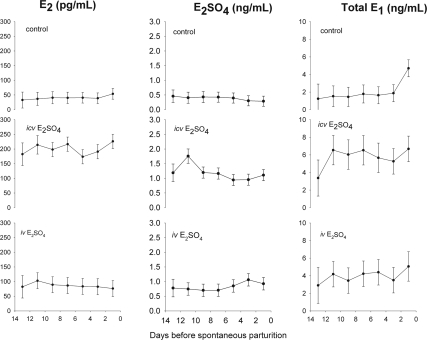
Plasma concentrations of E2, E2SO4, and total (sulfoconjugated plus unconjugated) E1, (Fig. 2) did not increase significantly before parturition as analyzed by one- and two-way ANOVA. Infusion of E2SO4 by both routes increased fetal plasma concentrations of E1, E2, and E2SO4 (P < 0.001 for main effect of group for all three hormones; Fig. 2). Plasma concentrations of E1, E2, and E2SO4 were significantly higher in the icv group than in the iv or control groups (P < 0.05 by Student-Newman-Keuls test), and plasma concentrations of E2 and E2SO4 were significantly higher in the iv group than in the control group (P < 0.05 by Student-Newman-Keuls test). Values of plasma E2 concentration in the icv group were approximately doubled compared with fetuses in the iv group (197.0 ± 9.9 vs. 82.3 ± 10.5 pg/ml) and increased approximately 6-fold compared with control fetuses (32.3 ± 8.5 pg/ml). Similar to E2, increases in plasma E2SO4 concentrations were highest in the icv group. Infusion of E2SO4 significantly reduced the ratio of E2SO4 to E2 (P < 0.001, two way ANOVA). Plasma progesterone (P4) concentrations were highest in the icv group (P < 0.001for main effect of group in ANOVA; P < 0.05 comparing control with icv for P4 by Student-Newman-Keuls test). E2SO4 infusion icv significantly increased plasma P4 concentrations beginning on d −15, peaking at d −9 (10.4 ± 1.9 ng/ml), and remained elevated relative to other experimental groups until term. The plasma E2 to P4 ratio was significantly different among groups (P < 0.001, two way ANOVA). The E2 to P4 ratio increased after the E2SO4 treatment iv (0.018 ± 0.0026) and icv (0.029 ± 0.0025) relative to control (0.0071 ± 0.0021) fetuses. The E2SO4 treatment significantly decreased the E2SO4 to E2 ratio (9.9 ± 1.2 iv and 5.4 ± 1.1 icv) relative to control fetuses (12.0 ± 1.0).
Fig. 2.
Plasma E2 (left panels), E2SO4 (middle panels), and total (conjugated plus unconjugated) E1 (right panels) concentrations in chronically catheterized singleton ovine fetuses throughout the last 13 d of gestation. Fetal sheep were treated with no steroid infusion (control, top panels), estradiol sulfate icv (middle panels), estradiol sulfate iv (bottom panels). Values are represented as mean ± sem.
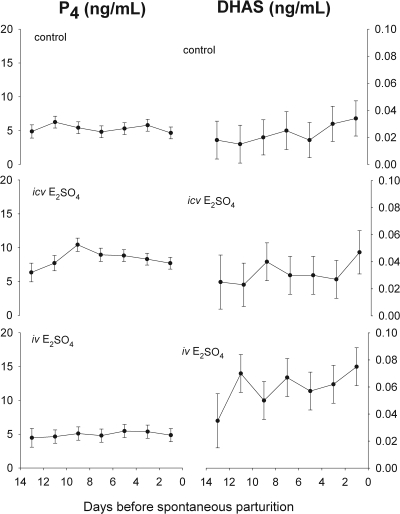
The plasma DHAS concentrations were significantly different among the experimental groups (P < 0.001, the main effect of group in two way ANOVA; Fig. 3). DHAS did not change as a function of gestational age (P = NS for gestational age in ANOVA) but was higher in the E2SO4 iv-treated fetuses (0.026 ± 0.003, 0.031 ± 0.003, and 0.058 ± 0.008 ng/ml in the control, icv, and iv groups, respectively; P < 0.05 by Student-Newman-Keuls test).
Fig. 3.
Plasma P4 (left panels) and DHAS (right panels) concentrations in chronically catheterized singleton ovine fetuses throughout the last 13 d of gestation. Fetal sheep were treated with no steroid infusion (control, top panels), estradiol sulfate icv (middle panels), estradiol sulfate iv (bottom panels). Values are represented as mean ± sem.
Fetal arterial blood gases and pH in all three experimental groups were consistent with known values for chronically catheterized fetuses (Table 1). There were no significant differences in PaO2 among groups. PaCO2 was decreased in the E2SO4 iv and icv groups (P < 0.001 for the main effect of the group in ANOVA; P < 0.05 control > icv > iv using Student-Newman-Keuls test). pHa was marginally significantly different among the experimental groups (P = 0.05 for main effect of group in ANOVA), but post hoc analysis by Student-Newman-Keuls test did not identify individual differences among groups.
Table 1.
Fetal blood gases and pHa
| Treatment group | PaO2 (mm Hg) | PaCO2 (mm Hg) | pHa |
|---|---|---|---|
| Control (n = 5) | 19.0 ± 0.7 | 59.6 ± 0.6 | 7.32 ± 0.01 |
| E2SO4 iv (n = 4) | 18.4 ± 0.8 | 55.4 ± 0.7b | 7.33 ± 0.01 |
| E2SO4 icv (n = 5) | 18.5 ± 0.8 | 57.9 ± 0.7 | 7.32 ± 0.01 |
Mean values ± sem were calculated using the mean value of arterial oxygen and carbon dioxide partial pressures and pH (PaO2, PaCO2, and pHa, respectively).
P < 0.001 compared with control.
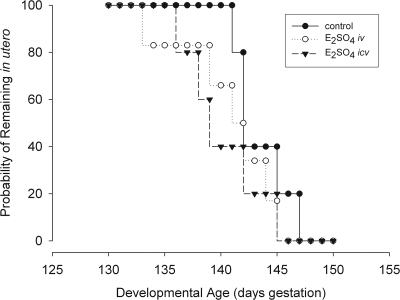
Treatment of fetal sheep with icv E2SO4 advanced the day of spontaneous parturition slightly but significantly (Fig. 4). Survival analysis of gestation length revealed a highly significant difference in parturition day among the three groups (P = 0.001). Lambs delivered on d 144 ± 1 (mean ± sem; control), 141 ± 2 (mean ± sem; E2SO4 icv; P < 0.001 compared with control by Wilcoxon test), and 142 ± 2 (E2SO4 iv; P = NS compared with control and P = 0.038 compared with E2SO4 icv by Wilcoxon test).
Fig. 4.
Survival plot of probability of remaining in utero (probability of nondelivery) in control- (●), E2SO4 iv- (○), and E2SO4 icv (▾)-treated fetuses.
Multiple linear regression analysis of plasma hormone and blood gas data reveals a statistically significant correlation (r2 = 0.59, n = 74, P < 0.001) between plasma concentration of cortisol (nanograms per milliliter) and plasma concentrations of ACTH1-39 (picograms per milliliter), and POMC (picomoles per liter), and blood concentrations of H+ (nanomoles per liter). The relationship among these variables is shown in the following equation:
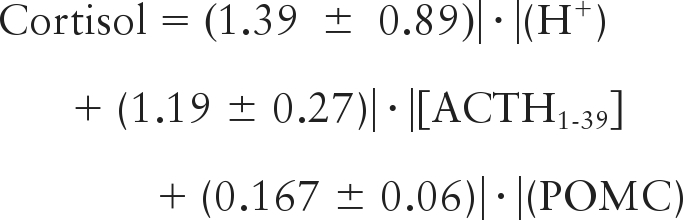 |
Discussion
The results of this study demonstrate the following: 1) E2SO4 increases fetal plasma cortisol but not ACTH or POMC; 2) administration of E2SO4 centrally and, perhaps iv, increases endogenous E2 and E2SO4 secretion; and 3) E2SO4 advances the timing of parturition. We propose that the physiological effects of sulfoconjugated E2 on the fetal endocrine environment observed in this study are more complicated than for E2 alone; understanding the complexity of E2SO4 action on fetal neuroendocrine control mechanisms will entail understanding the role of local conjugation/deconjugation enzymes, transporters, and receptors in the relevant brain regions.
E2SO4 effects on the fetal HPA axis
We measured fetal plasma ACTH1-39, POMC, and cortisol as a means of investigating HPA activity in this study. Many investigators have shown that HPA axis activity increases near term in this species (31–34), an observation we confirmed in our control fetuses (Fig. 1). Treatment with E2SO4, either iv or icv, attenuated the normal preparturient rise in ACTH. E2SO4 increased cortisol secretion but tended to not change or reduce POMC and ACTH1-39 secretion. Interestingly, despite attenuated ACTH secretion in treated fetuses, cortisol output near term increased in the iv and icv groups. These results are consistent with other recent results from our laboratory in which we compared plasma hormone concentrations and gene expression in twin fetuses, one of which received an icv infusion of E2SO4 (35). In that study, the infusion increased fetal plasma cortisol concentration and decreased the abundance of POMC mRNA in fetal pituitary as well as the abundance of CRH mRNA in hypothalamus.
Together with the present data, we speculate that there is an increase in fetal adrenal cortisol secretion (caused by increased adrenal sensitivity to the circulating ACTH, adrenal response to a novel adrenocorticotropic stimulus, or a direct action of E2 or E2SO4 at the adrenal cortex) and that hypothalamo-pituitary control of ACTH secretion are inhibited by negative feedback (36). It might also result from secondary influences on adrenal secretion. Multiple regression analysis revealed a statistically significant association between H+ and plasma cortisol concentrations. In adult animals, H+ stimulates aldosterone biosynthesis (37). Although controversial (38), some investigators have suggested that this direct effect of acid on adrenal steroidogenesis might be mediated by tandem of P domains in a weak inward rectifying K+ channel (TWIK)-related, acid-sensitive K+ channels, which are known to be expressed in the adrenal cortex of the adult rat (39). Similarly, it is possible that there is a direct E2 effect at the adrenal cortex (after deconjugation) (40).
With regard to the neuroendocrine regulation of ACTH secretion, the actions of E2 and E2SO4 are somewhat different. Chronic infusions of E2 stimulate fetal ACTH secretion chronically (8). Previous studies in this laboratory have demonstrated that infusions of E2SO4 for 5 d increase fetal ACTH and cortisol secretion (18). The present study demonstrates that the stimulation of fetal ACTH by E2SO4 is transient and that because of an apparent stimulatory effect on the fetal adrenal, long-term (>5 d) infusions actually inhibit the preparturient rise in fetal ACTH secretion. Nevertheless, it is possible that E2SO4 may be inhibitory to CRH and ACTH through neurosteroid-like interaction with γ-aminobutyric acid (GABA)A receptors or, perhaps less likely, by acting as an antagonist of estrogen receptor. The paraventricular nucleus (PVN) is known to have GABAergic innervation, with nearly half of all synapses in the medial parvocellular PVN containing GABA receptors (41). Specific GABAA receptor subunits can be found within hypophysiotrophic CRH neurons (42), and pharmacological studies both in vitro and in vivo have shown GABAergic mechanisms to be involved in the inhibition of CRH secretion (43–45). Activity of the HPA axis is reduced (as measured by ACTH secretion) after icv injection of GABAA antagonists (46). It is possible that, in the present experiments, E2SO4 either acted directly as a neurosteroid or modified endogenous neurosteroid levels in the PVN, consistent with known actions of neurosteroids, which are GABAA agonists (47–49).
E2SO4 effects on E2 and E2SO4 secretion
The data collected in this study allow us to estimate the secretion and clearance of E2SO4 and to estimate the changes in E2 and E2SO4 secretion rate in response to infusion of the sulfoconjugated steroid icv. Based on calculated MCR for E2SO4 and E2, we estimate that approximately 26% of the iv infused E2SO4 was converted to E2 and that when E2SO4 was infused directly into the brain, there was an increase in the endogenous synthesis of both E2 and, perhaps to a lesser extent, E2SO4. This increase in E2 synthesis is accompanied by an increase in the plasma P4 concentration, possibly the result of concomitant increases in the E2 and P4 secretion.
E2SO4 was approximately 10 times more abundant than E2 in plasma of control animals. The differences in concentration between circulating conjugated and unconjugated steroids was previously noted for E2 (18) and E1 (19). E2SO4 treatment, either icv or iv, significantly lowered the E2SO4 to E2 ratio compared with control fetuses, suggesting that infusion of E2SO4 may up-regulate STS activity, down-regulate estrogen STF activity, or preferentially stimulate E2 secretion. This would have the effect of lowering the E2SO4 to E2 ratio by increasing plasma E2, which is what we saw in both E2SO4 treatment groups. Recent data from this laboratory have confirmed that icv infusion of E2SO4 does up-regulate STS and down-regulate STF protein in the fetal brain (35). The up-regulation of STS is likely to be quantitatively more important because the abundance of the mRNA for this enzyme is far greater than for STF (35).
The site of biosynthesis of the various estrogens circulating in fetal blood has not been definitively identified. Because the placenta contains a relative abundance of aromatase, it has been assumed that the placenta is the major source of E1 and E2 (17, 50). The ovine placenta has a low activity of 17-hydroxylase and 17,20 lyase until late in gestation, suggesting that the placental E1 and E2 biosynthesis might depend on a supply of 17α-hydroxylated substrate from other steroidogenic tissues. Mitchell et al. (51) have proposed that androstenedione secretion by the fetal adrenal serves as a precursor for placental estrogen production. In the present study, icv infusion of E2SO4 increased fetal plasma P4 concentration. Because the major source of P4 in fetal blood is thought to be placenta (52), the data suggest a placental source of the E2 and E2SO4, perhaps from de novo synthesis from cholesterol. Interestingly, the plasma DHAS concentration was increased only in the iv group. We expected increases in DHAS concentrations in fetal plasma to be evident in groups in which steroidogenesis was stimulated (especially in groups in which there was any evidence of E2 or E2SO4 biosynthesis). In the present experiments, the only group in which we observed evidence of increased E2 synthesis was the icv group. Nevertheless, the nature of the signal from fetal brain to the site of E2 and E2SO4 synthesis is unclear. In a separate study of twin fetuses, one treated with icv infusion of E2SO4 and one treated with vehicle, we found that the only difference in pituitary gene expression of known pituitary tropic hormones was a decrease in the abundance of ovine FSH mRNA (35). This might have been the result and not the cause of the increased circulating E2 concentrations. Several investigators have proposed neural regulation of steroidogenesis in the fetal sheep (53). Such a mechanism could play a role in the present experiment, but we have no evidence either supporting or refuting it.
In summary, we have examined the effects of E2SO4 on HPA axis activity and on the timing of parturition in sheep. This sulfoconjugated estrogen has a neuroendocrine action to increase the secretion of cortisol, E2, and E2SO4 and also to advance parturition. E2SO4 inhibits fetal ACTH secretion, possibly through interaction as a neurosteroid. The stimulus to cortisol and E2 secretion, taking into account the reduction in ACTH secretion, is not revealed by the present study. We conclude that E2SO4 has effects on the HPA axis that are distinct from E2 and that this may be an important mechanism for modulating this activity of the axis during gestation.
Acknowledgments
I thank Dr. Christine Schlaerth for her work in performing many of these experiments, Ms. Xiaoying (Lisa) Fang for her expert technical assistance in the laboratory, and Dr. Maureen Keller-Wood for ongoing advice and collaboration.
This work was supported by Grant HD57561 from the National Institute of Child Health and Human Development.
Disclosure Summary: The author has nothing to disclose.
Footnotes
- DHAS
- Dehydroepiandrosterone sulfate
- E1
- estrone
- E2
- estradiol
- E1SO4
- estrone-3-sulfate
- E2SO4
- estradiol-3-sulfate
- GABA
- γ-aminobutyric acid
- HPA
- hypothalamus-pituitary-adrenal
- icv
- intracerebroventricular
- MCR
- metabolic clearance rate
- P4
- progesterone
- POMC
- proopiomelanocortin
- PVN
- paraventricular nucleus
- STF
- sulfotransferase
- STS
- steroid sulfatase.
References
- 1. Liggins GC, Fairclough RJ, Grieves SA, Forster CS, Knox BS. 1977. Parturition in the sheep. CIBA Found Symp 5–30 [DOI] [PubMed] [Google Scholar]
- 2. Wood CE. 1995. Baroreflex and chemoreflex control of fetal hormone secretion. Reprod Fertil Dev 7:479–489 [DOI] [PubMed] [Google Scholar]
- 3. Wood CE. 1999. Control of parturition in ruminants. J Reprod Fertil Suppl 54:115–126 [PubMed] [Google Scholar]
- 4. Wood CE, Keil LC, Rudolph AM. 1982. Hormonal and hemodynamic responses to vena caval obstruction in fetal sheep. Am J Physiol 243:E278–E286 [DOI] [PubMed] [Google Scholar]
- 5. Purinton SC, Wood CE. 2002. Oestrogen augments the fetal ovine hypothalamus-pituitary-adrenal axis in response to hypotension. J Physiol 544:919–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wood CE, Giroux D. 2003. Central nervous system prostaglandin endoperoxide synthase-1 and -2 responses to oestradiol and cerebral hypoperfusion in late-gestation fetal sheep. J Physiol 549:573–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wood CE, Giroux D. 2006. Expression of nitric oxide synthase isoforms in the ovine fetal brain: alteration by hormonal and hemodynamic stimuli. J Soc Gynecol Investig 13:329–337 [DOI] [PubMed] [Google Scholar]
- 8. Wood CE, Saoud CJ. 1997. Influence of estradiol and androstenedione on ACTH and cortisol secretion in the ovine fetus. J Soc Gynecol Investig 4:279–283 [PubMed] [Google Scholar]
- 9. Kitay JI. 1961. Sex differences in adrenal cortical secretion in the rat. Endocrinology 68:818–824 [DOI] [PubMed] [Google Scholar]
- 10. Carey MP, Deterd CH, de Konig J, Helmerhost F, de Kloet ER. 1995. The influence of ovarian steroids on hypothalamic-pituitary-adrenal regulation in the female rat. J Endocrinol 144:311–321 [DOI] [PubMed] [Google Scholar]
- 11. Nappi RE, Bonneau MJ, Rivest S. 1997. Influence of the estrous cycle on c-fos and CRH gene transcription in the brain of endotoxin-challenged female rats. Neuroendocrinology 65:29–46 [DOI] [PubMed] [Google Scholar]
- 12. Nappi RE, Rivest S. 1995. Ovulatory cycle influences the stimulatory effect of stress on the expression of corticotropin-releasing factor receptor messenger ribonucleic acid in the paraventricular nucleus of the female rat hypothalamus. Endocrinology 136:4073–4083 [DOI] [PubMed] [Google Scholar]
- 13. Pollard I, White BM, Bassett JR, Cairncross KD. 1975. Plasma glucocorticoid elevation and desynchronization of the estrous cycle following unpredictable stress in the rat. Behav Biol 14:103–108 [DOI] [PubMed] [Google Scholar]
- 14. Viau V, Meaney MJ. 1991. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology 129:2503–2511 [DOI] [PubMed] [Google Scholar]
- 15. Coyne MD, Kitay JI. 1969. Effects of ovariectomy on pituitary secretion of ACTH. Endocrinology 85:1097–1102 [DOI] [PubMed] [Google Scholar]
- 16. Kitay JI. 1963. Pituitary-adrenal function in the rat after gonadectomy and gonadal hormone replacement. Endocrinology 73:253–260 [DOI] [PubMed] [Google Scholar]
- 17. Nathanielsz PW, Elsner C, Magyar D, Fridshal D, Freeman A, Buster JE. 1982. Time trend analysis of plasma unconjugated and sulfoconjugated estrone and 3 β-Δ5-steroids in fetal and maternal sheep plasma in relation to spontaneous parturition at term. Endocrinology 110:1402–1407 [DOI] [PubMed] [Google Scholar]
- 18. Wood CE, Gridley KE, Keller-Wood M. 2003. Biological activity of 17β-estradiol-3-sulfate in ovine fetal plasma and uptake in fetal brain. Endocrinology 144:599–604 [DOI] [PubMed] [Google Scholar]
- 19. Carnegie JA, Robertson HA. 1978. Conjugated and unconjugated estrogens in fetal and maternal fluids of the pregnant ewe: a possible role for estrone sulfate during early pregnancy. Biol Reprod 19:202–211 [DOI] [PubMed] [Google Scholar]
- 20. Hobkirk R. 1985. Steroid sulfotransferases and steroid sulfate sulfatases: characteristics and biological roles. Can J Biochem Cell Biol 63:1127–1144 [DOI] [PubMed] [Google Scholar]
- 21. Reed MJ, Purohit A, Woo LW, Newman SP, Potter BV. 2005. Steroid sulfatase: molecular biology, regulation, and inhibition. Endocr Rev 26:171–202 [DOI] [PubMed] [Google Scholar]
- 22. Purinton SC, Newman H, Castro MI, Wood CE. 1999. Ontogeny of estrogen sulfatase activity in ovine fetal hypothalamus, hippocampus, and brain stem. Am J Physiol 276:R1647–R1652 [DOI] [PubMed] [Google Scholar]
- 23. Purinton SC, Wood CE. 2000. Ovine fetal estrogen sulfotransferase in brain regions important for hypothalamus-pituitary-adrenal axis control. Neuroendocrinology 71:237–242 [DOI] [PubMed] [Google Scholar]
- 24. Wood CE, Giroux D, Gridley K. 2003. Fetal brain regional responses to cerebral hypoperfusion: modulation by estrogen. Brain Res 993:84–89 [DOI] [PubMed] [Google Scholar]
- 25. Schaub CE, Keller-Wood M, Wood CE. 2008. Blockade of estrogen receptors decreases CNS and pituitary prostaglandin synthase expression in fetal sheep. Neuroendocrinology 87:121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. 2002. Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol 283:R281–R283 [DOI] [PubMed] [Google Scholar]
- 27. Saoud CJ, Wood CE. 1997. Modulation of ovine fetal adrenocorticotropin secretion by androstenedione and 17β-estradiol. Am J Physiol 272:R1128–R1134 [DOI] [PubMed] [Google Scholar]
- 28. Wood CE, Powers Fraites M, Keller-Wood M. 2009. Blockade of PGHS-2 inhibits the hypothalamus-pituitary-adrenal axis response to cerebral hypoperfusion in the sheep fetus. Am J Physiol Regul Integr Comp Physiol 296:R1813–R1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wood CE, Cudd TA, Kane C, Engelke K. 1993. Fetal ACTH and blood pressure responses to thromboxane mimetic U46619. Am J Physiol 265:R858–R862 [DOI] [PubMed] [Google Scholar]
- 30. Gehan EA. 1965. A generalized two-sample Wilcoxon test for doubly censored data. Biometrika 52:650–653 [PubMed] [Google Scholar]
- 31. Bassett JM, Thorburn GD. 1969. Foetal plasma corticosteroids and the initiation of parturition in sheep. J Endocrinol 44:285–286 [DOI] [PubMed] [Google Scholar]
- 32. Wintour EM, Brown EH, Denton DA, Hardy KJ, McDougall JG, Oddie CJ, Whipp GT. 1975. The ontogeny and regulation of corticosteroid secretion by the ovine foetal adrenal. Acta Endocrinol 79:301–316 [DOI] [PubMed] [Google Scholar]
- 33. Wood CE. 1988. Insensitivity of near-term fetal sheep to cortisol: possible relation to the control of parturition. Endocrinology 122:1565–1572 [DOI] [PubMed] [Google Scholar]
- 34. Wood CE. 1994. The function of the fetal pituitary-adrenal system. In: Thorburn GD, Harding R. eds. Textbook of fetal physiology. Oxford, UK: Oxford University Press; 351–358 [Google Scholar]
- 35. Winikor J, Schlaerth C, Rabaglino MB, Cousins R, Sutherland M, Wood CE. 2011. Complex actions of estradiol-3-sulfate in late gestation fetal brain. Reprod Sci 18:654–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wood CE. 1986. Sensitivity of cortisol-induced inhibition of ACTH and renin in fetal sheep. Am J Physiol 250:R795–R802 [DOI] [PubMed] [Google Scholar]
- 37. Kramer RE, Robinson TV, Schneider EG, Smith TG. 2000. Direct modulation of basal and angiotensin II-stimulated aldosterone secretion by hydrogen ions. J Endocrinol 166:183–194 [DOI] [PubMed] [Google Scholar]
- 38. Guagliardo NA, Yao J, Bayliss DA, Barrett PQ. 2011. TASK channels are not required to mount an aldosterone secretory response to metabolic acidosis in mice. Mol Cell Endocrinol 336:47–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Czirják G, Fischer T, Spät A, Lesage F, Enyedi P. 2000. TASK (TWIK-related acid-sensitive K+ channel) is expressed in glomerulosa cells of rat adrenal cortex and inhibited by angiotensin II. Mol Endocrinol 14:863–874 [DOI] [PubMed] [Google Scholar]
- 40. Figueiredo HF, Ulrich-Lai YM, Choi DC, Herman JP. 2007. Estrogen potentiates adrenocortical responses to stress in female rats. Am J Physiol Endocrinol Metab 292:E1173–E1182 [DOI] [PubMed] [Google Scholar]
- 41. Decavel C, Van den Pol AN. 1990. GABA: a dominant neurotransmitter in the hypothalamus. J Comp Neurol 302:1019–1037 [DOI] [PubMed] [Google Scholar]
- 42. Cullinan WE. 2000. GABA(A) receptor subunit expression within hypophysiotropic CRH neurons: a dual hybridization histochemical study. J Comp Neurol 419:344–351 [DOI] [PubMed] [Google Scholar]
- 43. Calogero AE, Gallucci WT, Chrousos GP, Gold PW. 1988. Interaction between gabaergic neurotransmission and rat hypothalamic corticotropin releasing hormone secretion in vitro. Brain Res 463:28–36 [DOI] [PubMed] [Google Scholar]
- 44. Hillhouse EW, Milton NG. 1989. Effect of noradrenaline and gamma-aminobutyric acid on the secretion of corticotrophin-releasing factor-41 and arginine vasopressin from the rat hypothalamus in vitro. J Endocrinol 122:719–723 [DOI] [PubMed] [Google Scholar]
- 45. Plotsky PM, Otto S, Sutton S. 1987. Neurotransmitter modulation of corticotropin releasing factor secretion into the hypophysial-portal circulation. Life Sci 41:1311–1317 [DOI] [PubMed] [Google Scholar]
- 46. Makara GB, Stark E. 1974. Effects of γ-aminobutyric acid (GABA) and GABA antagonist drugs on ACTH release. Neuroendocrinology 16:178–190 [DOI] [PubMed] [Google Scholar]
- 47. Belelli D, Lambert JJ. 2005. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci 6:565–575 [DOI] [PubMed] [Google Scholar]
- 48. Deutsch SI, Mastropaolo J, Hitri A. 1992. GABA-active steroids: endogenous modulators of GABA-gated chloride ion conductance. Clin Neuropharmacol 15:352–364 [PubMed] [Google Scholar]
- 49. Regelson W, Kalimi M. 1994. Dehydroepiandrosterone (DHEA)—the multifunctional steroid. II. Effects on the CNS, cell proliferation, metabolic and vascular, clinical and other effects. Mechanism of action? Ann NY Acad Sci 719:564–575 [DOI] [PubMed] [Google Scholar]
- 50. Yu HK, Cabalum T, Jansen CA, Buster JE, Nathanielsz PW. 1983. Androstenedione, testosterone, and estradiol concentrations in fetal and maternal plasma in late pregnancy in the sheep. Endocrinology 113:2216–2220 [DOI] [PubMed] [Google Scholar]
- 51. Mitchell BF, Lye SJ, Lukash L, Challis JRG. 1986. Androstenedione metabolism in the late gestation sheep fetus. Endocrinology 118:63–68 [DOI] [PubMed] [Google Scholar]
- 52. Anderson AB, Flint AP, Turnbull AC. 1975. Mechanism of activation of glucocorticoids in induction of ovine parturition: effect on placental steroid metabolism. J Endocr 66:61–70 [PubMed] [Google Scholar]
- 53. Myers DA, Robertshaw D, Nathanielsz PW. 1990. Effect of bilateral splanchnic nerve section on adrenal function in the ovine fetus. Endocrinology 127:2328–2335 [DOI] [PubMed] [Google Scholar]