Abstract
We hypothesized that hormonal therapy favors the development of the hormone-resistant phenotype through epigenetic mechanisms. Human prostate cancer tissues and in vitro and in vivo models were used to verify this hypothesis. We demonstrated that tumor cells continuously treated with bicalutamide (BCLT) or cultured in androgen-depleted medium progressively acquire higher DNA methyltransferase (DNMT) activity and expression than cells cultured in standard condition. Increased DNMT expression and activity also paralleled the up-regulation of truncated AR isoforms, which favors the development of the hormone-resistant phenotype. After androgen stimulation with 10−12 m dihydrotestosterone, DNMT activity was significantly reduced in comparison with hormonal therapy. Consistent with these observations, the silencing of DNMT3a and DNMT3b significantly decreased the DNMT activity levels. These findings were also directly correlated with phosphatase and tensin homolog down-regulation and activation of ERK and phosphatidylinositol 3-kinases/AKT8 virus oncogene cellular homolog pathways. The use of a pan-DNMT inhibitor (5-Azacitidine) greatly reduced the development of the hormone-resistant phenotype induced by long-term BCLT treatment, and this finding correlated with low DNMT activity. The regulation of DNMT activity was, in some measure, dependent on the androgen receptor, as small interfering RNA treatment targeting the androgen receptor greatly decreased the modulation of DNMT activity under androgenic and antiandrogenic stimulation. These observations were correlated in vivo in patients, as demonstrated by immunohistochemistry. Patients treated by BCLT before surgery had higher DNMT3a and DNMT3b expression than patients who had not undergone this treatment. Our findings provide evidence of a relationship between the castration-resistant phenotype and DNMT expression and activity in human prostate cancer.
The androgen receptor (AR) is involved in the development and maintenance of the normal prostate as well as in the progression of prostate cancer (Pca) (1–4). Therapeutic strategies targeting AR function are a valuable approach in the management of locally advanced or advanced Pca (5, 6). However, although initially effective, inducing a mixed response of cell cycle arrest and apoptosis, recurrent, incurable tumors ultimately arise as a result of inappropriately restored AR function (7, 8).
The molecular mechanisms by which Pca cells progress to become androgen-insensitive still remain largely unclear. It is believed that mutations, chromosomal translocations, and epigenetic modifications can alter AR gene expression and modify androgen sensitivity (5–13), and aberrant DNA methylation patterns have been detected in Pca (14, 15). The three main types responsible for establishing and differentiating DNA methylation patterns during development are DNA methyltransferase (DNMT)1, DNMT3a, and DNMT3b (14). A progressive increase in generalized DNMT enzymatic activity during malignant transformation has been demonstrated (16). Furthermore, several lines of evidence show a high incidence of hypermethylation in poorly differentiated tumors (16). In the transgenic mouse model of Pca, DNMT expression increases during the progressive stages of Pca, and this is associated with locus-specific nonrandom CpG island hypermethylation, as well as hypomethylation of repetitive DNA elements (17, 18). The DNMT inhibitor 5-Azacitidine (5-Aza) prevents prostatic disease progression and the development of lymph node metastases in this model (19).
Guided by these data, we performed several in vitro and in vivo experiments to investigate the relationship between DNMT expression/activity and Pca progression to androgen independent phenotype.
Materials and Methods
Cell cultures and reagents
The Pca human cell lines 22rv1 and LnCaP were obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany) and American Type Culture Collection (Rockville, MD), respectively. The LnCaP sublines (LnCaP-104-S, LnCaP-104-R1, and LnCaP-C-81), with decreasing androgen sensitivity (20, 21), were kindly provided by John M. Kokontis (University of Chicago, Chicago, IL) and Min-Fong Lin (University of Nebraska, Omaha, NB). The bicalutamide (BCLT)-resistant 22rv1 Pca cell line was generated by culturing 22rv1 parental cell line for 60 wk with 5 μm BCLT. The cell lines were cultured in DMEM supplemented with 10% fetal bovine serum, 50 IU penicillin, and 50 μg/ml streptomycin. 5-Aza (Vidaza) was obtained in collaboration with Celgene Corp. (Summit, NJ). BCLT and 6-Aza were purchased from Sigma (Milan, Italy). Antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) unless otherwise indicated.
Human tissues and immunohistochemistry (IHC)
A cohort of 90 patients with clinically localized Pca was studied retrospectively as already described in detail (22, 23). The tissue material used in this study concerned radical prostatectomy specimens. Of the 90 patients, 51 received preoperative BCLT (150 mg/d) therapy for 120 d. The remaining 39 patients were not treated with hormonal therapy (hormone naïve). Table 1 summarizes the patients' clinical and pathological characteristics. DNMT1, DNMT3a, and DNMT3b expression was evaluated on 4-μm tissue sections cut from blocks selected for the presence of representative tumor tissue. Negative controls were incubated only with universal negative control antibodies under identical conditions, processed, and mounted. The primary anti-DNMT antibodies were purchased from BioCarta LLC (San Diego, CA). All primary antibodies were used at the appropriate dilutions, according to the manufacturer's instructions. The pathologic evaluation and IHC results were interpreted by two uropathologists (Luca Ventura and Roberto Pomante). First, nuclear staining of DNMT1, DNMT3a, and DNMT3b in tumor tissue was scored blindly by eye with a semiquantitative immunoreactivity scoring (IRS) system. Category A scored the intensity of immunostaining as 0 (no immunostaining), 1 (weak immunostaining), 2 (moderate immunostaining), and 3 (strong immunostaining). Category B scored the percentage of immunoreactive cells as 0 (none), 1 (<10%), 2 (10–50%), 3 (51–80%), and 4 (>80%). Multiplication of A and B resulted in an IRS of from 0 to 12 for each tumor. An IRS of 7 or greater was considered as high for expression of DNMT.
Table 1.
Patients demography and clinical/pathological characteristics
| Variables | Treated with BCLT | Untreated with BCLT | P value |
|---|---|---|---|
| Patients, (N) | 51 | 39 | |
| Age (yr) | 67.5 ± 5.5 | 65.2 ± 7.9 | 0.0039a |
| PSA at biopsy (ng/ml) | 5.7 ± 5.1 | 6.4 ± 4.8 | 0.107a |
| Gleason score at biopsy | |||
| ≤6 | 29/51 (56.9%) | 20/39 (51.3%) | 0.753b |
| 7 | 19/51 (37.2) | 17/39 (43.6%) | 0.691b |
| 8–10 | 3/51 (5.9%) | 2/39 (5.1%) | 0.764b |
| Gleason score at radical prostatectomy | |||
| ≤6 | NA | 17/39 (43.6%) | |
| 7 | NA | 19/39 (48.8%) | |
| 8–10 | NA | 3/39 (7.7%) | |
| Pathological stage (TNM 2002) | |||
| pT2 | 14/51 (27.4%) | 16/39 (41.0%) | 0.258b |
| pT3 | 34/51 (66.7) | 20/39 (51.3%) | 0.207b |
| pT4 | 3/51 (5.9%) | 3/39 (7.7%) | 0.931b |
NA, Not assessable.
Unpaired Student's t test.
χ2 test.
Growth Assay
Briefly, Pca cells (3 × 104 cells/well) were seeded in 24-well tissue culture plates (Costar, Corning, NY) and left to attach and grow for 24 h. After this time, cells were maintained in the appropriate culture conditions, and the treatments were arranged as reported in the figures. Morphological controls were performed every day with an inverted phase-contrast photomicroscope (Nikon Diaphot, Tokyo, Japan), before cell trypsinization and counting. Cells trypsinized and resuspended in 1.0 ml of saline were counted using the NucleoCounter NC-100 (automated cell counter systems; Chemotec, Cydevang, Denmark). NucleoCounter NC-100 allows to determine also the number of death cells present in a cell sample. The effect on cell proliferation was measured by taking the mean cell number respect to controls in the time for the different treatment groups. All experiments were conducted in triplicate.
Immunoblotting analysis
Proteins were extracted, assessed using the Lowry method, and then separated on SDS-PAGE. The proteins were transferred to a nitrocellulose membrane by electroblotting. Immunoblottings were performed with the following antibodies: anti-DNMT1 (17), anti-DNMT3a (D15), anti-DNMT3b (4H84), and anti-β-actin (all from Santa Cruz Biotechnology, Inc.). Anti-AR (N-20) and (C-19) were purchased from Santa Cruz Biotechnology, Inc. Peroxidase-conjugate antimouse or antirabbit IgG [Amersham-Pharmacia Biotech (Newcastle upon Tyne, UK) or Santa Cruz Biotechnology, Inc.] were used for enhanced chemiluminescence detection.
DNMT activity assay
DNMT activity was evaluated by a colorimetric EpiQuik DNMT Activity Assay kit (BioVision, Mountain View, CA) in nuclear extracts of cells treated according to the treatment protocols and according to the manufacturer's instructions.
Small interfering RNA (siRNA) transfection
LNCaP clones (1.5 × 105 cells/well) and 22rv1 BCLT-R cells were plated in six-well plates and grown in phenol red-free DMEM containing 10% charcoal-stripped serum (CSS) for 2 d. LNCaP clones were transfected by AR siRNA: sc-29204 (Santa Cruz Biotechnology, Inc.) and 22rv1 BCLT-R cells were transfected by DNMT3-a [(h), sc-37757] and DNMT3-b siRNA [(h), sc-37759] (Santa Cruz Biotechnology, Inc.) according to the manufacturer's instructions. All siRNA duplexes were transfected using OligofectAMINE reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. After AR silencing, cells were grown in phenol red-free DMEM containing 10% CSS for 48 h and then treated with dihydrotestosterone (DHT) (10−12 m) and/or BCLT (5 μm) or vehicle control for 72 h. Before DNMT3a and DNMT3-b silencing, 22rv1 BCLT-R cells were cultured in phenol red-free DMEM containing 10% CSS supplemented with BCLT (5 μm) in association with DHT (10−12 m) for 48 h and then silenced for 72 h.
Analysis of apoptosis
After the appropriate treatments, cells (1 × 106) were fixed for 30 min in 70% ethanol and pelleted by centrifugation (720 g; 5 min). After removal of ethanol, cells were incubated and resuspended in 1 ml of DNA staining solution (PBS containing 200 mg/ml ribonuclease A and 20 mg/ml propidium iodide plus 0.1% Triton X-100) and left at room temperature for 60 min. Apoptosis was analyzed by using Annexin V staining (GenScript, Piscataway, NJ). All cells were then measured on a FACScan flow cytometer (FACScan; Becton Dickinson, Mountain View, CA) with an argon laser at 488 nm for excitation and analyzed using Cell Quest software (Becton Dickinson). Apoptotic cells were detected by the percentage of Annexin V-stained cells. The results were expressed as the percentage of death by apoptosis induced by a specific treatment. Experiments were performed in triplicate.
In vivo experiments
Male CD1 nude mice (Charles River, Milan, Italy) were kept in line with University guidelines (University of L'Aquila, Medical School and Science and Technology School Board Regulations, in compliance with Italian government regulation no. 116 of January 27, 1992 on the use of laboratory animals). Before any invasive manipulation, mice were anesthetized with a ketamine (25 mg/ml)/xylazine (5 mg/ml) mixture. All mice received sc flank injections of 1 × 106 in 250 μl of 12 mg/ml Matrigel (Becton Dickinson, Franklin Lakes, NJ) of 22Rv1 and 22Rv1-BCLT-R cells. Treatments were started when a mean tumor volume of 100 mm3 was reached.
In vivo treatment groups
Before tumor inoculation, mice were randomly assigned to four experimental groups of 12 mice each. For each cell line one control group received an ip injection of 100 μl of PBS for 21 consecutive days, and one group received 50 mg/kg · d BCLT by oral gavage for 21 consecutive days. The effects on tumor growth of different treatments were evaluated by: 1) tumor volume measured during and at the end of experiment by biweekly measurement of tumor diameters with a Vernier caliper (length × width), expressed in mm3 according to the formula 4/3πr3; and 2) tumor weight, measured at the end of experiment. Experiments were stopped 42 d after tumor inoculation, mice were killed by carbon dioxide inhalation, and tumors were removed surgically. Half of the tumor was directly frozen in liquid nitrogen for protein analysis, and the other half fixed in paraformaldehyde overnight for immunohystochemical analyses. CD31, Ki67, pAKT (AKT8 virus oncogene cellular homolog), pERK, and PTEN (phosphatase and tensin homolog) expression was evaluated on 4-μm tissue sections cut from blocks selected for the presence of representative tumor tissue. Negative controls were incubated only with universal negative control antibodies under identical conditions, processed, and mounted. The pathologic evaluation and IHC results were interpreted by two uropathologists (LV and RP).
Statistical analysis
Continuous variables were summarized as mean and sd and were compared by the unpaired Student's t test. Dichotomous variables were summarized by absolute and/or relative frequencies and were compared by the exact Fisher's test or χ2 test when appropriate. For multiple comparisons, the level of significance was corrected by multiplying the P value by the number of comparisons performed (n) according to Bonferroni correction. All tests were two-sided and were determined by Monte Carlo significance. P values of less than 0.05 were considered statistically significant. All statistical analyses were performed using the SPSS statistical analysis software package, version 10.0.
Results
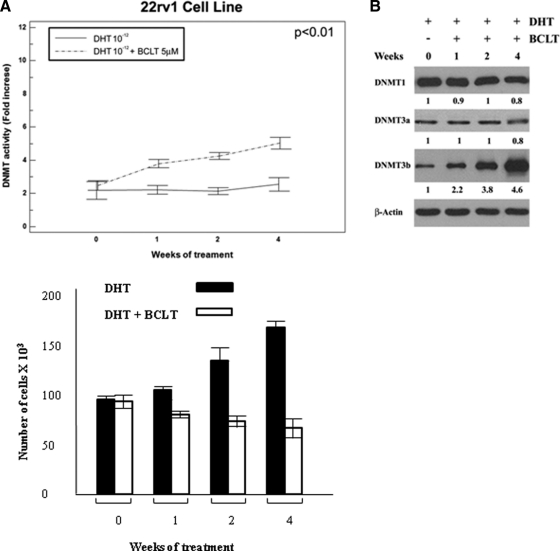
Short-term treatment with BCLT increases DNMT activity and DNMT3a/3b expression in 22rv1 cell line and human Pca tissues
The effects of BCLT and DHT on the expression and activity of DNMT1, DNMT3a, and DNMT3b were investigated on the 22rv1 tumor cell line. As shown in Fig. 1A, DNMT activity was unchanged upon DHT treatment, whereas BCLT gradually and significantly increased the enzymatic activity of these proteins. Modulation of enzymatic activity was associated with a significant increase in DNMT3b, whereas DNMT1 and DNMT3a remained unchanged (Fig. 1B). However, none of this resulted in the onset of a castration-resistant phenotype, as documented in Fig. 1C. The effects of short-term neoadjuvant BCLT treatment on DNMT expression were also investigated in human tissues (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org, and Table 2). The comparison of tissue samples from men with Pca treated or untreated with BCLT showed that BCLT treatment up-regulated DNMT3a [21/39 (53.9%) vs. 41/51 (80.4%) (P = 0.014)] and DNMT3b [9/39 (23.0%) vs. 24/51 (47.1%) (P = 0.034)]. No change was found in DNMT1 expression [25/39 (64.1%) vs. 37/51 (72.5%) (P = 0.530)]. DNMT expression also varied with tumor differentiation, with a change from Gleason less than or equal to 6 to Gleason 7 increasing expression as follows: DNMT1 from 52.9 to 68.4% (P = 0.543), DNMT3a from 29.4 to 68.4% (P = 0.045), and DNMT3b from 5.9 to 31.6% (P = 0.128). However, this difference was only significant for DNMT3a. With a Gleason score of 8–10, there was 100% expression of DNMT1 (P = 0.252) and DNMT3a (P = 0.016) and 66.7% of DNMT3b (P = 0.033).
Fig. 1.
BCLT treatment increases DNMT activity and expression, inhibiting the growth potential of the 22rv1 Pca cell line. DNMT activity (A) and protein expression (B) were assessed in the 22rv1 cell line. The values of fold increases over the control, arbitrarily set at 1, were obtained by densitometric analysis. Similar results were obtained in three experiments. C, Growth curve of 22rv1 cells DHT treated or DHT + BCLT treated. The data show the mean ± sd of triplicates of a representative experiment. Similar results were obtained in three experiments.
Table 2.
Immunohistochemical expression of DNMT1, DNMT3a, and DNMT3b in human tumor tissues
| Variables | DNMT1 | DNMT3a | DNMT3b |
|---|---|---|---|
| Men untreated | 25/39 (64.1%) | 21/39 (53.9%) | 9/39 (23.0%) |
| Men treated with BCLT | 37/51 (72.5%) | 41/51 (80.4%) | 24/51 (47.1%) |
| 0.530 | 0.014 | 0.034 | |
| Gleason score at radical prostatectomy | |||
| ≤6 | 9/17 (52.9%) | 5/17 (29.4%) | 1/17 (5.9%) |
| 7 | 13/19 (68.4%) | 13/19 (69.2%) | 6/19 (31.6%) |
| 8–10 | 3/3 (100%) | 3/3 (100%) | 2/3 (66.7%) |
| 0.543a | 0.045 | 0.128a | |
| 0.252b | 0.016b | 0.033b |
An IRS of 7 or greater was considered as high expression for DNMT as described in Material and Methods.
Gleason < 6 vs. Gleason 7.
Gleason 7 vs. Gleason 8–10.
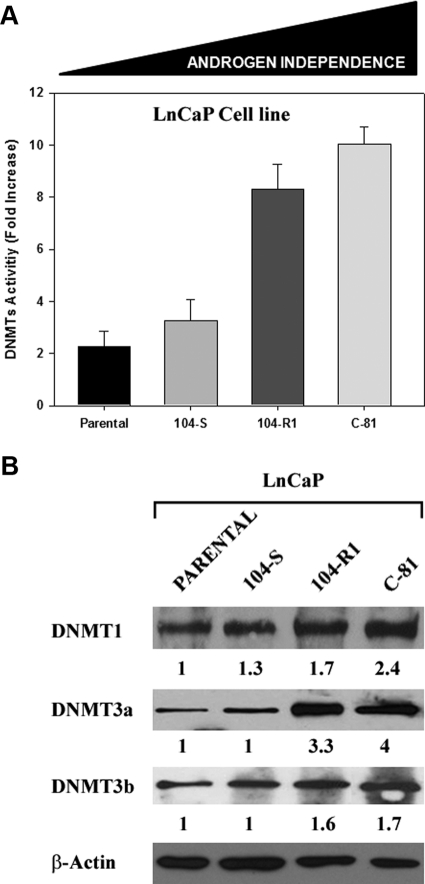
Prolonged hormonal therapy up-regulates full-length and truncated AR isoforms as well as DNMT activity and expression and correlates with the castration-resistant phenotype
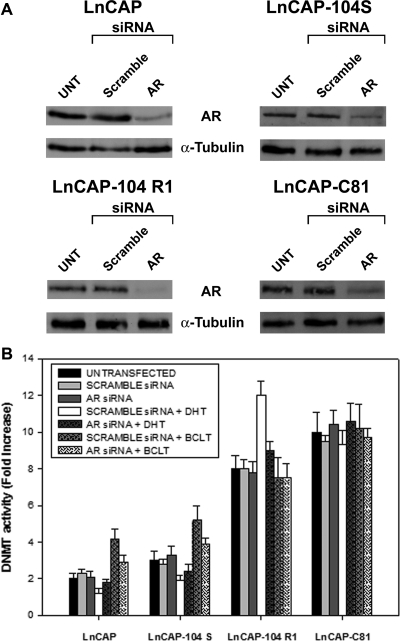
The LnCaP sublines (LnCaP, LnCaP-104-S, LnCaP-104-R1, and LnCaP-C-81) were used in these experiments, because they are a well-known cellular model with graded levels of androgen-independent phenotype. Enzymatic and Western blot analyses revealed that both DNMT activity (Fig. 2A) and DNMT1 and DNMT3a protein levels (Fig. 2B) were significantly higher in androgen-independent LnCaP-104-R1 and LnCaP-C-81 subclones than in androgen-dependent LnCaP and LnCaP-104-S cells. In these subclones, the acquisition of an androgen-independent phenotype was accompanied by the up-regulation of DNMT activity and expression. To determine whether AR modulates DNMT activity, LNCaP subclones with increasing androgen independence and high AR levels were silenced by siRNA for AR and then subjected to DHT (10−12 m) and/or BCLT (5 μm) treatment (Fig. 3). Seventy-two hours after transfection of LNCaP subclones with AR-specific siRNA, the levels of AR protein dropped by 80% in all subclones compared with control cells (Fig. 3A). In the presence of AR (scramble siRNA), DNMT activity was decreased after DHT treatment in the androgen-dependent LNCaP and LNCaP 104-S cells, whereas it was respectively increased and unchanged in androgen-independent LNCaP 104 R1 and LNCaP C-81 cells (Fig. 3B). BCLT increased DNMT activity in LNCaP and LNCaP 104-S, whereas levels were unchanged in LNCaP 104-S and LNCaP C-81. The transient AR silencing during DHT and BCLT treatment significantly lessened the modulation of DNMT activity in the three cell lines LNCaP, LNCaP 104-S, and LNCaP 104 R1, suggesting that AR is a key regulator of DNMT activity in these tumor models (Fig. 3B). However, because the modulation of DNMT activity in androgen-independent LNCaP C-81 cell was insensitive to AR knockdown, this implies that the higher level of DNMT expression in this cell line involves activation mechanisms not mediated by AR. The apparent mismatch in the modulation of DNMT activity by DHT was due to the well-known characteristics, in terms of androgenic control, of LNCaP subclones. As reported in the literature, the biological activity of the androgen-dependent tumor cells LNCaP and LNCaP 104-S is stimulated and repressed, respectively, by DHT and BCLT, whereas the androgen-independent subclone LNCaP 104 R1 is paradoxically repressed by DHT and insensitive to BCLT. Finally, the subclone LNCaP C-81 retains a significant insensitivity to both DHT and BCLT.
Fig. 2.
DNMT activity and expression increase during the passage of Pca cells to a castration-resistant phenotype. DNMT activity (A) and expression (B) in LnCaP, LnCaP-104-S, LnCaP-104R1, and LnCaP-C81 Pca cell lines. β-Actin shows the loading of samples. The values of fold increases over the controls, arbitrarily set at 1, were obtained by densitometric analysis. Similar results were obtained in three experiments.
Fig. 3.
DNMT activity in LNCaP clones after siRNA-mediated knockdown of AR. A, LNCaP clones were incubated in phenol red-free RPMI 1640 containing 10% CSS for 2 d and then mock transfected or transfected with AR siRNA directed against the coding region of the AR mRNA or the same amount of nonspecific siRNA. After 72 h, AR protein levels were measured by immunoblot analysis. The membrane was stripped and reprobed with an antiactin antibody. B, 72 h after AR siRNA or nonspecific siRNA transfection, LNCaP clones were treated with or without DHT (10–12 m) and/or BCLT (5 μm) for 24 h. B, DNMT activity was determined by the colorimetric EpiQuik DNMT Activity Assay 17 kit. The DNMT activity values were shown as fold increase respect to controls. Columns, Mean; bars, ±sd. Results shown are representative of three independent experiments. UNT, Untreated.
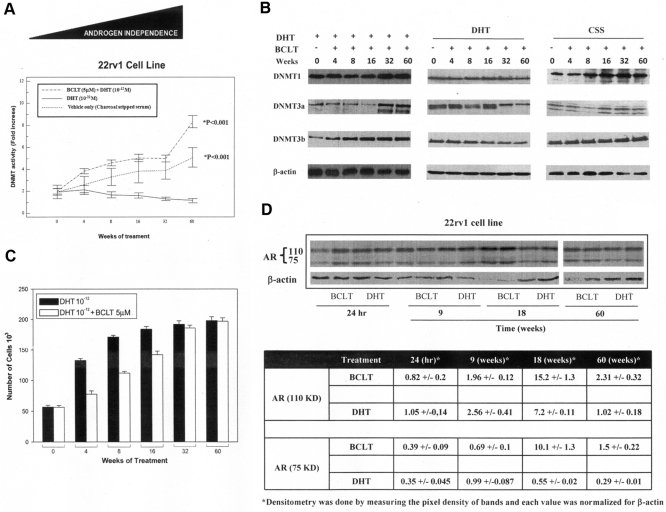
The correlation between DNMT activity and expression levels with the BCLT-resistant phenotype was examined. For this purpose, the 22rv1 cell line was continuously treated with BCLT, DHT, or vehicle only (medium supplemented with CSS) for 60 wk. DNMT activity (Fig. 4A) and DNMT3-a and DNMT3-b expression (Fig. 4B) were up-regulated upon BCLT or androgen depletion culture condition in a time-dependent manner. No significant change in DNMT activity (Fig. 4A) and expression (Fig. 4B) was documented upon DHT treatment. The growth curves showed that BCLT treatment made the cells less responsive to the growth-inhibiting action of BCLT (Fig. 4C). After 60 wk of BCLT treatment, the cells were refractory to BCLT, generating the 22rv1-BCLT-R cell line. Recent reports (20, 21) showed that alternatively spliced, truncated isoforms support androgen-independent phenotype in 22rv1 cells. We therefore examined whether these isoforms were modulated, in a time-dependent manner, during long-term treatment with BCLT. As shown in Fig. 4D, a substantial increase, as documented by densitometric analysis performed on three independent experiments, of both AR isoforms expression was documented after 60 wk of treatment with BCLT with a peak in the expression at 18 wk. This suggests that BCLT can partially sustain the production of truncated AR isoforms and contribute to the development of androgen-independent phenotype.
Fig. 4.
DNMT activity and expression parallel the acquisition of a more aggressive phenotype of the Pca cell line in vitro. DNMT activity (A) and expression (B) in 22rv1 cell line constantly treated in culture medium supplemented with BCLT, DHT, or CSS for 60 wk. The values of fold increases over the controls, arbitrarily set at 1, were obtained by densitometric analysis. C, Proliferation potential of 22rv1 cells untreated (control) or treated (BCLT) constantly with BCLT for 60 wk. Similar results were obtained in three independent experiments. D, Western blot and densitometric analyses of AR isoforms upon long-term treatment with BCLT in 22rv1 tumor cells. Densitometric analysis results shown are representative of three independent experiments.
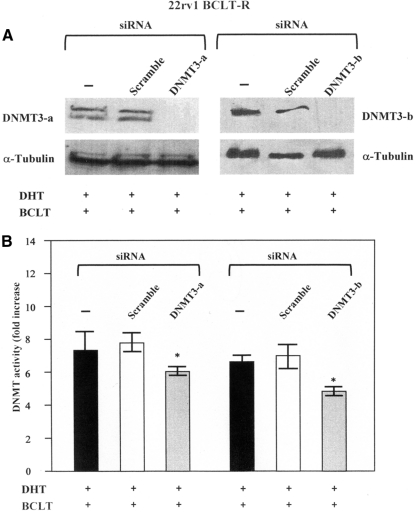
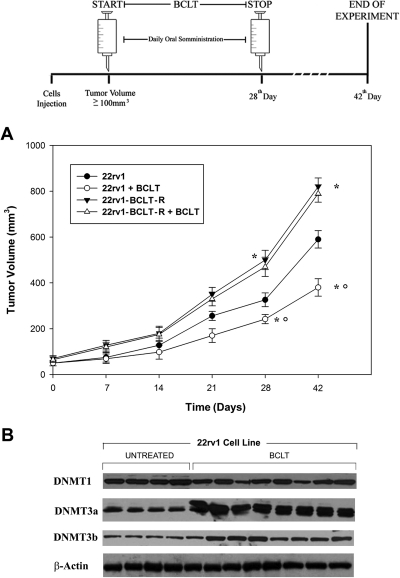
Because the long-term BCLT treatment results in a worsening of androgen-independent phenotype in 22Rv1 cell and because this phenomenon is associated with increased expression of DNMT3-a and DNMT3-b, we silenced these two enzymes to tested whether their knockdown decreased DNMT activity. Figure 5 displays a Western blot analysis of an experiment in which siDNMT3-a and siDNMT3-b were used to transfect 22rv1 BCLT-R cell lines upon BCLT and DHT treatment. A scramble siRNA was used as a control. We observed a complete disappearance of DNMT3-a and DNMT3-b 72 h after transfection (Fig. 5A). We next determined whether siRNA-targeted knockdown of DNMT3-a and DNMT3-b induced a decrease in the DNMT enzymatic activity. In the assay, single knockdown of either DNMT3-a or DNMT3-b resulted in a significant decrease in the DNMT activity levels respect to controls (Fig. 5 B), suggesting that these two enzymes significantly cooperate to maintain higher the DNMT activity levels in 22rv1-BCLT-R. The responsiveness of 22rv1-BCLT-R to BCLT (50 mg/kg · d) was further tested in vivo. The results showed that the tumor growth rate of 22rv1-BCLT-R was not reduced by BCLT treatment, in contrast with the parental cells (Fig. 6A). Next, the aggressiveness of 22rv1-BCLT-R was tested, and 22rv1-BCLT-R cells were found to be more tumorigenic than the parental line, as indicated by the faster tumor growth rate (Fig. 6A). In nude mice xenografted with 22rv1 cells, the antiandrogenic BCLT treatment (50 mg/kg · d) increased DNMT3a and DNMT3b expression with respect to the controls (Fig. 6B). In contrast, DNMT1 remained unchanged. 22rv1-BCLT-R xenografted tumors showed an increase in the proliferation index (Ki67) (Supplemental Fig. 2A) and blood vessel formation (CD31) (Supplemental Fig. 2B) in comparison with controls. Moreover, in these tumors, the expression of p-Akt (Supplemental Fig. 2C) and p-ERK (Supplemental Fig. 2D) was increased, whereas tumor-suppressor PTEN was decreased (Supplemental Fig. 2E).
Fig. 5.
DNMT3-a and DNMT3-b silencing decrease the DNMT activity in 22rv1 BCLT-R cell line. A, 22rv1 BCLT-R cell line was incubated in phenol red-free DMEM containing 10% CSS for 48 h. Before silencing cell were cultured under the following condition: medium supplemented with DHT (10−12 m) and BCLT (5 μm) or in a medium supplemented with 10% CSS for 48 h. Then tumor cells were mock transfected or transfected with DNMT3-a and DNMT3-b siRNA. After 72 h, DNMT3-a and DNMT3-b levels were measured by immunoblot analysis. The membrane was stripped and reprobed with an antiactin antibody. B, DNMT activity was determined by the colorimetric EpiQuik DNMT Activity Assay 17 kit. The DNMT activity values were shown as fold increase respect to controls. Columns, Mean; bars, ±sd. Results shown are representative of three independent experiments.
Fig. 6.
DNMT expression parallels the acquisition of a more aggressive phenotype of Pca cell line in vivo. Nude mice were xenografted with 22rv1 or 22rv1-BCLT cells. The mice received ip injections of 50 mg/kg · d BCLT for consecutive 21 d. A, Tumor growth in 22rv1 and 22rv1-BCLT-R xenografts in presence or absence of BCLT. *, P < 0.05; comparison vs. control; * and °, P < 0.05; comparison vs. 22rv1-BCLT-R + BCLT. Similar results were obtained in three experiments. B, DNMT expression checked by immunoblotting in 22rv1 xenografts treated or untreated by BCLT in nude mice.
The development of castration-resistant phenotype is mediated by epigenetic mechanisms in 22rv1 Pca cell line in vitro
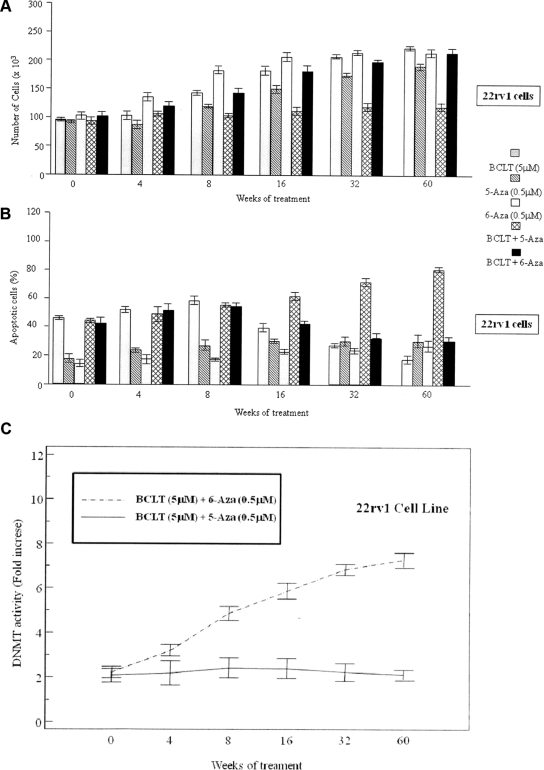
We determined whether a noncytotoxic concentration (0.5 μm) of 5-Aza was able to counteract the development of the BCLT-resistant phenotype in 22rv1 Pca cells. The cells were cultured for 60 wk in medium supplemented with 10% charcoal-stripped serum under the following conditions: 1) DHT (10−12 m) + BCLT (5 μm), 2) 5-Aza (0.5 μm), or 3) DHT (10−12 m) + BCLT (5 μm) + 5-Aza (0.5 μm). As shown in Fig. 7, the coadministration of 5-Aza (0.5 μm) + BCLT (5 μm) in presence of DHT (10−12 m) prevented the onset of the castration-resistant phenotype. As showed in Fig. 7, 22Rv1 cells show a biphasic response after incubation with DHT (10−12 m) + BCLT (5 μm) with an initial decrease in cell growth and increase in apoptosis, followed by total resistance. Interestingly, when BCLT was coadministered with 5-Aza, this biphasic response was absent, and there was a synergistic effect in terms of growth arrest (Fig. 7A) and apoptosis (Fig. 7B) in comparison with single treatments, suggesting that 5-Aza greatly reduces the onset of the BCLT-resistant phenotype. DNMT activity was strongly decreased by this combined treatment, suggesting that the onset of the castration-resistant phenotype is partially mediated by DNMT (Fig. 7C). Because 5-Aza has epigenetic and cytotoxic antitumor effects (24, 25), the sensitizing effect to BCLT might be theoretically due to its cytotoxic properties. For this reason, to establish whether cytotoxic or epigenetic mechanisms significantly contributed to delay the onset of the BCLT-resistant phenotype, specific experiments with a cytotoxic nucleoside analog (6-Aza) were carried out. 22Rv1 cells were cultured for 60 wk in medium supplemented with 10% CS FCS under the following conditions: DHT (10−12 m) + BCLT (5 μm) + 6-Aza (0.5 μm). The coadministration of 6-Aza with BCLT did not prevent the onset of the BCLT-resistant phenotype (Fig. 7, A and B) and was clearly associated with a significant increase in DNMT activity, confirming a direct correlation between high levels of DNMT activity and the BCLT-resistant phenotype (Fig. 7C).
Fig. 7.
5-Aza slows the insurgence of the castration-resistant phenotype in the 22rv1 Pca cell line. The 22rv1 Pca cell line was grown in the presence of BCLT for 60 wk with or without 5-Aza and 6-Aza. The responsiveness of tumor cells to BCLT treatment (A and B) and of DNMT activity (C) in the 22rv1 cell line was measured. The values of fold increases over the controls, arbitrarily set at 1, were obtained by densitometric analysis.
Discussion
The mechanisms that lead to the acquisition of an androgen-independent phenotype in Pca are multifactorial and still remain unclear. Many approaches have been proposed to improve the treatment outcome of this disease, but advanced Pca patients still have a dismal prognosis (1). Clarification of the molecular mechanisms that govern the passage toward the androgen-independent phenotype in Pca could lead to a new therapeutic strategy in treating this terminal disease. Here, we suggest that Pca may acquire androgen independence upon hormonal therapy through epigenetic mechanisms. When tumor cells were continuously cultured with BCLT or in androgen-depleted medium, they modified DNMT expression and activity with a direct correlation with androgen independence. These observations were correlated in vivo in patients. In human tissues, DNMT3a and DNMT3b expression was up-regulated by neoadjuvant treatment with BCLT. However mechanisms underlying Pca progression to androgen ablation resistance are complicated, and many factors may be involved. In this report, we also showed that alternative truncated AR isoforms may be one of the means to diversify its signaling and confer androgen-independent phenotype (20, 21). We show that there is a time-dependent increase in the expression levels of full and truncated AR upon long-term BCLT treatment. It is possible that the tumor cells use the truncated AR isoforms to escape from hormonal therapy, and the aberrant expression of the constitutively active truncated AR isoforms may contribute to ablation-independent growth (20, 21). In LNCaP subclones selected for increased androgen independence in androgen-depleted medium, DNMT expression, particularly of DNMT3a and DNMT3b, increased in proportion to androgen independence, and selective AR silencing greatly reduced the modulation of these enzymes. A main finding of the present work, which adds another dimension to the notions referred to above, is that the AR is a key regulator of DNMT expression in some Pca cell models. However, it is very interesting to point out that AR may not be invariably a key master of DNMT activity and expression modulation in all Pca models we studied. As we show, the modulation of DNMT activity in LNCaP-104-R1 and LNCaP-C81 is insensitive to AR knockdown, with a paradoxical effect in LNCaP-104-R1, where DHT functioned as antagonist increasing the DNMT activity levels. These data indicate that androgen-independent Pca are a heterogeneous array of biological conditions with many putative mechanisms and with a common leitmotiv, the relative insensitivity to DHT. So, alternative mechanisms, other than those involving AR, may be surely involved in the modulation of DNMT, but today, the mediators responsible for this process remain essentially unknown. However, the evidence that the modulation of DNMT activity upon DHT and BCLT treatments is affected by AR silencing indubitably indicates that AR is one of the key biological mediators of this phenomenon. Consistent with data on gene silencing of AR, the functional block of this receptor by BCLT led to increased DNMT expression and activity in cell lines chronically treated with this nonsteroidal antiandrogen BCLT, which progressively acquired higher levels of DNMT3a and DNMT3b and, more importantly, became progressively unresponsive to the antitumor effect of BCLT.
The relationship between DNMT and BCLT treatment was studied in vivo using a 22rv1-BCLT-R cell line xenograft model selected for resistance to BCLT. This model offered the opportunity to study the interaction between DNMT and antiandrogen response in a tumor environment closer to that of clinical patients. Cell lines that had lost the antiandrogen response were more aggressive than the parent cell line and more rapidly gave rise to increased tumor mass upon BCLT treatment. Increased vascularization, decreased tumor suppressor expression (PTEN), and activation of survival signal transduction pathways (AKT and ERK) were found in xenografts from antiandrogen unresponsive cell lines. Collectively, these results, which are comparable with those obtained in the cultured cell lines, support the findings on the role of BCLT in Pca progression.
The in vitro and in vivo results corresponded with the events taking place in patients during antiandrogenic treatment, suggesting that BCLT therapy for patients could select a new Pca cell population characterized by aberrant DNMT expression, which might be responsible for their dedifferentiated and proliferative phenotype.
Consistent with the idea that hormonal therapy might affect DNMT in Pca cells, treatment with 5-Aza recovered the antiproliferative responses of BCLT chronically treated cell lines. The results from Pca cells demonstrated that BCLT-mediated changes in DNA methylation status may play a major role in preventing the action of antiandrogen therapy. The possible benefit of 5-Aza treatment in androgen-independent Pca cell lines is demonstrated by their increased apoptosis when cultured in the presence of this drug. However, it is possible that the efficacy of 5-Aza, in recovering the antitumor response under BCLT treatment or upon androgen deprivation (culture in CSS), may be rather different irrespective of the fact that these two treatments, per se, may effectively increase DNMT activity and expression and so to result in an androgen-independent phenotype. The reason why we found a discrepancy in terms of antiproliferative and proapoptotic effects when 5-Aza is added to a medium supplemented with CSS (androgen deprivation culture condition) or when this inhibitor acts together with BCLT may be explained considering that Pca cells may use different mechanisms to compensate for low androgen levels. Among all the putative mechanisms, a ligand-independent AR transactivation by other signaling pathways may be responsible for our findings (26–29). Increased MAPK signaling, mediated by oncogenes such as epidermal growth factor receptor 1 or epidermal growth factor receptor 2, can cause ligand-independent activation of AR especially when its function is not blocked by specific antagonists, such as BCLT (26–29). So, we believe the best therapeutic setting, in which the DNMT functional knockdown may better act, is when DNMT inhibitors are administered in presence of competitive AR antagonists and androgen-deprivation condition rather than in presence of androgen-deprivation conditions only. These findings may to lead to new therapeutic approaches combining castration, AR, and DNMT inhibitors.
Data from human tissues deriving from radical prostatectomy reinforced the significance of the above-described data from in vitro cell lines and in vivo studies. Moreover, an increase in DNMT levels was noted in patients who underwent BCLT therapy administered as neoadjuvant hormone therapy to reduce the tumor mass and capsular involvement before surgery.
Fighting the insurgence of the antiandrogenic-resistant phenotype is an important goal in the treatment of Pca patients. Our data suggest that 5-Aza may differentially recovers the antitumor response, in terms of therapeutic efficacy, of competitive AR antagonists or androgen deprivation therapy providing a new treatment option to counteract disease progression and improve and prolong the effects under hormonal treatment.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health (NIH) Grants R01CA70896, R01CA75503, and R01CA107382 (to R.G.P.). The Kimmel Cancer Center was supported by the NIH Cancer Center Core Grant P30CA56036 (to R.G.P.). This work is also funded in part by the Dr. Ralph and Marian C. Falk Medical Research Trust and by a grant from Pennsylvania Department of Health (R.G.P.), Ministero dell'Istruzione, dell'Università e della Ricerca (B.M.Z.), the University of L'Aquila, and “Cassa Edile di Roma e Provincia” and in part by MINISTERO DELL'UNIVERSITA' E RICERCA, Progetto di Ricerca di Interesse Nazionale 2007, Grant XLTPTK (to M.M).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AKT
- AKT8 virus oncogene cellular homolog
- AR
- Androgen receptor
- 5-Aza
- 5-Azacitidine
- BCLT
- bicalutamide
- CSS
- charcoal-stripped serum
- DHT
- dihydrotestosterone
- DNMT
- DNA methyltransferase
- IHC
- immunohistochemistry
- IRS
- immunoreactivity scoring
- Pca
- prostate cancer
- PTEN
- phosphatase and tensin homolog
- siRNA
- small interfering RNA.
References
- 1. Jansen FH, Roobol M, Jenster G, Schröder FH, Bangma CH. 2009. Screening for prostate cancer in 2008 II: the importance of molecular subforms of prostate-specific antigen and tissue kallikreins. Eur Urol 55:563–574 [DOI] [PubMed] [Google Scholar]
- 2. Morgentaler A, Traish AM. 2009. AM Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol 55:310–320 [DOI] [PubMed] [Google Scholar]
- 3. Evans RM. 1988. The steroid and thyroid hormone receptor superfamily. Science 240:889–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Knudsen KE, Penning TM. 2010. Partners in crime: deregulation of AR activity and androgen synthesis in prostate cancer. Trends Endocrinol Metab 21:315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chi KN, Bjartell A, Dearnaley D, Saad F, Schröder FH, Sternberg C, Tombal B, Visakorpi T. 2009. Castration-resistant prostate cancer: from new pathophysiology to new treatment targets. Eur Urol 56:594–605 [DOI] [PubMed] [Google Scholar]
- 6. Loblaw DA, Mendelson DS, Talcott JA, Virgo KS, Somerfield MR, Ben-Josef E, Middleton R, Porterfield H, Sharp SA, Smith TJ, Taplin ME, Vogelzang NJ, Wade JL, Jr, Bennett CL, Scher HI. 2004. American society of clinical oncology recommendations for the initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer. J Clin Oncol 22:2927–2941 [DOI] [PubMed] [Google Scholar]
- 7. Craft N, Shostak Y, Carey M, Sawyers CL. 1999. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med 5:280–285 [DOI] [PubMed] [Google Scholar]
- 8. Feldman BJ, Feldman D. 2001. The development of androgen-independent prostate cancer. Nat Rev Cancer 1:34–45 [DOI] [PubMed] [Google Scholar]
- 9. Gelmann EP. 2002. Molecular biology of the androgen receptor. Amplification and co-regulators of androgen receptor gene in prostate cancer. J Clin Oncol 20:3001–3015 [DOI] [PubMed] [Google Scholar]
- 10. Golias Ch, Iliadis I, Peschos D, Charalabopoulos K. 2009. Amplification and co-regulators of androgen receptor gene in prostate cancer. Exp Oncol 31:3–8 [PubMed] [Google Scholar]
- 11. Lin C, Yang L, Tanasa B, Hutt K, Ju BG, Ohgi K, Zhang J, Rose DW, Fu XD, Glass CK, Rosenfeld MG. 2009. Nuclear receptor-induced chromosomal proximity and DNA breaks underlie specific translocations in cancer. Cell 139:1069–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Popov VM, Wang C, Shirley LA, Rosenberg A, Li S, Nevalainen M, Fu M, Pestell RG. 2007. The functional significance of nuclear receptor acetylation. Steroids 72:221–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leader JE, Wang C, Fu M, Pestell RG. 2006. Epigenetic regulation of nuclear steroid receptors. Biochem Pharmacol 72:1589–1596 [DOI] [PubMed] [Google Scholar]
- 14. Nelson WG, De Marzo AM, Yegnasubramanian S. 2009. Epigenetic alterations in human prostate cancers. Endocrinology 150:3991–4002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feinberg AP, Tycko B. 2004. The history of cancer epigenetics. Nat Rev Cancer 4:143–153 [DOI] [PubMed] [Google Scholar]
- 16. Morey Kinney SR, Smiraglia DJ, James SR, Moser MT, Foster BA, Karpf AR. 2008. Stage-specific alterations of DNA methyltransferase expression, DNA hypermethylation, and DNA hypomethylation during prostate cancer progression in the transgenic adenocarcinoma of mouse prostate model. Mol Cancer Res 6:365–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perry AS, Foley R, Woodson K, Lawler M. 2006. The emerging roles of DNA methylation in the clinical management of prostate cancer. Endocr Relat Cancer 13:357–377 [DOI] [PubMed] [Google Scholar]
- 18. Morey Kinney SR, Smiraglia DJ, James SR, Moser MT, Foster BA, Karpf AR. 2008. Stage-specific alterations of DNA methyltransferase expression, DNA hypermethylation, and DNA hypomethylation during prostate cancer progression in the transgenic adenocarcinoma of mouse prostate model. Mol Cancer Res 8:1365–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zorn CS, Wojno KJ, McCabe MT, Kuefer R, Gschwend JE, Day ML. 2007. 5-Aza-2′-deoxycytidine delays androgen-independent disease and improves survival in the transgenic adenocarcinoma of the mouse prostate mouse model of prostate cancer. Clin Cancer Res 13:2136–2143 [DOI] [PubMed] [Google Scholar]
- 20. Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, Han M, Partin AW, Vessella RL, Isaacs WB, Bova GS, Luo J. 2009. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res 69:16–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li Y, Alsagabi M, Fan D, Bova GS, Tewfik AH, Dehm SM. 2011. Intragenic rearrangement and altered RNA splicing of the androgen receptor in a cell-based model of prostate cancer progression. Cancer Res 71:2108–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Festuccia C, Gravina GL, Muzi P, Pomante R, Ventura L, Ricevuto E, Vicentini C, Bologna M. 2007. In vitro and in vivo effects of bicalutamide on the expression of TrkA and P75 neurotrophin receptors in prostate carcinoma. Prostate 67:1255–1264 [DOI] [PubMed] [Google Scholar]
- 23. Festuccia C, Gravina GL, Muzi P, Pomante R, Ventura L, Vessella RL, Vicentini C, Bologna M. 2007. Bicalutamide increases phospho-Akt levels through Her2 in patients with prostate cancer. Endocr Relat Cancer 14:601–611 [DOI] [PubMed] [Google Scholar]
- 24. Gravina GL, Festuccia C, Millimaggi D, Dolo V, Tombolini V, de Vito M, Vicentini C, Bologna M. 2008. Chronic azacitidine treatment results in differentiating effects, sensitizes against bicalutamide in androgen-independent prostate cancer cells. Prostate 68:793–801 [DOI] [PubMed] [Google Scholar]
- 25. Gravina GL, Marampon F, Di Staso M, Bonfili P, Vitturini A, Jannini EA, Pestell RG, Tombolini V, Festuccia C. 2010. 5-azacitidine restores and amplifies the bicalutamide response on preclinical models of androgen receptor expressing or deficient prostate tumors. Prostate 70:1166–1178 [DOI] [PubMed] [Google Scholar]
- 26. Gravina GL, Festuccia C, Angelucci A, Poletti A, Capuano D, Vicentini C, Motta M, Bologna M. 2004. Long-term presence of androgens and anti-androgens modulate EGF-receptor expression and MAP-kinase phosphorylation in androgen receptor-prostate positive cancer cells. Int J Oncol 25:97–104 [PubMed] [Google Scholar]
- 27. Festuccia C, Gravina GL, Muzi P, Millimaggi D, Dolo V, Vicentini C, Bologna M. 2008. Akt down-modulation induces apoptosis of human prostate cancer cells and synergizes with EGFR tyrosine kinase inhibitors. Prostate 68:965–974 [DOI] [PubMed] [Google Scholar]
- 28. Festuccia C, Gravina GL, Muzi P, Pomante R, Ventura L, Ricevuto E, Vicentini C, Bologna M. 2007. In vitro and in vivo effects of bicalutamide on the expression of TrkA and P75 neurotrophin receptors in prostate carcinoma. Prostate 67:1255–1264 [DOI] [PubMed] [Google Scholar]
- 29. Gravina GL, Festuccia C, Galatioto GP, Muzi P, Angelucci A, Ronchi P, Costa AM, Bologna M, Vicentini C. 2007. Surgical and biologic outcomes after neoadjuvant bicalutamide treatment in prostate cancer. Urology 70:728–733 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.