Abstract
Osteoporosis and age-related bone loss are important public health concerns. Therefore, there is a high level of interest in the development of medical interventions and lifestyle changes that reduce the incidence of osteoporosis and age-related bone loss. Decreased bone mineral density is associated with high cholesterol, and patients on statins have increased bone mineral densities, strongly implicating cholesterol as a negative regulator of bone homeostasis. In this study, using both molecular and pharmacological approaches, we have been able to demonstrate that the primary cholesterol metabolite, 27-hydroxycholesterol, through its actions on both estrogen receptors and liver X receptors, decreases osteoblast differentiation and enhances osteoclastogenesis, resulting in increased bone resorbtion in mice. Induction of the short heterodimer partner protein by estrogens in osteoblasts can attenuate the liver X receptor-mediated actions of 27-hydroxycholesterol in bone. These data establish a mechanistic link between cholesterol and bone quality, highlight an unexpected target of estrogens in osteoblasts, and define a signaling axis, the therapeutic exploitation of which is likely to yield novel antiosteoporotic drugs.
Meaningful stepwise reductions in the risk for cardiovascular disease can be achieved using increasingly potent 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) to lower low-density lipoprotein (LDL) cholesterol (LDL-C). However, it is remarkable, considering the essential role of cholesterol in cell growth and cell signaling, that the dramatic lowering of LDL-C achievable with statin intervention is so well tolerated. Indeed, it now appears that reducing circulating LDL-C may have unanticipated positive effects on cell physiology that are unrelated to cardiovascular function. Most notable in this regard are data that suggest that statin use is associated with increased bone mineral density (BMD) secondary to increased osteoblast function (1). Given the minimal extra hepatic exposure to statins, it is considered likely that the bone protective activities of these drugs relates to their ability to lower circulating LDL-C rather than a direct effect on osteoblast/osteoclast function. In support of this hypothesis is the observation that hypercholesteremia is a risk factor for decreased BMD and osteoporosis (2–5). Thus, it is apparent that pharmacological interventions that regulate cholesterol synthesis can have an unanticipated positive effect on bone homeostasis, the mechanism of which is not readily apparent.
Recent data from our laboratory have shown that genetic or pharmacological manipulations that increase serum levels of the cholesterol metabolite 27-hydroxycholesterol (27HC) in mice result in a profound decrease in both trabecular and cortical BMD (6). 27HC is synthesized predominantly in differentiated macrophages through the actions of CYP27A1. Importantly, a robust stoichiometric relationship has been established between circulating levels of 27HC in humans and those of total cholesterol (7). This suggests that 27HC may be an important link between circulating cholesterol and bone homeostasis. It was of particular significance, therefore, that we, and others, have recently determined that 27HC can bind to and modulate the transcriptional activity of the estrogen receptors (ER) (ERα and ERβ). Specifically, it was demonstrated that 1) 27HC interacted with the ligand binding domain of ERα, permitting it to adopt a unique conformational state; 2) the ERα-27HC complex exhibited a cofactor binding preference distinct from that observed when the receptor was occupied by 17β-estradiol (E2); and 3) the relative ERα agonist/antagonist activity of 27HC differed between cells and on different promoters within the same cell (8, 9). Thus, by all established criteria, 27HC exhibited the pharmacological characteristics of an endogenous selective ER modulator.
It is clear from our recently published studies that some of the negative impact of 27HC in bone results from its ability to interact with and modulate ER function. However, it was also evident from these initial studies performed in mice that, at least with respect to cortical bone, the negative impact of 27HC 1) was apparent in ovary intact animals, 2) was considerably accentuated upon ovariectomy, and 3) could not be reversed by the administration of supraphysiological doses of E2 (6). This suggested that there might be targets other than ER contributing to the pathophysiological activities of 27HC in bone. In this regard, we now report that in addition to ER, the liver X receptors (LXR) (LXRα and LXRβ) are targets of 27HC in bone. Notably, activation of LXR by 27HC decreases osteoblast differentiation and activity and increases the production of the osteoclastogenic factors TNFα and receptor activator of nuclear factor κB ligand (RANKL). Furthermore, through its actions on ERα, 27HC inhibits the expression of the LXR inhibitor small heterodimeric partner (SHP) in osteoblasts allowing for unchecked LXR activity. Thus, as a result of the dual actions of 27HC on ER and LXR, osteoblastogenesis and osteoclastogenesis are uncoupled, resulting in decreased bone density and reduction in bone quality. These important findings will be instructive with respect to the development of strategies to modulate the risk of osteoporosis by diet or by pharmaceutical interventions. Additionally, these results may also explain differences in response among patients to existing antiresorbtive drugs and provide new targets in bone, the regulation of which may have clinical utility.
Materials and Methods
All studies involving the use of animals were conducted after previous approval by the Duke Institutional Animal Care and Use Committee.
Primary calvarial (pre)osteoblast culture
Calvaria from 3- to 5-d-old pups were removed, cut into pieces, and washed (Hanks' balanced salt solution + 4 mm EDTA and pen/strep) three times at 37 C for 10 min each, then digested with collagenase (5 mg/ml in Hanks' balanced salt solution + pen/strep) for 20 min at 37 C, five times. The first digest was discarded, and the subsequent four digests were pooled and filtered (70 μm). Filtrate was pelleted, followed by resuspension in phenol red-free MEMα supplemented with 8% charcoal-stripped fetal bovine serum (CFS) and pen/strep. Cells were washed twice more, followed by plating.
Alkaline phosphatase activity
Primary osteoblasts were plated at 20,000 cells/well in phenol-red free media containing 8% CFS and pen/strep in a 48-well plate. After overnight incubation, the cells were treated with the indicated ligand for 36 h, at which time the cells were lysed with 0.1% Triton X-100. Lysate was incubated with substrate solution [0.1 m glycine, 1 mm MgCl2, 1 mm ZnCl2, and 1 mg/ml 4-nitrophenol phosphate (Sigma, St. Louis, MO)] for 30 min at 37 C. The reaction was stopped by addition of NaOH, and absorbance was read at 405 nm. Total protein was determined by the Bradford method (Bio-Rad, Hercules, CA). Absorbance values were normalized to total protein in each sample (6).
Proliferation assays
Primary calvarial preosteoblasts were seeded at 5000 cells/well in a 96-well plates, using a separate plate for each time point. After 12 h, ligands were added at indicated doses. At indicated time points, medium was aspirated and the plate frozen. Cells were lysed by freeze-thaw in water. As a proxy for cell number, DNA content was assessed by quantification of Hoescht 33258 staining (360-nm excitation/460-nm emission).
Mineral deposition
Murine bone marrow was flushed from the femora and tibiae with phenol-red free MEMα (15% CFS, pen/strep), filtered (70 μm), and plated on a surface modified polystyrene 24-well plate (Falcon, Franklin Lakes, NJ). Adherent cells were allowed to expand for 48–72 h, after which media were replaced with osteoblast differentiation media (MEMα, 50 mg/liter ascorbic acid, 10 mm β-glycerphosphate, 10−11 m dexamethasone, and 15% CFS, pen/strep), with indicated ligands. Cells were grown for 25 d, with 90% media and ligand change every 2–3 d.
Staining for mineral was based on Stanford et al. (10). Briefly, cells were then washed in PBS, fixed in 10% neutral buffered formalin for 10 min, washed again with PBS, and stained with 2% (wt/vol) alizarin red for 10 min. Cells were washed with deionized water followed by PBS and a final air dry stage. To quantify the staining, cells were destained with 10% (wt/vol) cetylpyridinium chloride in 10 mm sodium phosphate (pH 7) for 30 min. Alizarin red concentration was then quantified by absorbance at 570 nm. Bone marrow from each animal was run in triplicate. The mean of triplicate values was used as the alizarin concentration for each animal. Data shown represent mean absorbance across all animals ± sem with respect to mean control.
Osteoclast differentiation
RAW264.7 or dispersed murine bone marrow cells were seeded on 48-well plates and allowed to settle overnight. For coculture assays, primary calvaria preosteoblasts were also seeded at this time. Media were then changed to osteoclast differentiating media [MEMα, 8% CFS, pen/strep, 30 ng/ml RANKL (R&D Systems, Minneapolis, MN), and 20 ng/ml macrophage colony-stimulating factor (R&D Systems)] with indicated ligand. Ligands and media were changed every 2 d. After 8 d of differentiation, cells were stained for tartrate-resistant acid phosphatase (TRAcP) (Sigma) as previously described. TRAcP-positive cells with at least three nuclei were counted. Data represent the average percent relative to control. In a separate set of wells, TRAcP enzyme activity was determined by lysing cells with 0.1% Triton X-100 and using p-nitrophenyl as a substrate in a citrate buffer (pH 5.5) containing 0.4 m sodium tartrate (modified from Ref. 11). Absorbances (405 nm) were normalized with total protein as determined by the Bradford method.
Molecular biology
Quantitative real-time PCR (QPCR) was performed as previously described (6). Briefly, total RNA was harvested [cell culture, Aurum Total RNA Mini kit (Bio-Rad) or mouse tissues, TRIzol (Invitrogen, Carlsbad, CA)], reverse transcribed (Bio-Rad), and amplified with iQ SYBRGreen Supermix (Bio-Rad). The relative quantitation method (2−ΔΔCt) was used where all values were normalized to the cyclophilin housekeeping gene, and presented with respect to vehicle control values. Stealth small interfering RNA (siRNA) for ERα and LXR, and appropriate negative controls (scrambled siRNA) were purchased from Invitrogen. siRNA against SHP and relevant mock-control were from Sigma. Cells were transfected with Dharmafect (Thermo Scientific, Waltham, MA) and left for at least 48 h before treatment with ligand.
Migration assays
RAW 264.7 cells (mouse leukemic monocyte macrophage cell line) were seeded on six-well plates at approximately 105 cells/well in DMEM (8% CFS, pen/strep). After 48 h, cells were lifted with Accutase, washed, resuspended in DMEM (0.1% fatty acid-free BSA and 10 mm HEPES, pen/strep), counted, and seeded at 75,000 onto the top of a 8-μm Boyden Chamber (BD Biosciences, Palo Alto, CA). The bottom of the chamber contained media with 10% fetal bovine serum only or spent media from calvarial osteoblasts treated with indicated ligands for 24 h. RAW264.7 cells were allowed to migrate for 20 h, at which time the top chamber was swabbed three times, washed with PBS, and fixed for at least 18 h at 4 C with 2% formaldehyde and 0.1% glutaraldehyde in PBS. After three washes in PBS, fixed cells were stained with 0.5% crystal violet (Sigma-Aldrich, St. Louis, MO) in 20% methanol for 1 h at 25 C and then washed three more times with 1× PBS. Cells in three randomly selected fields were quantified under a light microscope (×100) and averaged. Data represents the mean of three chambers ± sem.
High-cholesterol diet (HCD), GW3965 treatment, and bone analysis
Mouse chow, including the HCD, was purchased from TestDiet (Richmond, IN). GW3965 was synthesized by the Duke University Small Molecule Synthesis Facility, dissolved in dimethylsulfoxide and diluted in a 2-hydroxypropyl-β-cyclodextrin solution. Female 6-wk-old C57BL/6 mice (Charles River Laboratories. Inc., Wilmington, MA) were allowed chow ad libitum and, if appropriate, treated daily by sc injection with 30 mg/kg GW3965 or placebo (dimethylsulfoxide diluted in 2-hydroxypropyl-β-cyclodextrin). Within the last 4 d of the study, mice were isolated in metabolic cages for a 12-h urine collection. After the study period, animals were deeply anesthetized by CO2, blood drawn by cardiac puncture, mice decapitated, and tissues harvested. Serum cholesterol and 27HC were determined on a randomly selected four to seven samples per group by liquid chromatography/mass spectrometry as previously described (9). Dual-energy x-ray absorptiometry was performed using the LUNARPIXIMUS bone densitometer (Lunar Corp., Madison, WI) as previously described (12). Femur microarchitectures at the diaphysis (trabecular) and midsection (cortical) were determined by micro-computed tomography (μCT) (high-resolution quantitative CT) (12). Histomorphometry was performed at the Mayo Clinic as previously described (6). Briefly, femora were fixed in 70% ethanol, dehydrated to 100% ethanol embedded in methylmethacrylate-2-hydroxyethyl and methylacrylate (12.5:1), and sectioned (5 μm) on a Reichert-Jung Supercut 2050 microtome using tungsten-carbide-tipped steel knives. Sections were then stained with Goldner Masson Trichrome, imaged using a light/epifluorescence microscope, and quantified with a digitizing table and the OsteoMeasure histomorphometry system (OsteoMetrics, Inc., Atlanta, GA).
Statistical analysis
For comparisons of two treatment groups, results were analyzed by the unpaired (two-tailed) Student's t test. For comparisons of more than two treatment groups, results (either raw or Ln-transformed) were analyzed by one-way ANOVA followed by the Student-Newman-Keuls multiple comparison test. For proliferation assays (see figure 3 below), a two-way ANOVA was preformed followed by Bonferroni post hoc tests, where groups were compared with control. In all cases, statistical significance was determined if P < 0.05.
Results
Cholesterol negatively impacts bone
Although an association between high cholesterol and poor bone quality in humans has been reported (2–5), and mice fed a high-fat, HCD (Western) develop an osteoporotic phenotype (13), to our knowledge, the effects of cholesterol alone on bone have not been demonstrated. Therefore, we fed female mice a HCD (2% cholesterol + 0.5% NaCholate), with the remainder of the nutritional content of the food being the same. Mice were fed ad libitum, and food intake between the two groups was not specifically controlled for. Mouse weight and serum triglycerides were not significantly altered between groups (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org), indicating that the energy balance and therefore nutritional status between the groups remained similar. However, a HCD for 30 or 90 d resulted in significant increases in both serum cholesterol and 27HC (27HC reaching a mean of 0.33 μm after 30 d and 1.91 μm after 90 d) (Supplemental Fig. 1). Although mice on a normal diet had 27HC concentrations that were below the detection limits in this study, previous work has reported that C57BL/6 mice have concentrations of approximately 0.1 μm (6, 7, 14). Thus, a HCD increased 27HC by approximately 3-fold over 30 d and 20-fold over 90 d. For comparison, human circulating 27HC concentrations can range from 0.075 to 0.8 μm (7, 15).
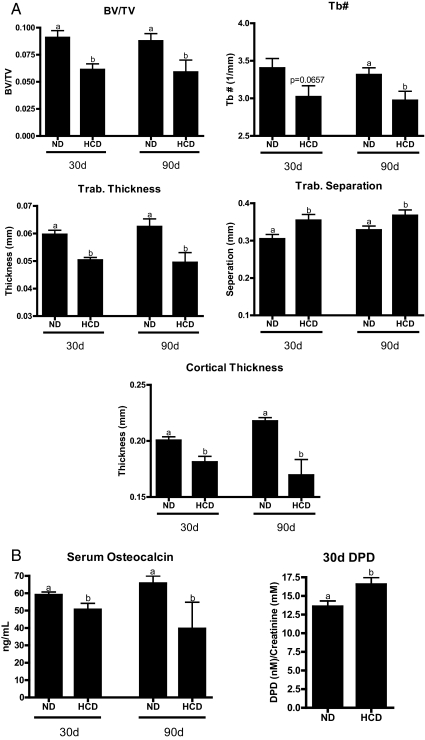
Importantly, mice fed a HCD showed significant loss of trabecular bone [bone volume compared with total volume (BV/TV)] as determined by μCT (Fig. 1). Trabecular number and thickness were reduced, whereas trabecular separation was increased. Cortical thickness was decreased after 30 d with a more pronounced loss after 90d on a HCD. Serum osteocalcin, a marker of osteoblast activity, was reduced, whereas urine deoxypyridinoline (DPD) cross-links, a marker of bone turnover (osteoclast activity) was elevated. Thus, dietary elevation of cholesterol does have a negative impact on bone quality. This finding is significant, given that the oxysterol, 27HC, has a negative impact on bone and that a robust stoichiometric relationship between serum levels of cholesterol and 27HC has been established in both humans and rodents and that the 27HC concentrations achieved in mice after 30 d on a HCD reflect values within the range observed in humans (7). These data suggest that 27HC may be a link between cholesterol metabolism and bone homeostasis.
Fig. 1.
HCD negatively impacts bone quality in vivo. Six-week-old female C57BL/6 mice were fed normal chow (ND) or chow supplemented with 2% cholesterol and 0.5% NaCholate for 30 or 90 d (HCD). A, μCT analysis of femurs indicating total BV/TV, trabecular number (Tb#), trabecular thickness, trabecular separation, and cortical thickness. B, Serum osteocalcin and urine DPD. Different letters denote statistical significance (P < 0.05).
27HC is a ligand for both ER and LXR
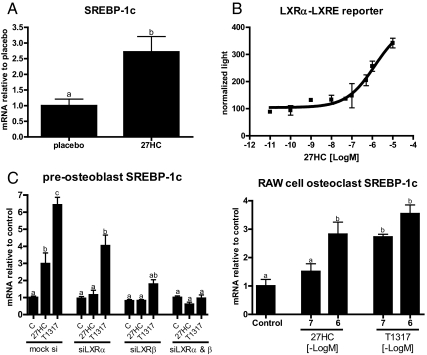
We have previously demonstrated that 27HC is an ERα partial agonist, and as such, it can, in mice, attenuate the positive effects of endogenous estrogens in bone. However, it was also observed that the actions of 27HC in bone were considerably accentuated upon ovariectomy and could not be completely reversed by the administration of supraphysiological doses of E2. Furthermore, in vivo 27HC treatment decreased the expression in calvaria of osteocalcin, a marker of osteoblast activity that is increased upon estrogen withdrawal, and thus would be expected to increase if 27HC solely antagonized ER. Taken together, these data suggested that although engagement of ERα was important for 27HC action, there were likely to be additional targets that were involved in mediating the pharmacological activity of this oxysterol in bone. It was of interest, therefore, that we determined that the mRNA encoding sterol regulatory element-binding protein-1c (SREBP-1c), a known target gene of the LXR, was robustly induced in the calvaria of 27HC-treated mice (Fig. 2A). Although somewhat controversial, it has been reported that 27HC can function as an LXR agonist (16, 17). Importantly, using an LXR response element (direct repeat 4) linked to a luciferase reporter, we confirmed that 27HC activates LXRα in HeLa cells (Fig. 2B). Furthermore, in two bone cell types (primary undifferentiated calvarial preosteoblasts and RAW264.7 cells differentiated into osteoclasts), it was determined that 27HC induces the expression of the LXR target genes SREBP-1c, fatty acid synthase, and ATP-binding cassette, sub-family A, member 1 (Fig. 2C and data not shown). Finally, the induction of SREBP-1c in preosteoblast cells by 27HC was shown to be LXR dependent, because siRNA-mediated knockdown of LXR (α and β) ablated the response. These data suggest that in addition to ERα, it was necessary to consider the LXR as targets of 27HC action in bone.
Fig. 2.
27HC activates the LXR in bone. A, Calvarial mRNA of the LXR target gene SREBP-1c in mice treated with placebo or 27HC for 28 d. B, HeLa cells were transfected with LXRα and a luciferase reporter downstream an LXRE (direct repeat 4) and then treated overnight with vehicle or increasing doses 27HC. Cells were then harvested and assayed for luciferase activity. Luciferase values were normalized to β-galactosidase control. C, mRNA expression of Sterol Regulatory Element-Binding Protein-1c (SREBP-1c) from cultured calvarial preosteoblasts or RAW264.7 cells that had been cultured in osteoclast differentiating media for 8 d and then treated with 27HC for 24 h. Different letters denote statistical significance (P < 0.05).
Activation of LXR by 27HC inhibits osteoblast differentiation and activity
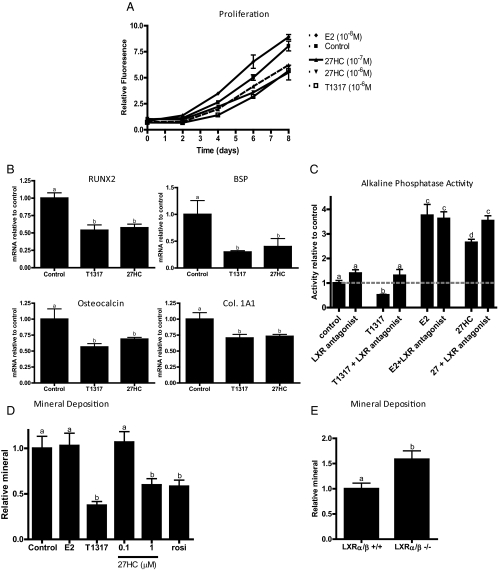
Having demonstrated that 27HC can interact with both ER and LXR, we next evaluated the activity of this oxysterol on preosteoblast proliferation and differentiation. With respect to proliferation, it was observed that treatment of mouse calvarial preosteoblasts with synthetic [T0901317 (T1317)] or endogenous LXR agonists (27HC) significantly inhibited proliferation when compared with vehicle alone or E2 treatment (Fig. 3A). Similar responses were observed in the osteosarcoma cell lines Saos-2 and MG-63 (data not shown). Furthermore, it was observed that treatment of preosteoblasts for 24 h with T1317 or 27HC reduced the expression of Runt-related transcription factor 2, bone sialoprotein, osteocalcin, collagen 1A1, and other markers associated with osteoblast differentiation (Fig. 3B and data not shown). We also assessed the impact of LXR and ER modulators on the expression of alkaline phosphatase activity, a marker of osteoblast activity. As shown in Fig. 3C, alkaline phosphatase activity in calvarial preosteoblast cells was decreased upon treatment with T1317, and this was reversed by cotreatment with the LXR antagonist, GSK2033 (Fig. 3C) (18). As expected, addition of E2 increased alkaline phosphatase activity in these cells in a manner that was unaffected by cotreatment with the LXR antagonist. It was observed under similar conditions that 27HC modestly induces alkaline phosphatase activity, and this induction can be reversed with a pure ER antagonist (Fig. 3C and data not shown). Interestingly, cotreatment of 27HC and the LXR antagonist further increased alkaline phosphatase activity. This suggests that by removing the inhibitory action of the 27HC:LXR complex, the estrogenic actions of this oxysterol are more pronounced. These findings highlight the complexity of 27HC pharmacology but also reveal an unexpected functional cross talk between the ER and LXR signaling pathways in preosteoblasts.
Fig. 3.
LXR activation impairs osteoblast differentiation and activity. A, Cellular proliferation of preosteoblasts. B, QPCR of osteoblast differentiation genes in murine calvarial preosteoblasts treated with indicated ligand (10−6 m) for 24 h. C, Alkaline phosphatase activity of preosteoblasts 36 h after treatment [LXR antagonist (GSK2033), 10−5 m; T1317 and 27HC, 10−6 m; and E2, 10−8 m]. D and E, Mineral deposition as determined by alizarin red staining of bone marrow mesenchymal stem cells after 25 d of differentiation in osteoblastogenic conditions (E2, 10−8 m; T1317, 27HC, and rosiglitazone, 10−6 m). Different letters denote statistical significance (P < 0.05). For 2A, at 8 d, control is significantly elevated compared with 27HC at either dose, and T1317. BSP, Bone sialoprotein.
The effects of 27HC, E2, and LXR agonists on the differentiation of osteoblasts from bone marrow-derived mesenchymal stem cells were also evaluated. Specifically, it was observed that continuous treatment for 25 d with either T1317 or 27HC, but not E2, resulted in the deposition of markedly less mineral (Fig. 3D). The magnitude of the decrease was similar to that observed in cells treated with rosiglitazone, a peroxisome proliferator-activated receptor γ agonist that has previously been shown to inhibit osteoblastogenesis in a robust manner. Conversely, increased mineral deposition was observed in mesenchymal stem cells isolated from LXRα/β double knockout animals that had been grown in osteogenic conditions for 25 d (Fig. 3E). Taken together, these data indicate that 27HC inhibits the proliferation and subsequent differentiation of osteoblast precursors through its ability to activate LXR and its ability to attenuate ER activation.
27HC modulates osteoclastogenesis through ER and LXR
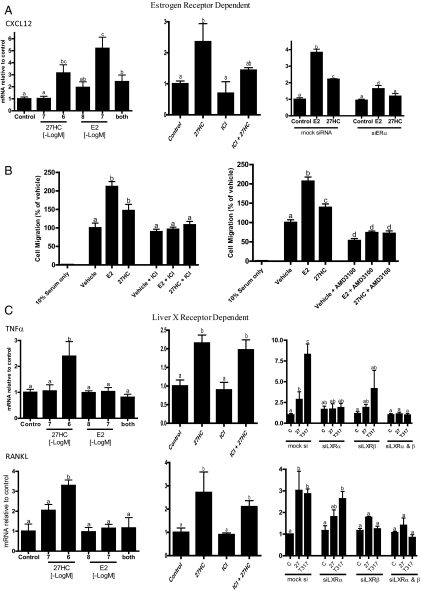
Estrogens inhibit osteoclast differentiation and function both directly and via paracrine signaling from osteoblasts (19, 20). Thus, we evaluated the effects of 27HC on the ability of osteoblasts to recruit monocytes to the bone surface. Using the calvarial-derived preosteoblast model described above, we demonstrated that the chemokine, CXCL12, was induced by E2 and that 27HC functioned as a partial ER agonist in this assay. As would be expected of a classical partial agonist, cotreatment with 27HC reduces the induction of E2 to that of 27HC alone (Fig. 4A). The ER dependence of the E2 and 27HC response was confirmed by showing that it was abrogated by the ER antagonist (ICI 182,780) (Fig. 4A, middle) or siRNA-mediated down-regulation of ERα expression (Fig 4A, right). The level of increased CXCL12 expression observed is functionally significant, because in a coculture model, increased monocyte migration toward 27HC-treated osteoblasts was observed when compared with osteoblasts treated with vehicle (Fig. 4B, left). The enhanced migration was both ER and CXCL12 dependent, because both ICI 182,780 and AMD3100 (a CXCR4 antagonist) attenuated the 27HC-mediated increase in migration (Fig. 4B, right). We infer from these in vitro studies that 27HC, acting through ER, has the potential to increase monocyte recruitment to the bone surface and next proceeded to examine whether this resulted ultimately in increased osteoclastogenesis.
Fig. 4.
Actions of 27HC in osteoblasts are mediated by ER and LXR. A, 27HC increases expression of CXCL12 in an ER-dependent manner. B, Spent media from osteoblasts treated with indicated ligands induce monocyte (RAW264.7 cells) chemotaxis. The 27HC-induced migration is ER and CXCL12-dependent [blocked by ICI 182,780 (ICI) and AMD3100, respectively]. C, LXR activation induces TNFα and receptor activator of nuclear factor κB ligand (RANKL), mediators of osteoclastogenesis in osteoblasts. Unless otherwise indicated, doses are as follows: E2, 10−8 m; and 27HC, ICI 182,780, and T1317, 10−6 m. Different letters denote statistical significance (P < 0.05).
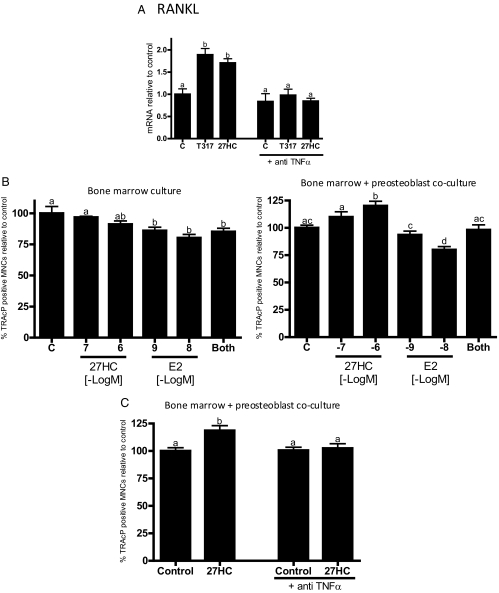
The osteoblast-derived differentiation factors TNFα and RANKL are key mediators of osteoclastogenesis. It was of interest, therefore, that 27HC up-regulated both TNFα and RANKL in primary osteoblasts (Fig. 4C). TNFα induction at the protein level was confirmed by ELISA analysis of spent media (data not shown). This activity of 27HC was ER independent, because neither ICI 182,780 nor siRNA knockdown of ERα had any impact on its ability to up-regulate the expression of TNFα or RANKL by 27HC (Fig. 4C and data not shown). However, it was observed that siRNA knockdown of both LXRα and LXRβ inhibited the 27HC- and T1317-mediated induction of both RANKL and TNFα expression (Fig. 4C). Importantly, and contrary to what is reported for ovariectomized mice (21), we demonstrated that 27HC had no impact on the production of TNFα by T cells, an ER expressing cell of importance to bone biology (data not shown).
It has previously been shown that TNFα is a direct target gene of LXR, and furthermore, it is known that RANKL expression is up-regulated by TNFα. We confirmed the linearity of this regulatory axis in preosteoblasts by showing that both 27HC- and T1317-mediated induction of RANKL expression could be blocked by a neutralizing antibody against secreted TNFα (Fig. 5A). Thus, we concluded that 27HC, acting through LXR, induces the expression of TNFα in osteoblasts, which in turn functions in an autocrine manner to induce the expression of RANKL.
Fig. 5.
LXR-mediated induction of RANKL in osteoblasts is dependent on TNFα and results in increased osteoclastogenesis. A, Calvarial preosteoblasts were treated with indicated ligands with or without a neutralizing TNFα antibody. B, Bone marrow extracts alone, or in coculture with calvarial preosteoblasts, were cultured in differentiating media with indicated ligands and then stained for TRAcP (tartrate-resistant acid phosphate). Positively stained cells with three or more nuclei were counted as osteoclasts. C, Bone marrow extracts were differentiated with or without 27HC, with or without a neutralizing TNFα antibody. Unless otherwise indicated, doses are as follows: 27HC and T1317, 10−6 m; E2, 10−9 m and 27HC 10−6 m in the combined treatment. Different letters denote statistical significance (P < 0.05).
The functional consequences of the increased TNFα and RANKL production observed in 27HC- and T1317-treated preosteoblasts was next assessed using a bone marrow coculture assay. In unmanipulated whole bone marrow cells, which contain few differentiated osteoblasts, E2 decreased osteoclastogenesis as expected, and although not statistically significant, 27HC appeared to function similarly (Fig. 5B). An analogous effect was observed using a RAW264.7 cell osteoclastogenesis assay (data not shown). However, when the same bone marrow cells were cocultured with osteoblasts, addition of 27HC resulted in a dose-dependent increase in the number of differentiated osteoclasts (Fig. 5B). Interestingly, in this model system, E2 inhibited the proosteoclastogenic actions of 27HC. Similar results were obtained when osteoclast activity, (TRAcP activity) was measured (data not shown). Finally, the osteoclastogenic activity of 27HC was abolished upon cotreatment with an anti-TNFα antibody (Fig. 5C). We conclude, therefore, that 27HC-activated LXR in osteoblasts induce TNFα, which subsequently facilitates the expression of RANKL, thereby increasing osteoclastogenesis, and that the ER agonist activity of E2, but not 27HC, is able to overcome this effect. These data highlight the functional importance of LXR/ER cross talk in maintaining osteoblast/osteoclast communication.
LXR agonists and 27HC have a similar impact on bone quality
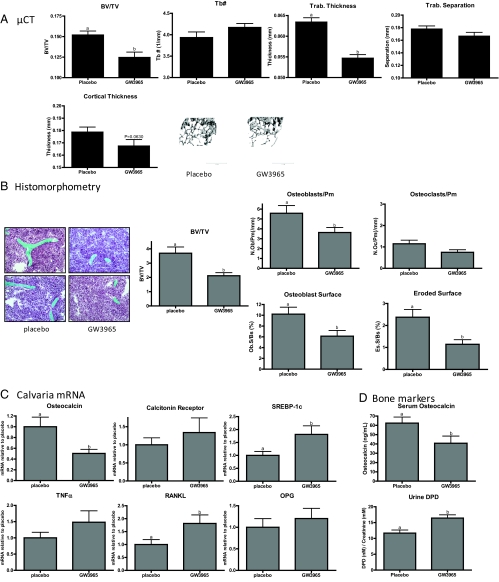
Our previous studies have shown that elevation of 27HC in mice results in an osteoporotic phenotype and that this activity is only partially reversed when animals are given supraphysiological doses of E2. It was of significance, therefore, that activation of LXR by 27HC, or a selective synthetic agonist, resulted in increased osteoclastogenesis in vitro. These data suggested that, in addition to ER, LXR may be a target of 27HC action in bone. Thus, to uncouple ER from LXR signaling and definitively examine the role of LXR in bone, we treated 6-wk-old, ovary intact female mice for 28 d with GW3965, a specific LXR agonist. Increased hepatic and calvarial mRNA expression of the LXR target genes SREBP-1c and fatty acid synthase confirmed that the treatment protocol resulted in adequate drug exposure (Fig. 6C and data not shown). Analysis by dual-energy x-ray absorptiometry identified decreases in BMD in the lumbar spine, midfemur, and midtibia (data not shown). Upon μCT analysis, it was observed that femora from mice treated with GW3965 had decreased bone volume fraction (BV/TV) and decreased trabecular thickness compared with placebo-treated mice (Fig. 6A). Femoral cortical thickness was also decreased in GW3965-treated mice (P = 0.0630, Mann-Whitney test). The decreased BV/TV was confirmed by histomorphometry (Fig. 6B). Importantly, the number of osteoblasts per perimeter and percent of bone surface covered by osteoblasts were reduced in GW3965-treated mice. Not unexpectedly, in these ovary intact animals, we did not see a statistically significant increase in the number of osteoclasts per bone perimeter (see below). However, DPD cross links, a biochemical marker of osteoclast activity, were found to be elevated in GW3965-treated mice (Fig. 6D). Significantly, GW3965 treatment also resulted in increased calvarial RANKL mRNA without a concurrent change in osteoprotegrin. However, the expression of TNFα or calcitonin receptor mRNA were not altered significantly (Fig. 6C). Finally, serum osteocalcin, a marker of osteoblast activity, was decreased, as was the expression of this mRNA in calvaria (Fig. 6, C and D).
Fig. 6.
Effect of the synthetic LXR agonist, GW3965, on bone, in vivo. Six-week-old female C57BL/6 mice were treated sc with 30 mg/kg·d for 28 d. A, μCT analysis of femurs indicating total BV/TV, trabecular number (Tb#), trabecular thickness, trabecular separation, and cortical thickness. B, Histomorphometric analysis of femora. Sections were stained with Goldner's trichrome stain and analyzed at ×20 magnification. C, Calvarial mRNA expression as determined by QPCR. D, Serum osteocalcin and urine DPD. OPG, Osteoprotegrin. Different letters denote statistical significance (P < 0.05).
E2 counteracts the negative effects of LXR in part by induction of SHP
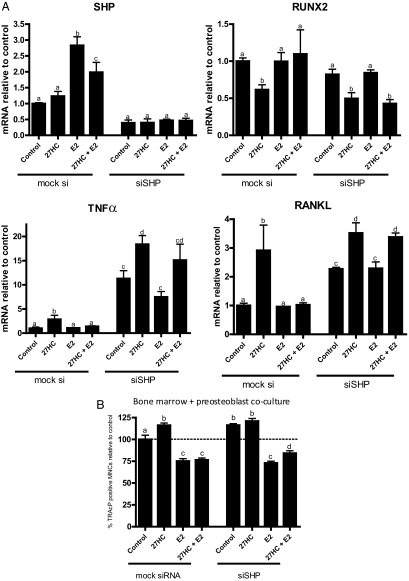
Although E2, acting through ER, can reverse LXR/27HC-mediated induction of TNFα/RANKL expression and subsequent osteoclastogenesis, the mechanism(s) underlying this functional cross talk between ER and LXR are unclear. It was of considerable significance, therefore, that we observed that the expression of the LXR repressor SHP was induced by E2 in osteoblasts (Fig. 7A). Therefore, we tested whether the pharmacology of the 27HC/LXR complex was altered by SHP knockdown. As expected, we observed that E2 treatment reversed 27HC-mediated suppression of RUNX2 expression in control siRNA transfected cells. However, the inhibitory effect of E2 in this assay was lost upon SHP knockdown (Fig. 7A). Similarly, when SHP expression was knocked down, the ability of E2 to attenuate LXR-mediated increases in TNFα and RANKL mRNA levels was also diminished. It is interesting that the basal expression of both TNFα and RANKL mRNA expression is dramatically elevated upon SHP knockdown, a result which may explain in part the osteoporotic phenotype observed in SHP knockout mice (22). In the bone marrow/preosteoblast coculture model (described above), introduction of a control siRNA into the preosteoblasts does not affect the ability of E2 to inhibit and 27HC to increase osteoclastogenesis (Fig. 7B). However, when SHP expression was ablated in the osteoblasts, 27HC attenuated the E2-mediated inhibition of osteoclastogenesis. We conclude from these experiments that the ability of E2 to inhibit osteoclastogenesis involves both its ability to modulate LXR signaling as well as activities that are independent of this receptor.
Fig. 7.
E2 reverses some effects of LXR via induction of SHP. Gene expression (A) or osteoclastogenesis (B) in the presence or absence of siRNA against SHP. Unless otherwise indicated, doses are as follows: E2, 10−8 m; 27HC and T1317, 10−6 m. Different letters denote statistical significance (P < 0.05).
Discussion
These studies were driven by the observations that elevated cholesterol in postmenopausal women is associated with decreased BMD, whereas statin use is associated with increased BMD. The potential link between cholesterol metabolism and bone is also suggested by recent studies in which it was shown in rodents that a “Western diet” resulted an osteoporotic phenotype (13). We found that when cholesterol alone was elevated by diet, bone quality was significantly decreased, reflecting our previous studies, which show that elevated 27HC also negatively impacts bone in vivo. Although there are some data suggesting that certain classes of statins have a direct effect on protein prenylation and that this impacts osteoblast function in vitro, it remains unclear as to how cholesterol and bone biology are linked (1, 23). In this study, we demonstrate that a primary metabolite of cholesterol, 27HC, negatively affects bone physiology through its actions on ER and LXR in osteoblasts. Importantly, the circulating levels of 27HC achieved after only 30 d on a HCD reflect concentrations found within the range of human values and are sufficient to activate the LXR. Together with our data, the robust association between the levels of serum cholesterol and 27HC suggests that this oxysterol may be a pathophysiologically relevant link between cholesterol metabolism and bone homeostasis.
It was demonstrated in this study that LXR activation by 27HC decreases osteoblast proliferation, differentiation, and activity. Together with our data, however, there are additional studies that support a role for LXR in bone biology. Notably, it has been demonstrated that 1) LXR agonists (T1317 and GW3965) inhibit sonic hedgehog-regulated osteoblastogenesis (24), 2) treatment of mouse calvarial osteoblasts with T1317 decreased secreted osteocalcin and alkaline phosphatase activity (25), 3) female LXRα−/− mice exhibit increased BMD compared with their wild-type counterparts, and 4) LXRβ−/− mice exhibit increased expression of markers indicative of increased osteoblast function (26). In this study, we show that GW3965 treatment of female ovary intact mice resulted in pathological changes in bone microarchitecture, reduced osteoblast numbers, a smaller osteoblast surface, and decreased serum osteocalcin levels. Counterposed to this hypothesis are the results of a recent study that failed to see any effect on the bones of female mice treated for 12 wk with either T1317 or GW3965 (25). However, given that the drugs were administered in mouse chow, it is unclear as to whether this regime allowed the delivery of an efficacious dose. When taken together, however, we believe that our data and complementary data from other studies provide strong support for a role of LXR as modulator of bone homeostasis and that through this receptor, 27HC is able to exert a regulatory effect on both osteoblastogenesis and the production of proosteoclastic factors.
In this study, we have shown that 27HC (and other LXR ligands) acts through LXR to induce TNFα expression in osteoblasts. This result seemed paradoxical given the general belief that LXR activation is antiinflammatory (27–29). However, as with most nuclear receptors, the activity of LXR is likely to be influenced heavily by context. For instance, it has been shown that 1) the livers from mice on a chronic HCD have increased expression of TNFα (27, 30), 2) hepatic TNFα expression was increased by T1317 in apoE−/− mice fed a high-fat diet/HCD (31), and 3) TNFα expression is increased in cholesterol-loaded macrophages. Furthermore, in monocytes and stimulated dendritic cells, LXR activation results in increased TNFα synthesis and secretion (32, 33). The recent demonstration of a specific, functional LXRE, within the human TNFα promoter, suggests that it is a direct target of LXR (32). In this study, we define an important autocrine loop, wherein LXR-mediated induction of TNFα results in the secondary production of RANKL, a primary mediator of osteoclastogenesis. Importantly, the activities of 27HC phenocopy those of the LXR agonists in this system, providing an explanation for its proresorbtive activities in bone.
One of the most interesting findings from our studies is that although LXR is clearly a primary target of 27HC, its actions are somewhat attenuated by cotreatment with E2. The mechanistic basis for this functional cross talk between ER and LXR was revealed in a series of studies that demonstrated that E2 induces the expression of the LXR repressor, SHP, in osteoblasts. Thus, through its actions on ER, 27HC can inhibit the induction of SHP, and through its actions on LXR, it initiates a signaling cascade that results ultimately in increased osteoclastogenesis. In support of this hypothesis is the observation that the expression of both ERα and SHP increases throughout osteoblast differentiation (22, 34), and that SHP−/− mice have decreased trabecular and cortical BMD (22). This important observation implies that postmenopausal women may be at a greater risk of the negative skeletal effects of 27HC, because a loss of E2 and subsequent decrease in SHP expression would leave LXR signaling unhindered. It also suggests that the actions of 27HC could be attenuated by inducing SHP expression using farnesoid X receptor ligands. Interestingly, we have recently shown that osteoblasts do indeed express farnesoid X receptor and, when activated, can induce the expression of SHP.
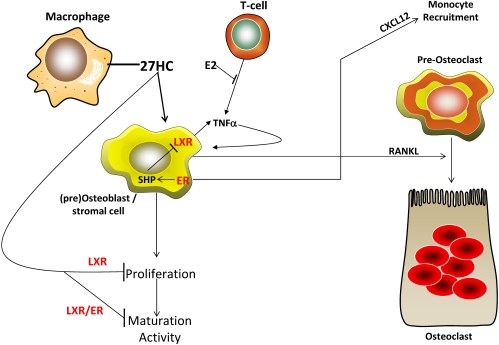
Collectively, our data provide strong support for the hypothesis that 27HC impairs bone deposition and enhances bone turnover both by 1) competing with E2 at the level of its cognate receptor, one consequence of which is to inhibit SHP expression while inducing CXCL12; and 2) by activation of LXR, which inhibits osteoblast differentiation and increases the expression of TNFα and RANKL (Fig. 8). These data suggest that 27HC is a key biochemical entity that links cholesterol metabolism to bone biology, a finding of considerable medical importance.
Fig. 8.
27HC negatively impacts bone via the ER and LXR. 27HC, produced in part by macrophages, activates LXR, which then inhibits subsequent proliferation, maturation, and activity. 27HC-activated osteoblast LXR concomitantly increase TNFα synthesis, which then feeds back to increase osteoblast expression of RANKL. RANKL increases osteoclastogenesis from monocyte precursors. Monocyte precursors are recruited to the bone surface by the actions of 27HC via the ER to stimulate CXCL12 expression in osteoblasts. Thus 27HC decreases bone deposition and increases bone resorption.
Supplementary Material
Acknowledgments
We thank Dr. D. J. Mangelsdorf (University of Texas Southwestern Medical Center) for the LXRα/β double knockout mice, Dr. W. J. Zuercher (GlaxoSmithKline, Research Triangle Park, NC) for the LXR antagonist, and members of the McDonnell laboratory for insightful discussions.
Present address for C.D.D.: Genentech, Inc., South San Francisco, California 94080.
This work was supported by National Institutes of Health Grants R37DK048807 (to D.P.M.) and AG004875 (to S.K.), National Institute of Diabetes and Digestive and Kidney Diseases Grant 1P30DK079328 (to M.U.), the Arthritis Foundation (D.G.-P.), and the Department of Defense Postdoctoral Award W81XWH-09-1-0613 (to E.R.N.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMD
- Bone mineral density
- BV/TV
- bone volume compared with total volume
- CFS
- charcoal-stripped fetal bovine serum
- μCT
- micro-computed tomography
- DPD
- deoxypyridinoline
- E2
- 17β-estradiol
- ER
- estrogen receptor
- 27HC
- 27-hydroxycholesterol
- HCD
- high-cholesterol diet
- LDL
- low-density lipoprotein
- LDL-C
- LDL cholesterol
- LXR
- liver X receptor
- QPCR
- quantitative real-time PCR
- RANKL
- receptor activator of nuclear factor κB ligand
- SHP
- small heterodimeric partner
- siRNA
- small interfering RNA
- SREBP-1c
- sterol regulatory element-binding protein-1c
- T1317
- T0901317
- TRAcP
- tartrate-resistant acid phosphatase.
References
- 1. Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, Boyce B, Zhao M, Gutierrez G. 1999. Stimulation of bone formation in vitro and in rodents by statins. Science 286:1946–1949 [DOI] [PubMed] [Google Scholar]
- 2. Orozco P. 2004. Atherogenic lipid profile and elevated lipoprotein (a) are associated with lower bone mineral density in early postmenopausal overweight women. Eur J Epidemiol 19:1105–1112 [DOI] [PubMed] [Google Scholar]
- 3. Tankó LB, Bagger YZ, Nielsen SB, Christiansen C. 2003. Does serum cholesterol contribute to vertebral bone loss in postmenopausal women? Bone 32:8–14 [DOI] [PubMed] [Google Scholar]
- 4. Tarakida A, Iino K, Abe K, Taniguchi R, Higuchi T, Mizunuma H, Nakaji S. 2011. Hypercholesterolemia accelerates bone loss in postmenopausal women. Climacteric 14:105–111 [DOI] [PubMed] [Google Scholar]
- 5. Yamaguchi T, Sugimoto T, Yano S, Yamauchi M, Sowa H, Chen Q, Chihara K. 2002. Plasma lipids and osteoporosis in postmenopausal women. Endocr J 49:211–217 [DOI] [PubMed] [Google Scholar]
- 6. DuSell CD, Nelson ER, Wang X, Abdo J, Mödder UI, Umetani M, Gesty-Palmer D, Javitt NB, Khosla S, McDonnell DP. 2010. The endogenous selective estrogen receptor modulator 27-hydroxycholesterol is a negative regulator of bone homeostasis. Endocrinology 151:3675–3685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karuna R, Holleboom AG, Motazacker MM, Kuivenhoven JA, Frikke-Schmidt R, Tybjaerg-Hansen A, Georgopoulos S, van Eck M, van Berkel TJ, von Eckardstein A, Rentsch KM. 2011. Plasma levels of 27-hydroxycholesterol in humans and mice with monogenic disturbances of high density lipoprotein metabolism. Atherosclerosis 214:448–455 [DOI] [PubMed] [Google Scholar]
- 8. DuSell CD, Umetani M, Shaul PW, Mangelsdorf DJ, McDonnell DP. 2008. 27-Hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol Endocrinol 22:65–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Umetani M, Domoto H, Gormley AK, Yuhanna IS, Cummins CL, Javitt NB, Korach KS, Shaul PW, Mangelsdorf DJ. 2007. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat Med 13:1185–1192 [DOI] [PubMed] [Google Scholar]
- 10. Stanford CM, Jacobson PA, Eanes ED, Lembke LA, Midura RJ. 1995. Rapidly forming apatitic mineral in an osteoblastic cell line (UMR 106-01 BSP). J Biol Chem 270:9420–9428 [DOI] [PubMed] [Google Scholar]
- 11. Voronov I, Heersche JN, Casper RF, Tenenbaum HC, Manolson MF. 2005. Inhibition of osteoclast differentiation by polycyclic aryl hydrocarbons is dependent on cell density and RANKL concentration. Biochem Pharmacol 70:300–307 [DOI] [PubMed] [Google Scholar]
- 12. Gesty-Palmer D, Flannery P, Yuan L, Corsino L, Spurney R, Lefkowitz RJ, Luttrell LM. 2009. A β-arrestin-biased agonist of the parathyroid hormone receptor (PTH1R) promotes bone formation independent of G protein activation. Sci Transl Med 1:1ra1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Demigné C, Bloch-Faure M, Picard N, Sabboh H, Besson C, Rémésy C, Geoffroy V, Gaston AT, Nicoletti A, Hagège A, Ménard J, Meneton P. 2006. Mice chronically fed a westernized experimental diet as a model of obesity, metabolic syndrome and osteoporosis. Eur J Nutr 45:298–306 [DOI] [PubMed] [Google Scholar]
- 14. Li-Hawkins J, Lund EG, Turley SD, Russell DW. 2000. Disruption of the oxysterol 7α-hydroxylase gene in mice. J Biol Chem 275:16536–16542 [DOI] [PubMed] [Google Scholar]
- 15. DuSell CD, McDonnell DP. 2008. 27-Hydroxycholesterol: a potential endogenous regulator of estrogen receptor signaling. Trends Pharmacol Sci 29:510–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fu X, Menke JG, Chen Y, Zhou G, MacNaul KL, Wright SD, Sparrow CP, Lund EG. 2001. 27-Hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol-loaded cells. J Biol Chem 276:38378–38387 [DOI] [PubMed] [Google Scholar]
- 17. Song C, Liao S. 2000. Cholestenoic acid is a naturally occurring ligand for liver X receptor α. Endocrinology 141:4180–4184 [DOI] [PubMed] [Google Scholar]
- 18. Zuercher WJ, Buckholz RG, Campobasso N, Collins JL, Galardi CM, Gampe RT, Hyatt SM, Merrihew SL, Moore JT, Oplinger JA, Reid PR, Spearing PK, Stanley TB, Stewart EL, Willson TM. 2010. Discovery of tertiary sulfonamides as potent liver X receptor antagonists. J Med Chem 53:3412–3416 [DOI] [PubMed] [Google Scholar]
- 19. Krum SA, Miranda-Carboni GA, Hauschka PV, Carroll JS, Lane TF, Freedman LP, Brown M. 2008. Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. EMBO J 27:535–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S. 2007. Estrogen prevents bone loss via estrogen receptor α and induction of Fas ligand in osteoclasts. Cell 130:811–823 [DOI] [PubMed] [Google Scholar]
- 21. Roggia C, Gao Y, Cenci S, Weitzmann MN, Toraldo G, Isaia G, Pacifici R. 2001. Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci USA 98:13960–13965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jeong BC, Lee YS, Bae IH, Lee CH, Shin HI, Ha HJ, Franceschi RT, Choi HS, Koh JT. 2010. The orphan nuclear receptor SHP is a positive regulator of osteoblastic bone formation. J Bone Miner Res 25:262–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weivoda MM, Hohl RJ. 2011. Effects of farnesyl pyrophosphate accumulation on calvarial osteoblast differentiation. Endocrinology 152:3113–3122 [DOI] [PubMed] [Google Scholar]
- 24. Kim WK, Meliton V, Park KW, Hong C, Tontonoz P, Niewiadomski P, Waschek JA, Tetradis S, Parhami F. 2009. Negative regulation of Hedgehog signaling by liver X receptors. Mol Endocrinol 23:1532–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prawitt J, Beil FT, Marshall RP, Bartelt A, Ruether W, Heeren J, Amling M, Staels B, Niemeier A. 2011. Short-term activation of liver X receptors inhibits osteoblasts but long-term activation does not have an impact on murine bone in vivo. Bone 48:339–346 [DOI] [PubMed] [Google Scholar]
- 26. Robertson KM, Norgård M, Windahl SH, Hultenby K, Ohlsson C, Andersson G, Gustafsson JA. 2006. Cholesterol-sensing receptors, liver X receptor α and β, have novel and distinct roles in osteoclast differentiation and activation. J Bone Miner Res 21:1276–1287 [DOI] [PubMed] [Google Scholar]
- 27. Wang YY, Dahle MK, Steffensen KR, Reinholt FP, Collins JL, Thiemermann C, Aasen AO, Gustafsson JA, Wang JE. 2009. Liver X receptor agonist GW3965 dose-dependently regulates lps-mediated liver injury and modulates posttranscriptional TNF-α production and p38 mitogen-activated protein kinase activation in liver macrophages. Shock 32:548–553 [DOI] [PubMed] [Google Scholar]
- 28. Castrillo A, Joseph SB, Marathe C, Mangelsdorf DJ, Tontonoz P. 2003. Liver X receptor-dependent repression of matrix metalloproteinase-9 expression in macrophages. J Biol Chem 278:10443–10449 [DOI] [PubMed] [Google Scholar]
- 29. Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. 2003. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med 9:213–219 [DOI] [PubMed] [Google Scholar]
- 30. Li Y, Schwabe RF, DeVries-Seimon T, Yao PM, Gerbod-Giannone MC, Tall AR, Davis RJ, Flavell R, Brenner DA, Tabas I. 2005. Free cholesterol-loaded macrophages are an abundant source of tumor necrosis factor-α and interleukin-6: model of NF-κB- and map kinase-dependent inflammation in advanced atherosclerosis. J Biol Chem 280:21763–21772 [DOI] [PubMed] [Google Scholar]
- 31. Dai X, Ou X, Hao X, Cao D, Tang Y, Hu Y, Li X, Tang C. 2007. Effect of T0901317 on hepatic proinflammatory gene expression in apoE−/− mice fed a high-fat/high-cholesterol diet. Inflammation 30:105–117 [DOI] [PubMed] [Google Scholar]
- 32. Landis MS, Patel HV, Capone JP. 2002. Oxysterol activators of liver X receptor and 9-cis-retinoic acid promote sequential steps in the synthesis and secretion of tumor necrosis factor-α from human monocytes. J Biol Chem 277:4713–4721 [DOI] [PubMed] [Google Scholar]
- 33. Töröcsik D, Baráth M, Benko S, Széles L, Dezso B, Póliska S, Hegyi Z, Homolya L, Szatmári I, Lányi A, Nagy L. 2010. Activation of liver X receptor sensitizes human dendritic cells to inflammatory stimuli. J Immunol 184:5456–5465 [DOI] [PubMed] [Google Scholar]
- 34. Bodine PV, Henderson RA, Green J, Aronow M, Owen T, Stein GS, Lian JB, Komm BS. 1998. Estrogen receptor-α is developmentally regulated during osteoblast differentiation and contributes to selective responsiveness of gene expression. Endocrinology 139:2048–2057 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.