Abstract
Vitamin D exerts important regulatory effects on the endocrine and immune systems. Autoimmune type 1 diabetes (T1D) development in the inbred NOD mouse strain can be accelerated by vitamin D insufficiency or suppressed by chronic treatment with high levels of 1α,25-dihydroxyvitamin D3. Consequently, a report that T1D development was unaffected in NOD mice genetically lacking the vitamin D receptor (VDR) was unexpected. To further assess this result, the mutant stock was imported to The Jackson Laboratory, backcrossed once to NOD/ShiLtJ, and progeny rederived through embryo transfer. VDR-deficient NOD mice of both sexes showed significant acceleration of T1D. This acceleration was not associated with alterations in immune cells targeting pancreatic β-cells. Rather, the capacity of β-cells to produce and/or secrete insulin was severely impaired by the hypocalcaemia developing in VDR-deficient NOD mice fed a standard rodent chow diet. Feeding a high-lactose calcium rescue diet that circumvents a VDR requirement for calcium absorption from the intestine normalized serum calcium levels, restored β-cell insulin secretion, corrected glucose intolerance, and eliminated accelerated T1D in VDR-deficient NOD mice. These findings suggest that calcium and/or vitamin D supplementation may improve disease outcomes in some T1D-prone individuals that are calcium deficient.
Vitamin D insufficiency has been identified as a potential environmental factor that increases the risk for autoimmune-mediated type 1 diabetes (T1D) (1). Sunlight is the primary induction source of circulating vitamin D. T1D frequency is lowest in humans in equatorial regions, where exposure to sunlight is high, and greatest in more polar regions with reduced sunlight exposure (2). Plasma 25-hydroxyvitamin D3, the predominant circulating form of vitamin D, is significantly lower in T1D patients compared with similarly aged subjects. Moreover, increased T1D susceptibility has been associated with polymorphisms in four vitamin D metabolism genes that regulate plasma 25-hydroxyvitamin D3 levels (3). Inbred NOD mice have provided a model system for dissecting genetic and environmental interactions regulating T1D development (4). Additional support for vitamin D as an environmental factor modulating T1D development is a report that its elimination from diet and UV light sources accelerates disease onset in NOD mice (5). Furthermore, T1D is inhibited in NOD mice supplemented with the metabolically active form of vitamin D3, 1α,25-dihydroxyvitamin D3, or various 1α,25-dihydroxyvitamin D3 synthetic analogs (6–9). The reasons why these compounds inhibit T1D development in NOD mice remain unclear. However, several studies have suggested that they do so by initiating vitamin D receptor (VDR)-mediated signaling events normalizing immunological tolerance induction processes, which are impaired in NOD mice, and also possibly human T1D patients. These include reducing the capacity of professional antigen presenting dendritic cells to activate pathogenic T cells (10), increasing the frequency of regulatory T cells (Treg) that suppress autoimmune responses (9, 11), and by skewing the effector CD4 T cells toward a T helper 2 cytokine production phenotype that putatively inhibits T1D in NOD mice (12–14).
Less attention has focused on how 1α,25-dihydroxyvitamin D3 or related analogs may reduce T1D risk through their effects on calcium homeostasis. 1α,25-dihydroxyvitamin D3 interacts with the VDR in cells of the intestines, kidneys, and bone to maintain serum calcium levels by controlling calcium absorption, retention, and resorption, respectively, by these organs. Vitamin D deficiency-induced calcium insufficiency causes dysregulation of many tissues and organ systems, including those that control the metabolism and absorption of glucose (15). Indeed, calcium is necessary for both glucose-induced insulin secretion by pancreatic β-cells (16) and insulin-mediated glucose uptake by skeletal muscle (17, 18). This requirement has been clearly demonstrated in rats, which become glucose intolerant when deprived of calcium (19, 20). Similar states of hypocalcaemia-induced glucose intolerance may accelerate disease in NOD mice and T1D-prone humans by either limiting the capacity of β-cells surviving autoimmune destruction to secrete insulin and/or by increasing their insulin requirements for glucose uptake. Some evidence for a link between calcium status and risk for T1D exists for humans. Hyppönen et al. (21) found that children with suspected rickets in their first year of life were three times more likely to develop T1D compared with healthy infants. T1D patients also have decreased bone mineral density and a greater risk of fractures compared with the general population (22).
Because vitamin D is required for efficient calcium absorption it is often difficult to differentiate the effects of a vitamin D vs. a calcium deficiency from the available literature. Thus, NOD mice carrying a null allele for the VDR gene (Vdr−/−) were previously generated at The Catholic University in Leuven to dissect components of vitamin D-mediated T1D protection. Surprisingly, NOD. Vdr−/− mice produced in Leuven did not develop accelerated diabetes compared with their standard NOD strain (23). However, the current work shows that after rederivation into The Jackson Laboratory (JAX) that involved a single outcross to the NOD/ShiLtJ inbred substrain, both male and female mice of the resultant stock that were homozygous for the disrupted Vdr gene (hereafter designated VDR−/−) were more glucose intolerant and susceptible to T1D than wild-type and heterozygous Vdr-expressing control segregants (hereafter respectively designated VDR+/+ and VDR−/+). Accelerated T1D onset in these latter Vdr−/− NOD genetic background mice does not appear to be due to an increase in the pathogenicity of β-cell-directed autoimmune responses but rather as a consequence of a hypocalcaemia-induced reduction in β-cell function.
Materials and Methods
Mice and diet
Male NOD/Cmat genetic background mice heterozygous for a Vdr gene deletion (23) were imported from The Catholic University to the Type 1 Diabetes Repository at JAX. At JAX, the targeted Vdr mutation was backcrossed for one generation to the inbred NOD/ShiLtJ substrain and pups rederived by embryo transfer. A sibling intercross strategy was then employed to generate a new NOD genetic background stock either homozygous for the Vdr gene deletion (formal designation NOD.Cg-Vdrtm1Ska/CmatJ, JAX no. 6956 hereafter noted as VDR−/−) as well as heterozygous segregants used as controls (hereafter noted VDR−/+). An intercross strategy employing VDR−/+ mice and the previously described NOD.Cg-PrkdcscidEmv30−/−/Dvs strain (24) was used to generate a VDR-deficient NOD background stock that also lacks B and T cells (JAX no. 2313 and here designated SCID.VDR−/−). Colonies were maintained under specific pathogen-free (SPF) conditions. Mice were fed a standard National Institutes of Health rat and mouse 6% fat chow purchased from Purina LabDiet (5K52) (Purina, St. Louis, MO) with calcium and phosphorus levels of 1.17 and 0.93%, respectively. In some studies, a semipurified high-lactose calcium rescue (HiCal) diet (TD.96348) containing 2.0% calcium and 1.25% phosphorus purchased from Harlan Teklad (Madison, WI) was fed to the indicated mice.
Assessment of diabetes and insulitis
Diabetes was assessed by daily monitoring of glycosuria with Ames Diastix (Bayer, Diagnostics Division, Elkhart, IN), with disease onset defined by two consecutive values of more than or equal to 3. A previously described metric (25) was used to assign insulitis scores ranging from 0 (normal β-cell mass with no leukocytic infiltration) to 4 (complete destruction) for the indicated mice.
Metabolic studies
For the glucose tolerance test, blood samples were analyzed after overnight fasting at 0, 15, 30, 60, and 120 min after ip injection with 2 g/kg dextrose. For the insulin tolerance test, blood glucose levels were analyzed from fed mice at 0, 15, 30, and 60 min after ip injection with 0.75 U/kg of purified porcine insulin (Novo Nordisk, Bagsvaerd, Denmark). For the glucose-stimulated insulin secretion test, blood samples were obtained at 0 and 2 min after ip injection of 3 g/kg dextrose. Blood glucose levels were determined from whole venous blood taken from the tail using an automatic glucose monitor (One Touch Ultra; Lifescan, Mountain View, CA). Insulin levels were measured in serum from retro-orbital bleeds by ELISA (Crystal Chem, Chicago, IL) using mouse insulin as a standard. Serum calcium levels were determined by dilution with Arsenazo III that combines with available calcium to form calcium-Arsenazo, which can be monitored for changes in absorbance at 650 nm using a Beckman SYNCHRON CX5 DELTA atomic absorption spectrometer. Serum concentrations of 25-hydroxyvitamin D3 were assessed using 125I RIA kits (DiaSorin, Inc., Stillwater, MN).
Immunological studies
For the adoptive transfer of T1D, 5-wk-old male and female SCID.VDR−/−, SCID.VDR−/+, and SCID.VDR+/+ recipients were injected iv with 1 × 107 splenocytes from 6- to 7-wk-old NOD VDR−/− or VDR+/+ donors. In a separate experiment, proportions of various leukocyte populations within pancreatic lymph nodes, spleens, and thymii of 14-wk-old VDR−/− and VDR−/+ female NOD mice were compared by flow cytometry using antibodies specific for the immune cell surface molecules CD4 (RM4-5), CD8 (53-6.72), T cell receptor β (H57-597), B220 (RA3-6B2), CD80 (16-10A1), CD86 (GL-1), CD11c (N418), Gr-1 (RB6-8C5), CD11b (M1/70), and plasmacytoid DC Ag-1 (eBio927). Natural killer T (NKT) cells were detected by staining with a phycoerythrin-conjugated CD1d tetramer loaded with PBS57, an α-galactosylceramide (α-GalCer) analog, provided by the National Institutes of Health Tetramer Facility. For intracellular NKT cell staining, splenocytes from mice injected iv 2 h previously with 4 μg per mouse of α-GalCer or vehicle were surface labeled with CD1d tetramer, anti-CD4, and anti-TCRβ and restained with antibodies specific for interferon γ (XMG1.2) and IL-4 (11B11) after fixation and permeabilization with solutions from the BD Biosciences intracellular staining kit (BD Biosciences, San Jose, CA). For intracellular FoxP3 staining, cells were surface stained using antibodies for CD4 and CD25 (PC61) molecules before they were fixed and permeabilized with solutions contained within the eBiosciences FoxP3 staining set (eBiosciences, Inc., San Diego, CA). The recommended manufacturer staining protocol was followed using an antimouse/rat FoxP3 antibody (FJK-16s). A Treg suppression assay was performed as previously described (26). Briefly, Treg were obtained by first purifying CD4+ T cells from spleens of VDR−/− and VDR+/+ mice by depleting B220+, CD8+, CD11b+, and CD11c+ cells using a magnetic bead system. The CD25+ fraction was isolated from the eluted CD4 T cells by staining with biotinylated anti-CD25 (clone 7D4; BD Biosciences) followed by selection with streptavidin-conjugated microbeads (Miltenyi Biotec, Auburn, CA). CD4+CD25− T cells from VDR+/+ mice were purified as described above and labeled with carboxyfluorescein succinimidyl ester. These cells were then cocultured (5 × 104 cells/well) in triplicate with indicated ratios of Treg in round-bottomed 96-well tissue culture plates with 5 μg/ml anti-CD3 (clone 145-2C11; BD Biosciences) and 2 × 105/well NOD.scid splenocytes. T-cell proliferation was assessed after incubation at 37 C for 3 d by carboxyfluorescein succinimidyl ester dilution.
Results
VDR deficiency accelerates T1D and promotes glucose intolerance in NOD mice
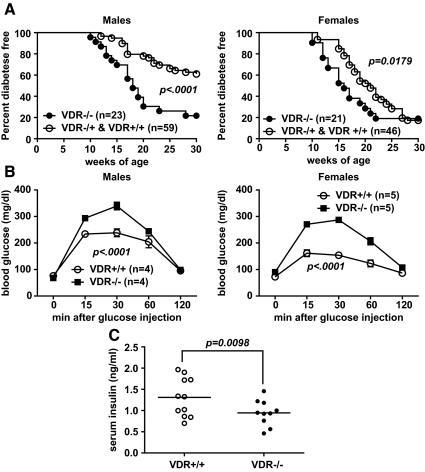
When fed a standard rodent chow diet, VDR−/− males developed hyperglycemia at an accelerated rate and at higher incidence [80% diabetic by 30 wk of age compared with 40% of wild-type and heterozygous mice (survival curves for wild-type and heterozygous mice overlapped and were thus combined)] (Fig. 1A). Diabetes onset was also accelerated in VDR−/− females, although their final disease incidence at 30 wk was similar to control mice (Fig. 1A). The VDR−/− mice displayed anticipated abnormalities in vitamin D3 metabolism, including low levels of serum 25-hydroxyvitamin D3, which results from the extremely high 1α-hydroxylase activity in these animals (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org) (23). Both male and female VDR−/− mice demonstrated impaired glucose tolerance compared with VDR+/+ controls when analyzed at 6–7 wk of age (Fig. 1B). Although fasting blood glucose values were similar, peak levels at 30 min after injection were significantly higher in VDR−/− mice. Glucose values for both VDR−/− and VDR+/+ groups returned to near fasting levels by 120 min. To determine whether the accelerated T1D in VDR−/− mice was the result of reduced levels of circulating serum insulin, this hormone was assayed in unmanipulated 16-wk-old male VDR−/− and VDR+/+ mice fed the standard chow diet ad libitum. Significantly lower average serum insulin concentrations were detected in the VDR−/− mice compared with controls (Fig. 1C). In contrast to the observed disease acceleration, glucose intolerance, and reduced serum insulin values, we were unable to detect an earlier development of more severe insulitis in either sex of VDR−/− mice compared with controls when scored at 7 wk of age (Supplemental Fig. 2). Insulitis levels also did not differ in the 16-wk-old VDR−/− (2.63 ± 0.35) and VDR+/+ (2.42 ± 0.27) mice used to measure fed serum insulin levels (Fig. 1C).
Fig. 1.
VDR ablation accelerates T1D and promotes glucose intolerance in NOD mice. A, Incidence of T1D in male and female NOD mice carrying an inactive form of the VDR gene (VDR−/−) compared with littermate controls carrying one (VDR−/+) or both copies (VDR+/+) of the functional allele. Survival curves were compared according to the Log-rank test. B, Glucose tolerance was tested by ip injection of 6- to 7-wk-old fasted VDR−/− and VDR+/+ mice with 2 g/kg of body weight of dextrose. Analysis of interactions between different VDR genotypes and blood glucose levels at various time points after dextrose injection were determined by two-way ANOVA. C, Levels of serum insulin from unmanipulated 16-wk-old male VDR−/− and VDR+/+ mice analyzed according to the Wilcoxon signed rank test.
Immune effects are not responsible for Vdr deficiency-dependent glucose intolerance and T1D acceleration
We next conducted experiments to determine how VDR status in immune cells might affect the development of T1D. Earlier studies reported that T1D in VDR-intact NOD mice is accelerated by vitamin D3 deprivation (5) and suppressed by 1α,25-dihydroxyvitamin D3 analog supplementation (6, 7). It was hypothesized that these effects may result because depriving or oversupplying NOD mice with vitamin D3 metabolites, respectively, exacerbates or corrects many of the immune defects that characterize this strain (reviewed in Ref. 27). Thus, we initially enumerated different immune cell types in both VDR−/− and VDR−/+ mice known to contribute to T1D in the NOD strain (Table 1). VDR−/− female mice had lower levels of CD4+ T cells, but a higher frequency of B cells, in pancreatic lymph nodes and spleen compared with VDR−/+ controls. Although low in both cases, the proportion of B cells in the thymus was also significantly higher in VDR−/− than VDR−/+ females (Table 1). CD8+ T-cell levels were lower only in spleens of VDR−/− females. VDR−/− females were also found to have a higher frequency of CD11c+ dendritic cells in pancreatic lymph nodes, and these expressed, respectively, higher and unchanged levels of the T cell costimulatory molecules CD80 and CD86 (Table 1). It was previously reported that NKT cells and CD25+CD4+ Treg, which both inhibit T1D in the NOD disease model (reviewed in Ref. 4), were numerically and functionally deficient in mice lacking VDR (23, 28, 29). However, we found no differences in the frequency of either cell type between VDR−/− and VDR−/+ female mice (Table 1). Furthermore, as assessed by interferon γ and IL-4 production, respectively, in response to activation by the NKT cell superagonist α-GalCer (Supplemental Fig. 3) and the ability of Treg to suppress effector T cells in an in vitro assay (Supplemental Fig. 4), neither of these immuno-modulatory populations appear to functionally differ in VDR−/− and VDR−/+ mice.
Table 1.
Flow cytometric analysis of cell surface proteins from leukocytes obtained from pancreatic lymph node, spleen, and thymus of VDR−/− and VDR−/+ NOD mice
| Pancreatic lymph node |
Spleen |
Thymus |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| VDR−/− | VDR−/+ | P value | VDR−/− | VDR−/+ | P value | VDR−/− | VDR−/+ | P value | |
| CD4+CD8+ | 82.2 ± 1.0 | 84.3 ± 0.8 | NS | ||||||
| CD4+CD8− | 41.5 ± 2.9 | 47.6 ± 1.1 | 0.0143 | 23.5 ± 2.1 | 29.5 ± 3.2 | 0.0143 | 9.9 ± 0.5 | 9.3 ± 0.5 | NS |
| CD4−CD8+ | 19.9 ± 2.1 | 21.6 ± 2.3 | NS | 10.9 ± 0.4 | 13.2 ± 1.4 | 0.0143 | 3.1 ± 0.2 | 2.5 ± 0.2 | NS |
| CD4+FoxP3+ | 4.7 ± 0.5 | 5.4 ± 0.5 | NS | 4.8 ± 0.3 | 4.6 ± 0.3 | NS | 1.0 ± 0.2 | 0.8 ± 0.1 | 0.0090 |
| (CD25 MFI) | 6.1 ± 1.1 | 6.2 ± 1.0 | NS | 8.3 ± 1.1 | 8.7 ± 0.4 | NS | 16.9 ± 2.5 | 16.1 ± 2.5 | NS |
| CD1d tetramer+ | 0.4 ± 0.3 | 0.4 ± 0.1 | NS | 0.4 ± 0.1 | 0.5 ± 0.1 | NS | 0.3 ± 0.1 | 0.3 ± 0.1 | NS |
| (%CD4+CD8−) | 71.3 ± 2.8 | 61.0 ± 8.7 | NS | 82.2 ± 2.9 | 78.7 ± 4.5 | NS | 43.2 ± 7.6 | 42.6 ± 8.1 | NS |
| B220+ | 33.0 ± 2.2 | 24.1 ± 2.4 | 0.0143 | 54.1 ± 2.7 | 46.2 ± 3.2 | 0.0143 | 1.1 ± 0.5 | 0.6 ± 0.1 | 0.0163 |
| CD11c+ | 0.8 ± 0.1 | 0.6 ± 0.1 | 0.0143 | 2.2 ± 0.2 | 2.5 ± 0.3 | NS | 0.2 ± 0.04 | 0.2 ± 0.03 | NS |
| (CD80 MFI) | 2354 ± 277 | 1726 ± 323 | 0.0472 | 2039 ± 175 | 1764 ± 254 | NS | 1242 ± 476 | 1025 ± 125 | NS |
| (CD86 MFI) | 414 ± 66 | 415 ± 167 | NS | 187 ± 11 | 180 ± 14 | NS | 111 ± 10 | 122 ± 16 | NS |
| (%CD11b+) | 50.1 ± 3.9 | 41.7 ± 6.7 | NS | 68.7 ± 1.8 | 68.7 ± 2.9 | NS | 33.5 ± 9.8 | 32.0 ± 6.3 | NS |
| CD11b+GR-1+ | 0.01 ± 0.005 | 0.01 ± 0.003 | NS | 1.75 ± 0.38 | 1.75 ± 0.27 | NS | 0.01 ± 0.01 | 0.02 ± 0.02 | NS |
| PDCA-1+CD11c+ | 0.24 ± 0.02 | 0.19 ± 0.06 | NS | 0.87 ± 0.04 | 0.95 ± 0.10 | NS | 0.10 ± 0.04 | 0.12 ± 0.02 | NS |
Table values represent the variable mean ± sem for viable cells. Five litter-matched female VDR−/− and VDR−/+ NOD mice were compared at 14 wk of age. NS indicates no significant difference (P ≥ 0.05) between VDR−/− and VDR−/+ mice when analyzed by the Wilcoxon rank test. MFI indicates mean fluorescence intensity.
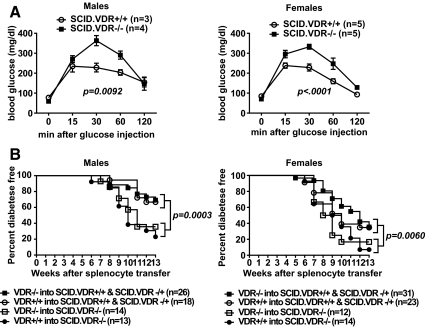
To further evaluate whether VDR deficiency accelerates T1D through immune-mediated effects, VDR-deficient NOD.scid (SCID.VDR−/−) mice were generated that lack B and T cells required for disease development. We initially used this stock to test whether the impaired glucose tolerance described above for the NOD.Vdr−/− mice was the result of increased islet cell destruction by autoreactive lymphocytes. As observed for immuno-competent VDR−/− and VDR+/+ mice, glucose tolerance was significantly impaired in lymphocyte-deficient SCID.VDR−/− mice of both sexes compared with SCID.VDR+/+ controls (Fig. 2A). Next, we tested whether T1D development is accelerated in NOD mice with VDR absent in hematopoietic or somatic cell types or both compartments. This was performed by adoptively transferring splenocytes from VDR−/− or VDR+/+ mice into SCID.VDR−/− vs. SCID.VDR−/+ and SCID.VDR+/+ recipients in a reciprocal fashion. Diabetes development was accelerated in male and female SCID.VDR−/− recipients compared with SCID.VDR−/+ and SCID.VDR+/+ mice irrespective of whether they were engrafted with VDR−/− or VDR+/+ splenocytes (Fig. 2B). These data collectively indicate that the increased T1D susceptibility of genetically VDR-deficient NOD mice used in the current study that were held under environmental conditions present at JAX is due to the mutational effects on nonimmune cell types.
Fig. 2.
VDR deficiency does not affect T1D development or glucose intolerance by altering β-cell autoreactive immune cells. A, Glucose tolerance was tested by ip injection of 6- to 7-wk-old fasted SCID.VDR−/− and SCID.VDR+/+ mice with 2 g/kg of body weight of dextrose. B, Diabetes development after the reciprocal transfer of 1 × 107 splenocytes from VDR−/− and VDR+/+ mice into SCID.VDR−/− vs. SCID.VDR−/+ and SCID.VDR+/+ recipients. Combined survival curves of SCID.VDR−/− vs. SCID.VDR−/+ and SCID.VDR+/+ recipients were compared according to the Log-rank test.
Disrupting the VDR gene leads to pancreatic β-cell dysfunction in vivo
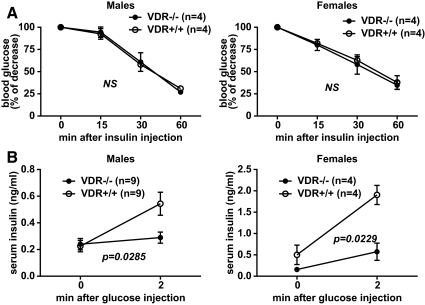
Impaired glucose tolerance in VDR-deficient NOD mice may be due to a lower capacity of this stock to produce and/or respond to insulin. We assessed these possibilities in vivo by performing physiological studies on insulin signaling using SCID. VDR−/− mice. The disparate glucose tolerances observed were not due to differences in insulin sensitivity, because both SCID.VDR−/− and SCID. VDR+/+ mice responded similarly when injected with exogenous insulin (Fig. 3A). We also evaluated pancreatic β-cell function by measuring acute-phase insulin secretion in response to glucose. In this experiment, the serum levels of fasted male and female SCID. VDR−/− mice increased only marginally compared with SCID.VDR+/+ control animals after glucose injection (Fig. 3B). This indicates that β-cells of VDR-deficient NOD mice are impaired in their ability to produce and/or secrete insulin.
Fig. 3.
Disrupting the VDR gene in vivo impairs insulin secretion but not insulin sensitivity. A, Insulin tolerance test where glucose levels were measured from fed 7-wk-old SCID.VDR−/− and SCID.VDR+/+ mice injected ip with 0.75 U/kg body weight of purified porcine insulin. Values are expressed as the percentage of glucose levels at the 0-min time point. B, Acute-phase insulin secretion in response to glucose. Serum insulin levels of fasted mice at 0 and 2 min after ip injection with 3 g/kg body weight of dextrose. Analysis of interactions between VDR genotypes and time after treatment for both the insulin tolerance and acute-phase insulin secretion tests were determined by two-way ANOVA. NS, Not significant.
Impaired glucose tolerance and accelerated T1D in VDR-deficient NOD mice can be corrected by calcium
Previous studies demonstrated that glucose intolerance develops in rats rendered hypocalcaemic by vitamin D depletion (30) and that calcium supplementation alone is sufficient to correct this defect (19, 20). Furthermore, supplementation of vitamin D3 or 22-oxa-1,25-dihydroxyvitamin D3, a synthetic 1α,25-dihydroxyvitamin D3 analog, could not normalize glucose tolerance in the absence of calcium (19, 20). This suggested that the glucose intolerance associated with vitamin D deficiency predominantly results from the subsequent induction of a hypocalcaemic state. Thus, we evaluated how correcting hypocalcaemia in VDR−/− mice alters their glucose intolerance using a HiCal diet, from which calcium can be absorbed through VDR-independent mechanisms (31).
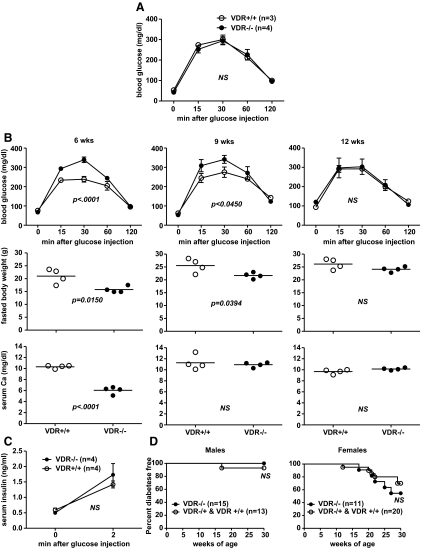
VDR−/− mice fed the HiCal diet from weaning were no longer glucose intolerant compared with VDR+/+ controls when tested at 6–7 wk of age (Fig. 4A). Furthermore, we discovered that established glucose intolerance in a cohort of hypocalcaemic VDR−/− mice could be gradually reversed by switching them from standard chow to the HiCal diet from 6 to 12 wk of age (Fig. 4B). This was accompanied by increases in weight gain and serum calcium concentrations from typically rachitic levels to those of nonhypocalcaemic VDR+/+ control mice fed the chow diet (Fig. 4B). It is likely that the described corrections in glucose tolerances were the result of improved β-cell function in response to normalized circulating calcium levels. This conclusion is supported by the finding that acute-phase insulin secretion responses for SCID.VDR−/− mice fed the HiCal diet from weaning to 12 wk of age were normal compared with SCID.VDR+/+ controls (Fig. 4C). Finally, both VDR−/− and VDR+/+ mice were fed the HiCal diet from weaning until 30 wk of age to evaluate whether ameliorating hypocalcaemia in VDR-deficient animals influenced their susceptibility to T1D. Feeding HiCal diet significantly reduced the disease incidence in both groups of mice (Fig. 4D). This was likely, in part, because its ingredients do not include vegetable protein sources present in standard rodent chow diets normally needed to support robust T1D development in standard NOD mice (32, 33). Nevertheless, both VDR−/− and VDR+/+ mice were equally well protected from disease by the HiCal diet. This indicates that when systemic calcium concentrations are normalized, VDR-deficient animals no longer have an enhanced susceptibility to T1D. The pathogenic basis for the cases of T1D that did occur in HiCal-fed VDR−/− mice is more likely to be due to the autoimmune processes, underlying disease development in unmanipulated standard NOD mice rather than hypocalcaemic-induced defects in insulin production/secretion. For still unclear reasons, in unmanipulated standard NOD mice, there is a female bias in development of autoimmune-mediated T1D (34). This likely accounts for the higher rate of T1D in HiCal-fed female than male VDR−/− mice.
Fig. 4.
Impaired glucose tolerance, reduced insulin secretion, and accelerated T1D in VDR-deficient NOD mice can be corrected by feeding a HiCal diet. A, Glucose tolerance test performed on 6- to 7-wk-old male VDR−/− mice fed the HiCal diet from weaning vs. VDR+/+ controls fed a standard rodent chow diet. B, Six-, 9-, and 12-wk glucose tolerance test (upper panels), fasted body weight (middle panels), and serum calcium concentration (lower panels) results from four male VDR−/− and VDR+/+ mice, respectively, fed HiCal vs. standard chow diet from 6 to 12 wk of age. C, Acute-phase insulin secretion response for male SCID.VDR−/− and SCID.VDR+/+ mice fed HiCal diet from weaning to 12 wk of age. D, Incidence of T1D in male and female VDR−/− vs. VDR−/+ and VDR+/+ mice fed HiCal diet. NS, Not significant.
Discussion
The current work documents that when housed under vivarium conditions extant at JAX, NOD mice homozygous for a targeted Vdr gene deletion are characterized by enhanced T1D susceptibility. Our results differ from a previous study finding a similar stock of NOD background VDR−/− mice housed at The Catholic University had unaltered T1D presentation (23). Several possible reasons for this disparity exist. Firstly, after 14 backcross cycles of the inactivated Vdr allele from an original outbred donor to NOD/Cmat at Leuven, upon importation of the stock to JAX at N14F7, the mutation underwent an additional backcross to NOD/ShiLtJ allowing production of pups for rederivation through embryo transfer. Rederived heterozygous mice were intercrossed to produce VDR−/− experimental and VDR−/+ control segregants used in studies at JAX. Genetic quality control conducted by the Type 1 Diabetes Resource (http://type1diabetes.jax.org/) confirmed that the colony established at JAX was homozygous for NOD alleles at 15 loci associated with diabetes susceptibility as well markers on chromosome 15 flanking the targeted Vdr mutation. Nevertheless, it is possible that before rederivation, there may have been some residual heterozygosity at sites other than the targeted Vdr allele (due to a new mutation in the NOD/Cmat stock in Leuven or to some genomic residuum from the original donor stock of the targeted Vdr allele). The additional backcross to NOD/ShiLtJ at JAX could have also eliminated any such unrecognized genetic heterozygosity at sites removed from the targeted Vdr gene. Backcrossing the Leuven VDR−/− mice to the JAX NOD/ShiLtJ stock could also have shortened the congenic interval containing the targeted Vdr mutation on chromosome 15, because this region encompasses many immune and regulatory genes. However, this possibility is unlikely, because genotyping results were the same for selected markers around the Vdr gene for VDR−/− mice from both institutes.
We favor an explanation that environmental factors are more likely to have affected the differential T1D susceptibility of NOD.Vdr−/− mice housed at JAX and Leuven. Several possible candidates exist, including variability in the ingredient and nutrient levels of the diets fed. The Leuven and JAX studies employed chow diets purchased from two different sources (The Carfil Quality Labofood, Leuven, Belgium, and Purina LabDiet, JAX). Both contained protein from wheat and soy, which is required for robust T1D development in NOD mice (32, 33). However, we were unable to assess whether differences in these and other ingredients possibly contributed to the disparity in the observed T1D rates, because The Carfil Quality Ltd. Co. does not divulge specifics of constituents in their open formula diet. For the same reason, little is known about the nutrient content of the Carfil diet with the exception that it contained similar levels of calcium and phosphorus compared with the Purina LabDiet used at JAX (23). However, the bioavailability of both minerals depends on the form in which they are added to the diet as well as how they interact with other nutrients (35). Future experiments are planned to determine whether feeding VDR−/− mice at Leuven the LabDiet alters their disease susceptibility. This will ultimately resolve whether variation between diets contributed to the different rates of T1D onset observed.
Another factor that may have added to the different disease responses is varying microbial exposure in the vivaria where the mice were housed for each study, which may have altered the microbiome. It is well documented that T1D incidence in NOD mice is influenced by stimuli from microbes or microbial products (36–38). High levels of microbial exposure usually result in suppressing disease penetrance. However, it has also been shown that certain microorganisms are likely required for efficient disease development (39). Although breeders and progeny at JAX were maintained under SPF conditions, it was not a full barrier facility, whereas in Leuven, breeders were maintained in a high-barrier SPF isolator unit and progeny were raised in a separate installation.
VDR−/− mice at JAX only developed accelerated T1D when hypocalcaemic. This effect was reversed if VDR−/− mice were switched to the HiCal diet. Our results are consistent with other published data showing that calcium is essential for normal β-cell function (reviewed in Ref. 16) and that calcium insufficiency results in impaired glucose tolerance that develops independently of a deficiency in vitamin D (19, 20). Our data did not indicate that pathogenic responses against β-cells were exacerbated by a loss of a functional VDR in immune cells. No obvious further exacerbations of deficiencies in the frequency or function of immunoregulatory Treg or NKT cells known to contribute to T1D were detected in VDR−/− mice. VDR−/− splenocytes did not transfer T1D more efficiently into NOD.SCID recipients compared with those from VDR+/+ donors. Also, hypocalcaemic NOD. SCID.VDR−/− mice that lack lymphocytes required for T1D progression still develop impaired glucose tolerance responses compared with NOD.SCID.VDR+/+ controls. Hence, T1D acceleration in hypocalcaemic VDR−/− mice is best explained by pancreatic β-cells with impaired insulin secretory capacity undergoing an autoimmune attack not differing from that in VDR-intact NOD mice. Most human T1D patients are not hypocalcaemic. However, our results would indicate that the onset of a hypocalcaemic state in humans carrying T1D risk genes could further exacerbate the possibility of actually developing disease.
Deleting the VDR had the greatest impact on male T1D incidence. T1D in male NOD mice is more slowly progressive than in females and is characterized by a high proportion of mice that develop severe insulitis but retain sufficient β-cell mass to avoid hyperglycaemia. Further, male mice are less capable than female mice of maintaining glucose homeostasis under conditions of chronic β-cell stress (40). Insulin sensitivity is also less in male than female mice (41). Due to these collective factors, the moderate stresses imposed on β-cells by reduced levels of serum calcium may result in more obvious changes to disease progression in males rather than in females, where a loss in insulin production capacity sufficient to elicit overt hyperglycemia is more rapidly induced by heightened autoimmune responses.
Our results do not explain why VDR-intact NOD mice that do not develop hypocalcaemia are protected from T1D by 1α,25-dihydroxyvitamin D3. It may be that 1α,25-dihydroxyvitamin D3, through binding the VDR, directly promotes expression of genes that normalize immunological tolerance induction processes, underlying T1D susceptibility (reviewed in Ref. 27). This may account for why some synthetic analogs of 1α,25-dihydroxyvitamin D3 that induce only mild increases in serum calcium concentrations still block T1D (11, 42–44). Although some 1α,25-dihydroxyvitamin D3 analogs that increase serum calcium concentrations do not alter T1D development in NOD mice (Gysemans, C., and C. Mathieu, unpublished data), there is also evidence that the positive association between hypercalcaemia and vitamin D analog-mediated amelioration of disease may not be simply coincidental. We previously reported that T1D development was more strongly suppressed in NOD mice fed the alphacalcidol analog in place of 1α,25-dihydroxyvitamin D3 (9). The enhanced disease protection was associated with significantly more severe hypercalcaemia in alphacalcidol vs. 1α,25-dihydroxyvitamin D3-fed mice (9). An interaction between dietary calcium and 1α,25-dihydroxyvitamin D3 levels has been described for experimental autoimmune encephalitis (EAE), a mouse model for multiple sclerosis in humans (45). Feeding C57BL/6J female mice low levels of 1α,25-dihydroxyvitamin D3 (6 ng/d) protected completely against EAE when the diet contained a high level of Ca (1%) inducing severe hypercalcaemia. However, even very high levels of 1α,25-dihydroxyvitamin D3 (200 ng/d) did not elicit EAE protection when dietary calcium was low (0.2%). The reason for this interaction is as yet unclear. However, because T1D and EAE both have an autoimmune etiology, the possibility exists that high serum calcium levels contribute to effective vitamin D-analog-mediated inhibition of both diseases.
In conclusion, we have found that VDR disruption in NOD mice accelerates T1D by reducing their capacity to supply β-cells with sufficient calcium to maintain normal insulin secretion. These data suggest that calcium and/or vitamin D insufficiency may also accelerate disease onset in humans at risk of T1D (and possibly type 2 diabetes). Future treatment of humans at risk for T1D with vitamin D and calcium supplements may help to delay or even prevent the disease in some individuals.
Supplementary Material
Acknowledgments
We thank Racheal Wallace for curation and genetic quality control of the NOD.Cg-Vdrtm1Ska/CmatJ, JAX no. 6956 mouse strain.
This work was supported by Type 1 Diabetes Repository at The Jackson Laboratory (National Institutes of Health Grant DK-75000), National Institutes of Health Grant DK-46266, Cancer Center Support Grant CA34196, and grants from the Juvenile Diabetes Research Foundation International. J.P.D. is supported by an advanced postdoctoral fellowship from the Juvenile Diabetes Research Foundation International (JDRF489 JD-01). C.G. is recipient of a Flemish Research Foundation postdoctoral fellowship and C.M. of a Flemish Research Foundation clinical fellowship.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- EAE
- Experimental autoimmune encephalitis
- α-GalCer
- α-galactosylceramide
- HiCal
- high-lactose calcium rescue
- JAX
- The Jackson Laboratory
- NKT
- natural killer T
- SPF
- specific pathogen free
- T1D
- type 1 diabetes
- Treg
- regulatory T cell
- VDR
- vitamin D receptor.
References
- 1. Hyppönen E. 2010. Vitamin D and increasing incidence of type 1 diabetes-evidence for an association? Diabetes Obes Metab 12:737–743 [DOI] [PubMed] [Google Scholar]
- 2. Mohr SB, Garland CF, Gorham ED, Garland FC. 2008. The association between ultraviolet B irradiance, vitamin D status and incidence rates of type 1 diabetes in 51 regions worldwide. Diabetologia 51:1391–1398 [DOI] [PubMed] [Google Scholar]
- 3. Cooper JD, Smyth DJ, Walker NM, Stevens H, Burren OS, Wallace C, Greissl C, Ramos-Lopez E, Hyppönen E, Dunger DB, Spector TD, Ouwehand WH, Wang TJ, Badenhoop K, Todd JA. 2011. Inherited variation in vitamin D genes is associated with predisposition to autoimmune disease type 1 diabetes. Diabetes 60:1624–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Driver JP, Serreze DV, Chen YG. 2011. Mouse models for the study of autoimmune type-1 diabetes: a NOD to similarities and differences to human disease. Semin Immunopathol 33:67–87 [DOI] [PubMed] [Google Scholar]
- 5. Giulietti A, Gysemans C, Stoffels K, van Etten E, Decallonne B, Overbergh L, Bouillon R, Mathieu C. 2004. Vitamin D deficiency in early life accelerates type 1 diabetes in non-obese diabetic mice. Diabetologia 47:451–462 [DOI] [PubMed] [Google Scholar]
- 6. Mathieu C, Laureys J, Sobis H, Vandeputte M, Waer M, Bouillon R. 1992. 1, 25-Dihydroxyvitamin D3 prevents insulitis in NOD mice. Diabetes 41:1491–1495 [DOI] [PubMed] [Google Scholar]
- 7. Mathieu C, Waer M, Laureys J, Rutgeerts O, Bouillon R. 1994. Prevention of autoimmune diabetes in NOD mice by 1, 25 dihydroxyvitamin D3. Diabetologia 37:552–558 [DOI] [PubMed] [Google Scholar]
- 8. Mathieu C, Waer M, Casteels K, Laureys J, Bouillon R. 1995. Prevention of type I diabetes in NOD mice by nonhypercalcemic doses of a new structural analog of 1, 25-dihydroxyvitamin D3, KH1060. Endocrinology 136:866–872 [DOI] [PubMed] [Google Scholar]
- 9. Driver JP, Foreman O, Mathieu C, van Etten E, Serreze DV. 2008. Comparative therapeutic effects of orally administered 1,25-dihydroxyvitamin D(3) and 1α-hydroxyvitamin D(3) on type-1 diabetes in non-obese diabetic mice fed a normal-calcaemic diet. Clin Exp Immunol 151:76–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Penna G, Adorini L. 2000. 1α, 25-dihydroxyvitamin D3 inhibits differntiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol 164:2405–2411 [DOI] [PubMed] [Google Scholar]
- 11. Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L. 2002. 1a, 25-dihydroxyvitamin D3 analog enhances regulatory T cells and arrests autoimmune diabetes in NOD mice. Diabetes 51:1367–1374 [DOI] [PubMed] [Google Scholar]
- 12. Lemire JM, Archer DC, Beck L, Spiegelberg HL. 1995. Immuno-suppressive actions of 1, 25-dihydroxyvitamin D3; preferential inhibition of Th1 functions. J Nutr 125:1704S–1708S [DOI] [PubMed] [Google Scholar]
- 13. Overbergh L, Decallonne B, Waer M, Rutgeerts O, Valckx D, Casteels KM, Laureys J, Bouillon R, Mathieu C. 2000. 1a, 25-dihydroxyvitamin D3 induces an autoantigen-specific T-helper 1/T -helper 2 immune shift in NOD mice immunized with GAD65 (p524–543). Diabetes 49:1301–1307 [DOI] [PubMed] [Google Scholar]
- 14. Takeuchi A, Reddy GS, Kobayashi T, Okano T, Park J, Sharma S. 1998. Nuclear factor of activated T cells (NFAT) as a molecular target for 1α, 25-dihydroxyvitamin D3-mediated effects. J Immunol 160:209–218 [PubMed] [Google Scholar]
- 15. Peterlik M, Cross HS. 2009. Vitamin D and calcium insufficiency-related chronic diseases: molecular and cellular pathophysiology. Eur J Clin Nutr 63:1377–1386 [DOI] [PubMed] [Google Scholar]
- 16. Islam MS. 2010. Calcium signaling in the islets. Adv Exp Med Biol 654:235–259 [DOI] [PubMed] [Google Scholar]
- 17. Lanner JT, Katz A, Tavi P, Sandström ME, Zhang SJ, Wretman C, James S, Fauconnier J, Lännergren J, Bruton JD, Westerblad H. 2006. The role of Ca2+ influx for insulin-mediated glucose uptake in skeletal muscle. Diabetes 55:2077–2083 [DOI] [PubMed] [Google Scholar]
- 18. Lanner JT, Bruton JD, Katz A, Westerblad H. 2008. Ca(2+) and insulin-mediated glucose uptake. Curr Opin Pharmacol 8:339–345 [DOI] [PubMed] [Google Scholar]
- 19. Beaulieu C, Kestekian R, Havrankova J, Gascon-Barré M. 1993. Calcium is essential in normalizing intolerance to glucose that accompanies vitamin D depletion in vivo. Diabetes 42:35–43 [DOI] [PubMed] [Google Scholar]
- 20. Ismail A, Namala R. 2000. Impaired glucose tolerance in vitamin D deficiency can be corrected by calcium. J Nutr Biochem 11:170–175 [DOI] [PubMed] [Google Scholar]
- 21. Hyppönen E, Läärä E, Reunanen A, Järvelin MR, Virtanen SM. 2001. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet 358:1500–1503 [DOI] [PubMed] [Google Scholar]
- 22. Khazai NB, Beck GR, Jr, Umpierrez GE. 2009. Diabetes and fractures: an overshadowed association. Curr Opin Endocrinol Diabetes Obes 16:435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gysemans C, van Etten E, Overbergh L, Giulietti A, Eelen G, Waer M, Verstuyf A, Bouillon R, Mathieu C. 2008. Unaltered diabetes presentation in NOD mice lacking the vitamin D receptor. Diabetes 57:269–275 [DOI] [PubMed] [Google Scholar]
- 24. Serreze DV, Leiter EH, Hanson MS, Christianson SW, Shultz LD, Hesselton RM, Greiner DL. 1995. Emv30null NOD-scid mice: an improved host for adoptive transfer of autoimmune diabetes and growth of human lymphohematopoietic cells. Diabetes 44:1392–1398 [DOI] [PubMed] [Google Scholar]
- 25. Takaki T, Marron MP, Mathews CE, Guttmann ST, Bottino R, Trucco M, DiLorenzo TP, Serreze DV. 2006. HLA-A*0201-restricted T cells from humanized NOD mice recognize autoantigens of potential clinical relevance to type 1 diabetes. J Immunol 176:3257–3265 [DOI] [PubMed] [Google Scholar]
- 26. Scheuplein F, Rissiek B, Driver JP, Chen YG, Koch-Nolte F, Serreze DV. 2010. A recombinant heavy chain antibody approach blocks ART2 mediated deletion of an iNKT cell population that upon activation inhibits autoimmune diabetes. J Autoimmun 34:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takiishi T, Gysemans C, Bouillon R, Mathieu C. 2010. Vitamin D and diabetes. Endocrinol Metab Clin North Am 39:419–446, table of contents [DOI] [PubMed] [Google Scholar]
- 28. Yu S, Cantorna MT. 2008. The vitamin D receptor is required for iNKT cell development. Proc Natl Acad Sci USA 105:5207–5212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu S, Cantorna MT. 2011. Epigenetic reduction in invariant NKT cells following in utero vitamin D deficiency in mice. J Immunol 186:1384–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cade C, Norman AW. 1986. Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology 119:84–90 [DOI] [PubMed] [Google Scholar]
- 31. Pansu D, Bellaton C, Bronner F. 1979. Effect of lactose on duodenal calcium-binding protein and calcium absorption. J Nutr 109:508–512 [DOI] [PubMed] [Google Scholar]
- 32. Beales PE, Elliott RB, Flohé S, Hill JP, Kolb H, Pozzilli P, Wang GS, Wasmuth H, Scott FW. 2002. A multi-centre, blinded international trial of the effect of A(1) and A(2) β-casein variants on diabetes incidence in two rodent models of spontaneous type I diabetes. Diabetologia 45:1240–1246 [DOI] [PubMed] [Google Scholar]
- 33. Flohé SB, Wasmuth HE, Kerad JB, Beales PE, Pozzilli P, Elliott RB, Hill JP, Scott FW, Kolb H. 2003. A wheat-based, diabetes-promoting diet induces a Th1-type cytokine bias in the gut of NOD mice. Cytokine 21:149–154 [DOI] [PubMed] [Google Scholar]
- 34. Fitzpatrick F, Lepault F, Homo-Delarche F, Bach JF, Dardenne M. 1991. Influence of castration, alone or combined with thymectomy, on the development of diabetes in the nonobese diabetic mouse. Endocrinology 129:1382–1390 [DOI] [PubMed] [Google Scholar]
- 35. Gropper SS, Smith JL, Groff JL. 2008. Advanced nutrition and human metabolism. Adams P, Lustig A. eds. Belmont, CA: Wadsworth, Cengage Learning; 431–446 [Google Scholar]
- 36. McInerney MF, Pek SB, Thomas DW. 1991. Prevention of insulitis and diabetes onset by treatment with complete Freund's adjuvant in NOD mice. Diabetes 40:715–725 [DOI] [PubMed] [Google Scholar]
- 37. Sadelain MW, Qin HY, Lauzon J, Singh B. 1990. Prevention of type I diabetes in NOD mice by adjuvant immunotherapy. Diabetes 39:583–589 [DOI] [PubMed] [Google Scholar]
- 38. Pozzilli P, Signore A, Williams AJ, Beales PE. 1993. NOD mouse colonies around the world—recent facts and figures. Immunol Today 14:193–196 [DOI] [PubMed] [Google Scholar]
- 39. Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, Gordon JI, Chervonsky AV. 2008. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature 455:1109–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Leiter EH. 1982. Multiple low-dose streptozotocin-induced hyperglycemia and insulitis in C57BL mice: influence of inbred background, sex, and thymus. Proc Natl Acad Sci USA 79:630–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leiter EH. 1989. The genetics of diabetes susceptibility in mice. FASEB J 3:2231–2241 [DOI] [PubMed] [Google Scholar]
- 42. Pedullà M, Desiderio V, Graziano A, d'Aquino R, Puca A, Papaccio G. 2007. Effects of a vitamin D3 analog on diabetes in the bio breeding (BB) rat. J Cell Biochem 100:808–814 [DOI] [PubMed] [Google Scholar]
- 43. Giarratana N, Penna G, Amuchastegui S, Mariani R, Daniel KC, Adorini L. 2004. A vitamin D analog down-regulates proinflammatory chemokine production by pancreatic islets inhibiting T cell recruitment and type 1 diabetes development. J Immunol 173:2280–2287 [DOI] [PubMed] [Google Scholar]
- 44. Casteels KM, Mathieu C, Waer M, Valckx D, Overbergh L, Laureys JM, Bouillon R. 1998. Prevention of type I diabetes in nonobese diabetic mice by late intervention with nonhypercalcemic analogs of 1,25-dihydroxyvitamin D3 in combination with a short induction course of cyclosporin A. Endocrinology 139:95–102 [DOI] [PubMed] [Google Scholar]
- 45. Cantorna MT, Humpal-Winter J, DeLuca HF. 1999. Dietary calcium is a major factor in 1,25-dihydroxycholecalciferol suppression of experimental autoimmune encephalomyelitis in mice. J Nutr 129:1966–1971 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.