Abstract
Mutations in the GALNT3 gene cause tumoral calcinosis characterized by ectopic calcifications due to persistent hyperphosphatemia. We recently developed Galnt3 knockout mice in a mixed background, which had hyperphosphatemia with increased bone mineral density (BMD) and infertility in males. To test the effect of dietary phosphate intake on their phenotype, Galnt3 knockout mice were generated in the C57BL/6J strain and fed various phosphate diets: 0.1% (low), 0.3% (low normal), 0.6% (normal), and 1.65% (high). Sera were analyzed for calcium, phosphorus, alkaline phosphatase, creatinine, blood urine nitrogen, 1,25-dihydroxyvitamin D, osteocalcin, tartrate-resistant acid phosphatase 5b, and fibroblast growth factor 23 (Fgf23). Femurs were evaluated by dual-energy x-ray absorptiometry, dynamic histomorphometry, and/or microcomputed tomography. Galnt3 knockout mice in C57BL/6J had the same biochemical phenotype observed in our previous study: hyperphosphatemia, inappropriately normal 1,25-dihydroxyvitamin D level, decreased alkaline phosphatase activity, and low intact Fgf23 concentration but high Fgf23 fragments. Skeletal analyses of their femurs revealed significantly high BMD with increased cortical bone area and trabecular bone volume. On all four phosphate diets, Galnt3 knockout mice had consistently higher phosphorus levels and lower alkaline phosphatase and intact Fgf23 concentrations than littermate controls. The low-phosphate diet normalized serum phosphorus, alkaline phosphatase, and areal BMD but failed to correct male infertility in Galnt3 knockout mice. The high-phosphate diet did not increase serum phosphorus concentration in either mutant or control mice due to a compensatory increase in circulating intact Fgf23 levels. In conclusion, dietary phosphate restriction normalizes biochemical and skeletal phenotypes of Galnt3 knockout mice and, thus, can be an effective therapy for tumoral calcinosis.
Tumoral calcinosis (also referred to as hyperphosphatemic familial tumoral calcinosis, OMIM #211900) is characterized by persistent hyperphosphatemia, leading to ectopic calcifications in soft tissues. Although large calcifications found around major joints are a prototypical feature of tumoral calcinosis, the phenotype can vary significantly between patients and sometimes includes dental and ophthalmological abnormalities (1–5). There is also a variant form of tumoral calcinosis, hyperostosis-hyperphosphatemia syndrome, which manifests with recurrent swelling of the long bones (diaphysitis and cortical hyperostosis). However, coexistence of ectopic calcifications and cortical hyperostosis (6–8), as well as the recent data showing the same genetic etiology of both conditions (2, 9–11), indicate that they are different manifestations of the same disease.
Tumoral calcinosis can be caused by inactivating mutations in the FGF23 gene (12–15), encoding a peptide hormone that regulates phosphate homeostasis (16–18), as well as the Klotho (KL) gene (19), encoding a coreceptor required for fibroblast growth factor 23 (FGF23) signaling through cognate FGF receptors (20, 21). However, by far, the majority of the patients have mutations in GALNT3 (2, 22, 23), which encodes a Golgi-associated glycosyltransferase, UDP-N-acetyl-α-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase 3 (ppGalNAc-T3), involved in the posttranslational modification of the subtilisin-like proprotein convertase recognition sequence (176RHTR179) in FGF23 (24, 25).
In the physiological state, FGF23 inhibits reabsorption of phosphate by suppressing sodium-phosphate cotransporters NaPi-IIa and NaPi-IIc in the kidney (26–28). FGF23 also inhibits renal biosynthesis of 1,25-dihydroxyvitamin D [1,25(OH)2D] by both decreasing production and increasing metabolism of 1,25(OH)2D (16, 26–29). However, in tumoral calcinosis due to GALNT3 mutations, FGF23 lacks O-glycosylation by ppGalNAc-T3, making FGF23 proteins susceptible to proteolysis by subtilisin-like proprotein convertases. Thus, the increased cleavage leads to low or undetectable levels of circulating intact FGF23, a biologically active form of FGF23 (24, 25, 30, 31). This inability to produce sufficient intact FGF23 is the main molecular defect responsible for increased renal phosphate reabsorption as well as increased renal 1,25(OH)2D synthesis, which in turn enhances intestinal absorption of phosphate and calcium. These increases in circulating calcium and phosphate levels are thought to cause calcium-phosphate deposits in soft tissues in patients with tumoral calcinosis.
To better understand the pathogenesis of tumoral calcinosis, we recently created Galnt3 knockout mice and characterized their phenotype at 3, 6, and 12 wk (32). As in patients with tumoral calcinosis, these animals were hyperphosphatemic and had low intact Fgf23 levels in the blood, despite increased Fgf23 expression in the bone. In addition, male Galnt3 knockout mice had increased bone mineral density (BMD) and infertility. In this study, we evaluated the phenotype of Galnt3 knockout mice at an advanced age (24 wk). We also fed Galnt3 knockout mice various phosphate diets to test the hypothesis that dietary phosphate intake alters biochemical and skeletal phenotypes of these mice. The findings from the present study have clinical implications for treatment of patients with tumoral calcinosis.
Materials and Methods
Generation and maintenance of experimental mice
The initial characterization of our Galnt3 knockout mice used animals on a C57BL/6J-129SvEv hybrid background (32). To eliminate the potential effect of background strains, these mice were backcrossed to the C57BL/6J strain for 10 generations before all experiments. For phenotyping of 24-wk-old Galnt3 knockout mice, experimental mice were generated by mating heterozygous parents (Galnt3+/−) and maintained on a regular rodent diet, which contained 1.01% calcium, 0.65% phosphorus, and 2.05 IU/g vitamin D3 (Teklad Global 18% Protein Extruded Rodent Diet, 2018SX; Harlan, Indianapolis, IN).
For dietary phosphate load experiments, heterozygous males (Galnt3+/−) were crossed to homozygous females (Galnt3−/−). At 3 wk of age, their male offspring (heterozygotes and homozygotes) were weaned to diets containing various phosphate contents: 0.1% (low), 0.3% (low normal), 0.6% (normal), and 1.65% (high) (T.09048, T.07153, T.07152, and TD.88345, respectively; Harlan). All four diets were based on egg whites and contained similar nutritional compositions, including approximately 1.0% calcium and 2.0 IU/g vitamin D3. When the males reached 5 wk of age, they were paired with either heterozygous or homozygous females (mean, 6.2 wk old; range, 4.4–10.9 wk old at the start of breeding) and allowed to breed for 12 wk on the same diets.
Animals had access to the diets and tap water ad libitum. The study was approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee.
Serum biochemistry measurements
The mice for phenotyping in the congenic strain were euthanized at 24 wk, and the males for the phosphate loading were euthanized at 17 wk. Blood samples were collected under anesthesia by cardiac puncture and stored at −80 C until analysis. Routine serum biochemistries (calcium, phosphorus, creatinine, blood urine nitrogen, and alkaline phosphatase) were determined by Roche COBAS Mira S (Roche Diagnostics, Indianapolis, IN). Serum 1,25(OH)2D and tartrate-resistant acid phosphatase 5b concentrations were measured using 1,25-Dihydroxy Vitamin D EIA and MouseTRAP Assay (TRACP 5b Mouse), respectively (Immunodiagnostic Systems Ltd., Fountain Hills, AZ). Serum osteocalcin concentrations were measured using Mouse Osteocalcin EIA Kit (Biomedical Technologies, Inc., Stoughton, MA). Serum Fgf23 concentrations were determined using two different ELISA kits. FGF23 ELISA kit (Kainos Laboratories Inc., Tokyo, Japan) measures only intact FGF23 (33). In contrast, Mouse FGF-23 (C-Term) ELISA (Immutopics International, San Clemente, CA) detects both intact FGF23 and C-terminal fragments of FGF23 and, thus, measures total FGF23 in circulation.
Skeletal phenotyping
Femurs were harvested from Galnt3 knockout mice and their age-matched littermate controls and fixed in 10% neutral-buffered formalin for 2 d. Areal BMD and bone mineral content (BMC) were measured by dual-energy x-ray absorptiometry (DXA), using a PIXImus2 densitometer (LUNAR Corp., Madison, WI). Coefficient of variation from 11 measurements of a frozen mouse specimen was 0.57% for BMD.
Microcomputed tomography (micro-CT; Skyscan 1172, Kontich, Belgium) of the distal femur metaphysis was performed using a 6-μm voxel size, as previously described (32). Trabecular bone properties, including bone volume/tissue volume (BV/TV), trabecular number, and trabecular thickness, and bone material density were assessed on 1 mm of tissue (∼166 slices) in the distal metaphysis beginning at 0.5 mm from the growth plate. For cortical bone parameters, bone area, cross-sectional moment of inertia, cortical thickness, and bone material density were assessed on a single slice of the diaphysis. Bone material density is the average gray scale value of the bone tissue and represents a measure of tissue mineralization.
For dynamic histomorphometric analysis, mice were injected ip with 0.6% calcein (30 mg/kg; Sigma-Aldrich, St. Louis, MO) solution 14 and 4 d before animals were killed. The fixed femurs were embedded in plastic (methyl methacrylate) using standard methods. Four-micrometer-thick sections were cut from the distal femurs and left unstained for assessment of calcein labeling on trabecular surfaces using standard methods. Outcome parameters included BV/TV, mineral apposition rate, mineralizing surface/bone surface, and bone formation rate/bone surface.
Statistical analysis
Means, sd, and sem were calculated for all outcome measures by genotype. ANOVA was used to test for overall differences among genotypes; where appropriate, subgroup analyses (e.g. within sex or same diet) were conducted. Unpaired t tests were then used to test differences between two genotypic groups. P values <0.05 were considered significant for all analyses.
Results
Phenotypic characterization of 24-wk-old Galnt3 knockout mice
Serum biochemistries
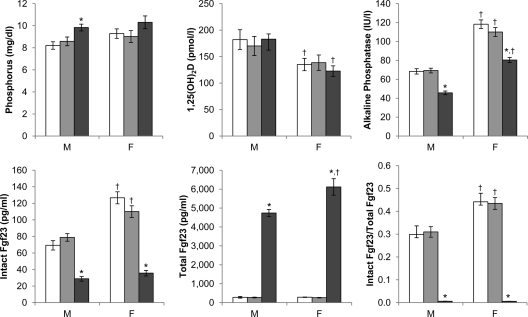
Galnt3 knockout mice described previously (32) were in a C57BL/6J-129SvEv hybrid background. Thus, to eliminate the potential background strain effects, Galnt3 knockout mice were regenerated in the homogeneous C57BL/6J strain. There were differences in serum biochemical values between males and females (Fig. 1), which were not apparent in the initial characterization of these mice. Compared with male mice of the same Galnt3 genotypes, female mice had consistently higher levels of intact Fgf23 (on average, +49% compared with same-genotype males) and alkaline phosphatase (+69%), but lower 1,25(OH)2D (−26%). Although not statistically significant, serum phosphorus was also higher in females.
Fig. 1.
Serum biochemistries of 24-wk-old mice (n = 9–18 per group). White bars, wild type; light gray bars, heterozygote; dark gray bars, homozygote. P values <0.05 (by t test) are indicated: *, compared with same-sex wild-type control; †, compared with same-genotype male. F, Females; M, males.
Comparisons between wild-type and heterozygous mice of the same sex showed no differences in serum biochemistries (Fig. 1 and Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). Compared with normal mice, both male and female Galnt3 knockout mice on the C57BL/6J background exhibited normocalcemia, hyperphosphatemia, inappropriately normal 1,25(OH)2D level (for the degree of hyperphosphatemia), decreased alkaline phosphatase activity, and low intact Fgf23 concentration, which is similar to what was observed on the mixed background (32). However, serum phosphorus was not statistically different between female wild-type and Galnt3 knockout mice (Fig. 1). Because serum creatinine and BUN in Galnt3 knockout mice were normal, homozygous loss of Galnt3 did not affect renal function (Supplemental Fig. 1).
The amounts of intact Fgf23 and C-terminal fragments measured by the Mouse FGF-23 (C-Term) ELISA were 20 times higher in Galnt3 knockout mice than wild-type or heterozygous mice (Fig. 1). However, the apparent increase in Fgf23 concentrations was entirely due to production of inactive fragments because less than 1% of the measured Fgf23 was biologically active, intact protein in Galnt3 knockout mice (Fig. 1).
Skeletal phenotype
At 24 wk of age, male Galnt3 knockout mice were heavier, but the females were lighter than corresponding wild-type mice (data not shown). Bone areas of femurs determined by DXA were similar between wild-type and Galnt3 knockout mice. However, femoral BMD and weight-adjusted BMC were significantly higher in both male and female Galnt3 knockout mice (Table 1). Males had 10% higher BMD, whereas females had 7%. The BMC was 9 and 16% higher in males and females, respectively.
Table 1.
Skeletal analysis of the femur of 24-wk-old mice
| Male |
Female |
|||
|---|---|---|---|---|
| +/+ | −/− | +/+ | −/− | |
| DXA | ||||
| BMD (g/cm2) | 0.0531 ± 0.0006 | 0.0587 ± 0.0008a | 0.0529 ± 0.0004 | 0.0563 ± 0.0012a |
| Weight-adjusted BMC | 0.00100 ± 0.00001 | 0.00110 ± 0.00002a | 0.00114 ± 0.00001 | 0.00132 ± 0.00004a |
| Micro-CT (trabecular bone) | ||||
| BV/TV (%) | 7.80 ± 0.33 | 10.16 ± 0.65a | 3.28 ± 0.23 | 4.52 ± 0.40a |
| Trabecular thickness (mm) | 0.0463 ± 0.0018 | 0.0493 ± 0.0008 | 0.0393 ± 0.0007 | 0.0417 ± 0.0010 |
| Trabecular number (1/mm) | 1.689 ± 0.061 | 2.053 ± 0.118a | 0.834 ± 0.055 | 1.085 ± 0.087a |
| Bone material density, gray scale (pixel intensity) | 130.8 ± 0.9 | 132.9 ± 0.8 | 127.1 ± 0.6 | 129.6 ± 0.9a |
| Micro-CT (cortical bone) | ||||
| Mean total cross-sectional bone area (mm2) | 0.819 ± 0.019 | 0.924 ± 0.019a | 0.810 ± 0.014 | 0.879 ± 0.021a |
| Mean polar moment of inertia (mm4) | 0.425 ± 0.017 | 0.570 ± 0.031a | 0.351 ± 0.011 | 0.406 ± 0.018a |
| Cortical thickness (mm) | 0.162 ± 0.004 | 0.167 ± 0.003 | 0.182 ± 0.002 | 0.192 ± 0.003a |
| Bone material density, gray scale (pixel intensity) | 170.6 ± 1.3 | 169.0 ± 1.7 | 177.9 ± 1.1 | 178.6 ± 1.5 |
| Histomorphometry | ||||
| BV/TV (%) | 10.03 ± 1.06 | 11.59 ± 1.25 | 4.35 ± 0.52 | 5.93 ± 0.43a |
| Mineral apposition rate (μm/d) | 1.20 ± 0.06 | 1.28 ± 0.07 | 1.89 ± 0.11 | 1.80 ± 0.09 |
| Mineralizing surface/bone surface (%) | 39.9 ± 2.5 | 34.8 ± 2.5 | 31.3 ± 1.3 | 31.9 ± 1.6 |
| Bone formation rate/bone surface (μm3/μm2 · yr) | 178 ± 19 | 162 ± 12 | 217 ± 16 | 211 ± 19 |
The data are presented as mean ± sem (n = 8–13 per group).
P < 0.05 compared with same-sex wild-type control mice.
Micro-CT analysis of femoral cortical bone showed that the bone area of Galnt3 knockout mice was 13% higher in males and 9% higher in females, compared with the wild-type counterparts (Table 1). In addition, male affected mice had significantly higher polar moment of inertia (34% increase) with females having a less, yet still significant, difference of +16% compared with wild-type controls. Analysis of trabecular bone at the distal metaphysis also revealed higher bone volume and trabecular number in affected males and females. Specifically, BV/TV in Galnt3 knockout mice was more than 30% higher than wild-type mice. Although the magnitude of difference in affected males was generally greater than that of females, females had a greater number of properties that were significantly affected (Table 1).
Consistent with the micro-CT analysis, bone volume measured by histomorphometry was also higher in Galnt3 mutant mice (although it was statistically significant only for affected females) (Table 1). There were no other significant differences between mutant and control mice for any of the histomorphometry parameters.
The effects of dietary phosphate loads on Galnt3 knockout mice
Serum biochemistries
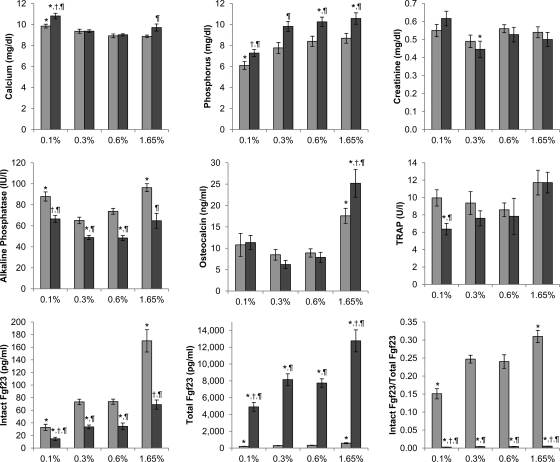
Upon confirming that Galnt3 knockout mice retained their phenotype in the congenic strain, we evaluated how these mice respond to alterations in dietary phosphate. Because there were no discernable differences between wild-type and heterozygous mice, heterozygotes were used as littermate controls for this experiment. On all four diets, Galnt3 knockout mice had significantly higher serum phosphorus (1.2–2.0 mg/dl) than heterozygous control littermates, with the smallest difference on the low-phosphate diet (Fig. 2). The low-phosphate diet made heterozygous littermates severely hypophosphatemic. Serum phosphorus in Galnt3 knockout mice on the same diet were reduced by an average of 3.0 mg/dl; phosphorus in these mice was at the low level of normal because it was not statistically different from that of controls on the normal diet (P = 0.07). In contrast, the high-phosphate diet did not have a significant effect on serum phosphorus levels in either Galnt3 knockout mice or their littermate controls; the levels increased only 0.3 mg/dl on the high-phosphate diet.
Fig. 2.
Serum biochemistries of male mice on various phosphate diets (n = 6–12 per group). Light gray bars, heterozygote; dark gray bars, homozygote. P values <0.05 are indicated: *, compared with heterozygous control on the 0.6% diet; †, compared with Galnt3 knockout mice on the 0.6% diet (comparisons limited to Galnt3 knockout mice); ¶, compared with heterozygous control on the same diet. TRAP, Tartrate-resistant acid phosphatase 5b.
With increasing dietary phosphate content, there was a compensatory increase in both intact and total Fgf23 concentrations (Fig. 2). The increase was not limited to wild-type mice; Galnt3 knockout mice also had a similar response to dietary phosphate loads. Galnt3 knockout mice on the high-phosphate diet achieved intact Fgf23 levels comparable to wild-type mice on the normal-phosphate diet by increasing overall production of Fgf23. However, it should be noted that the level of intact Fgf23 in Galnt3 knockout mice was still markedly inappropriate in the face of hyperphosphatemia. Furthermore, in both wild-type and Galnt3 knockout mice, the fraction of intact Fgf23 within the total immunoreactive Fgf23 increased as the phosphate load increased, and the fraction decreased on the low-phosphate diet (Fig. 2), indicating that these mice can modulate proteolytic cleavage of intact Fgf23 protein to some extent.
In heterozygous mice, there was a general trend toward decreased serum calcium as phosphate contents increased (Fig. 2). Compared with mice on the normal diet, serum calcium was significantly elevated only on the low-phosphate diet. Although Galnt3 knockout mice showed a similar response to lower-phosphate diets, serum calcium had a marginally significant increase on the high-phosphate diet (P = 0.05). Compared with the littermate control, Galnt3 knockout mice had approximately 10% higher serum calcium on the low- or high-phosphate diets, but there was no difference between the two genotypic groups on the low-normal- or normal-phosphate diets. Serum creatinine levels fluctuated considerably (Fig. 2). However, there were no major differences between diets and genotypes.
As in the 24-wk-old mice (Fig. 1), alkaline phosphatase concentrations were consistently lower in male Galnt3 knockout mice at 17 wk of age than in heterozygous littermates (24–34% decrease) (Fig. 2). Regardless of genotypes of the mice, alkaline phosphatase had a biphasic pattern with higher levels on the low- and high-phosphate diets. Levels of another bone formation marker, osteocalcin, showed a similar biphasic pattern. However, unlike alkaline phosphatase, osteocalcin levels were different between the two genotypic groups only on the high-phosphate diet with Galnt3 knockout mice having higher osteocalcin levels (Fig. 2). Compared with control heterozygotes, serum levels of a bone resorption maker, tartrate-resistant acid phosphatase 5b, tended to be lower in Galnt3 knockout mice, particularly on the low-phosphate diet (Fig. 2).
Skeletal phenotype
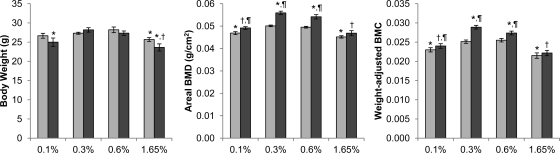
As in the biochemical values, the low-normal (0.3%) phosphate diet had no effect on areal BMD (Fig. 3). However, compared with same-genotype mice on the normal-phosphate diet, femurs from those fed the two extreme diets had significantly lower areal BMD, particularly on the high-phosphate diet. It is worth noting that this biphasic pattern is an opposite of alkaline phosphatase and osteocalcin (and to some extent, tartrate-resistant acid phosphatase 5b) concentrations (Fig. 2).
Fig. 3.
Body weight and femoral BMD and BMC of male mice on various phosphate diets (n = 10–12 per group for body weight). Light gray bars, heterozygote; dark gray bars, homozygote. Bone specimens that were broken or missing pieces were excluded from analysis (n = 6–10 per group for BMD and BMC). P values <0.05 are indicated: *, compared with heterozygous control on the 0.6% diet; †, compared with Galnt3 knockout mice on the 0.6% diet (comparisons limited to Galnt3 knockout mice); ¶, compared with heterozygous control on the same diet.
Comparisons between Galnt3 knockout mice and heterozygous littermates revealed that on all diets except the high-phosphate diet, femurs of Galnt3 knockout mice had significantly higher BMD (up to 11%) than those of heterozygous littermates (Fig. 3). More importantly, the low-phosphate diet essentially normalized areal BMD in Galnt3 knockout mice because there was no difference from heterozygous controls on the normal diet. Compared with mice on the normal diet, those on the two extreme diets tended to be smaller. Thus, weight-adjusted BMC were also compared between genotypes and diets (Fig. 3). The findings were identical to those in areal BMD.
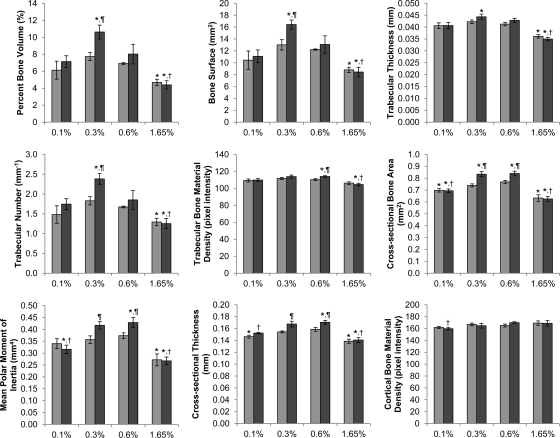
Consistent with the DXA data, almost all variables measured by micro-CT analysis were higher for mice on the low-normal- and normal-phosphate diets and lower for those on the low- and high-phosphate diets (Fig. 4 and Supplemental Fig. 2). In addition, the differences between the two genotypic groups that were often apparent on the low-normal and normal diets disappeared in the mice on the two extreme diets. It should also be noted that trabecular bone parameters for Galnt3 knockout mice on the low-phosphate diet were similar to heterozygous controls on the normal diet, but in the same comparison, cortical bone parameters were significantly lower in Galnt3 knockout mice on the low-phosphate diet.
Fig. 4.
Micro-CT analysis of male mice on various phosphate diets. Light gray bars, heterozygote; dark gray bars, homozygote. Bone specimens that were broken or missing pieces were excluded from analysis (n = 5–6 per group). P values <0.05 are indicated: *, compared with heterozygous control on the 0.6% diet; †, compared with Galnt3 knockout mice on the 0.6% diet (comparisons limited to Galnt3 knockout mice); ¶, compared with heterozygous control on the same diet.
Fertility
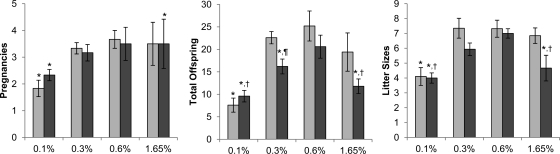
On the same four diets, Galnt3 knockout mice and their heterozygous littermates were allowed to reproduce freely for 12 wk. Regardless of Galnt3 genotypes in females, those placed in the same cages with male Galnt3 knockout mice had no sign of pregnancy or produced no offspring (data not shown). In contrast, all females in the same cages with heterozygous males became pregnant and produced offspring at least once. However, overall fecundity and fertility in these females were affected by dietary phosphate loads. Compared with those on the normal-phosphate diet, the number of pregnancies per female was significantly reduced in mice on the low-phosphate diet and in Galnt3 knockout mice on the high-phosphate diet (Fig. 5). In addition, the same groups had small litters, contributing to decreased total offspring born to each female.
Fig. 5.
Fertility of female mice bred with heterozygous males (n = 5 per group). Light gray bars, heterozygote; dark gray bars, homozygote. P values <0.05 are indicated: *, compared with heterozygous control on the 0.6% diet; †, compared with Galnt3 knockout mice on the 0.6% diet (comparisons limited to Galnt3 knockout mice); ¶, compared with heterozygous control on the same diet.
Compared with heterozygous littermates, female Galnt3 knockout mice generally had reduced numbers of pregnancies and offspring and had smaller litter sizes (Fig. 5). However, only total number of pups on the low-normal-phosphate diet was significantly different between the two genotypes.
Discussion
To better understand the pathophysiology of tumoral calcinosis, we recently developed an animal model of the disease by disrupting the Galnt3 gene in mice (32). The initial phenotypic characterization of Galnt3 knockout mice in the mixed background revealed biochemical characteristics of tumoral calcinosis as well as increased BMD in males, which may be reminiscent of hyperostosis-hyperphosphatemia syndrome. In this study, we extended phenotypic characterization of the animals in a more homogeneous background of C57BL/6J and on various dietary phosphate loads. Biochemical phenotypes of Galnt3 knockout mice in the new strain were essentially identical to those found in the mixed background (32): normocalcemia, hyperphosphatemia, inappropriately normal 1,25(OH)2D level, decreased alkaline phosphatase activity, and low intact Fgf23 concentration. However, this study revealed that Galnt3 knockout mice have markedly elevated C-terminal Fgf23 fragments, recapitulating the low intact, high C-terminal pattern observed in patients with tumoral calcinosis (24, 30, 31, 34, 35). Similarly, Galnt3 knockout mice on the four different phosphate diets had consistently high phosphorus and C-terminal Fgf23 fragments but low intact Fgf23 and alkaline phosphatase concentrations.
Because of a compensatory increase in Fgf23 production and decrease in intact Fgf23 processing, serum phosphorus levels within same genotypic groups did not differ significantly for either Galnt3 knockout mice or their littermate controls on the low-normal-, normal-, and high-phosphate diets. This finding suggests that given the increased stimulus, Galnt3 knockout mice (and human counterparts) can secrete more intact Fgf23 protein and, thus, may be able to achieve normal phosphorus levels. However, we cannot rule out that at least in Galnt3 knockout mice, the lack of increased phosphorus may be due to the fact that excess phosphate is precipitated as calcium-phosphate deposits in soft tissues as seen in some mice on the high-phosphate diet (unpublished observations).
Hypercalcemia seen in some of the younger Galnt3 knockout mice in our previous study (32) also manifested in mice on the low-phosphate diet. It is likely that low intact Fgf23 levels secondary to the severe hypophosphatemia enhanced renal 1,25(OH)2D synthesis, which in turn increased intestinal absorption of phosphate as well as calcium. In contrast, the increased calcium in Galnt3 knockout mice on the high-phosphate diet (compared with littermate controls) is more difficult to explain. On the high-phosphate diet, there is a higher demand for intact Fgf23 protein to reduce intestinal phosphate absorption and enhance renal phosphate excretion. However, because Galnt3 knockout mice lag behind the controls in intact Fgf23 production, this may lead to increased 1,25(OH)2D synthesis and calcium absorption in these mice. It is also possible that Pth, which has a similar phosphaturic effect as Fgf23, may be elevated in Galnt3 knockout mice such that phosphate remains normal at the expense of hypercalcemia.
In anticipation of a more pronounced skeletal phenotype, we investigated Galnt3 knockout mice at 24 wk of age. Although in the previous study (32) only 12-wk-old males had increased BMD, in this study, both male and female Galnt3 knockout mice showed increased femoral BMD likely due to the combination of increased sample size and the homogeneous background. Femoral BMD in Galnt3 knockout mice were also consistently higher than those in heterozygous littermates on the low, low-normal, and normal diets. Micro-CT analysis, which was previously done only on a small number of males (32), revealed that both male and female Galnt3 knockout mice have increased cortical and trabecular bone properties. This finding may reflect the unique skeletal feature (i.e. hyperostosis) seen in some patients carrying GALNT3 mutations. Because there was no evidence for increased bone formation rate by histomorphometric analysis, the higher bone volume in Galnt3 knockout mice could be due to reduced osteoclast activity as indicated by somewhat lower serum tartrate-resistant acid phosphatase 5b levels or higher bone formation rates earlier in life that have normalized in these skeletally mature aged animals. Alternatively, it is possible that small changes in bone formation exist in these mice, yet the differences are not sufficiently large enough to be detected by histomorphometry.
Because Galnt3 knockout mice had hyperphosphatemia on the low-normal- or normal-phosphate diets, they tolerated the low-phosphate diet reasonably well. In fact, most biochemical and skeletal phenotypes in these mice were normalized by the low-phosphate diet. However, as indicated by various bone parameters measured by DXA and micro-CT, the low- or high-phosphate diets had adverse effects on bone quality of normal mice. In addition, femurs in Galnt3 knockout mice on the high-phosphate diet were particularly prone to breakage during the extraction. Because mice fed the low- or high-phosphate diets had generally higher serum bone turnover markers (alkaline phosphatase, osteocalcin, and tartrate-resistant acid phosphatase 5b), increased bone remodeling is a likely explanation for bone loss in these animals.
Mice lacking Fgf23 or Kl, the two other genes associated with tumoral calcinosis, also have hyperphosphatemia and abnormal bone development. Kl knockout mice have low-turnover osteopenia, accompanied by decreased cortical bone thickness, but increased trabecular bone (36–39). Similarly, mice lacking the Fgf23 gene have significantly decreased mineralized bone with reduced osteoblast and osteoclast surface areas, indicative of suppressed bone turnover (40, 41). Their skeletal phenotypes are strikingly different from the increased bone in Galnt3 knockout mice. The differences could be due to the severity of hyperphosphatemia affecting overall health of these animals. In this regard, Galnt3 knockout mice can suppress hyperphosphatemia (as observed on the high-phosphate diet) because of small amounts of intact Fgf23 in the blood and have a normal lifespan, whereas the other two animals develop severe hyperphosphatemia due to no Fgf23 or its signaling, leading to a short lifespan.
Because male infertility was one of the prominent features of Galnt3 knockout mice (32), we tested the hypothesis that hyperphosphatemia causes infertility in these mice. None of the females placed in the same cages with male Galnt3 knockout mice produced offspring even on the low-phosphate diet, which normalized serum phosphorus in the males. In contrast, infertility caused by hypogonadism in Kl-null mice was reversed by introducing homozygous loss of NaPi-IIa in the Kl-null background (i.e. NaPi-IIa/Kl double-knockout mice) and, thus, genetically reducing serum phosphate levels (42). However, the double-knockout mice became infertile again once fed a high-phosphate diet (1.2%). These data suggest that male infertility in Galnt3 knockout mice is not mediated by hyperphosphatemia like in Kl-null mice, but rather it is a direct consequence of lack of O-glycosylation by ppGalNAc-T3.
All females placed with heterozygous males produced offspring. However, total number of pregnancies, total offspring born, and mean litter size were all reduced in females on the low-phosphate diet as well as Galnt3 knockout mice on the high-phosphate diet. Although these data imply the relationship between phosphate and fertility, it is difficult to estimate how much of the reduced fertility and fecundity is attributable to overall health or serum phosphorus levels of experimental mice.
To further our understanding of tumoral calcinosis, we generated Galnt3 knockout mice in the congenic C57BL/6J background and tested the effect of dietary phosphate intake on their phenotype. The biochemical and skeletal findings observed in this study are of significant clinical importance. Regardless of phosphate load, Galnt3 knockout mice had consistently higher phosphorus levels and lower alkaline phosphatase and intact Fgf23 concentrations than littermate controls. Although high phosphate intake may not increase serum phosphorus in Galnt3 knockout mice due to a compensatory increase in circulating intact Fgf23 levels, our data suggest that it may induce hypercalcemia, likely contributing to the overall increase in calcium-phosphate products and subsequent ectopic calcifications. On the other hand, the low-phosphate diet normalized serum phosphorus and alkaline phosphatase levels and areal BMD in Galnt3 knockout mice. Therefore, dietary phosphate restriction, as well as the use of phosphate binders, should be considered when treating patients with familial tumoral calcinosis.
Supplementary Material
Acknowledgments
We thank Drew Brown and Chris Newman for their assistance with micro-CT scanning and analysis.
This work was supported by Indiana University School of Medicine Biomedical Research Grant (to S.I.), Showalter Biomedical Trust Grant (to S.I.), and National Institutes of Health Grant R01 AR042228 (to M.J.E.). The preparation of this report was partially supported by a KL2 career development award (to S.I.) from the Indiana Clinical and Translational Sciences Institute funded in part by National Institutes of Health Grant RR025760.
Current affiliation for A.M.A.: Department of Pediatrics, Washington University School of Medicine, St. Louis, Missouri 63110.
Disclosure Summary: M.J.E. receives royalties from and is a consultant for Kyowa Hakko Kirin Co. Ltd. All other authors state that they have no conflicts of interest.
Footnotes
- BMC
- Bone mineral content
- BMD
- bone mineral density
- BV/TV
- bone volume/tissue volume
- DXA
- dual-energy x-ray absorptiometry
- FGF23
- fibroblast growth factor 23
- micro-CT
- microcomputed tomography
- 1,25(OH)2D
- 1,25-dihydroxyvitamin D
- ppGalNAc-T3
- UDP-N-acetyl-α-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase 3.
References
- 1. Carmichael KD, Bynum JA, Evans EB. 2009. Familial tumoral calcinosis: a forty-year follow-up on one family. J Bone Joint Surg Am 91:664–671 [DOI] [PubMed] [Google Scholar]
- 2. Ichikawa S, Baujat G, Seyahi A, Garoufali AG, Imel EA, Padgett LR, Austin AM, Sorenson AH, Pejin Z, Topouchian V, Quartier P, Cormier-Daire V, Dechaux M, Malandrinou FCh, Singhellakis PN, Le Merrer M, Econs MJ. 2010. Clinical variability of familial tumoral calcinosis caused by novel GALNT3 mutations. Am J Med Genet A 152A:896–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McPhaul JJ, Jr, Engel FL. 1961. Heterotopic calcification, hyperphosphatemia and angioid streaks of the retina. Am J Med 31:488–492 [DOI] [PubMed] [Google Scholar]
- 4. Ghanchi F, Ramsay A, Coupland S, Barr D, Lee WR. 1996. Ocular tumoral calcinosis. A clinicopathologic study. Arch Ophthalmol 114:341–345 [DOI] [PubMed] [Google Scholar]
- 5. Lyles KW, Burkes EJ, Ellis GJ, Lucas KJ, Dolan EA, Drezner MK. 1985. Genetic transmission of tumoral calcinosis: autosomal dominant with variable clinical expressivity. J Clin Endocrinol Metab 60:1093–1096 [DOI] [PubMed] [Google Scholar]
- 6. Clarke E, Swischuk LE, Hayden CK., Jr 1984. Tumoral calcinosis, diaphysitis, and hyperphosphatemia. Radiology 151:643–646 [DOI] [PubMed] [Google Scholar]
- 7. Narchi H. 1997. Hyperostosis with hyperphosphatemia: evidence of familial occurrence and association with tumoral calcinosis. Pediatrics 99:745–748 [DOI] [PubMed] [Google Scholar]
- 8. Wilson MP, Lindsley CB, Warady BA, Johnson JA. 1989. Hyperphosphatemia associated with cortical hyperostosis and tumoral calcinosis. J Pediatr 114:1010–1013 [DOI] [PubMed] [Google Scholar]
- 9. Dumitrescu CE, Kelly MH, Khosravi A, Hart TC, Brahim J, White KE, Farrow EG, Nathan MH, Murphey MD, Collins MT. 2009. A case of familial tumoral calcinosis/hyperostosis-hyperphosphatemia syndrome due to a compound heterozygous mutation in GALNT3 demonstrating new phenotypic features. Osteoporos Int 20:1273–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Joseph L, Hing SN, Presneau N, O'Donnell P, Diss T, Idowu BD, Joseph S, Flanagan AM, Delaney D. 2010. Familial tumoral calcinosis and hyperostosis-hyperphosphataemia syndrome are different manifestations of the same disease: novel missense mutations in GALNT3. Skeletal Radiol 39:63–68 [DOI] [PubMed] [Google Scholar]
- 11. Frishberg Y, Topaz O, Bergman R, Behar D, Fisher D, Gordon D, Richard G, Sprecher E. 2005. Identification of a recurrent mutation in GALNT3 demonstrates that hyperostosis-hyperphosphatemia syndrome and familial tumoral calcinosis are allelic disorders. J Mol Med 83:33–38 [DOI] [PubMed] [Google Scholar]
- 12. Araya K, Fukumoto S, Backenroth R, Takeuchi Y, Nakayama K, Ito N, Yoshii N, Yamazaki Y, Yamashita T, Silver J, Igarashi T, Fujita T. 2005. A novel mutation in fibroblast growth factor 23 gene as a cause of tumoral calcinosis. J Clin Endocrinol Metab 90:5523–5527 [DOI] [PubMed] [Google Scholar]
- 13. Benet-Pagès A, Orlik P, Strom TM, Lorenz-Depiereux B. 2005. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet 14:385–390 [DOI] [PubMed] [Google Scholar]
- 14. Chefetz I, Heller R, Galli-Tsinopoulou A, Richard G, Wollnik B, Indelman M, Koerber F, Topaz O, Bergman R, Sprecher E, Schoenau E. 2005. A novel homozygous missense mutation in FGF23 causes familial tumoral calcinosis associated with disseminated visceral calcification. Hum Genet 118:261–266 [DOI] [PubMed] [Google Scholar]
- 15. Larsson T, Yu X, Davis SI, Draman MS, Mooney SD, Cullen MJ, White KE. 2005. A novel recessive mutation in fibroblast growth factor-23 causes familial tumoral calcinosis. J Clin Endocrinol Metab 90:2424–2427 [DOI] [PubMed] [Google Scholar]
- 16. Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. 2001. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA 98:6500–6505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saito H, Kusano K, Kinosaki M, Ito H, Hirata M, Segawa H, Miyamoto K, Fukushima N. 2003. Human fibroblast growth factor-23 mutants suppress Na+-dependent phosphate co-transport activity and 1α,25-dihydroxyvitamin D3 production. J Biol Chem 278:2206–2211 [DOI] [PubMed] [Google Scholar]
- 18. Shimada T, Muto T, Urakawa I, Yoneya T, Yamazaki Y, Okawa K, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. 2002. Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology 143:3179–3182 [DOI] [PubMed] [Google Scholar]
- 19. Ichikawa S, Imel EA, Kreiter ML, Yu X, Mackenzie DS, Sorenson AH, Goetz R, Mohammadi M, White KE, Econs MJ. 2007. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest 117:2684–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M. 2006. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 281:6120–6123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. 2006. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444:770–77417086194 [Google Scholar]
- 22. Ichikawa S, Lyles KW, Econs MJ. 2005. A novel GALNT3 mutation in a pseudoautosomal dominant form of tumoral calcinosis: evidence that the disorder is autosomal recessive. J Clin Endocrinol Metab 90:2420–2423 [DOI] [PubMed] [Google Scholar]
- 23. Topaz O, Shurman DL, Bergman R, Indelman M, Ratajczak P, Mizrachi M, Khamaysi Z, Behar D, Petronius D, Friedman V, Zelikovic I, Raimer S, Metzker A, Richard G, Sprecher E. 2004. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet 36:579–581 [DOI] [PubMed] [Google Scholar]
- 24. Frishberg Y, Ito N, Rinat C, Yamazaki Y, Feinstein S, Urakawa I, Navon-Elkan P, Becker-Cohen R, Yamashita T, Araya K, Igarashi T, Fujita T, Fukumoto S. 2007. Hyperostosis-hyperphosphatemia syndrome: a congenital disorder of O-glycosylation associated with augmented processing of fibroblast growth factor 23. J Bone Miner Res 22:235–242 [DOI] [PubMed] [Google Scholar]
- 25. Kato K, Jeanneau C, Tarp MA, Benet-Pagès A, Lorenz-Depiereux B, Bennett EP, Mandel U, Strom TM, Clausen H. 2006. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J Biol Chem 281:18370–18377 [DOI] [PubMed] [Google Scholar]
- 26. Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. 2004. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19:429–435 [DOI] [PubMed] [Google Scholar]
- 27. Larsson T, Marsell R, Schipani E, Ohlsson C, Ljunggren O, Tenenhouse HS, Jüppner H, Jonsson KB. 2004. Transgenic mice expressing fibroblast growth factor 23 under the control of the α1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology 145:3087–3094 [DOI] [PubMed] [Google Scholar]
- 28. Bai X, Miao D, Li J, Goltzman D, Karaplis AC. 2004. Transgenic mice overexpressing human fibroblast growth factor 23 (R176Q) delineate a putative role for parathyroid hormone in renal phosphate wasting disorders. Endocrinology 145:5269–5279 [DOI] [PubMed] [Google Scholar]
- 29. Shimada T, Yamazaki Y, Takahashi M, Hasegawa H, Urakawa I, Oshima T, Ono K, Kakitani M, Tomizuka K, Fujita T, Fukumoto S, Yamashita T. 2005. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol 289:F1088–F1095 [DOI] [PubMed] [Google Scholar]
- 30. Ichikawa S, Guigonis V, Imel EA, Courouble M, Heissat S, Henley JD, Sorenson AH, Petit B, Lienhardt A, Econs MJ. 2007. Novel GALNT3 mutations causing hyperostosis-hyperphosphatemia syndrome result in low intact fibroblast growth factor 23 concentrations. J Clin Endocrinol Metab 92:1943–1947 [DOI] [PubMed] [Google Scholar]
- 31. Ichikawa S, Imel EA, Sorenson AH, Severe R, Knudson P, Harris GJ, Shaker JL, Econs MJ. 2006. Tumoral calcinosis presenting with eyelid calcifications due to novel missense mutations in the glycosyl transferase domain of the GALNT3 gene. J Clin Endocrinol Metab 91:4472–4475 [DOI] [PubMed] [Google Scholar]
- 32. Ichikawa S, Sorenson AH, Austin AM, Mackenzie DS, Fritz TA, Moh A, Hui SL, Econs MJ. 2009. Ablation of the Galnt3 gene leads to low-circulating intact fibroblast growth factor 23 (Fgf23) concentrations and hyperphosphatemia despite increased Fgf23 expression. Endocrinology 150:2543–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, Takeuchi Y, Fujita T, Nakahara K, Yamashita T, Fukumoto S. 2002. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab 87:4957–4960 [DOI] [PubMed] [Google Scholar]
- 34. Garringer HJ, Fisher C, Larsson TE, Davis SI, Koller DL, Cullen MJ, Draman MS, Conlon N, Jain A, Fedarko NS, Dasgupta B, White KE. 2006. The role of mutant UDP-N-acetyl-α-d-galactosamine-polypeptide N-acetylgalactosaminyltransferase 3 in regulating serum intact fibroblast growth factor 23 and matrix extracellular phosphoglycoprotein in heritable tumoral calcinosis. J Clin Endocrinol Metab 91:4037–4042 [DOI] [PubMed] [Google Scholar]
- 35. Garringer HJ, Mortazavi SMJ, Esteghamat F, Malekpour M, Boztepe H, Tanakol R, Davis SI, White KE. 2007. Two novel GALNT3 mutations in familial tumoral calcinosis. Am J Med Genet A 143:2390–2396 [DOI] [PubMed] [Google Scholar]
- 36. Kawaguchi H, Manabe N, Miyaura C, Chikuda H, Nakamura K, Kuro-o M. 1999. Independent impairment of osteoblast and osteoclast differentiation in klotho mouse exhibiting low-turnover osteopenia. J Clin Invest 104:229–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. 1997. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390:45–51 [DOI] [PubMed] [Google Scholar]
- 38. Yamashita T, Nabeshima Y, Noda M. 2000. High-resolution micro-computed tomography analyses of the abnormal trabecular bone structures in klotho gene mutant mice. J Endocrinol 164:239–245 [DOI] [PubMed] [Google Scholar]
- 39. Yamashita T, Nifuji A, Furuya K, Nabeshima Y, Noda M. 1998. Elongation of the epiphyseal trabecular bone in transgenic mice carrying a klotho gene locus mutation that leads to a syndrome resembling aging. J Endocrinol 159:1–8 [DOI] [PubMed] [Google Scholar]
- 40. Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. 2004. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113:561–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sitara D, Razzaque MS, Hesse M, Yoganathan S, Taguchi T, Erben RG, Jüppner H, Lanske B. 2004. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol 23:421–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ohnishi M, Razzaque MS. 2010. Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging. FASEB J 24:3562–3571 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.