Abstract
Beyond their classical role as a class of female sex hormones, estrogens (e.g. 17β-estradiol) exert important biological actions, both protective and undesirable. We have investigated the ability of estradiol to protect the lung in three models of acute injury induced by 1) oxidant stress due to the herbicide paraquat; 2) excitotoxicity, caused by glutamate agonist N-methyl-d-aspartate; and 3) acute alveolar anoxia. We also assessed the role of estrogen receptors (ER) ERα and ERβ and the neuropeptide vasoactive intestinal peptide (VIP) in mediating this protection. Isolated guinea pig or rat lungs were perfused in situ at constant flow and mechanically ventilated. The onset and severity of lung injury were monitored by increases in pulmonary arterial and airway pressures, wet/dry lung weight ratio, and bronchoalveolar lavage fluid protein content. Estradiol was infused into the pulmonary circulation, beginning 10 min before induction of injury and continued for 60–90 min. Lung injury was marked by significant increases in the above measurements, with paraquat producing the most severe, and excitotoxicity the least severe, injury. Estradiol significantly attenuated the injury in each model. Both ER were constitutively expressed and immunohistochemically demonstrable in normal lung, and their selective agonists reduced anoxic injury, the only model in which they were tested. As it protected against injury, estradiol rapidly and significantly stimulated VIP mRNA expression in rat lung. Estradiol attenuated acute lung injury in three experimental models while stimulating VIP gene expression, a known mechanism of lung protection. The up-regulated VIP expression could have partially mediated the protection by estrogen.
In addition to their primary function as reproductive hormones, estrogens (e.g. 17β-estradiol) influence a wide range of other physiological processes in humans and other mammals, including cardiovascular, respiratory, and neuronal function, and bone density. By the same token, estrogens are involved in the pathogenesis or amelioration of a variety of disorders, including different types of cancer, certain cardiovascular and respiratory diseases, and osteoporosis (1–3). With special respect to the cardiorespiratory system, estrogens promote lung development and differentiation (4–6) and exhibit pulmonary and cardiovascular protective properties (7, 8).
Estrogens exerts their actions by at least two different mechanisms: 1) the classical or genomic mechanism, in which these hormones diffuse into the cell and bind to the estrogen receptor (ER), located in the nucleus, a mechanism that occurs over a period of hours, and 2) the nongenomic mechanism, which occurs through activation of receptors located at or near the plasma membrane and requires only seconds or minutes.
In this study, we focused on a topic that has been of special interest to our laboratory for over two decades, namely acute lung injury. We examined the ability of 17β-estradiol to protect the lung against different forms of acute injury: two models of oxidative stress, one induced by paraquat (methyl violagen) in guinea pig lungs and the other by the glutamate agonist N-methyl-d-aspartate (NMDA) in rat lungs, as well as by alveolar anoxia, also induced in rat lungs. Guinea pigs were chosen for the paraquat model because of their particular susceptibility to lung injury by this toxin (9).
A second and related objective was to investigate the presence, localization, and role of ER in the lung. We examined the mRNA expression and immunohistochemical localization of ERα and ERβ in normal lung tissue and assessed their possible role in lung protection by the use of selective receptor agonists.
Finally, in view of reports that exogenously administered estradiol elicits overexpression of mRNA levels of vasoactive intestinal polypeptide (VIP) in certain organs, and the knowledge that VIP protects against acute lung injury in a variety of experimental models, including two of those tested here (10), we considered the possibility that protection by estradiol might be attributable, at least in part, to stimulated VIP gene expression in lung tissue.
We report that 1) 17β-estradiol effectively protected against lung injury leading to acute high-permeability pulmonary edema in all three experimental models tested: paraquat-induced and excitotoxic oxidant stress as well as alveolar anoxia. 2) ERα and -β were normally expressed, and immunohistochemically demonstrable, in both airways and peripheral lung tissue. 3) Selective agonists of both receptors provided equal protection to estradiol itself in the only model in which they were tested, that of acute anoxia. 4) These effects were receptor mediated, by rapid, nongenomic mechanisms. 5) Estradiol increased VIP gene expression in the lung, within the same time frame, suggesting that the increased expression of VIP, which has proven lung-protective properties, was a significant contributor to estradiol-induced protection.
Materials and Methods
Animals
All experiments were conducted on males of each species. Sprague-Dawley rats (300–350 g) and guinea pigs (300–500 g), respectively, were from Taconic (Germantown, NY) and Hilltop (Scottdale, PA). All experiments and animal care procedures were approved by our Institutional Animal Care and Use Committee and were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Chemicals
Paraquat (methyl viologen), NMDA, and 17β-estradiol were from Sigma (St. Louis, MO). Selective ERα agonist (WAY-2033306) and selective ERβ agonist (WAY-200070-3) were kindly provided by Heather Harris, Ph.D., Women's Health Research Institute, Wyeth Research (Collegeville, PA).
Expression of ER in rat lung
RNA was extracted from major airways and peripheral lung samples taken from normal male rats after perfusion with saline for 15 min to remove blood. RT-PCR was performed using specific primers for the ERα and ERβ isoforms, normalized for the ribosomal protein L19 (Table 1) from Invitrogen (Carlsbad, CA) (11). RT-PCR products were electrophoresed on 1.5% agarose gels and stained with ethidium bromide. Images were captured and analyzed with an AlphaImager 2000 system, using the linear range of the camera. ER samples were subjected to 40 PCR cycles (96 C for 1.5 min, 55 C for 1.5 min, and 72 C for 3 min); L19 levels were determined after 30 cycles.
Table 1.
ER primers and predicted RT-PCR product sizes
| mRNA | Sense primer (5′–3′) | Antisense primer (5′–3′) | Product size (bp) |
|---|---|---|---|
| ERα | AAT TCT GAC AAT CGA CGC CAG | GTG CTT CAA CAT TCT CCC TCC TC | 344 |
| ERß | TTC CCG GCA GCA CCA GTA ACC | TCC CTC TTT GCG TTT GGA CTA | 263 |
| L19 | GAA ATC GCC AAT GCC AAC TC | ACC TTC AGG TAC AGG CTG TG | 290 |
Isoform-specific primers were used to amplify the two known ER subtypes by RT-PCR. Primer pairs were designed to span over intronic sequences to exclude potential amplification of contaminant genomic DNA (10). The integrity of the PCR process was verified by the presence of the ribosomal protein L18 mRNA.
Immunohistochemical localization of ER
Sections (5 μm) were prepared from formalin-fixed, paraffin-embedded samples of rat lungs. Endogenous peroxidases were inactivated by 30 min incubation in methanol plus 0.3% hydrogen peroxide. Samples were antigen unmasked by three cycles of heating and cooling in 10 mm citrate (pH 6). After blocking in serum for 30 min at 37 C, samples were incubated for 1 h at 37 C with primary antibodies (Santa Cruz Biotechnology Inc., Santa Cruz, CA) against either ERα (rabbit polyclonal, 1:100) or ERβ (goat polyclonal, 1:100). After washing in PBS, samples were incubated with biotinylated secondary antibodies (Vectastain horse antirabbit for ERα or Vectastain rabbit antigoat for ERβ) for 30 min at 37 C, washed in PBS, and incubated with Vectastain ABC reagent for 30 min at 37 C. Color was developed by incubation with diaminobenzidine with metal enhancer (Sigma) for 4 min at room temperature.
Isolated rat and guinea pig lung preparations
Isolated lung experiments were conducted as previously reported (9, 12). Rats were ventilated with 95% O2/5% CO2 at 60 breaths/min and a tidal volume of 5 ml/kg. Perfusion was started at the rate of 8 ml/min and then adjusted to reach an initial mean pulmonary artery (PA) pressure (PPA) of 5–8 cm H2O. Guinea pigs were ventilated with 95% O2/5% CO2 at 50 breaths/min and a tidal volume of 10 ml/kg. Perfusion was started at the rate of 10 ml/min and adjusted to maintain a pulmonary arterial pressure (PPA) at or just below 10 cm H2O.
Physiological measurements
Peak airway pressure (PAW) and PPA were continuously monitored by pressure transducers attached to the tracheal cannula and to a catheter in the main PA, respectively, and were recorded. Left atrial pressure was maintained at 2 cm H2O by placing the tip of the outflow cannula 2 cm above the level of the atrium. At the conclusion of the experiment, the left lung was lavaged with 3 ml saline for measurement of bronchoalveolar lavage (BAL) fluid protein content as an index of protein leakage due to alveolar-microvascular membrane injury. The right lung was removed, gently blotted, weighed (wet weight), and then oven-dried at 85 C for 3 d to a constant weight (dry weight). The wet to dry lung weight ratio (W/D) was a measure of the presence and severity of pulmonary edema.
Experimental lung injury groups
Oxidative injury by paraquat in guinea pigs
As we previously described (9), paraquat (100 mg/kg of the dichloride salt, dissolved in 0.5 ml 0.9% NaCl) was infused directly into the PA over 20 sec by an infusion pump (Harvard Apparatus; model 940-S; Holliston, MA). Lung perfusion was continued for 60 min (n = 14). Paraquat produced sufficiently severe lung injury to permit a clear demonstration of potential protection by estrogen. In a control group of guinea pigs, a 0.9% saline solution was infused instead of paraquat into the PA, at the same flow rate, followed by a 1-h observation period (n = 9).
Excitotoxic injury by NMDA in rats
As previously described (12), NMDA (1 mm) in the presence of l-arginine (10 mm) was infused directly into the PA over 20 min, and perfusion was continued for 60 min (n = 20). In another group of rats, a 0.9% saline solution instead of NMDA was infused into the PA at the same flow rate and for the same duration (n = 15).
Anoxia-induced injury in rats
Rat lungs were initially ventilated with air and 5% CO2 for 20 min and then with a mixture of 0% O2 plus 5% CO2 in 95% N2 for 90 min (n = 7). In a separate group of rats, the lungs were ventilated with air and 5% CO2 during the whole experiment (n = 5).
Possible attenuation of injury by estradiol and its agonists: experimental groups
Oxidative injury by paraquat: paraquat plus estradiol
At 10 min before the paraquat infusion, estradiol (1 μm) dissolved in 0.9% saline was infused at 1 pg/kg·min into the PA by an infusion pump, and the infusion was continued for 60 min (n = 8).
Excitotoxic injury by NMDA: NMDA plus l-arginine plus estradiol
At 10 min before the NMDA infusion, estradiol (1 μm) dissolved in 0.9% saline was infused at 1 pg/kg·min into the PA by an infusion pump, and the infusion was continued for 60 min (n = 7).
Anoxia-induced injury: anoxia plus estradiol
At 10 min before exposure to anoxia, estradiol (1 μm) dissolved in 0.9% saline was infused at 1 pg/kg·min into the PA by an infusion pump, and the infusion was continued for the balance of the experiment (90 min, n = 4). In two separate experiments (n = 4), selective ERα agonist (WAY-2033306; Wyeth Research) or selective ERβ agonist (WAY-200070-3; Wyeth Research) was infused into the PA in the same concentration and for the same duration as estrogen.
Effect of estradiol on the expression of VIP mRNA
A group of six rats received 17β-estradiol in sesame oil (100 mg/kg ip). Another group of six rats received vehicle only, as a control. After euthanasia, lung tissue was collected from each group at 0, 0.5, 1, 3, 6, and 24 h after injection. The tissues were fresh-frozen in liquid nitrogen, and kept at −80 C until RNA was extracted and subjected to RT-PCR, as described above, with specific VIP and β-actin primers.
Statistical analysis
All results are expressed as means ± sem. In each group, the significance of differences in mean PAW and PPA, before and after injury, was analyzed by the two-tailed unpaired Student's t test. Differences in PAW, peak PPA, W/D, and BAL protein content among the different groups were first assessed by ANOVA, followed by Tukey's post hoc test for paired comparison.
Results
Both ER are normally expressed in rat lung
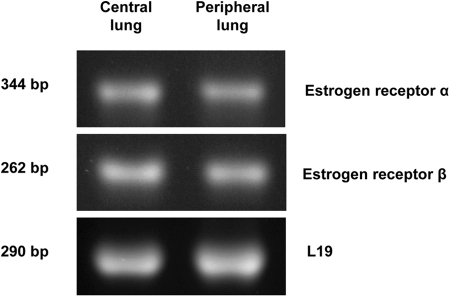
RT-PCR studies showed that mRNA for both ER, ERα and ERβ, are constitutively expressed in peripheral and central regions of male rat lung (Fig. 1). When normalized to expression of the ribosomal protein L19, the relative expression of either receptor was comparable in both lung regions.
Fig. 1.
Localization of ER by RT-PCR. RNA was extracted from central and peripheral regions of male rat lung and converted to cDNA with reverse transcriptase. Isoform-specific primers were used to amplify the two known ER subtypes by PCR. Integrity of PCR process was verified by the presence of the ribosomal protein L19 mRNA. ER samples were subjected to 40 PCR cycles; L19 levels were determined after 30 cycles. RT-PCR products were electrophoresed on 1.5% agarose gels and stained with ethidium bromide. Images were captured and analyzed with an AlphaImager HP Imaging System.
Immunohistochemical localization of ER
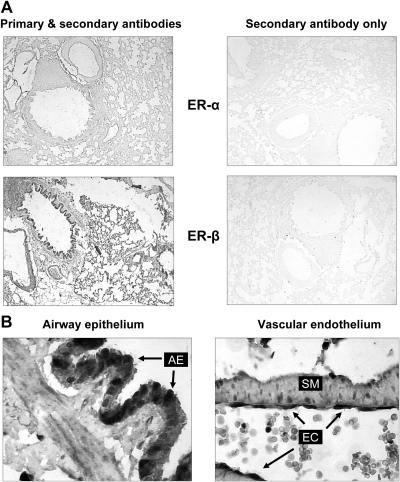
There was marked staining for both ERα and ERβ in the male rat lung. Vascular endothelium and airway epithelium as well as smooth muscle cells in smaller pulmonary arteries showed the most intense staining (Fig. 2, A and B).
Fig. 2.
Immunohistochemical localization of ER in rat lungs. A, low-magnification view (×40) of immunohistochemical staining for ERα and ERβ in male rat lung. Sections were incubated in the presence (left panel) or absence (right panel) of primary antibody to demonstrate specificity of staining. B, Strong immunohistochemical staining of ERα is noted in airway epithelial cells (AE, arrows, left panel), and endothelial cells (EC, arrows, right panel), and smooth muscle cells (SM). Original magnification, ×400.
Estradiol protected against oxidant lung injury by paraquat
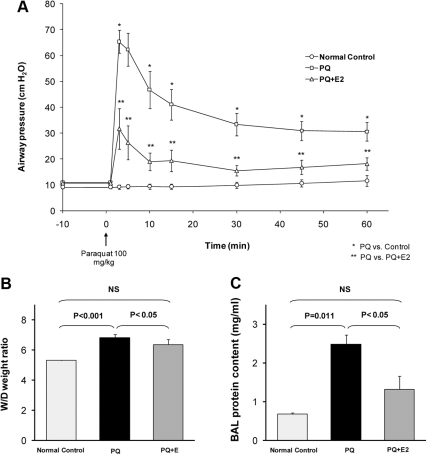
In isolated, perfused guinea pig lungs, the infusion of paraquat into the PA induced marked increases in PAW, PPA, W/D, and protein content in BAL fluid. PAW increased rapidly from a baseline value of 10.9 ± 0.7 cm H2O, reaching a peak of 65.4 ± 4.4 cm H2O at about 2 min, then declining rapidly to 41.1 ± 5.9 cm H2O at 15 min and remaining elevated for the duration of the experiment (n = 14; P < 0.001; Fig. 3A). PPA increased moderately from 6.7 ± 0.6 cm H2O to a peak of 18.3 ± 3.4 cm H2O at 5 min, then gradually declined to 12.7 ± 2.0 cm H2O at 60 min (n = 14; P < 0.01). Mean W/D of paraquat-injured lungs (n = 14) was 6.80 ± 0.24, compared with normal control lungs (n = 9; P < 0.001; Fig. 3B). BAL fluid protein content in paraquat-treated lungs was 2.48 ± 0.64 vs. 0.67 ± 0.18 mg/ml in the control group (P = 0.011; Fig. 3C). Pretreatment with estradiol reduced the peak increase in PAW by more than 50% to 31.7 ± 7.8 cm H2O (P = 0.001). Lung weight gain was also attenuated, with a mean W/D of 5.91 ± 0.14 (P < 0.05), and BAL protein content was reduced to 0.96 ± 0.19 mg/ml (P < 0.05). The decrease in the level of PPA was not statistically significant (Fig. 3, A–C).
Fig. 3.
Induction of oxidative lung injury by paraquat (PQ) and its prevention by estradiol. In the presence of estradiol (E2), the peak increase in PAW was markedly attenuated (A), and there was no increase in the lung weight gain or BAL protein content (B and C). NS, Not significant.
Estradiol attenuated excitotoxic lung injury
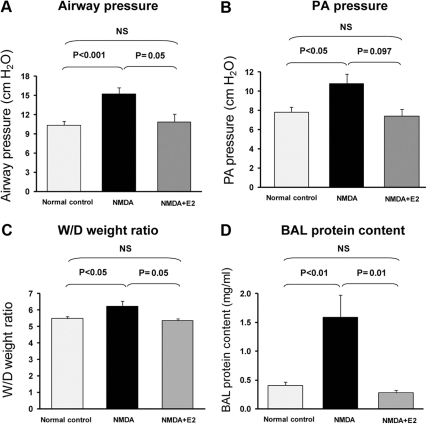
The excitotoxic agonist NMDA (1 mm) induced acute oxidant injury in rat lungs within 60 min. PAW increased to 15.25 ± 0.96 from 8.85 ± 0.23 cm H2O (n = 20; P < 0.001); PPA increased to 10.78 ± 1 from 6.89 ± 0.22 cm H2O (n = 18; P < 0.005); W/D increased to 6.22 ± 0.31 (n = 10) from a control value of 5.47 ± 0.12 (n = 6; P < 0.05); and protein content of BAL fluid increased to 1.59 ± 0.42 mg/ml (n = 10) from a control value of 0.40 ± 0.06 mg/ml (n= 6; P < 0.01; Fig. 4, A–D).
Fig. 4.
Excitotoxic lung injury in rats and its prevention by estradiol. In the presence of estradiol (E2), none of the manifestations of lung injury, i.e. increase in PAW (A), PPA (B), W/D (C), and BAL (D) protein content was observed. Each panel shows values in normal control (left bar), NMDA alone (middle bar), and NMDA plus estrogen (right bar). NS, Not significant.
Addition of estradiol to the perfusate prevented all manifestations of injury. Peak PAW was reduced (10.86 ± 1.22 vs. 15.25 ± 1.22, P < 0.05), and PPA increased from 5.8 ± 0.37 to 7.4 ± 0.68, which was similar to the control untreated group (6.47 ± 0.22 to 7.8 ± 0.53). W/D and BAL protein content remained at normal levels (5.36 ± 0.12 vs. 5.47 ± 0.12 and 0.27 ± 0.05 mg/ml vs. 0.40 ± 0.6 mg/ml, respectively) (Fig. 4, A–D).
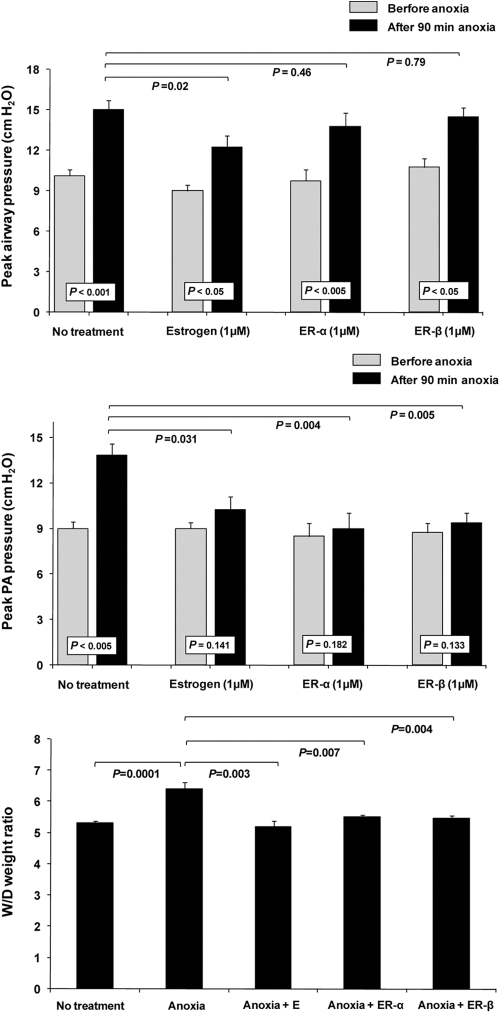
Estradiol effectively prevented acute anoxic lung injury
Within 90 min of exposure to anoxia, PAW increased from 10.1 ± 0.45 to 14.6 ± 0.71 cm H2O (P < 0.001; n = 7), PPA increased from 9.0 ± 0.58 to 13.86 ± 0.93 cm H2O (P < 0.005; n = 7), and W/D weight increased to 6.4 ± 0.2 from a normal value of 5.3 ± 0.1 (P < 0.0001; n = 7). In the presence of estradiol (1 μm), PPA increased to 10.25 ± 0.8 and PAW increased to 12.25 ± 0.8, both less than without estrogen (P < 0.03 and 0.02, respectively; n = 4), and lung weight gain (W/D) remained normal at 5.2 ± 0.2 (n = 4) (Fig. 5, A–C).
Fig. 5.
Anoxic lung injury was moderately attenuated by estradiol or by selective agonists of ERα or -β. Shown are changes induced by anoxia vs. normoxia in PAW, peak PPA, and W/D under four conditions: 1) control, no further treatment; 2) plus 1 μm estrogen; 3) plus 1 μm ERα, and 4) plus 1 μm ERβ. Abbreviations as in Figs. 3 & 4. E, Estrogen.
Attenuation of anoxic injury by ER agonists
In the presence of ERα agonist, WAY-2033306 (n = 4), anoxia caused a slight but insignificant increase in PPA (9.00 ± 0.4 vs. 8.50 ± 0.3); the increase was significantly attenuated (P < 0.05). PAW increased from 9.75 ± 0.9 to 13.75 ± 1.00; i.e. there was no significant change in PAW in the presence of this agonist. Lung weight gain was abolished (P < 0.01). In the presence of ERβ agonist WAY-200070-3 (n = 4), values for PPA, PAW, and W/D were as observed with the ERα agonist (Fig. 5, A–C).
Stimulation of VIP expression by estradiol
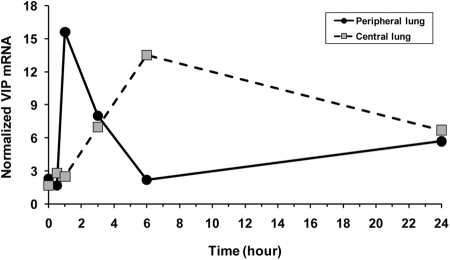
In peripheral lung tissue, which includes the alveolar-microvascular area, the main site of acute injury, VIP mRNA, normalized to β-actin, increased sharply within minutes of the injection of estradiol, decreasing gradually within 6 h but remaining elevated for over 24 h. In major airways, VIP mRNA reached a peak in 6 h and decreased to the same level as in peripheral lung (Fig. 6).
Fig. 6.
Estradiol stimulated the expression of VIP in lung tissue. Estradiol was injected at time zero. In peripheral lung, VIP mRNA, normalized to β-actin, increased sharply within minutes of the injection of estradiol, then stabilized at an elevated but reduced level, decreasing gradually over the next 24 h. In central lung (airways), VIP mRNA increased to a peak in 6 h but remained elevated as in peripheral lung.
Discussion
Cell and tissue protection by estrogens
The protection against lung injury demonstrated in these experiments is one example of cell and tissue protection by estrogens. Additional examples include neuroprotection (1, 13), strengthening of bone density (3), and attenuation of several forms of pulmonary and cardiovascular alterations (7, 13).
Amelioration of lung disorders by estrogens
Numerous reports document the favorable effects of estrogens on the lung under normal conditions, and in a variety of experimental settings. Thus, estrogens have been shown to regulate pulmonary alveolar formation, loss, and regeneration (4, 5). In studies related to ours, other investigators have described attenuation of lung injury induced by trauma-hemorrhage (14–16), endotoxemia (17), and inflammation (18). Estrogens also reduce hypoxic pulmonary vasoconstriction (19) and, at least in some species, protect against the development of pulmonary hypertension (20, 21). In this study, we focused on the potential lung-protective properties of estrogen, specifically its ability to reduce or prevent acute high-permeability lung edema. The results show that estradiol attenuated or prevented this injury in all three experimental models, two of which result from oxidant stress due to the herbicide paraquat or the excitotoxic agonist glutamate, and the third caused by acute anoxia.
The three models expressed similar features of lung injury, including increased PAW at the same tidal volume, indicating increased airway resistance; elevated PPA at the same lung perfusion flow rate, indicating increased pulmonary vascular resistance; and higher lung W/D, with protein leakage in BAL fluid, indicating high-permeability pulmonary edema. Judging by these physiological measurements, the paraquat-induced injury was the most severe and was particularly noted for the pronounced increase in peak PAW, which largely subsided within 30 min. Such a sharp and largely reversible elevation in airway resistance strongly suggests a significant degree of airway constriction, possibly combined with the presence of pulmonary edema foam in the airways.
How did estradiol attenuate lung injury in these models? There were probably several mechanisms. First, there had to be a decrease in pulmonary microvascular permeability, reducing the tendency to alveolar fluid leakage and pulmonary edema. In addition, there was probably a contribution from relaxation of pulmonary vascular and airway smooth muscle cells, as strongly suggested by the quick reversal of the initial sharp peak of PAW with estradiol therapy (Fig. 3) and by observations in related studies demonstrating the ability of estrogens to relax airway and pulmonary vascular smooth cells (7, 22). Estrogen has even been reported to decrease acetylcholine-elicited airway reactivity in ovariectomized rats (23) and to reduce asthma severity (24).
ER localization in the lung
Although one group of investigators (6) reported their inability to demonstrate ERα in mouse lungs, two other groups have confirmed our demonstration of the presence of both α- and β-receptors. One report (25) described the expression of both receptor subtypes in human lung tissue and tumor cell lines, whereas the other concluded that ERα, but not ERβ, mediates estrogen-induced reduction of the inflammatory response in mouse lungs and may be a drug target to guard against lung inflammation (11).
Molecular mechanisms and pathways of protection by estrogens
Several lines of evidence converge to suggest that the protective effects of estradiol demonstrated here were mediated by its nongenomic mechanism, via both ERα and ERβ receptors. 1) Both receptors were expressed at the mRNA level, and their presence was confirmed at the protein level by immunohistochemical localization (Figs. 1 and 2), as supported by other investigators (26, 27). 2) In the only injury model in which they were tested, anoxic ventilation, selective ERα and ERβ agonists were as effective as estrogen in attenuating the injury, although they were less effective in reducing the elevated airway pressure. 3) The rapidity with which estradiol exerted its protection was characteristic of its nongenomic nature (13). The relative mediator roles of ERα and ERβ have been evaluated in different experimental models by the use of selective receptor antagonists or the study of mice with targeted deletion of either receptor gene (8, 26, 28). Our use of either of these approaches in this study would have provided useful confirmation.
Investigations of the mechanisms by which estradiol exerts its salutary effects have revealed a number of possible mechanisms. In this investigation, we concentrated on one particular possibility, namely the role of overexpression of VIP. As noted in Results, and illustrated in Fig. 6, estradiol rapidly and markedly up-regulated VIP gene expression in the lung. Other investigators have reported similar up-regulation, although in other tissues (29, 30). Because VIP has multiple, well-recognized, anti-injury, including antioxidant, properties (9, 10, 12), its accentuated expression in the peripheral (i.e. alveolar and microvascular structures) and central lung (i.e. airways) was arguably a significant factor in inhibiting both the high-permeability edema and the airway constriction.
The dose of estradiol used to elicit the up-regulation of VIP was large enough to be considered in the pharmacological range. Nevertheless, the VIP response serves to highlight an important potential mechanism by which the peptide with established protective property may come to the aid of estrogens in responding to inflammation and injury.
Additional mechanisms known to contribute to protection by estrogens in general probably participated in these experiments. These include 1) activation of endothelial NO synthase via the phosphatidylinositol 3-kinase-Akt pathway in endothelial cells (31–33); 2) nongenomic stimulation of adenylate cyclase and cAMP-regulated gene transcription (34), an action that could be mediated, at least in part, by augmented VIP production; 3) an antiinflammatory effect resulting from inhibition of nuclear factor-κB by ER (35–37); and 4) attenuation of the inflammatory, vasoconstrictor, and injury-promoting effects of endothelin-1 (38, 39).
Conclusions
Estradiol effectively protected guinea pig and rat lungs against acute high-permeability edema caused by paraquat, by excitotoxic oxidant stress, or by alveolar anoxia. Selective ERα and ERβ agonists were as effective as estradiol in attenuating the acute injury, but were less effective in reducing the increase in airway pressure. Several pathways probably mediated this protection, including estradiol stimulation of the expression of VIP, which has proven protective properties against lung and other vital organ injury.
Acknowledgments
This work was supported by National Institutes of Health Grant HL-68188 (to S.I.S.) and the Department of Veterans Affairs.
Disclosure Summary: None of the coauthors declares potential conflict of interest.
Footnotes
- BAL
- Bronchoalveolar lavage
- ER
- estrogen receptor
- NMDA
- N-methyl-d-aspartate
- PA
- pulmonary artery
- PAW
- airway pressure
- PPA
- pulmonary arterial pressure
- VIP
- vasoactive intestinal polypeptide
- W/D
- wet to dry lung weight ratio.
References
- 1. Deroo BJ, Korach KS. 2006. Estrogen receptors and human disease. J Clin Invest 116:561–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. 2007. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids 72:381–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Krum SA, Miranda-Carboni GA, Hauschka PV, Carroll JS, Lane TF, Freedman LP, Brown M. 2008. Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. EMBO J 27:535–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Massaro D, Massaro GD. 2004. Estrogen regulates pulmonary alveolar formation, loss, and regeneration in mice. Am J Physiol Lung Cell Mol Physiol 287:L1154–L1159 [DOI] [PubMed] [Google Scholar]
- 5. Massaro D, Massaro GD. 2006. Estrogen receptor regulation of pulmonary alveolar dimensions: alveolar sexual dimorphism in mice. Am J Physiol Lung Cell Mol Physiol 290:L866–L870 [DOI] [PubMed] [Google Scholar]
- 6. Patrone C, Cassel TN, Pettersson K, Piao YS, Cheng G, Ciana P, Maggi A, Warner M, Gustafsson JA, Nord M. 2003. Regulation of postnatal lung development and homeostasis by estrogen receptor β. Mol Cell Biol 23:8542–8552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carey MA, Card JW, Voltz JW, Germolec DR, Korach KS, Zeldin DC. 2007. The impact of sex and sex hormones on lung physiology and disease: lessons from animal studies. Am J Physiol Lung Cell Mol Physiol 293:L272–L278 [DOI] [PubMed] [Google Scholar]
- 8. Pare G, Krust A, Karas RH, Dupont S, Aronovitz M, Chambon P, Mendelsohn ME. 2002. Estrogen receptor-α mediates the protective effects of estrogen against vascular injury. Circ Res 90:1087–1092 [DOI] [PubMed] [Google Scholar]
- 9. Pakbaz H, Foda HD, Berisha HI, Trotz M, Said SI. 1993. Paraquat-induced lung injury: prevention by vasoactive intestinal peptide and related peptide helodermin. Am J Physiol 265:L369–L373 [DOI] [PubMed] [Google Scholar]
- 10. Said SI. 2000. The Viktor Mutt Memorial Lecture. Protection by VIP and related peptides against cell death and tissue injury. Ann NY Acad Sci 921:264–274 [DOI] [PubMed] [Google Scholar]
- 11. Vegeto E, Cuzzocrea S, Crisafulli C, Mazzon E, Sala A, Krust A, Maggi A. 2010. Estrogen receptor-α as a drug target candidate for preventing lung inflammation. Endocrinology 151:174–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Said SI, Berisha HI, Pakbaz H. 1996. Excitotoxicity in the lung: N-methyl-d-aspartate-induced, nitric oxide-dependent, pulmonary edema is attenuated by vasoactive intestinal peptide and by inhibitors of poly(ADP-ribose) polymerase. Proc Natl Acad Sci USA 93:4688–4692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hall JM, Couse JF, Korach KS. 2001. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem 276:36869–36872 [DOI] [PubMed] [Google Scholar]
- 14. Yu HP, Hsieh YC, Suzuki T, Shimizu T, Choudhry MA, Schwacha MG, Chaudry IH. 2006. Salutary effects of estrogen receptor-β agonist on lung injury after trauma-hemorrhage. Am J Physiol Lung Cell Mol Physiol 290:L1004–L1009 [DOI] [PubMed] [Google Scholar]
- 15. Kan WH, Hsu JT, Schwacha MG, Choudhry MA, Bland KI, Chaudry IH. 2008. Estrogen ameliorates trauma-hemorrhage-induced lung injury via endothelial nitric oxide synthase-dependent activation of protein kinase G. Ann Surg 248:294–302 [DOI] [PubMed] [Google Scholar]
- 16. Doucet D, Badami C, Palange D, Bonitz RP, Lu Q, Xu DZ, Kannan KB, Colorado I, Feinman R, Deitch EA. 2010. Estrogen receptor hormone agonists limit trauma hemorrhage shock-induced gut and lung injury in rats. PLoS One 5:e9421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nolan JP. 1967. Protective action of oestrogen against the lethal effect of endotoxin in the rat. Nature 213:201–202 [DOI] [PubMed] [Google Scholar]
- 18. Cuzzocrea S, Bruscoli S, Crisafulli C, Mazzon E, Agostini M, Muià C, Esposito E, Di Virgilio R, Meli R, Vegeto E, Maggi A, Riccardi C. 2007. Estrogen receptor antagonist fulvestrant (ICI 182,780) inhibits the anti-inflammatory effect of glucocorticoids. Mol Pharmacol 71:132–144 [DOI] [PubMed] [Google Scholar]
- 19. Lahm T, Crisostomo PR, Markel TA, Wang M, Wang Y, Weil B, Meldrum DR. 2008. Exogenous estrogen rapidly attenuates pulmonary artery vasoreactivity and acute hypoxic pulmonary vasoconstriction. Shock 30:660–667 [DOI] [PubMed] [Google Scholar]
- 20. Hansmann G, Wagner RA, Schellong S, Perez VA, Urashima T, Wang L, Sheikh AY, Suen RS, Stewart DJ, Rabinovitch M. 2007. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator-activated receptor-γ activation. Circulation 115:1275–1284 [DOI] [PubMed] [Google Scholar]
- 21. Said SI, Hamidi SA, Dickman KG, Szema AM, Lyubsky S, Lin RZ, Jiang YP, Chen JJ, Waschek JA, Kort S. 2007. Moderate pulmonary arterial hypertension in male mice lacking the vasoactive intestinal peptide gene. Circulation 115:1260–1268 [DOI] [PubMed] [Google Scholar]
- 22. Freay AD, Curtis SW, Korach KS, Rubanyi GM. 1997. Mechanism of vascular smooth muscle relaxation by estrogen in depolarized rat and mouse aorta. Role of nuclear estrogen receptor and Ca2+ uptake. Circ Res 81:242–248 [DOI] [PubMed] [Google Scholar]
- 23. Degano B, Prévost MC, Berger P, Molimard M, Pontier S, Rami J, Escamilla R. 2001. Estradiol decreases the acetylcholine-elicited airway reactivity in ovariectomized rats through an increase in epithelial acetylcholinesterase activity. Am J Respir Crit Care Med 164:1849–1854 [DOI] [PubMed] [Google Scholar]
- 24. Haggerty CL, Ness RB, Kelsey S, Waterer GW. 2003. The impact of estrogen and progesterone on asthma. Ann Allergy Asthma Immunol 90:284–291; quiz 291–293, 347 [DOI] [PubMed] [Google Scholar]
- 25. Mollerup S, Jørgensen K, Berge G, Haugen A. 2002. Expression of estrogen receptors α and β in human lung tissue and cell lines. Lung Cancer 37:153–159 [DOI] [PubMed] [Google Scholar]
- 26. Harris HA, Katzenellenbogen JA, Katzenellenbogen BS. 2002. Characterization of the biological roles of the estrogen receptors, ERα and ERβ, in estrogen target tissues in vivo through the use of an ERα-selective ligand. Endocrinology 143:4172–4177 [DOI] [PubMed] [Google Scholar]
- 27. Saunders PT, Maguire SM, Gaughan J, Millar MR. 1997. Expression of oestrogen receptor β (ER β) in multiple rat tissues visualised by immunohistochemistry. J Endocrinol 154:R13–R16 [DOI] [PubMed] [Google Scholar]
- 28. Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. 1998. Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci USA 95:15677–15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gozes I, Werner H, Fawzi M, Abdelatty A, Shani Y, Fridkin M, Koch Y. 1989. Estrogen regulation of vasoactive intestinal peptide mRNA in rat hypothalamus. J Mol Neurosci 1:55–61 [DOI] [PubMed] [Google Scholar]
- 30. Kasper S, Popescu RA, Torsello A, Vrontakis ME, Ikejiani C, Friesen HG. 1992. Tissue-specific regulation of vasoactive intestinal peptide messenger ribonucleic acid levels by estrogen in the rat. Endocrinology 130:1796–1801 [DOI] [PubMed] [Google Scholar]
- 31. Haynes MP, Sinha D, Russell KS, Collinge M, Fulton D, Morales-Ruiz M, Sessa WC, Bender JR. 2000. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ Res 87:677–682 [DOI] [PubMed] [Google Scholar]
- 32. Hisamoto K, Ohmichi M, Kurachi H, Hayakawa J, Kanda Y, Nishio Y, Adachi K, Tasaka K, Miyoshi E, Fujiwara N, Taniguchi N, Murata Y. 2001. Estrogen induces the Akt-dependent activation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem 276:3459–3467 [DOI] [PubMed] [Google Scholar]
- 33. Sugden PH, Clerk A. 2001. Akt like a woman: gender differences in susceptibility to cardiovascular disease. Circ Res 88:975–977 [DOI] [PubMed] [Google Scholar]
- 34. Aronica SM, Kraus WL, Katzenellenbogen BS. 1994. Estrogen action via the cAMP signaling pathway: stimulation of adenylate cyclase and cAMP-regulated gene transcription. Proc Natl Acad Sci USA 91:8517–8521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kalaitzidis D, Gilmore TD. 2005. Transcription factor cross-talk: the estrogen receptor and NF-κB. Trends Endocrinol Metab 16:46–52 [DOI] [PubMed] [Google Scholar]
- 36. McKay LI, Cidlowski JA. 1999. Molecular control of immune/inflammatory responses: interactions between nuclear factor-κB and steroid receptor-signaling pathways. Endocrine Reviews 20:435–459 [DOI] [PubMed] [Google Scholar]
- 37. Vegeto E, Belcredito S, Etteri S, Ghisletti S, Brusadelli A, Meda C, Krust A, Dupont S, Ciana P, Chambon P, Maggi A. 2003. Estrogen receptor-α mediates the brain antiinflammatory activity of estradiol. Proc Natl Acad Sci USA 100:9614–9619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Earley S, Resta TC. 2002. Estradiol attenuates hypoxia-induced pulmonary endothelin-1 gene expression. Am J Physiol Lung Cell Mol Physiol 283:L86–L93 [DOI] [PubMed] [Google Scholar]
- 39. Tan Z, Wang TH, Yang D, Fu XD, Pan JY. 2003. Mechanisms of 17β-estradiol on the production of ET-1 in ovariectomized rats. Life Sci 73:2665–2674 [DOI] [PubMed] [Google Scholar]