Abstract
Orexins/hypocretins heavily innervate the posterior division of the paraventricular nucleus of the thalamus (pPVT), which expresses both orexin receptor types. The pPVT is important for adaptations to repeated stress, particularly the ability to facilitate to novel stress after repeated stress exposure. Here, we examined how orexins acting in the pPVT regulate facilitation of hypothalamic-pituitary-adrenal (HPA) responses to novel restraint after 4 d of repeated swim stress. Blockade of orexin receptors in the pPVT with SB334867 before novel restraint did not change the facilitated HPA response. However, blockade of orexin receptors before each of four daily swim exposures prevented the facilitated ACTH and facilitated hypothalamic c-Fos response to restraint as well as the repeated swim stress-induced increase in CRH mRNA in the paraventricular hypothalamus. These results suggest that orexin actions in the pPVT during the 4 d of swim, but not during restraint, are necessary for the facilitated HPA response to heterotypic restraint. Exposure to the fourth swim produced a shift in orexin1 receptors from membrane to cytosolic fractions. OrexinA also changed the firing patterns of pPVT cells to be more responsive in repeatedly swim stressed rats compared with nonstressed rats. Together, the results suggest that orexin actions in the pPVT, mediated by orexin1 receptors, are important for the ability to adapt to repeated stress.
Orexins (also called hypocretins) are synthesized from the precursor molecule preproorexin that is exclusively localized in cells of the lateral and posterior hypothalamus (1, 2). Preproorexin is cleaved into two highly structurally related and highly conserved peptides, orexinA and orexinB (1, 2), which bind to two G protein-coupled receptors, orexin1 receptor (orexin1R) and orexin2 receptor (orexin2R). Stimulation of orexin receptors promotes arousal or a heightened responsiveness to sensory inputs (3) and increased wakefulness (4). An obvious extension of the role of orexins in initiating arousal is in mediating defensive responses to stressful and aversive stimuli, which requires a transition from a basal state to an aroused state. Intraventricular or systemic administration of orexins increases anxiety-related behavior (5, 6), depolarizes cells, and increases CRH and arginine vasopressin (AVP) mRNA in the paraventricular hypothalamus (PVN), the hypothalamic arm of the hypothalamic-pituitary-adrenal (HPA) axis (7, 8), and increases plasma ACTH and corticosterone (8–10). However, little is known about specific sites at which orexins act to regulate responses to either acute or repeated stress.
The paraventricular nucleus of the thalamus (PVT) is an interface between sensory inputs and limbic and cortical structures of the extended amygdala (11–16). Our previous studies indicated that the posterior division of the PVT (pPVT) is important for neuroendocrine and behavioral adaptations to repeated stress (17, 18). The PVT contains moderate to high densities of both orexin receptors (19–22) and receives among the densest orexin innnervation in the brain in rodents and primates (12, 23–26). There is little orexin innervation of adjacent thalamic nuclei or the habenulae (12, 27). Within the PVT, the posterior division receives the densest inputs (12). Orexins depolarize neurons of the PVT (28, 29) and increase anxiety-related behavior (30). However, whether orexin inputs to the pPVT are important for adaptation to stress is not known. Animals can exhibit two adaptations to repeated stress: habituation to familiar, homotypic stressors and facilitation to novel, heterotypic stressors (17, 31, 32). We have previously shown that there are two phases to habituation. During the development phase (the first phase), the effects of repeated stress become established allowing habituation to be expressed (the second phase) in response to the homotypic stressor. Our previous work suggests that different neural substrates mediate development vs. expression phases (18, 33). In the studies presented here, we examined facilitation of HPA activity to novel restraint after repeated swim stress and asked whether orexin inputs to the pPVT play a role in the development (during repeated swim) and/or expression (during restraint) of facilitation.
Materials and Methods
Animals
Male Sprague Dawley rats (Charles River, Kingston, NY) weighing between 225 and 250 g were given ad libitum access to food and water and individually housed in plastic tub cages on a 12-h light, 12-h dark cycle with lights on at 0600 h. A 5- to 7-d acclimation period was allowed before start of experimentation or surgery. All stress procedures and experimentation occurred before 1200 h. Naïve cohorts of animals were used for each experiment/subexperiment. All procedures were approved by the Institutional Animal Care and Use Committee at the Children's Hospital of Philadelphia Research Institute.
Experimental Design
Experiment 1. Effect of acute administration of orexinA in the pPVT
Experiment 1a. HPA response to acute restraint.
Rats were administered 0, 0.1, or 0.5 ng of orexinA (Tocris, Ellisville, MO) in 250 nl of dimethylsulfoxide (DMSO) directly into the pPVT 30 min before a 30-min restraint in Plexiglas cylinders. Doses in the nanogram range were chosen based on previous literature (34). Histological verification of cannulae placements was conducted (see below) and also confirmed no overt alterations in cellular morphology or gliosis produced by DMSO. Blood samples were taken for ACTH and corticosterone, as described in Ref. 35, via tail snip at 0, 15, and 30 min of restraint. Rats were returned to their home cage, and 30 min later, at the 60-min time point, rats were decapitated and trunk blood collected. Plasma ACTH and corticosterone were measured using kits from MP Biomedicals (Orangeburg, NY). The minimum levels of detection for ACTH and corticosterone were 5.7 pg/ml and 0.6 μg/dl, respectively. Intra- and interassay variability was less than 10%.
Experiment 1b. Behavior in the elevated plus maze (EPM).
Rats were injected with vehicle or 0.1 ng of orexinA (250 nl) directly into the pPVT 30 min before EPM exposure for 10 min. Behavior was recorded and analyzed using EthovisionPro 3.1, and time in open and closed arms was expressed as percent of total time (36). EPM testing for experiments 1 and 2 were performed in different facilities, which led to differences in baseline open arm times. Therefore, data were converted to percent of vehicle-injected control.
Experiment 2. Effect of acute administration of the orexin receptor antagonist SB334867
Experiment 2a. HPA response to acute restraint.
On test day, rats were injected with 0 or 0.1 μg of SB334867 (Tocris) in 250 nl of DMSO into the pPVT 30 min before restraint. SB334867 is a nonpeptidic antagonist with 50-fold higher selectivity for the orexin1R over orexin2R (37, 38). Effective doses are in the microgram range for intracerebral injections (39, 40). Blood was sampled as in experiment 1a.
Experiment 2b. Behavior in the EPM.
Rats were injected with vehicle or 0.1 μg in SB334867 (250 nl) into the pPVT 30 min before 10 min of EPM exposure. Testing was performed as in experiment 1b, except it was conducted in a different facility.
Experiment 3. Effects of orexinA and/or SB334867 on pPVT orexin receptor cellular distribution and on pERK
Rats were injected with vehicle (DMSO), 0.1 μg of SB334867, 0.1 ng of orexinA, or a combination of SB334867 (0.1 μg) and orexinA (0.1 ng) in 250 nl into the pPVT 30 min before killing. In a separate cohort (Western blot analyses run separately), animals were injected with 0.5 ng of orexinA or 0.5 ng of orexinA in combination with 0.1 μg of SB334867. Circular 1-mm PVT punches were collected for Western blot analysis of orexin receptor cellular distribution and of pERK, described below.
Experiment 4. Effects of orexinB on pPVT orexin receptor cellular distribution and on pERK
Rats were injected, as in experiment 3, with vehicle (DMSO), orexinB at 0.3 μg, or orexinB at 3.0 μg in 250 nl into the pPVT 30 min before killing. pPVT punches were collected (as in experiment 3) for Western blot analysis of orexin receptor cellular distribution and pERK, described below.
Experiment 5. Effect of SB334867 administration before novel restraint in repeatedly swim-stressed rats
Rats were exposed to no stress (handled daily) or 4 d of daily 15-min swim (in a glass chromatography jar filled two-thirds with 25 C water). On d 5, all rats received an injection (250 nl) of vehicle or SB334867 (0.1 μg) directly into the pPVT at 30 min before a 30-min restraint, and blood was sampled as in experiment 1a. Brains were collected at 60 min after onset of restraint for immunocytochemistry for c-Fos in the parvocellular PVN (pPVN).
Experiment 6. Effect of SB334867 before daily repeated swim stress on the facilitatory response to novel restraint
Rats were injected (250 nl) with vehicle, 0.1, or 1.0 μg of SB334867 directly into the pPVT at 30 min before a daily 15-min swim for 4 d. Control rats were injected with vehicle or SB334867 at the same time of day as stressed rats. On d 5, all rats were exposed to a 30-min restraint without any drug injection. Blood samples were collected as in experiment 1a. Brains were collected for immunocytochemistry for c-Fos in the pPVN and in situ hybridization for CRH and AVP in the pPVN.
Experiment 7. Effects of orexinA on membrane properties of pPVT neurons
One group of nonstressed rats and one group of rats previously exposed to 4 d of repeated swim were decapitated under basal conditions on d 5. No drugs were administered. Visualized whole-cell patch clamp recording techniques of pPVT cells were used to record active and passive membrane properties (as described in Refs. 38, 39) and the response to orexin (100 nm). At the end of data collection, the cells were visualized using immunocytochemistry for biocytin to confirm localization within the pPVT and for morphological analysis (see Supplemental data, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org) using confocal xyz stacks and the Neurolucida software program (Microbrightfield, Williston, VT).
Experiment 8. Effects of acute vs. repeated swim on pPVT orexin receptor cellular distribution and pERK
Rats were exposed to either one 15-min swim stress or daily 15-min swim stress for 4 d. Rats were then killed 30 min after the end of the first or fourth swim. pPVT punches were taken for Western blot analysis of orexin receptors and pERK. No drugs were administered.
Experiment 9. Effects of repeated swim on pPVT orexin receptor cellular distribution and on pERK after restraint
Rats were exposed to no stress or repeated daily 15-min swim stress for 4 d and then killed on d 5 under basal conditions. Other rats were exposed to no stress or repeated 15-min swim stress for 4 d and then restrained for 30 min on d 5 and killed 30 min later. pPVT punches were collected for Western blot analysis of orexin receptors and pERK. No drugs were administered.
Stereotaxic surgery and confirmation of cannulae placement in the pPVT
Unilateral guide cannulae (22 gauge) were implanted in the pPVT to target the posterior division: anterior-posterior, −2.8; medial-lateral, 0.0 mm; dorsal-ventral, −6.4 mm. Drug was administered by inserting a 7-mm injector cannula connected to polyethylene tubing and injecting a volume of 250 nl. In all experiments, except those requiring collection of pPVT tissue punches, cannulae placement was confirmed. The pPVT was defined as extending from −2.8 to −3.3 mm from bregma (31). Using previously described criteria (31, 41), the success rate was 75% (see figure 5 below).
In situ hybridization
Brains were collected, flash frozen, and sectioned on a cryostat at 14 μm. Antisense and sense RNA probes for CRH and AVP were generated from cut cDNA (generously donated by Drs. Bob Thompson and Audrey Seasholtz; University of Michigan, Ann Arbor, MI). Additional details are in Ref. 42. Data for each animal represents an average of two to four sections through the pPVN.
c-Fos immunocytochemistry
Slides were incubated with primary c-Fos antibody (sc-52; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at a 1:5000 dilution (as in Ref. 43). Quantification of c-Fos positive cells was done by trained observers blind to the treatment groups using ImageJ software (http://rsbweb.nih.gov/ij/). Two to four sections per brain region were analyzed and averaged per animal.
Western blot analysis
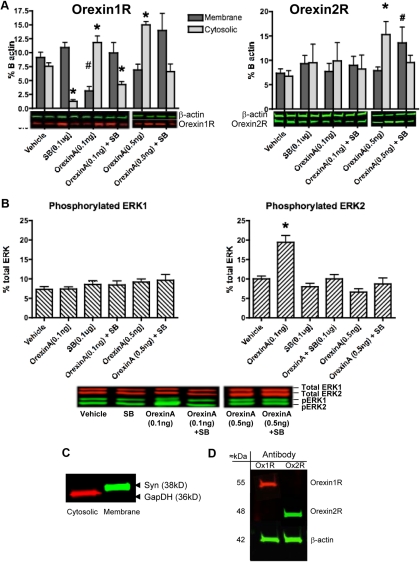
Circular 1-mm punches of pPVT were flash frozen on dry ice. An average of 20 μg of protein was recovered from pPVT samples. Orexin1R and orexin2R protein was quantified in cytosolic and membrane fractions of pPVT punch homogenates separated through centrifugation in a sucrose gradient. Previous studies have used similar methods for quantifying the distribution of cytosolic and membrane proteins (44, 45). Orexin1R was visualized using sc-8073 (Santa Cruz Biotechnology, Inc.) at a concentration of 1:500. Orexin2R was visualized using ab43445 (Abcam, Cambridge, MA) at 1:1000. Orexin receptor antibody specificity was tested by comparison of two separate blots that had been treated with the individual antibodies (see figure 2 below). Effectiveness of the sucrose gradient to enrich membrane and cytosolic fractions was validated via Western blot analysis of the membrane-bound protein synaptophysin, which was only observed in the membrane fraction and the cytosolic protein glyceraldehyde-3-phosphate dehydrogenase, which was only observed in the cytosolic fraction (see figure 2 below). Blots were visualized on a LI-COR Odyssey infrared imaging system (LI-COR, Lincoln, NE). All groups were evenly represented on each gel to compensate for intergel variations. Orexin1R yielded a band at 55 kDa and the orexin2R yielded a band at 48 kDa, which is consistent with the range seen in previously published work (46). Receptors were quantified as a percent of total β-actin (A2228; Sigma, St. Louis, MO) in their respective fractions. Phosphorylated ERK was quantified in the cytosolic fractions. Incubation with primary antibodies for pERK and total ERK (Santa Cruz Biotechnology, Inc.) yielded two bands at 44 and 42 kDa for ERK1 and ERK2, respectively, and quantified as a percent of total ERK.
Statistical analyses
For analysis in experiments 1 and 2, one-way ANOVA were performed to test the effect of drug dose. Cytosolic and membrane fractions were analyzed separately in experiment 3, 4, 8, and 9. For analysis of hormone and immunocytochemistry data in experiment 5, stress (control vs. repeated swim) × drug (vehicle vs. 0.1 μg of SB334867) ANOVA were tested at each time point. In experiment 6, two-way ANOVA were performed [stress (control vs. repeated swim) × drug (vehicle vs. 0.1 vs. 1.0 μg of SB334867)] at each time point. In experiment 7, data were analyzed by stress × drug ANOVA before and after orexinA application for cell characteristics; χ2 analysis was used to test overall differences in the proportion of cells exhibiting the three types of firing patterns between stress groups before and after orexin application. The Stuart-Maxwell test of marginal homogeneity assessed specific differences in the proportion of cells that changed their firing patterns after orexinA. The P value was set at P < 0.05, and Fisher's post hoc tests were performed in the event of significant main or interaction effects.
Results
Experiment 1
Experiment 1a. OrexinA in the pPVT increases the HPA response to acute restraint
Administration of the 0.1 ng dose, but not the 0.5 ng dose, of orexinA into the pPVT significantly increased the ACTH response to acute restraint compared with vehicle-treated rats at 0 (F2, 22 = 5.110, P = 0.015), 15 (F2,22 = 4.949, P = 0.017), and 30 min (F2,22 = 7.838, P = 0.0027) (Fig. 1A) and produced increases in corticosterone at 60 min (F2,22 = 9.553, P = 0.001). When injected into the lateral ventricle, these doses of orexinA were without effect on HPA activity, indicating that the effects of orexinA in the pPVT were not due to its leakage into the ventricles (data not shown). Because effects of orexinA on ACTH secretion were only seen at the 0.1 ng dose, subsequent experiments were conducted using that dose.
Fig. 1.
A, Plasma ACTH (left panel) and plasma corticosterone (right panel) responses to a 30-min restraint in rats that were given microinjections of vehicle (n = 7) or 0.1 (n = 4) or 0.5 ng (n = 14) of orexinA directly into the pPVT 30 min before restraint. B, Percent of total time spent in the open arms or closed arms and total distance traveled in a 10-min EPM test in rats given microinjections of vehicle (n = 4) or 0.1 ng orexinA (n = 7) into the pPVT 30 min before testing. C, Plasma ACTH (left panel) and plasma corticosterone (right panel) responses to a 30-min restraint in rats given microinjections of vehicle (n = 10) or 0.1 μg of the orexin receptor antagonist SB334867 (n = 11) directly into the pPVT 30 min before restraint. D, Percent of total time spent in the open arms or closed arms and total distance traveled in a 10-min EPM test in rats given vehicle (n = 9) or 0.1 μg of the orexin receptor antagonist SB334867 (n = 13) administration into the pPVT 30 min before testing. *, P < 0.05 vs. vehicle.
Experiment 1b. OrexinA in the pPVT increases anxiety-related behavior
Compared with vehicle, orexinA (0.1 ng) decreased percent of total time spent in the open arms (F1,9 = 30.94, P = 0.0004) (Fig. 1B), increased percent of total time spent in the closed arms (F1,9 = 15.159, P = 0.0037) with no effect on total distance traveled.
Experiment 2
Experiment 2a. SB334867 in the pPVT has no effect on the HPA response to acute restraint
Orexin receptor blockade in the pPVT with SB334867 did not significantly alter either ACTH or corticosterone responses to acute restraint compared with vehicle (Fig. 1C).
Experiment 2b. SB334867 decreases anxiety-related behavior
When compared with vehicle-treated animals, there was a trend for SB334867 to increase the percent of total time spent in the open arms of the plus maze (P = 0.094) (Fig. 1D) and a significant main effect of SB334867 to decrease time spent in the closed arms (F2,20 = 4.899, P = 0.039). There was no effect on total distance traveled.
Experiment 3. OrexinA and/or SB334867 into the pPVT alter orexin receptor cellular distribution and ERK phosphorylation
There was a main effect of drug treatment on orexin1R protein in both the cytosolic (F5,44 = 37.187, P < 0.0001) and membrane (F5,44 = 5.06, P = 0.006) fractions (Fig. 2A, left panel). Post hoc tests indicated that, compared with vehicle, orexinA increased cytosolic expression and decreased membrane expression of orexin1R at both 0.1 and 0.5 ng doses. SB334867 alone decreased the cytosolic expression of the orexin1R and also prevented orexinA-induced increase in orexin1R in the cytosolic fraction. Neither orexinA at 0.1 ng nor SB334867 had any effect on orexin2R cytosolic or membrane expression when compared with vehicle (Fig. 2A, right panel). However, 0.5 ng of orexinA increased the cytosolic fraction of orexin2R compared with vehicle, which was blocked by SB334867.
Fig. 2.
A, Orexin1R distribution (left panel) and orexin2R distribution (right panel) in cytosolic and membrane fractions of pPVT punches taken from rats given microinjections of vehicle (n = 11), 0.1 ng of orexinA (n = 10), 0.1 μg of SB334867 (n = 9), a combination of 0.1 ng of orexinA and 0.1 μg of SB334867 (n = 8), 0.5 ng of orexinA (n = 5), or a combination of 0.5 ng of orexinA and 0.1 μg of SB334867 (n = 7) 30 min before killing. The inset is a composite of different representative Western blots; representative blots for the higher dose of orexinA are shown separately, because they were run at a different time. *, P < 0.05 vs. vehicle in cytosolic fraction; #, P < 0.05 vs. vehicle in membrane fraction. B, Phosphorylated ERK1 (left panel) and ERK2 (right panel) in the cytosolic fraction of pPVT punches taken from rats in A. *, P < 0.05 vs. all other groups. C, A sucrose gradient was used to enrich membrane and cytosolic fractions. A Western blot analysis shows localization of the membrane protein synaptophysin (Syn) uniquely in the membrane fraction of pPVT punches and the localization of the cytoplasmic protein glyceraldehyde-3-phosphate dehydrogenase (GapDH) uniquely in the cytosolic fraction. This provides validation for the ability to detect shifts in orexin receptors from membrane to cytosolic fractions after drugs (in A) and after stress (Figs. 7 and 8). D, Composite figure of two different Western blot demonstrating specificity of the orexin1R antibody (left lane) and the orexin2R antibody (right lane) and their approximate molecular masses in kilodaltons.
There was no effect of drug treatment on ERK1 phosphorylation (Fig. 2B, left panel). However, there was a main effect of drug on ERK2 (F3,32 = 19.080, P < 0.0001) (Fig. 2B, left panel). Post hoc tests indicated that, when compared with vehicle, the 0.1 ng dose but not the 0.5 ng dose, of orexinA increased the density of phosphorylated ERK2 (P < 0.0001) (Fig. 2B, right). This effect was blocked by SB334867, which had no effects on its own.
Experiment 4. OrexinB injected into the pPVT alters orexin1R and orexin2R cellular distribution but has no effect on ERK phosphorylation
Results are expressed as percent of β-actin. There was a significant effect of orexinB on density of orexin1R in the membrane fraction (F2,21 = 4.440, P < 0.0455), with orexinB decreasing the orexin1R in membrane fractions (vehicle, 9.126 ± 0.92, n = 8; 0.3 μg of orexinB, 4.707 ± 0.775, n = 8; 3.0 μg of orexinB, 2.746 ± 0.464, n = 8). There was no effect on the cytosolic fraction of orexin1R. OrexinB also significantly increased density of orexin2R in the cytosolic fraction of pPVT punches (F2,21 = 4.835, P < 0.0375; vehicle, 6.944 ± 0.619, n = 8; 0.3 μg of orexinB, 11.050 ± 1.479, n = 8; 3.0 μg orexinB, 10.857 ± 1.860, n = 8). There were no effects on pERK1/2 (data not shown).
Experiment 5. SB334867 before novel restraint has no effect on the facilitatory response to restraint
There was a significant main effect of stress at the 15-min time point (F1,14 = 12.717, P < 0.0031), with ACTH responses to restraint being higher in swim-stressed rats compared with nonstressed rats regardless of drug (Fig. 3A, left panel). There was no other significant effect on ACTH or on corticosterone (Fig. 3A, right panel).
Fig. 3.
A, Plasma ACTH (left panel) and plasma corticosterone (right panel) responses to a 30-min novel restraint in rats administered vehicle or the orexin receptor antagonist SB334867 (0.1 μg) into the pPVT half an hour before restraint on d 5. Rats were either nonstressed or exposed to 4 d of 15-min swim per day before restraint on d 5. Group sizes are nonstressed: vehicle, n = 4–5; nonstressed SB334867, n = 4–6; vehicle swim stressed, n = 5; SB334867 swim stressed, n = 5–6, depending on time point. Rats were killed at 60 min after the beginning of restraint and brains collected for immunocytochemistry. *, P < 0.05; repeatedly swim-stressed rats significantly different from nonstressed controls regardless of drug treatment. B, Immunocytochemical staining of c-Fos in the pPVN in brains of rats in A. Data are expressed as number of c-Fos-stained cells. Group sizes are: nonstressed vehicle, n = 7; nonstressed SB334867, n = 4; vehicle swim stressed, n = 7; SB334867 swim stressed, n = 5. *, P < 0.05; repeatedly swim-stressed rats significantly different from nonstressed controls regardless of drug treatment.
In the pPVN, there was a significant main effect of stress (F1,19 = 7.737, P < 0.012) (Fig. 3B), with swim-stressed rats exhibiting a greater number of c-Fos-immunoreactive cells in response to restraint compared with nonstressed rats exposed to restraint regardless of drug treatment.
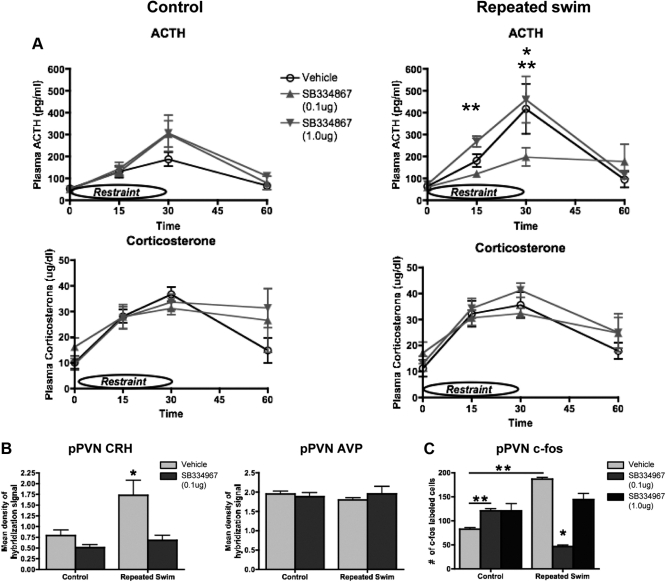
Experiment 6. Intra-pPVT SB334867 before daily swim inhibits the facilitatory response to novel restraint
There were no effects at 0 or 60 min. There was a significant stress × drug interaction at 15 min (F2,41 = 3.726, P < 0.0326) and at 30 min (F2,37 = 3.262, P < 0.049). Post hoc tests showed that vehicle-injected repeatedly swim-stressed rats exhibited a higher ACTH response than vehicle-injected nonstressed rats at both 15 and 30 min, providing evidence for a facilitated ACTH response to restraint in swim-stressed rats. At 30 min, post hoc tests also indicated that the ACTH response in swim-stressed rats injected with 0.1 μg of SB334867 was lower than in swim-stressed rats injected with vehicle or 1.0 μg or SB334867 and not different than vehicle-injected controls. These results indicate that 0.1 μg of SB334867 prevented the facilitated ACTH response to restraint (Fig. 4A, right top panel). There was no effect of SB334867 on ACTH in control rats (Fig. 4A, top left panel), and there were no significant effects on corticosterone (Fig. 4A, bottom panels) in any groups.
Fig. 4.
A, Plasma ACTH (top two panels) and plasma corticosterone (bottom two panels) responses to restraint on d 5 in nonstressed rats or rats exposed to a daily 15-min swim for 4 d that were injected with vehicle or 0.1 or 1.0 μg of orexin receptor antagonist SB334867 directly into the pPVT before each of the 4 d of no stress or swim. All rats were exposed to a 30-min restraint without any drug injection on d 5. Vehicle-injected repeatedly swim-stressed rats exhibited facilitated ACTH responses to restraint compared with vehicle-injected control rats exposed to restraint at 15 and 30 min. At 30 min, the ACTH response to restraint was lower after 0.1 μg of SB334867 treatment in repeatedly swim-stressed rats compared with all control rats, indicating that blocking orexin receptors during repeated swim prevented facilitation to novel restraint. Group sizes were: nonstressed vehicle, n = 8; nonstressed 0.1 μg of SB334867, n = 6–8; nonstressed 1.0 μg of SB334867, n = 7–8; vehicle swim stressed, n = 5; 0.1 μg of SB334867 swim stressed, n = 9; 1.0 μg of SB334867 swim stressed, n = 7 for both ACTH and corticosterone. *, P < 0.05 0.1 μg dose of SB334867 significantly different from vehicle in repeatedly swim-stressed rats. **, P < 0.05 swim-stressed vehicle significantly different from control vehicle. B, Rats in A were killed 60 min after the beginning of restraint. In situ hybridization analysis of pPVN CRH (left panel) and AVP (right panel) was conducted in the subset of rats in A that received vehicle or the effective 0.1 μg dose of SB334867. Data are expressed as integrated density of the hybridization signal. Group sizes are: nonstressed vehicle, n = 4; nonstressed 0.1 μg of SB334867, n = 5; vehicle swim stressed, n = 4; 0.1 μg of SB334867 swim stressed, n = 5. *, P < 0.05 significantly different than all other groups. C, Immunocytochemical staining for the c-Fos protein in the PVN in rats from A that were injected with vehicle, 0.1 μg of SB334867 or 1.0 μg of SB334867. Data are expressed as number of c-Fos-stained cells. Group sizes are: nonstressed vehicle, n = 7; nonstressed 0.1 μg of SB334867, n = 7; nonstressed 1.0 μg of SB334867, n = 6; swim-stressed vehicle, n = 4; swim stressed 0.1 μg of SB334867, n = 8; swim stressed 1.0 μg of SB334867, n = 6. *, P < 0.05 significantly different than all other groups. **, P < 0.05 groups indicated by the bar are different from each other.
In situ hybridization analysis of pPVN CRH and AVP was performed on the subset of rats in this experiment treated with vehicle or 0.1 μg of SB334867. There was a significant main effect of stress on CRH mRNA (F1,14 = 9.187, P < 0.009) (Figs. 4B, left panel, and 5A) with swim-stressed rats exhibiting higher CRH mRNA than nonstressed animals. There was a significant main effect of drug (F1,14 = 13.292 P < 0.0026), and post hoc tests indicated that SB334867 decreased CRH mRNA compared with vehicle treatment. There was a significant interaction (F1,14 = 4.45, P < 0.05). Post hoc tests indicated that vehicle-injected repeatedly stressed rats exhibited increased CRH mRNA compared with control rats. Further, SB334867 decreased CRH mRNA in swim-stressed rats to the level of control rats. There was no effect on pPVN AVP mRNA (Fig. 4B, right panel).
Fig. 5.
A, Representative examples of PVN CRH in situ hybridization signal from the different treatment groups in experiment 5 (shown in Fig. 4B). The groups are control non-stressed (Ctrl) rats and repeatedly swim stressed rats (Swim) injected with vehicle or the orexin receptor antagonist SB334867 (SB) prior to daily no stress or swim stress. B, Representative examples of PVN c-Fos immunohistochemical staining from treatment groups in experiment 5 (shown in Fig. 4C). C, Representative pPVT cannulae placements. In the top two panels, accurate cannulae placements in the pPVT are shown, with the placement in the top left panel shown in higher magnification on the top right. On the bottom left, an unacceptable (too ventral) placement is shown. On the bottom right, the rat brain atlas (Paxinos and Watson, Ref. 52) outlines the posterior level of PVT (−2.8 to −3.3 mm from bregma for orientation) targeted in these studies.
Analysis of the number of c-Fos-expressing cells in the pPVN (Figs. 4C and 5B) indicated a significant effect of stress (F1,21 = 16.04, P < 0.001) and of drug (F1,21 = 186.6, P < 0.0001) and a significant interaction effect (F1,21 = 577.3, P < 0.0001). Post hoc tests revealed that vehicle-treated swim-stressed rats exhibited increased numbers of c-Fos-expressing cells compared with vehicle-injected nonstressed animals exposed to restraint, providing evidence for a facilitated c-Fos response. SB334867 (0.1 μg) treatment before swim for 4 d decreased the number of c-Fos-expressing cells, but the 1.0 μg dose was not different from vehicle. SB334867 (0.1 μg) treatment daily for 4 d without concurrent stress increased the number of c-Fos-expressing cells in response to restraint compared with vehicle-injected nonstressed rats.
Experiment 7. Effects of orexinA on membrane properties of pPVT neurons
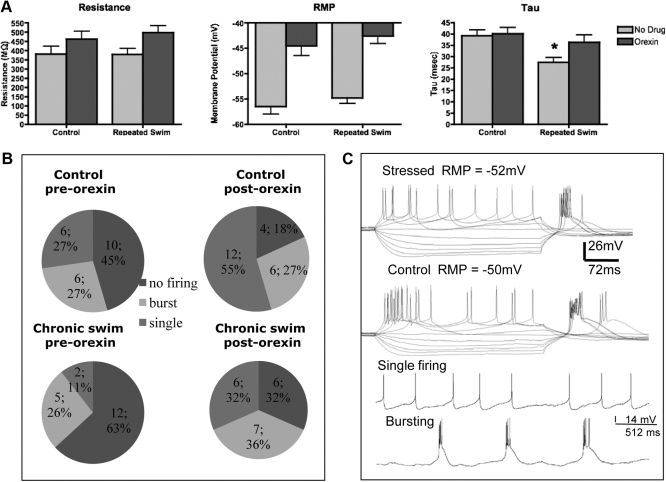
pPVT neurons responded with either tonic firing or a bursting response to depolarizing current pulses and a rebound depolarization after hyperpolarizing current injection when tested at their resting membrane potentials or when held at −75 mV. Representative traces are shown in Fig. 6C. There was no effect of repeated stress on resting membrane potential. OrexinA depolarized the membrane potential similarly in nonstressed and swim-stressed rats (significant drug effect, F1,39 = 2.58; P < 0.0001) (Fig. 6A, middle panel). For membrane resistance (Fig. 6A, left panel), there was no effect of stress, but there was an increase in resistance after orexinA (significant drug effect, F1,37 = 2.44; P < 0.002) in both nonstressed and repeatedly swim-stressed animals. There was a significant stress effect (F1,34 = 3.18, P = 0.018), a significant drug effect (F1,34 = 4.87, P = 0.034), and a tendency for an interaction (P < 0.083) with τ (Fig. 6A, right panel). The main effects indicated a lower τ in stressed compared with nonstressed rats and an increase in τ after orexinA. Because the interaction effect was approaching significance, a t test was conducted and indicated a significantly lower τ in the swim-stressed rats without drug that was increased by orexinA (P < 0.038). The response to orexinA was blocked by pretreatment with the orexin1R antagonist SB334867 in both groups of rats (data not shown). χ2 analysis revealed that before orexinA application, there was no significant difference in the firing pattern of pPVT cells between control, nonstressed and swim-stressed rats with most of the cells not firing (under basal conditions on d 5) (Fig. 6B). The Stuart-Maxwell test of marginal homogeneity revealed an unequal effect of orexinA in the nonstressed compared with swim-stressed rats. OrexinA shifted the firing pattern of pPVT cells in nonstressed rats to a predominantly single firing pattern [6.55 (2); P < 0.0379]. In swim-stressed rats, orexinA shifted the firing pattern of pPVT cells to equally single firing and burst firing [7.23 (2); P < 0.027] (Fig. 6B).
Fig. 6.
A, Resting membrane potential (RMP) (left panel), resistance (middle panel), and τ (right panel) of pPVT cells before and after orexinA bath application (100 nm) from rats exposed to no stress (control, n = 22 cells) or 4 d of repeated swim stress (swim stress, n = 19 cells). *, P < 0.05 vs. control. B, Firing patterns of the pPVT cells mentioned above indicating the number of cells and the total percentage of cells exhibiting no firing, single firing, or burst firing before and after application of orexinA. C, Voltage current traces recorded from a stressed and control rat in response to hyperpolarizing and depolarizing current pulses increasing in 10-pA increments. Bottom two traces depict the spontaneous firing of a cell exhibiting single firing and a second cell exhibiting burst firing.
Experiment 8. Acute and repeated swim stress effects on orexin1R cellular distribution and ERK phosphorylation
There was a significant effect on orexin1R in the membrane fraction (F2,27 = 18.01, P < 0.0001) with decreased expression of membrane orexin1R in both stressed groups (Fig. 7A, left panel). There was also a significant effect orexin1R in the cytosolic fraction (F2,26 = 11.95, P = 0.0002) (Fig. 7A, left panel). Post hoc tests indicated that both stressed groups had higher cytosolic orexin1R than the no swim group and that orexin1R expression in the cytosolic fraction of repeated swim rats was significantly lower than in acute swim rats (P < 0.047). There were no significant effects on orexin2R (Fig. 7A, right panel). There was a significant effect on ERK2 phosphorylation with acute swim increasing pERK2 compared with the other two groups (F2,26 = 6.317, P = 0.0062) (Fig. 7B).
Fig. 7.
A, Orexin1R distribution (left panel) and orexin2R distribution (right panel) in membrane and cytosolic fractions of pPVT punches taken from rats that were subjected to no stress (n = 10), 1 d of swim stress (acute swim, n = 10), or 4 d of swim stress (repeated swim, n = 10). The inset is a composite of representative Western blots for all groups. *, P < 0.05 vs. vehicle in cytosolic fraction; #, P < 0.05 vs. vehicle in membrane fraction; **, P < 0.05 acute swim vs. repeated swim in cytosolic fraction. B, Phosphorylated ERK1 (left panel) and ERK2 (right panel) in the cytosolic fraction of pPVT punches taken from rats in A. *, P < 0.05 significantly different pERK2 than in other groups.
Experiment 9. Restraint increases ERK1 and ERK2 phosphorylation in rats exposed to repeated swim
There was a significant effect of treatment on both the cytosolic (F3,31 = 5.559, P = 0.0036) and membrane (F3,31 = 10.373, P = 0.001) orexin1R receptor expression. Post hoc tests indicated that 4 d of repeated swim stress followed by restraint on d 5 decreased the expression of orexin1R in the membrane fraction (P = 0.002) (Fig. 8A, left panel) and increased orexin1R in the cytosolic fraction when compared with nonstressed animals (P < 0.0001) (Fig. 8A, left panel). There was no effect at 24 h after the fourth swim or of acute restraint alone on either cytosolic or membrane levels of orexin1R. There was a significant effect of stress group on the cytosolic (F3,33 = 3.33, P = 0.032) but not membrane expression of the orexin2R (Fig. 8A, right panel). Post hoc tests indicated that the cytosolic fraction of the orexin2R was significantly elevated in swim-stressed rats exposed to restraint compared with restraint alone and compared with no stress. Cytosolic and membrane fractions were summed to provide an indicator of total density of receptors. There were no differences between groups (data not shown), suggesting no change in total density of orexin1R or orexin2R by repeated stress.
Fig. 8.
A, Orexin1R distribution (left panel) and orexin2R distribution (right panel) in cytosolic and membrane fractions of pPVT punches taken from rats that were subjected to no stress and killed basally (n = 10), 4 d of swim stress and killed basally on d 5 (4-d swim, n = 9), 30-min restraint stress alone and killed at 60 min (restraint, n = 6), or 4-d swim followed by a 30-min restraint on d 5 and killed at 60 min (4-d swim + restraint, n = 10). The inset is a composite of representative Western blots for all groups. *, P < 0.05 vs. vehicle in cytosolic fraction; #, P < 0.05 vs. vehicle in membrane fraction. B, Phosphorylated ERK1 (left panel) and ERK2 (right panel) in the cytosolic fraction of pPVT punches taken from rats in A. *, P < 0.05 significantly different from all other groups.
A significant stress effect was observed with both pERK1 (F3,31 = 38.65, P = 0.001) and pERK 2 (F3,31 = 28.05, P = 0.001) (Fig. 8B). Post hoc tests indicated that 4 d of repeated swim-stressed rats exposed to restraint exhibited increased pERK1 (P = 0.001) and pERK2 (P < 0.0001) compared with all other groups.
Discussion
The effect of orexinA to shift orexin1R to the cytosol and of SB33486 to prevent this shift is consistent with the known pharmacological actions of these drugs and supports the idea that increases of the receptor in cytosolic fractions reflect activation or internalization of the receptor. Although the lower dose of orexinA stimulated HPA activity, the lack of effect by the higher dose (0.5 ng) of orexinA may be explained by the effect of this higher dose to increase orexin2R in the cytosolic fraction of the pPVT, which may oppose the effects of orexin1R, as recently suggested (46). Furthermore, orexin1R has almost a 2-fold greater affinity for orexinA than does the orexin2R (2). As a result, the lower and higher doses of orexinA may produce differential levels of activation of the two receptors in the pPVT resulting in the actions of the higher dose of orexinA at the orexin2R, thereby preventing effects at the orexin1R. OrexinA (0.1 ng) also increased pERK2 in the pPVT, and SB334867 blocked this effect. These data are consistent with previously published data showing that orexin receptors are internalized through interactions with β-arrestin-2 that are necessary for the activation of the MAPK cascade as measured by ERK phosphorylation (47). However, the high dose of orexinA (0.5 ng) had no effect on ERK1 or ERK2 or on HPA activity, supporting an important relationship between increased pPVT ERK phosphorylation and HPA activity. Thus, these results suggest that, in an aversive environment, orexinA in the pPVT increases HPA activity via activation of orexin1R and ERK2 phosphorylation.
Although orexinA administration increased the HPA response to restraint, SB334867 did not decrease the HPA response. These results suggest that endogenous orexins are not released during restraint in quantities that alter HPA activity. The finding that neither orexin receptor distribution in the membrane nor cytosol was shifted by acute restraint favors this conclusion. However, because orexinA increased, and SB334867 decreased, anxiety-related behavior in the EPM, it is possible that orexins are released in higher quantities during EPM or that that behavior in the EPM and HPA activity are regulated by distinct mechanisms requiring different modulatory inputs to the pPVT. Together, these results suggest that pPVT orexin does not regulate HPA activity acutely. Because our previous work demonstrates an important role for the pPVT in regulating HPA responses to repeated stress (18, 31), we explored this possibility next.
We first examined the role of pPVT orexins in the expression of facilitation by blocking orexin receptors during novel restraint in control or repeatedly swim-stressed rats. SB334867 did not alter the facilitated ACTH response to restraint or the facilitated increase in pPVN c-Fos expression, indicating that orexins acting in the pPVT during novel stress are not important for the expression of HPA facilitation. We then examined the role of pPVT orexins during the development (i.e. during the 4 d of exposure to 15-min swim) of facilitation. SB334867 administered into the pPVT before daily swim on four consecutive days blocked the facilitated ACTH response to restraint on d 5, prevented repeated swim-induced increases in CRH mRNA, and inhibited restraint-induced increases in pPVN c-Fos expression. These effects occurred only with the lower dose of SB334867. One explanation for the differential effect of the two doses is that the lower dose is primarily blocking orexin1R and marginally blocking orexin2R, whereas the higher dose is blocking both receptors. Alternatively, the higher dose of SB334867 may have some off-target effects that were not assessed here. In sum, these results indicate that orexins act in the pPVT during development, but not during expression, to regulate facilitation of HPA activity.
Orexins acting in the pPVT in repeatedly stressed rats regulated changes at the hypothalamic and pituitary levels but not at the adrenal. Assessment of adrenal sensitivity indicated no differences produced by repeated swim or by repeated SB334867 administration (see Supplemental data). It is possible that orexins in the pPVT regulate sympathetic inputs to the adrenals that decrease sensitivity to ACTH. The adrenals express orexin receptors, and orexinA produces secretion of corticosterone from human adrenocortical cells (48). Alternatively or additionally, a longer duration of repeated stress may be required for facilitation at the adrenal level, as seems to be the case for habituation to repeated homotypic stress (33, 49).
We further examined the orexin-related intracellular events taking place during the 4 d of swim. We observed that orexin1R but not orexin2R shift from the membrane to the cytosol during the first and fourth swim exposures, although the magnitude of this internalization decreases over exposures. In response to the first swim, there is a significant increase in pERK2 in pPVT, and this increase declines to control levels by the fourth swim exposure, suggesting habituation of ERK signaling despite release of orexins. It is possible that other intracellular signaling pathways become activated while phosphorylation of ERK declines or that there is a shift in dynamics of ERK phosphorylation so that changes in phosphorylation occur earlier or later than the time points assessed here. One way that ERK phosphorylation could decline with repeated stress is via MAPK phosphatase-1, which plays an important role in feedback control of MAP kinase cascades (50). Thus, it is possible that MAPK phosphatase-1 is up-regulated during the development of the facilitation, resulting in the decline of pERK2. When repeatedly swim-stressed rats were exposed to novel restraint, both pERK1 and pERK2 were markedly increased in the pPVT. Interestingly, orexinA administration in the pPVT increased only pERK2 but not pERK1 in the pPVT, demonstrating an isoform-specific effect on ERK by orexins and suggesting that repeated stress induction of both pERK1 and pERK2 involves inputs in addition to orexinA. This increase in phosphorylation of both ERK1 and ERK2 by restraint was accompanied by a shift in orexin1R from membrane to cytosol. Interestingly, this internalization and presumable activation of orexin receptors occurred even though orexin actions during restraint are not important for facilitation. This activation of orexin1R during the expression of facilitation might regulate non-HPA responses in facilitated animals, a question that will be addressed in future studies.
We further examined possible intracellular mechanisms that mediate the effects of orexins in the pPVT that lead to facilitation. In control nonstressed rats, orexinA increased the proportion of cells that were single firing. In swim-stressed rats, similar proportions of cells became single or burst firing after orexinA, suggesting a greater responsiveness of pPVT cells to orexinA that could underlie facilitation. τ is directly related to resistance and capacitance. Because resistance was unchanged, the decrease in τ by repeated swim was probably the result of a decreased capacitance that is related to the neuron surface area, i.e. morphology. Consistent with this, the number of nodes and ends was decreased by repeated swim, suggesting a decreased dendritic morphology by 4 d of stress (see Supplemental data). Further work is required to determine whether SB334867 could reverse the effects of stress on pPVT cellular morphology.
To conclude, the results have identified neural underpinnings of both the development and expression of facilitation. During development, orexin actions in the pPVT as a result of repeated swim stress are necessary for facilitation to occur, these are primarily mediated by orexin1R and associated with increases in pERK2 but not pERK1. With repeated swim, phosphorylation of ERK2 declines, suggesting possible activation of other intracellular signaling pathways or a change in dynamics of ERK2 phosphorylation. During expression of facilitation, orexin actions in the pPVT are not necessary for facilitation to occur, although the observed activation of orexin receptors suggests that orexin is released during restraint and may be important for regulation of other stress-induced endpoints. Facilitation is also accompanied by increases in phosphorylation of both ERK1 and ERK2, suggesting that nonorexinergic inputs to the pPVT are important in regulating the expression of facilitation. Thus, orexins released during repeated stress may prepare the pPVT for additional inputs during exposure to subsequent novel stressors. These could include previously identified regulators of stress adaptations, including cholecystokinin (31), glucocorticoids (18), and endocannabanoids (51).
Supplementary Material
Acknowledgments
We thank Dr. Harry Ischiropoulos for helpful discussions.
This work was supported, in part, by the National Institute of Mental Health (NIMH) Grant 067651 and the Army Research Office Grant DARPA 58077 LSDRP (to S.Bh.), T32 (Principal Investigator: Dr. Irwin Lucki) (to W.H.) and the NIMH Grant 075407 (to S.Be.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AVP
- Arginine vasopressin
- DMSO
- dimethylsulfoxide
- EPM
- elevated plus maze
- HPA
- hypothalamic-pituitary-adrenal
- orexin1R
- orexin1 receptor
- orexin2R
- orexin2 receptor
- pPVN
- parvocellular PVN
- pPVT
- posterior division of the PVT
- PVN
- paraventricular hypothalamus
- PVT
- paraventricular nucleus of the thalamus.
References
- 1. de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. 1998. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA 95:322–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. 1998. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92:573–585 [DOI] [PubMed] [Google Scholar]
- 3. Adamantidis A, de Lecea L. 2008. Physiological arousal: a role for hypothalamic systems. Cell Mol Life Sci 65:1475–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. 1998. Forebrain afferents to the rat dorsal raphe nucleus demontrated by retrograde and anterograde tracing methods. Neuroscience 82:443–468 [DOI] [PubMed] [Google Scholar]
- 5. Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, Träskman-Bendz L, Goddard AW, Brundin L, Shekhar A. 2010. A key role for orexin in panic anxiety. Nat Med 16:111–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Suzuki M, Beuckmann CT, Shikata K, Ogura H, Sawai T. 2005. Orexin-A (hypocretin-1) is possibly involved in generation of anxiety-like behavior. Brain Res 1044:116–121 [DOI] [PubMed] [Google Scholar]
- 7. Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M. 1999. Orexins, orexinergic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci USA 96:748–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Samson WK, Taylor MM, Follwell M, Ferguson AV. 2002. Orexin actions in hypothalamic paraventricular nucleus: physiological consequences and cellular correlates. Regul Peptides 104:97–103 [DOI] [PubMed] [Google Scholar]
- 9. Chang H, Saito T, Ohiwa N, Tateoka M, Deocaris CC, Fujikawa T, Soya H. 2007. Inhibitory effects of an orexin-2 receptor antagonist on orexin A- and stress-induced ACTH responses in conscious rats. Neurosci Res 57:462–466 [DOI] [PubMed] [Google Scholar]
- 10. Kuru M, Ueta Y, Serino R, Nakazato M, Yamamoto Y, Shibuya I, Yamashita H. 2000. Centrally administered orexin/hypocretin activates HPA axis in rats. Neuroreport 11:1977–1980 [DOI] [PubMed] [Google Scholar]
- 11. Berendse HW, Groenewegen HJ. 1991. Restricted cortical termination fields of the midline and intralaminar thalamic nuclei in the rat. Neuroscience 42:73–102 [DOI] [PubMed] [Google Scholar]
- 12. Kirouac GJ, Parsons MP, Li S. 2005. Orexin (hypocretin) innervation of the paraventricular nucleus of the thalamus. Brain Res 1059:179–188 [DOI] [PubMed] [Google Scholar]
- 13. Moga MM, Weis RP, Moore RY. 1995. Efferent projections of the paraventricular thalamic nucleus in the rat. J Comp Neurol 359:221–238 [DOI] [PubMed] [Google Scholar]
- 14. Su HS, Bentivoglio M. 1990. Thalamic midline cell populations projecting to the nucleus accumbens, amygdala, and hippocampus in the rat. J Comp Neurol 297:582–593 [DOI] [PubMed] [Google Scholar]
- 15. Turner BH, Herkenham M. 1991. Thalamoamygdaloid projections in the rat: a test of the amygdalaís role in sensory processing. J Comp Neurol 313:295–325 [DOI] [PubMed] [Google Scholar]
- 16. Dong HW, Swanson LW. 2006. Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J Comp Neurol 494:142–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bhatnagar S, Huber R, Nowak N, Trotter P. 2002. Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. J Neuroendocrinol 14:403–410 [DOI] [PubMed] [Google Scholar]
- 18. Jaferi A, Bhatnagar S. 2006. Corticosterone can act at the posterior paraventricular thalamus to inhibit hypothalamic-pituitary-adrenal activity in animals that habituate to repeated stress. Endocrinology 147:4917–4930 [DOI] [PubMed] [Google Scholar]
- 19. Cluderay JE, Harrison DC, Hervieu GJ. 2002. Protein distribution of the orexin-2 receptor in the rat central nervous system. Regul Peptides 104:131–144 [DOI] [PubMed] [Google Scholar]
- 20. Lu XY, Bagnol D, Burke S, Akil H, Watson SJ. 2000. Differential distribution and regulation of OX1 and OX2 orexin/hypocretin receptor messenger RNA in the brain upon fasting. Horm Behav 37:335–344 [DOI] [PubMed] [Google Scholar]
- 21. Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. 2001. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435:6–25 [DOI] [PubMed] [Google Scholar]
- 22. Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. 1998. Distribution of orexin receptor mRNA in the rat brain. Fed Eur Biochem Soc 438:71–75 [DOI] [PubMed] [Google Scholar]
- 23. Hsu DT, Price JL. 2009. Paraventricular thalamic nucleus: subcortical connections and innervation by serotonin, orexin and corticotropin-releasing hormone in macaque monkeys. J Comp Neurol 512:825–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kerman IA, Bernard R, Rosenthal D, Beals J, Akil H, Watson SJ. 2007. Distinct populations of presympathetic premotor neurons express orexin or melanin-concentrating hormone in the rat lateral hypothalamus. J Comp Neurol 505:586–601 [DOI] [PubMed] [Google Scholar]
- 25. Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. 1999. Distribution of orexin neurons in the adult rat brain. Brain Res 827:243–260 [DOI] [PubMed] [Google Scholar]
- 26. Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. 1998. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18:9996–10015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baldo BA, Daniel RA, Berridge CW, Kelley AE. 2003. Overlapping distributions of orexin/hypocretin- and dopamine-(-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol 464:220–237 [DOI] [PubMed] [Google Scholar]
- 28. Ishibashi M, Takano S, Yanagida H, Takatsuna M, Nakajima K, Oomura Y, Wayner MJ, Sasaki K. 2005. Effects of orexins/hypocretins on neuronal activity in the paraventricular nucleus of the thalamus in rats in vitro. Peptides 26:471–481 [DOI] [PubMed] [Google Scholar]
- 29. Kolaj M, Doroshenko P, Yan Cao X, Coderre E, Renaud LP. 2007. Orexin-induced modulation of state-dependent intrinsic properties in thalamic paraventricular nucleus neurons attenuates action potential patterning and frequency. Neuroscience 147:1066–1075 [DOI] [PubMed] [Google Scholar]
- 30. Li Y, Li S, Wei C, Wang H, Sui N, Kirouac GJ. 2010. Orexins in the paraventricular nucleus of the thalamus mediate anxiety-like responses in rats. Psychopharmacology 212:251–265 [DOI] [PubMed] [Google Scholar]
- 31. Bhatnagar S, Viau V, Chu A, Soriano L, Meijer OC, Dallman MF. 2000. A cholecystokinin-mediated pathway to the paraventricular thalamus is recruited in chronically stressed rats and regulates hypothalamic-pituitary-adrenal function. J Neurosci 20:5564–5573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dallman MF, Jones MT. 1973. Corticosteroid feedback control of ACTH secretion on subsequent stress responses in the rat. Endocrinology 92:1367–1375 [DOI] [PubMed] [Google Scholar]
- 33. Grissom NM, Bhatnagar S. 2011. The basolateral amygdala regulates adaptation to stress via β-adrenergic receptor-mediated reductions in phosphorylated extracellular signal-regulated kinase. Neuroscience 178:108–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paranjape S, Vavaiya K, Kale A, Briski K. 2007. Role of dorsal vagal motor nucleus orexin-receptor-1 in glycemic responses to acute versus repeated insulin administration. Neuropeptides 41:111–116 [DOI] [PubMed] [Google Scholar]
- 35. Akana SF, Hanson ES, Horsley CJ, Strack AM, Bhatnagar S, Bradbury MJ, Milligan ED, Dallman MF. 1996. Clamped corticosterone (B) reveals the effect of endogenous B on both facilitated responsivity to acute restraint and metabolic responses to chronic stress. Stress 1:33–49 [DOI] [PubMed] [Google Scholar]
- 36. Lapiz-Bluhm MD, Bondi CO, Doyen J, Rodriguez GA, Bédard-Arana T, Morilak DA. 2008. Behavioural assays to model cognitive and affective dimensions of depression and anxiety in rats. J Neuroendocrinol 20:1115–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Langmead CJ, Jerman JC, Brough SJ, Scott C, Porter RA, Herdon HJ. 2004. Characterisation of the binding of [3H]-SB-674042, a novel nonpeptide antagonist, to the human orexin-1 receptor. Br J Pharmacol 141:340–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smart D, Sabido-David C, Brough SJ, Jewitt F, Johns A, Porter RA, Jerman JC. 2001. SB-334867-A: the first selective orexin-1 receptor antagonist. Br J Pharmacol 132:1179–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G. 2007. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behav Brain Res 183:43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bai YJ, Li YH, Zheng XG, Han J, Yang XY, Sui N. 2009. Orexin A attenuates unconditioned sexual motivation in male rats. Pharmacol Biochem Behav 91:581–589 [DOI] [PubMed] [Google Scholar]
- 41. Bhatnagar S, Vining C, Iyer V, Kinni V. 2006. Changes in hypothalamic-pituitary-adrenal function, body temperature, body weight and food intake with repeated social stress exposure in rats. J Neuroendocrinol 18:13–24 [DOI] [PubMed] [Google Scholar]
- 42. Wood SK, Walker HE, Valentino RJ, Bhatnagar S. 2010. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology 151:1795–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bhatnagar S, Dallman M, Dallman M.F. 1998. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience 84:1025–1039 [DOI] [PubMed] [Google Scholar]
- 44. Castro MG, Morrison E, Perone MJ, Brown OA, Murray CA, Ahmed I, Perkins AV, Europe-Finner G, Lowenstein PR, Linton EA. 1996. Corticotropin-releasing hormone receptor type 1: generation and characterization of polyclonal antipeptide antibodies and their localization in pituitary cells and cortical neurones in vitro. J Neuroendocrinol 8:521–531 [DOI] [PubMed] [Google Scholar]
- 45. Chen MH, Malbon CC. 2009. G-protein-coupled receptor-associated A-kinase anchoring proteins AKAP5 and AKAP12: differential trafficking and distribution. Cell Signal 21:136–142 [DOI] [PubMed] [Google Scholar]
- 46. Karteris E, Randeva HS. 2003. Orexin receptors and G-protein coupling: evidence for another “promiscuous” seven transmembrane domain receptor. J Pharmacol Sci 93:126–128 [DOI] [PubMed] [Google Scholar]
- 47. Milasta S, Evans NA, Ormiston L, Wilson S, Lefkowitz RJ, Milligan G. 2005. The sustainability of interactions between the orexin-1 receptor and β-arrestin-2 is defined by a single C-terminal cluster of hydroxy amino acids and modulates the kinetics of ERK MAPK regulation. Biochem J 387(Pt 3):573–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ramanjaneya M, Conner AC, Chen J, Stanfield PR, Randeva HS. 2008. Orexins stimulate steroidogenic acute regulatory protein expression throughmultiple signaling pathways in human adrenal H295R cells. Endocrinology 149:4106–4115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gray M, Bingham B, Viau V. 2010. A comparison of two repeated restraint stress paradigms on hypothalamic-pituitary-adrenal axis habituation, gonadal status and central neuropeptide expression in adult male rats. J Neuroendocrinol 22:92–101 [DOI] [PubMed] [Google Scholar]
- 50. Duric V, Banasr M, Licznerski P, Schmidt HD, Stockmeier CA, Simen AA, Newton SS, Duman RS. 2010. A negative regulator of MAP kinase causes depressive behavior. Nat Med 16:1328–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ward RJ, Pediani JD, Milligan G. 2011. Ligand-induced internalization of the orexin OX(1) and cannabinoid CB(1) receptors assessed via N-terminal SNAP and CLIP-tagging. Br J Pharmacol 162:1439–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Paxinos G, Watson C. 2005. The rat brain in stereotaxic coordinates: the new coronal set. 5th Edition, San Diego: Elsevier Academic Press [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.