Abstract
GH receptor (GHR) mediates the anabolic and metabolic effects of GH. We previously characterized a monoclonal antibody (anti-GHRext-mAb) that reacts with subdomain 2 of the rabbit GHR extracellular domain (ECD) and is a conformation-specific inhibitor of GH signaling in cells bearing rabbit or human GHR. Notably, this antibody has little effect on GH binding and also inhibits inducible metalloproteolysis of the GHR that occurs in the perimembranous ECD stem region. In the current study, we demonstrate that anti-GHRext-mAb inhibits GH-dependent cellular proliferation and also inhibits hepatic GH signaling in vivo in mice that adenovirally express rabbit GHR, as assessed with our noninvasive bioluminescence hepatic signaling assay. A separate monoclonal antibody (anti-GHRmAb 18.24) is a sister clone of anti-GHRext-mAb. Here, we demonstrate that anti-GHRmAb 18.24 also inhibits rabbit and human GHR signaling and inducible receptor proteolysis. Further, we use a random PCR-generated mutagenic expression system to map the three-dimensional epitopes in the rabbit GHR ECD for both anti-GHRext-mAb and anti-GHRmAb 18.24. We find that each of the two antibodies has similar, but nonidentical, discontinuous epitopes that include regions of subdomain 2 encompassing the dimerization interface. These results have fundamental implications for understanding the role of the dimerization interface and subdomain 2 in GHR activation and regulated GHR metalloproteolysis and may inform development of therapeutics that target GHR.
GH is a multifunctional peptide hormone with anabolic, proproliferative, antiapoptotic, and metabolic effects in various target tissues (1, 2). Orchestration of these actions is incompletely understood, but structural and functional knowledge of the GH receptor (GHR) is critical for deciphering GH biology (3). GH is a four helix bundle “cytokine” with structural similarity to prolactin, erythropoietin, leptin, and several IL and other cytokines (4). Human GH (hGH)R (and rabbit GHR) is a 620-residue cell surface transmembrane glycoprotein with similarly sized extracellular and intracellular domains (3, 5). GHR is a member of the cytokine receptor superfamily that includes prolactin receptor, erythropoietin receptor, leptin receptor, and others (6).
The GHR extracellular domain (ECD) contains two subdomains (1 and 2). Each of the two subdomains is composed of a series of β strands arranged into two antiparallel β sheets (7). A 4-residue hinge separates subdomain 1 (residues 1–123) and subdomain 2 (residues 128–238) and the remaining ECD residues (239–246) form the juxtamembrane stem. Structural and mutagenesis studies indicate that GH binding to GHR ECD is mainly via residues in subdomain 1 and the hinge, although tryptophan 169 in subdomain 2 also contributes to binding. Subdomain 2 harbors the “dimerization interface” involving several residues that form noncovalent intermolecular bonds between GHR monomers within the GH(GHR)2 complex (7, 8). These residues are essential for signal transduction but not for hormone binding (9, 10). Although dimerization domain interaction is enhanced by GH, there is also a degree of “predimerization” of GHR in GH's absence, which may be attributed to transmembrane domain and other interactions (11–13). In addition to inducing noncovalent GHR-GHR interactions, GH induces formation of disulfide-linked GHR in a variety of cell lines; this disulfide linkage is mediated by Cys241 in the juxtamembrane stem (13–17).
GH-dependent signaling is triggered by GHR's adoption of a dimerized configuration that activates the receptor-associated cytoplasmic tyrosine kinase, Janus kinase 2 (JAK2), and other kinases and subsequent engagement of the signal transducer and activator of transcription (STAT), particularly STAT5A/B, ERK, phosphatidylinositol-3 kinase, and other pathways (18–25). Because it is desirable to inhibit GH action in situations of GH excess (e.g. acromegaly) and possibly in malignancies, there is interest in developing GH antagonists (26–29). GH bears two regions (sites 1 and 2) that sequentially engage the two monomeric GHR to form the activated GHR dimer (8). The prototype GH antagonist, Pegvisomant, has mutations that enhance site 1 affinity and diminish site 2 affinity, blocking the ability of normal GH to productively engage GHR (26).
Another approach to inhibit surface receptor signaling is with antireceptor antibodies that block either ligand binding or receptor activation (30, 31). This approach is both therapeutically relevant and instructive for understanding receptor activation mechanisms. We initially characterized a mouse monoclonal antibody, anti-GHRext-mAb, raised against the ECD of the rabbit GHR and cross-reactive with human, bovine, and porcine GHR but not mouse or rat GHR (14, 17). We found that anti-GHRext-mAb reacts with subdomain 2, but not subdomain 1. However, finer mapping was not possible. Furthermore, this antibody or its Fab fragment, when applied to intact cells in vitro, inhibited subsequent GH-dependent GHR conformational changes and GH signaling and prevented inducible (non-GH-dependent) metalloproteolysis of GHR and shedding of the receptor ECD (GH binding protein) (17). Thus, anti-GHRext-mAb is a conformation-sensitive GHR antagonist and, as such, may have therapeutic potential.
We now further characterize anti-GHRext-mAb and find that it is able to inhibit GH-dependent cellular proliferation in vitro and hepatic GH signaling in vivo. Further, we characterize an independently derived sister monoclonal antibody, anti-GHRmAb-18.24, and find that it displays similar GHR inhibitory properties. Three-dimensional epitope mapping of the two antibodies reveals that each has similar, but nonidentical, discontinuous epitopes that include regions of subdomain 2 encompassing the dimerization interface. These results have important implications for understanding the role of the dimerization interface and subdomain 2 in GHR activation and regulated GHR metalloproteolysis.
Materials and Methods
Materials
Phorbol 12-myristate 13-acetate (PMA) and routine reagents were purchased from Sigma-Aldrich Corp. (St. Louis, MO) unless otherwise noted. Fetal bovine serum, gentamicin sulfate, penicillin, and streptomycin were purchased from BioFluids (Rockville, MD). Recombinant hGH was kindly provided by Eli Lilly & Co. (Indianapolis, IN).
Antibodies
Polyclonal antiphospho-STAT5 was purchased from Zymed Laboratories (San Francisco, CA). Anti-STAT5 and anti-prolactin receptor were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-p-JAK2 and antiglutathione-S-transferase (GST) were purchased from Upstate Biotechnology, Inc. (Lake Placid, NY). Polyclonal anti-GHRcyt-AL47 against the intracellular domain of GHR (32) and anti-JAK2AL33 were described previously (33). Anti-GHRcyt-mAb (14) is a mouse monoclonal antibody (IgG2bκ) against a bacterially expressed GST fusion protein incorporating hGHR residues 271–620. Anti-GHRext-mAb is a mouse monoclonal antibody (IgG1κ) against a bacterially expressed GST fusion protein incorporating rabbit GHR residues 1–246 (14, 17, 34–36). Anti-GHRmAb 18.24 is a mouse monoclonal antibody (IgG1κ) that was derived as a sister clone of anti-GHRext-mAb (37). Anti-GHRmAb 238 was also a sister isolate (IgG1κ) of the same immunization. Each monoclonal anti-GHR antibody was protein G-Sepharose-purified from hybridoma supernatant [University of Alabama at Birmingham (UAB) Epitope Recognition and Immunoreagent Core Facility, UAB Arthritis and Musculoskeletal Center].
Cells, cell culture, and proliferation assay
32D-GHR cells were derived by stable transfection of rabbit GHR and cultured as described (38). Human γ2A fibrosarcoma cells stably expressing JAK2 and either wild-type rabbit GHR (C14 cells), GHR124–620 (lacking extracellular subdomain 1), or GHRΔ128–238 (lacking extracellular subdomain 2), and their culture conditions were described (39, 40). Serum-deprived 32D-GHR cells were subjected to the 3-(4, 5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) Cell Proliferation Assay (American Type Culture Collection, Manassas, VA), as described (38), but for 48 h in the presence or absence of monoclonal antibodies, as in figure legends.
Cell starvation, cell stimulation, protein extraction, electrophoresis, and immunoblotting
Serum starvation was accomplished by substitution of 0.5% (wt/vol) BSA (fraction V; Roche Molecular Biochemicals, Indianapolis, IN) for serum in culture media for 16–20 h. hGH (indicated concentrations) and PMA (1 μg/ml) treatment protocols have been described (32, 41, 42). Briefly, adherent cells were stimulated in binding buffer [consisting of 25 mm Tris-HCl (pH 7.4), 120 mm NaCl, 5 mm KCl, 1.2 mm MgCl2, 0.1% (wt/vol) BSA, and 1 mm dextrose] or DMEM (1 g/liter glucose) with 0.5% (wt/vol) BSA. Stimulations were terminated by washing cells with ice-cold PBS in the presence of 0.4 mm sodium orthovanadate (PBS-vanadate) and then harvesting by scraping in cold PBS-vanadate. Pelleted cells were collected by brief centrifugation. For each cell type, pelleted cells were solubilized for 15 min at 4 C in lysis buffer [1% (vol/vol) Triton X-100, 150 mm NaCl, 10% (vol/vol) glycerol, 50 mm Tris-HCl (pH 8.0), 100 mm NaF, 2 mm EDTA, 1 mm phenylmethylsulfonylfluoride, 1 mm sodium orthovanadate, 10 mm benzamidine, and 10 μg/ml aprotinin]. After centrifugation at 15,000 × g for 15 min at 4 C, the detergent extracts were electrophoresed under reducing conditions. To test effects of monoclonal antibodies on cell signaling and GHR proteolysis, purified antibodies were added directly to serum-starved cells at 37 C for the indicated pretreatment durations. Resolution of proteins by SDS-PAGE, Western transfer of proteins, and blocking of Hybond-ECL (Amersham, Inc., Buckinghamshire, UK) with 2% BSA were as described (13, 14, 17, 36, 40–42). Immunoblotting using horseradish peroxidase-conjugated antimouse or antirabbit secondary antibodies (1:10,000–1:15,000) and detection reagents (SuperSignal West Pico Chemiluminescent Substrate) (all from Pierce), and stripping and reprobing of blots were accomplished according to manufacturers' suggestions. Immunoblots were scanned using a high-resolution scanner (Hewlett-Packard Co., Palo Alto, CA).
Plasmid construction and preparation of GST fusion proteins
Plasmids encoding GST/GHR1–246 [GST N terminus to residues 1–246 of the rabbit GHR (the entire ECD)], analogous plasmids encoding residues 1–128, 129–246, 129–169, 169–202, and 202–246, and plasmids encoding rabbit GHR124–620 and rabbit GHRΔ128–238 were described (14, 17, 34, 35, 40). GST fusion proteins were expressed in Escherichia coli and purified as described (18).
In vivo hepatic GH signaling via adenovirally expressed GHRE-Luc reporter with Ad-GHR
Techniques and reagents (adenovirus constructs, luciferin) used for this were as described in detail (43–45). Briefly, 6-wk-old female C57BL/6 mice (n = 5 per group) were used. Each was administered Ad-GHRE-luc (1 × 108 plaque-forming unit iv) and 2 d later Ad-GHR (1 × 108 plaque-forming unit) and subjected to imaging studies 6 d thereafter, as in Ref. 46. After overnight fast, a baseline bioluminescence image was obtained 3 h after either anti-GHRext-mAb or isotype-matched control were injected (100 μg iv). GH (1 μg/g iv) was injected, and images were obtained 3 and 18 h later. For imaging, mice were injected with luciferin ip (2.5 mg) and imaged after 10 min with the IVIS-100 Imaging System. Image acquisition, display, and statistical analysis were as described (43–45).
Creation of random PCR mutagenesis yeast expression library and screening monoclonal antibody epitopes against the GHR ECD
To determine the precise epitopes for anti-GHRext-mAb and anti-GHRmAb 18.24, a comprehensive library of 5200 clones of rabbit GHR ECD with single or double mutations and a carboxy-tag (NLVSGPEH) was established in the BJ3505 yeast host, as described (47). The cDNA encoding the rabbit GHR ECD (residues 1–240) was cloned into the YEpFLAG-1 yeast expression vector (082394; Eastman Kodak Co., Rochester, NY) by EcoRI and BamHI. YEp confers tryptophan prototrophy on the BJ3505 yeast host strain and provides an ADH2 promoter for driving target protein expression (inducible by glucose deprivation).
Colonies from this library were cultured in 96-well microplates with complete supplement mixture minus tryptophan (BIO 101; Qbiogene, Solon, OH) with 2% glucose at 30 C for 3 d and then replica plated onto cellulose acetate filters in 144-mm culture dishes using a 96-channel replica plater. After 3 d in culture, the filters with yeast colonies were placed onto two sheets of Hybond-C Extra membrane (RPN137E; Amersham, Inc., Buckinghamshire, UK) on complete supplement mixture without glucose to allow the rabbit GHR ECD protein secreted by the yeast colonies on the filter to be transferred onto the Hybond membrane. Separate sheets of Hybond membrane were used for anti-GHRext-mAb and for anti-GHRmAb 18.24, and these were screened by immunoblot.
For this, the membranes were blocked with 6% skim milk in PBS and 0.03% Tween 20, with 4 μg/ml of anti-GHRext-mAb or anti-GHRmAb 18.24, and the bound antibody was detected using a horseradish peroxidase-conjugated antimouse antibody (GE Healthcare, Princeton, NJ) by the ECL plus Western Blotting Detection System (RPN 2106; Amersham, Inc.). The luminescence images were captured using a Fusion FX7 image acquisition system (Vilber, Marne-la-Vallée, France). The negative dots in the image of each membrane were assigned with Scion Image software (Scion Corp., Frederick, MD). In the program, the average integrated gray density of three negative control dots was taken as a threshold, from which the software recognized dots with an integrated gray density higher than the threshold value as “positive.” The negative colonies were then selected to confirm with anti-GHRext-mAb or anti-GHRmAb 18.24 again and to screen with a monoclonal antibody against C-terminal tag (8E7/55; Queensland Institute of Medical Research, Queensland, Australia) to eliminate prematurely terminated or unexpressed mutants from the screen.
The rabbit GHR ECD in the colonies negative for anti-GHRext-mAb or anti-GHRmAb 18.24 and positive for C-terminal tag was then amplified by PCR with the primers (YaN21, 5′-AGCACA AAT AAC GGG TTA TTG-3′ and YcC21, 5′-TAC AGACGC GTG TAC GCA TGT-3′; Sigma Chemical Co., St. Louis, MO) and the template from the above clone in yeast after heating in the hot block for 10 min, freezing at −80 C for 40 min and vortex with glass beads for 5 min. The PCR products were sequenced by ABI autosequencing (Applied Biosystems, Inc., Columbia, MD). The resulting sequences were analyzed using DNAMAN version 6 to identify the specific residues responsible for the anti-GHRext-mAb or anti-GHRmAb 18.24 epitope after eliminating clones with residue mutations X to Cys or Cys to X that may cause breaking or creation of disulfide bonds.
Mapping of monoclonal antibody epitopes onto the crystal structure model of the GHR ECD
Anti-GHRext-mAb or anti-GHRmAb 18.24 epitope residues were mapped onto a homology-modeled crystal structure of the GH(rabbit GHR)2 trimeric complex. The model was created by homology modeling from the structure of the human counterpart (PDB 3HHR) using the First Approach Mode and then the Optimize Mode of SWISS-MODE after manual alignment with SWISSpdb Viewer version 4.7.1.
Results
Anti-GHRext-mAb inhibits GH-dependent proliferation in vitro and hepatic GH signaling in vivo
Anti-GHRext-mAb is a mouse monoclonal antibody raised against the entire rabbit GHR ECD (residues 1–246) (14, 34, 35). This antibody immunoprecipitates rabbit, human, porcine, and bovine GHR but not rodent GHR (14, 17, 35) (data not shown). Pretreating intact cells with anti-GHRext-mAb or equimolar amounts of its Fab fragment inhibits subsequent GH-induced JAK2 and STAT5 activation and prevents GH-induced GHR disulfide linkage (17), suggesting that anti-GHRext-mAb impairs GH-induced attainment of the activated conformation of GHR dimers. Consistent with this, the antibody's antagonism is not accounted for by reduced 125I-GH binding to the receptor (17).
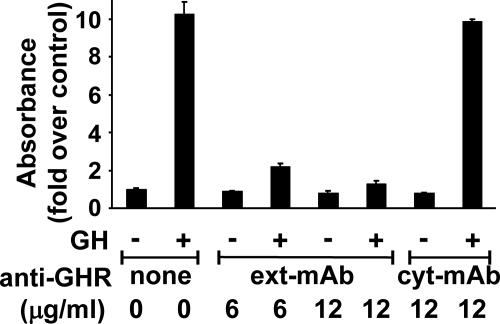
To further characterize anti-GHRext-mAb's effects on GH action, we first asked whether it impacts GH's ability to promote a cellular response downstream of acute receptor activation. For this, we examined GH-dependent cellular proliferation. 32D-GHR is a mouse promonocyte that stably expresses rabbit GHR (38, 48). GH acutely activates JAK2, STAT5, ERK, and Akt signaling and over 24–48 h increases 32D-GHR cell number (38, 48). In the experiment shown in Fig. 1, GH (100 ng/ml) treatment for 48 h increases 32D-GHR cell number by roughly 10-fold, as estimated by the MTT assay. Coincubation with anti-GHRext-mAb (6 or 12 μg/ml) dramatically inhibited the GH-dependent MTT signal. In contrast, anti-GHRcyt-mAb (12 μg/ml), which reacts with the GHR intracellular domain (17), did not affect the GH-induced increase in MTT signal. Thus, anti-GHRext-mAb specifically inhibited GH-dependent 32D-GHR proliferation.
Fig. 1.
Anti-GHRext-mAb specifically inhibits GH-induced proliferation. 32D-GHR cells (25,000 per well) were seeded in triplicate in 96-well plates and then serum starved. Cells were treated ± GH (100 ng/ml) for 48 h ± anti-GHRext-mAb (6 or 12 μg/ml) or anti-GHRcyt-mAb (12 μg/ml), as indicated. Viable cell number was then estimated by MTT assay, as in Materials and Methods. Data (mean ± sem of triplicate determinations) for this experiment are indicated as the GH-induced fold increase in OD490 relative to the value determined when no GH was added. Note that GH-induced proliferation is specifically inhibited by anti-GHRext-mAb and not by anti-GHRcyt-mAb. The experiment shown is representative of three such experiments.
We next examined the effect of anti-GHRext-mAb on in vivo GH actions. Ad-GHR is an adenovirus that directs expression of rabbit GHR in adenovirally infected cells (43). Administered iv, Ad-GHR directs rabbit GHR expression preferentially to the liver (43–45). Adenovirally expressed hepatic GHR can mediate GH-induced signaling in vivo. We previously developed a noninvasive signaling assay using Ad-GHRE-luc, which encodes the firefly luciferase gene under the control of a STAT5-responsive promoter element (43). In this system, GH-induced hepatic luciferase activity is monitored by bioluminescence imaging; in both C3H and nude mice, peak bioluminescence signal is observed 3 h after iv-administered GH (43, 44).
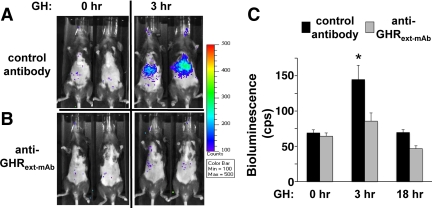
In the experiment in Fig. 2, we extended this system to C57BL/6 mice. Mice infected with Ad-GHR and Ad-GHRE-luc were pretreated with either isotype-matched control antibody (Fig. 2A) or anti-GHRext-mAb (Fig. 2B). Bioluminescence images obtained at baseline and 3 h after GH administration (1 μg/g iv) showed that in control antibody-treated mice, GH induced robust hepatic bioluminescence, consistent with findings in nude and C3H mice (43, 44). Importantly, anti-GHRext-mAb pretreatment markedly and significantly reduced GH-induced hepatic bioluminescence (∼70% reduced vs. control) (Fig. 2C). Thus, as in in vitro experiments, anti-GHRext-mAb inhibited GH-dependent hepatic signaling in vivo.
Fig. 2.
Anti-GHRext-mAb specifically inhibits hepatic GH signaling in vivo. A and B, Bioluminescence imaging. As described in Materials and Methods, C57BL/6 mice (6-wk-old female; n = 5 per group) were infected with Ad-GHRE-luc and Ad-GHR. Baseline (0 h) hepatic luciferase activity was assessed by bioluminescence imaging 3 h after administration of either isotype-matched control antibody (A) or anti-GHRext-mAb (B), and GH was then administered. Three hours thereafter, GH-induced (3 h) hepatic luciferase activity was again assessed by bioluminescence imaging. Note that GH-induced hepatic signal is markedly reduced in mice pretreated with anti-GHRext-mAb but not the isotype-matched control antibody. C, Quantitation (as in Materials and Methods) of the data in A and B and of data obtained 18 h (data not shown) after GH injection for n = 5 in each condition. Mean ± sem of bioluminescence signal counts per second (cps) obtained at baseline (0 h) and at 3 and 18 h after GH treatment are plotted, as indicated. As indicated by the asterisk, the mean GH-stimulated bioluminescence in the control antibody-treated mice was different from all other values at P < 0.0001 by two-way ANOVA evaluating the effect of antibody treatment and time. In particular, basal values did not differ statistically between the control- and anti-GHRext-mAb-treated mice. Average GH-induced increase in signal in anti-GHRext-mAb-treated mice (signal at 3 h minus that at 0 h) was reduced by approximately 70% compared with the average GH-induced increase in signal in control antibody-treated mice.
Anti-GHRmAb 18.24 reacts with GHR extracellular subdomain 2
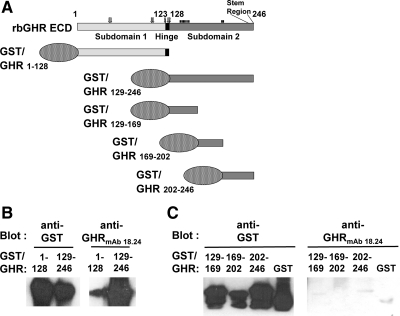
To better understand the properties of anti-GHRext-mAb, we characterized anti-GHRmAb 18.24, an independent hybridoma clone from the immunization with a GST fusion protein linked to the rabbit GHR ECD. Although it arose from the same immunization as anti-GHRext-mAb, anti-GHRmAb 18.24 has some distinct properties. For example, anti-GHRmAb 18.24 better recognizes by immunoblotting the hGHR and the GH binding protein (the circulating ECD of GHR) (37) (data not shown). To determine which ECD elements are recognized by anti-GHRmAb 18.24, we prepared GST fusion proteins incorporating ECD fragments for testing by immunoblotting. As diagrammed in Fig. 3A, GST/GHR1–128 contains the first 128 residues of the ECD, including subdomain 1 and the short hinge region between the two subdomains. GST/GHR129–246 contains subdomain 2, which harbors the residues that form the interface between receptor dimer partners (17). Each bacterially expressed fusion was glutathione purified, and equal protein aliquots were resolved by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with either anti-GST or anti-GHRmAb 18.24 (Fig. 3B). As expected, both fusions were detected by anti-GST. However, anti-GHRmAb 18.24 recognized nearly exclusively the fusion harboring subdomain 2 and not subdomain 1. To determine whether elements within subdomain 2 that are recognized by anti-GHRmAb 18.24 could be further defined, we prepared fusion proteins that divide this subdomain into three similarly sized components. GST/GHR129–169, GST/GHR169–202, and GST/GHR202–246 are diagrammed in Fig. 3A. Although each fusion was recognized by immunoblotting with anti-GST, none was detected by anti-GHRmAb 18.24, suggesting that the epitope, like that of anti-GHRext-mAb, requires the proper three-dimensional shape inherent in a nonfragmented subdomain for antibody recognition (more below).
Fig. 3.
Anti-GHRmAb 18.24 recognizes GHR extracellular subdomain 2 but not subdomain 1 in the context of GST fusion proteins. A, GST/GHR fusion proteins. The rabbit GHR ECD (residues 1–246) is diagrammed along with GST fusion proteins GST/GHR1–128, GST/GHR129–246, GST/GHR129–246, GST/GHR129–169, GST/GHR169–202, and GST/GHR202–246. Subdomain 1, subdomain 2, the hinge region, and the stem region are indicated. Also indicated are residues involved in GH binding (open arrows above) and the receptor dimerization interface (closed bars above). B and C, Immunological detection of GST fusion proteins by anti-GHRmAb 18.24. Each of the fusion proteins diagrammed in A was expressed in E. coli. Aliquots of the indicated extracted proteins were resolved by SDS-PAGE and immunoblotted with anti-GST or anti-GHRmAb 18.24, as indicated. Note that anti-GHRmab 18.24 reacts with subdomain 2 but not with fragments within that subdomain or with GST. The data shown are representative of two such experiments. rc, Rabbit.
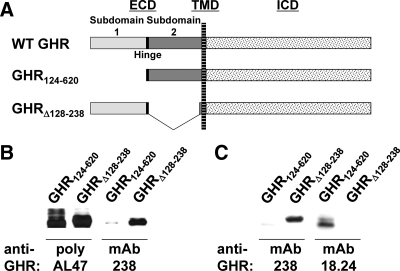
We also assessed the ability of anti-GHRmAb 18.24 to recognize full-length GHR deleted of subdomain 1 vs. subdomain 2. In Fig. 4A, the wild-type rabbit GHR is diagrammed compared with the two mutant receptors. GHR124–620 lacks extracellular subdomain 1 but retains the interdomain hinge, subdomain 2, and the transmembrane and intracellular domains; in contrast, GHRΔ128–238 lacks subdomain 2 (40). Each receptor mutant was expressed in γ2A-JAK2 cells, which express JAK2, but not GHR (40), and detergent cell extracts were resolved by SDS-PAGE and immunoblotted with anti-GHRcyt-AL47 (“poly AL47”), reactive with the GHR intracellular domain (Fig. 4B, first two lanes). As expected, this serum detected both mutant GHR (40). mAb238 is another sister clone from the immunization that yielded anti-GHRext-mAb and anti-GHRmAb 18.24. In other experiments, we found that mAb238, unlike both anti-GHRext-mAb and anti-GHRmAb 18.24, reacts with subdomain 1 and not subdomain 2 (data not shown). Consistent with this, mAb238 reacted with GHRΔ128–238 but not with GHR124–620 (Fig. 4, B, lanes 3 and 4, and C, first two lanes). In contrast to both anti-GHRcyt-AL47 and mAb238, anti-GHRmAb 18.24 failed to recognize GHRΔ128–238 but did react with GHR124–620 (Fig. 4C, lanes 3 and 4), verifying in the context of the full-length GHR that anti-GHRmAb 18.24 recognizes subdomain 2 of the GHR.
Fig. 4.
Anti-GHRmAb 18.24 recognizes GHR extracellular subdomain 2 but not subdomain 1 in the context of cellular GHR expression. A, GHR ECD deletion mutants. The rabbit GHR (residues 1–620) is diagrammed with mutants that delete extracellular subdomain 1 (GHR124–620) or extracellular subdomain 2 (GHRΔ128–238). TMD, Transmembrane domain; ICD, intracellular domain. Subdomain 1 and subdomain 2 are indicated. B and C, Anti-GHRmAb 18.24 reactivity. Extracts of human fibrosarcoma cells transfected with GHR124–620 or GHRΔ138–238 were immunoblotted with anti-GHRcyt-AL47 (poly AL47), anti-GHRmAb 238, or anti-GHRmAb 18.24, as indicated. Note that anti-GHRmAb 238 reacts with subdomain 1 and anti-GHRmAb 18.24 reacts with subdomain 2. In contrast, anti-GHRcyt-AL47, which reacts with the intracellular domain, recognizes both deletion mutant GHR. WT, Wild type.
Anti-GHRmAb 18.24 inhibits GH signaling and inducible GHR metalloproteolysis in cells expressing rabbit or hGHR
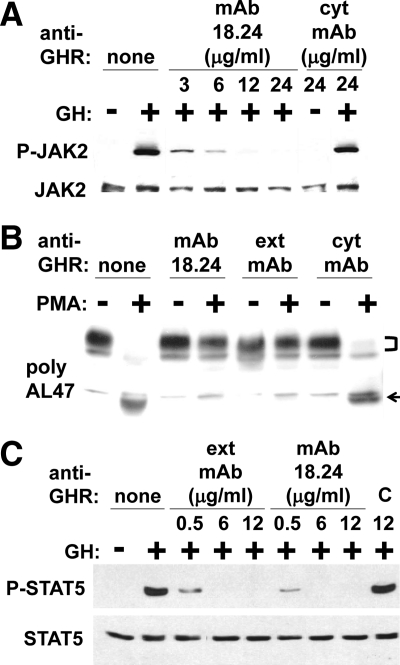
Because anti-GHRmAb 18.24, like anti-GHRext-mAb, reacts with extracellular subdomain 2 of GHR, we explored effects of anti-GHRmAb 18.24 on acute GH signaling. C14 is a human fibrosarcoma cell line that expresses JAK2 and the stably transfected rabbit GHR and responds to GH in terms of JAK2 and STAT5 activation (13, 16, 17, 40, 42, 49, 50). As previously observed, GH acutely induced JAK2 tyrosine phosphorylation in C14 cells (Fig. 5A, lane 2 vs. 1). This was unaffected when the cells were pretreated with the control monoclonal antibody, anti-GHRcyt-mAb (Fig. 5A, lanes 7 and 8). However, pretreatment with anti-GHRmAb 18.24 potently and dose dependently inhibited GH-induced JAK2 tyrosine phosphorylation, similar to the effect of anti-GHRext-mAb pretreatment on acute GH signaling previously seen (17).
Fig. 5.
Anti-GHRmAb 18.24 inhibits GH-induced signaling and PMA-induced GHR proteolysis. A and C, Serum-starved human fibrosarcoma cells stably expressing rabbit GHR (A) and human LNCaP prostate cancer cells (C) were treated ± GH (500 ng/ml; 15 min) ± anti-GHRmAb 18.24, anti-GHRext-mAb, anti-GHRcyt-mAb, or an isotype-matched control antibody (C) at the indicated concentrations. Detergent extracts were sequentially immunoblotted with anti-P-JAK2 and anti-JAK2 (A) and anti-P-STAT5 and anti-STAT5 (C). Note that anti-GHRmAb 18.24, but not control antibodies, inhibits GH-induced signaling through both rabbit and hGHR. B, Serum-starved human fibrosarcoma cells stably expressing rabbit GHR were pretreated ± anti-GHRmAb 18.24, anti-GHRext-mAb, or anti-GHRcyt-mAb and then treated ± PMA (1 μg/ml; 30 min), as detailed in Materials and Methods and as indicated. Detergent extracts were immunoblotted with anti-GHRcyt-AL47. The positions of GHR (bracket) and remnant (arrow) are indicated. Note that PMA-induced GHR proteolysis is specifically inhibited by anti-GHRmAb 18.24 and anti-GHRext-mAb.
GHR from various species is susceptible to inducible proteolysis in the ECD stem by the transmembrane metalloprotease, tumor necrosis factor-α converting enzyme (TACE) (A Disintegrin and Metalloprotease-17) (13, 17, 32, 35, 41, 44, 50–52). In cell culture, the phorbol ester, PMA, acutely promotes this proteolysis, yielding the shed soluble GH binding protein and the transmembrane/intracellular domain “remnant” protein that remains membrane associated. As seen in Fig. 5B, treatment of serum-deprived C14 cells with PMA promoted marked loss of full-length GHR (Fig. 5B, bracket) and appearance of the remnant (Fig. 5B, arrow), as monitored by anti-GHRcyt-AL47 immunoblotting of detergent cell extracts (Fig. 5B, lanes 1 and 2). As we previously demonstrated (17), pretreatment with anti-GHRext-mAb, but not anti-GHRcyt-mAb, prevented PMA-induced GHR receptor loss and remnant accumulation. Notably, anti-GHRmAb 18.24 was similarly able to inhibit inducible GHR metalloproteolysis.
The data in Figs. 4 and 5, A and B, demonstrate that these two distinct antibodies (anti-GHRmAb 18.24 and anti-GHRext-mAb), each directed at extracellular subdomain 2, are each inhibitory of GH-induced rabbit GHR signaling and (GH independent) inducible rabbit GHR metalloproteolysis. We further tested whether anti-GHRmAb 18.24, like anti-GHRext-mAb, also affects GH signaling via the hGHR (Fig. 5C). LNCaP cells were treated with GH in the presence of varying doses of the antibodies or a control antibody. GH-induced STAT5 tyrosine phosphorylation was unaffected by pretreatment with control antibody. In contrast, both anti-GHRext-mAb and GHRmAb 18.24 strongly inhibited GH-induced STAT5 activation in this human cancer cell line.
Three-dimensional epitope mapping of anti-GHRext-mAb and anti-GHRmAb 18.24
Mapping with GST fusions indicated that both anti-GHRext-mAb and anti-GHRmAb 18.24 interact with subdomain 2 and not subdomain 1 but that neither recognizes any of the fusions that divide subdomain 2 into three similarly sized fragments (Figs. 3 and 4) (17). Thus, we reasoned that the conformational epitopes for each antibody might be contributed to by discontinuously distributed residues localized to subdomain 2. To gain further insight into inhibitory mechanisms of these antibodies, we sought to map their epitopes by screening of a yeast expression library of PCR-mutagenized GHR ECD. This method was previously used (47) to map the conformational epitope of MAb 263, a stimulatory monoclonal antibody against rat GHR and cross-reactive with human and rabbit GHR (53, 54).
As previously (47), we generated by PCR a library of rabbit GHR ECD (residues 1–240) mutants with single mutations that was cloned into a yeast expression vector. In two separate experiments, transformed yeast clones (>5000) were screened by immunoblotting with either anti-GHRext-mAb or anti-GHRmAb 18.24 (2.5 μg/ml), focusing on nonreactive clones. Reasoning that mutations rendered them nonreactive, these clones were sequenced to detect the mutation. Mutants with premature stop codons or with changes that may break or create disulfide bonds or of residues known to affect subdomain 2's β-sandwich structure stability (i.e. 139W and 225F) (55) were not considered, so as to not artifactually implicate these residues.
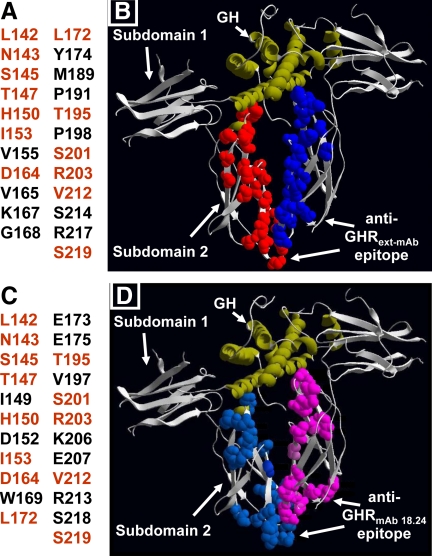
Results of the three-dimensional mapping are shown in Table 1 and Fig. 6. Residues implicated as forming the epitopes are listed in Fig. 6, A (anti-GHRext-mAb) and C (anti-GHRmAb 18.24), and detailed in Table 1. In Fig. 6, residues in common between the two epitopes are in orange. For ease of visualization, side chains of the implicated residues are shown in Fig. 6, B (anti-GHRext-mAb) and D (anti-GHRmAb 18.24), as space-filling balls (red or light blue for one receptor; blue or pink for the second receptor in the dimer) in the context of the crystal structure of the GH-engaged GHR dimer (7). Remarkably, 23 residues were implicated as forming the epitope for each antibody. Although discontinuous, residues forming each epitope are in subdomain 2 and include the surface nearest the dimer partner interface in the GH-(GHR ECD)2 crystal structure (i.e. the “lower” part of subdomain 2). Neither epitope includes subdomain 1 residues or regions of contact with GH. Notably, 13 of the 23 residues found for each of the monoclonal antibody are shared, and eight of these 13 are part of or are in close proximity to the dimerization interface. Furthermore, in general, the residues in both epitopes preferentially line the (inner) face of subdomain 2 that is disposed toward the dimer partner, rather than the (outer) face away from the dimer partner. Collectively, these epitope mapping data are consistent with our previous findings (for anti-GHRext-mAb) that antibody pretreatment inhibits GH binding far less than it inhibits GH signaling (17). Furthermore, the overlap in the epitopes for the two inhibitory antibodies suggests that they can interact with the unliganded predimerized GHR in such a way as to interfere with GH's ability to induce receptor conformational changes that reflect the activated arrangement of ECD and which rely on correct association of the dimerization domain.
Table 1.
Epitope residues for anti-GHRext-mAb and anti-GHRmAb 18.24 in rabbit GHR ECD determined by random PCR and expression screening
| Anti-GHRext-mAb |
Anti-GHRmAb 18.24 |
||
|---|---|---|---|
| Determined epitope residues in GHR sequence | Single-residue mutants with negative immunoresponse | Determined epitope residues in GHR sequence | Single-residue mutants with negative immunoresponse |
| L142 | L142P | L142 | L142S, L142P |
| N143 | N143K | N143 | N143S (2), N143Y |
| S145 | S145G, S145N | S145 | S145R, S145N |
| T147 | T147A | T147 | T147A, T147G |
| H150 | H150Q, H150R | I149 | I149T, I149S |
| I153 | I153V | H150 | H150Q, H150R |
| V155 | V155E | D152 | D152G, D152N |
| D164 | D164N | I153 | I153T |
| V165 | V165I (2) | D164 | D164N, D164Y, D164V |
| K167 | K167R | W169 | W169R (2), W169K |
| G168 | G168S | L172 | L172P, L172S |
| L172 | L172P | E173 | E173K, E173G |
| Y174 | Y174H | E175 | E175G (2) |
| M189 | M189T | T195 | T195L, T195S |
| P191 | P191S | V197 | V197F |
| T195 | T195P | S201 | S201P, S201L, S201E |
| P198 | P198L | R203 | R203G, R203S |
| S201 | S201P, S201L | K206 | K206I, K206R, K206E (2) |
| R203 | R203G, R203S | E207 | E207G |
| V212 | V212E | V212 | V212L, V212F |
| S214 | S214P | R213 | R213K |
| R217 | R217N | S218 | S218G |
| S219 | S219P (2) | S219 | S219F, S219P |
Numbers in parentheses are the frequency for the same residue.
Fig. 6.
Anti-GHRext-mAb and anti-GHRmAb 18.24 epitopes. A and C, Residues in subdomain 2 that when mutated prevent recognition of rabbit GHR by anti-GHRext-mAb (A) and anti-GHRmAb 18.24 (C). Shared residues in both epitopes are shown in orange. B and D, Side chains of residues identified in A and C are shown as space-filling balls (red or light blue for one receptor, blue or pink for the second receptor in the dimer) in the context of the crystal structure of the GH-engaged GHR dimer. GH ribbon structure is shown in green.
Discussion
Mechanisms of activation of GHR and other cytokine receptor superfamily members have been intensely studied over the past three decades. For GHR, the active signaling conformation of the receptor induced by GH is comprised of GH bound to the ECD of two GHR in a 1:2 GH:GHR stoichiometry (7, 8). This arrangement features sequential interactions between GH and each of the two receptors via elements in receptor subdomain 1 and between each receptor via their dimerization interface in subdomain 2. It was originally thought that each receptor was monomeric until GH binding brought them together as a homodimer that productively engaged intracellular signaling enzymes. More recent findings suggest that GHR exist in a dimeric assemblage even in the absence of ligand; this predimerization appears mediated by the transmembrane domain and possibly elements of subdomain 2 (11–13, 25, 56). Despite receptor predimerization in the absence of ligand, GH changes the conformation of the dimer to convert the GHR to its activated form.
Although mechanisms underlying this GH-induced transition are incompletely understood, we have described two biochemically detectable correlates of the GH-induced conformational change. First, GH induces formation of a disulfide linkage between GHR that is mediated by a Cys residue (Cys241) in the ECD stem below subdomain 2 (14, 15, 17). Second, brief treatment of several cell types with PMA (or some other protein kinase C activators) causes rapid TACE-mediated metalloproteolysis of GHR at a site within the stem (Fig. 5B) (13, 17, 32, 35, 41, 44, 50–52). However, previous exposure to GH (but not a mutated GH with defective site 2 binding) prevents PMA-induced GHR proteolysis (32). We previously reported that anti-GHRext-mAb and its Fab fragment inhibit GH-induced GHR disulfide linkage and GH signaling with similar concentration dependences (17). Although with a different concentration dependence than for inhibition of GH signaling, anti-GHRext-mAb and its Fab also prevent PMA-induced GHR proteolysis (17). These findings and others (13, 14) indicate that anti-GHRext-mAb, by virtue of interacting with extracellular subdomain 2, prevents both GH-induced GHR conformational changes required for signaling and a conformation-dependent inducible proteolysis of the GHR in its non-GH-liganded state.
Here, we extended our findings with anti-GHRext-mAb in important ways. We first tested its ability to block downstream cellular consequences of acute GH-induced signaling activation. GH-dependent cell proliferation over 48 h in the 32D-GHR model system was dramatically and specifically inhibited by a single coaddition of anti-GHRext-mAb with GH. This finding solidifies our interpretation that biologically meaningful inhibitory effects of this antibody on GH action are evident even without repeated antibody administration over this time period in vitro. We further demonstrated that in vivo anti-GHRext-mAb administration specifically inhibited GH-induced hepatic signaling in our noninvasive model signaling system. In concert with our previous findings in mice of sustained, robust, and specific interaction of radiolabeled anti-GHRext-mAb with hepatically expressed rabbit GHR (44), these new in vivo data embolden us to consider further development of conformationally dependent inhibitory GHR antibodies as potential therapeutics. Along these lines, we consider it encouraging that anti-GHRext-mAb also inhibits GH-dependent signaling in LNCaP human prostate cancer cells (Fig. 5C) (36) and RL-95–2 human endometrial carcinoma cells (data not shown).
We broadened our observations by further characterizing anti-GHRmAb 18.24, an independent clone from the immunization that yielded anti-GHRext-mAb. We now show that anti-GHRmAb 18.24, like anti-GHRext-mAb, recognizes subdomain 2, but not subdomain 1, both in the context of GST fusion proteins and of ECD deletion mutants of the full-length rabbit GHR expressed in human cells. We found that anti-GHRmAb 18.24 pretreatment also dramatically and specifically inhibited acute GH-induced signaling via both rabbit and hGHR as well as PMA-induced GHR proteolysis. These effects paralleled those of anti-GHRext-mAb, suggesting that the two antibodies have a common mechanism of inhibition and that this may reflect similarity in their epitopes within subdomain 2.
Indeed, comparative epitope mapping yielded compelling information about the three-dimensional composition of the epitopes for anti-GHRext-mAb and anti-GHRmAb 18.24. Although they have differences, both epitopes reside in discontinuous patches within subdomain 2 predominantly on the face of the subdomain that lies between the receptor dimer partners. More than half of the GHR residues implicated as forming the epitopes for each antibody are shared between the two antibodies, and most of these shared residues reside in and around the dimerization interface per se. Crystallographic data indicate that residues N143, S145, L146, T147, H150, D152, Y200, and S201 are engaged in intermolecular bonding between receptors in the GH-engaged complex (7). Notably, five of these residues (N143, S145, T147, H150, and S201) reside in the epitopes for both antibodies, and D152 is in the anti-GHRmAb 18.24 epitope.
Our previous studies indicated that the Fab fragment of anti-GHRext-mAb, like the full divalent immunoglobulin, inhibited GH signaling and PMA-inducible GHR proteolysis and that the Fab exhibits an equimolar concentration dependence compared with the divalent immunoglobulin for both of these inhibitory actions. Thus, the mechanism of inhibition by our antibodies likely does not rely on the divalent nature of the intact immunoglobulin. Informed by our functional data and epitope mapping, we propose a model for the inhibition effects of our antibodies as a point of departure. If one assumes that unliganded GHR, at least at the cell surface, can exist as dimers (via transmembrane domain and possibly extracellular subdomain 2) (11–13) in equilibrium with monomers, it is conceivable that the high affinity interaction of either anti-GHRext-mAb or anti-GHRmAb 18.24 (or their monovalent Fab fragments) with their targets (extracellular subdomain 2 of GHR monomers) might favorably compete against GHR-GHR interaction to push the equilibrium toward GHR monomers. Furthermore, the bound antibody would then prevent GH from causing the conformational change that enables productive locking of the dimerization domains and signaling. Alternatively, the antibody may be able to access exposed parts of a dimerization domain within the preformed dimer and, in so doing, prevent the conformational change that locks the key dimerization domain residues and initiates signaling in response to GH binding.
In terms of GHR proteolysis, we previously demonstrated that a dimerization interface mutant (GHRH150D) exhibits diminished predimerization and is unresponsive to GH in terms of inducible GHR disulfide linkage and signaling (13, 17). However, PMA promotes proteolysis of GHRH150D (17). Thus, monomeric GHR, although incompetent for signaling, is likely a suitable substrate for TACE. However, even if the antibody promotes formation of GHR monomers that might otherwise be susceptible to cleavage, we envision that in the presence of the antibody, TACE's access to its cleavage site in the wild-type GHR ECD stem is diminished. As noted previously (17), the IC50 for anti-GHRext-mAb (or its Fab) to inhibit GHR proteolysis is right shifted compared with its effect on GH-dependent signaling, indicating that the inhibitory mechanisms differ. Our model suggests that the antibody's enhanced inhibitory potency for GH signaling is explained by more complete prevention of GH-induced GHR conformational changes compared with its indirect impingement on the stem region cleavage site.
In addition to mechanistic implications, our characterization of anti-GHRext-mAb and anti-GHRmAb 18.24 and mapping results may have therapeutic implications. Existing GH antagonists are mutated forms of GH with changes in sites 1 and 2 that augment site 1 binding and reduce site 2 binding (57, 58). They do not activate GHR signaling but compete with wild-type GH to inhibit its actions. Thus, these agents are useful in states of GH excess, such as acromegaly. Our inhibitory antibodies or Fab similarly hold promise as GH antagonists. Like the site1/2 GH mutants, neither anti-GHRext-mAb nor anti-GHRmAb 18.24 by themselves activate GHR (Refs. 17, 36 and this study). Furthermore, we show that downstream GH-induced effects are blocked and antibody injection blocks in vivo signaling. However, whether our antibodies would be suitable inhibitors in the setting of high GH concentrations (such as acromegaly) is not yet known.
It is now believed that blockade of GH action could be beneficial even when GH levels are not elevated. For example, experimentally induced diabetic nephropathy is lessened by GH antagonism (59). In addition, onset and progression of certain experimental cancers in rodents are reduced in the absence of an intact GH axis (27–29). Future studies will be needed to determine whether our inhibitory GHR antibodies will be therapeutically active in such situations, either by themselves or in combination with site1/2 GH mutants. Furthermore, our epitope mapping may also provide information for design of other nonantibody inhibitors.
Acknowledgments
We thank helpful conversations with Dr. L. Deng, Dr. L. Liu, Dr. Y. Gan, Dr. D. Sung, Dr. P. Berry, Dr. K. Loesch, Dr. Y. Zhang, and Dr. X. Li in the Frank Laboratory and the expert assistance of the UAB Epitope Recognition and Immunoreagent Core Facility, UAB Arthritis and Musculoskeletal Center, and Dr. M.A. Accavitti-Loper.
Parts of this work were presented at the 91st Annual Meeting of The Endocrine Society, Washington, D.C., 2009.
This work was supported by the National Institutes of Health Grant R01-DK58259 (to S.J.F.), a Veterans Affairs Merit Review award (S.J.F.), the National Healthy Marriage Resource Center Project Grant 511120 (to M.J.W.), the NNSF of China Grant 30870924 (to Y.W.), and the Chinese Academy of Sciences Visiting Professorship for Senior International Scientists Grant 2010T2S03 (to P.E.L.).
Disclosure Summary: J.J., Y.W., X.W., J.X., Y.Z., K.R.Z., M.J.W., and S.J.F. have nothing to disclose. P.E.L. consults for Perseis Therapeutics, Ltd.
Footnotes
- ECD
- Extracellular domain
- GHR
- GH receptor
- GST
- glutathione-S-transferase
- hGH
- human GH
- JAK2
- Janus kinase 2
- MTT
- 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide
- STAT
- signal transducer and activator of transcription
- TACE
- tumor necrosis factor-α converting enzyme
- UAB
- University of Alabama at Birmingham.
References
- 1. Isaksson OG, Edén S, Jansson JO. 1985. Mode of action of pituitary growth hormone on target cells. Annu Rev Physiol 47:483–499 [DOI] [PubMed] [Google Scholar]
- 2. Møller N, Jørgensen JO. 2009. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev 30:152–177 [DOI] [PubMed] [Google Scholar]
- 3. Frank SJ, Messina JL. 2002. Growth hormone receptor. In: Oppenheim JJ, Feldman M. eds. Cytokine reference on-line. London: Academic Press, Harcourt; 1–21 [Google Scholar]
- 4. Nicola NA, Hilton DJ. 1998. General classes and functions of four-helix bundle cytokines. Adv Protein Chem 52:1–65 [DOI] [PubMed] [Google Scholar]
- 5. Leung DW, Spencer SA, Cachianes G, Hammonds RG, Collins C, Henzel WJ, Barnard R, Waters MJ, Wood WI. 1987. Growth hormone receptor and serum binding protein: purification, cloning and expression. Nature 330:537–543 [DOI] [PubMed] [Google Scholar]
- 6. Wang X, Lupardus P, Laporte SL, Garcia KC. 2009. Structural biology of shared cytokine receptors. Annu Rev Immunol 27:29–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Vos AM, Ultsch M, Kossiakoff AA. 1992. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science 255:306–312 [DOI] [PubMed] [Google Scholar]
- 8. Cunningham BC, Ultsch M, De Vos AM, Mulkerrin MG, Clauser KR, Wells JA. 1991. Dimerization of the extracellular domain of the human growth hormone receptor by a single hormone molecule. Science 254:821–825 [DOI] [PubMed] [Google Scholar]
- 9. Chen C, Brinkworth R, Waters MJ. 1997. The role of receptor dimerization domain residues in growth hormone signaling. J Biol Chem 272:5133–5140 [DOI] [PubMed] [Google Scholar]
- 10. Bernat B, Pal G, Sun M, Kossiakoff AA. 2003. Determination of the energetics governing the regulatory step in growth hormone-induced receptor homodimerization. Proc Natl Acad Sci USA 100:952–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gent J, van Kerkhof P, Roza M, Bu G, Strous GJ. 2002. Ligand-independent growth hormone receptor dimerization occurs in the endoplasmic reticulum and is required for ubiquitin system-dependent endocytosis. Proc Natl Acad Sci USA 99:9858–9863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown RJ, Adams JJ, Pelekanos RA, Wan Y, McKinstry WJ, Palethorpe K, Seeber RM, Monks TA, Eidne KA, Parker MW, Waters MJ. 2005. Model for growth hormone receptor activation based on subunit rotation within a receptor dimer. Nat Struct Molecular Biol 12:814–821 [DOI] [PubMed] [Google Scholar]
- 13. Yang N, Wang X, Jiang J, Frank SJ. 2007. Role of the growth hormone (GH) receptor transmembrane domain in receptor predimerization and GH-induced activation. Mol Endocrinol 21:1642–1655 [DOI] [PubMed] [Google Scholar]
- 14. Zhang Y, Jiang J, Kopchick JJ, Frank SJ. 1999. Disulfide linkage of growth hormone (GH) receptors (GHR) reflects GH-induced GHR dimerization. Association of JAK2 with the GHR is enhanced by receptor dimerization. J Biol Chem 274:33072–33084 [DOI] [PubMed] [Google Scholar]
- 15. Frank SJ, Gilliland G, Van Epps C. 1994. Treatment of IM-9 cells with human growth hormone (GH) promotes rapid disulfide linkage of the GH receptor. Endocrinology 135:148–156 [DOI] [PubMed] [Google Scholar]
- 16. Deng L, He K, Wang X, Yang N, Thangavel C, Jiang J, Fuchs SY, Frank SJ. 2007. Determinants of growth hormone receptor down-regulation. Mol Endocrinol 21:1537–1551 [DOI] [PubMed] [Google Scholar]
- 17. Jiang J, Wang X, He K, Li X, Chen C, Sayeski PP, Waters MJ, Frank SJ. 2004. A Conformationally-sensitive GHR (growth hormone (GH) receptor) antibody: impact on GH signaling and GHR proteolysis. Mol Endocrinol 18:2981–2996 [DOI] [PubMed] [Google Scholar]
- 18. Frank SJ, Gilliland G, Kraft AS, Arnold CS. 1994. Interaction of the growth hormone receptor cytoplasmic domain with the JAK2 tyrosine kinase. Endocrinology 135:2228–2239 [DOI] [PubMed] [Google Scholar]
- 19. Sotiropoulos A, Perrot-Applanat M, Dinerstein H, Pallier A, Postel-Vinay MC, Finidori J, Kelly PA. 1994. Distinct cytoplasmic regions of the growth hormone receptor are required for activation of JAK2, mitogen-activated protein kinase, and transcription. Endocrinology 135:1292–1298 [DOI] [PubMed] [Google Scholar]
- 20. VanderKuur JA, Wang X, Zhang L, Campbell GS, Allevato G, Billestrup N, Norstedt G, Carter-Su C. 1994. Domains of the growth hormone receptor required for association and activation of JAK2 tyrosine kinase. J Biol Chem 269:21709–21717 [PubMed] [Google Scholar]
- 21. Argetsinger LS, Campbell GS, Yang X, Witthuhn BA, Silvennoinen O, Ihle JN, Carter-Su C. 1993. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell 74:237–244 [DOI] [PubMed] [Google Scholar]
- 22. Frank SJ, Yi W, Zhao Y, Goldsmith JF, Gilliland G, Jiang J, Sakai I, Kraft AS. 1995. Regions of the JAK2 tyrosine kinase required for coupling to the growth hormone receptor. J Biol Chem 270:14776–14785 [DOI] [PubMed] [Google Scholar]
- 23. Tanner JW, Chen W, Young RL, Longmore GD, Shaw AS. 1995. The conserved box 1 motif of cytokine receptors is required for association with JAK kinases. J Biol Chem 270:6523–6530 [DOI] [PubMed] [Google Scholar]
- 24. Zhu T, Goh EL, LeRoith D, Lobie PE. 1998. Growth hormone stimulates the formation of a multiprotein signaling complex involving p130(Cas) and CrkII. Resultant activation of c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK). J Biol Chem 273:33864–33875 [DOI] [PubMed] [Google Scholar]
- 25. Rowlinson SW, Yoshizato H, Barclay JL, Brooks AJ, Behncken SN, Kerr LM, Millard K, Palethorpe K, Nielsen K, Clyde-Smith J, Hancock JF, Waters MJ. 2008. An agonist-induced conformational change in the growth hormone receptor determines the choice of signalling pathway. Nat Cell Biol 10:740–747 [DOI] [PubMed] [Google Scholar]
- 26. Kopchick JJ, Parkinson C, Stevens EC, Trainer PJ. 2002. Growth hormone receptor antagonists: discovery, development, and use in patients with acromegaly. Endocr Rev 23:623–646 [DOI] [PubMed] [Google Scholar]
- 27. Shen Q, Lantvit DD, Lin Q, Li Y, Christov K, Wang Z, Unterman TG, Mehta RG, Swanson SM. 2007. Advanced rat mammary cancers are growth hormone dependent. Endocrinology 148:4536–4544 [DOI] [PubMed] [Google Scholar]
- 28. Wang Z, Luque RM, Kineman RD, Ray VH, Christov KT, Lantvit DD, Shirai T, Hedayat S, Unterman TG, Bosland MC, Prins GS, Swanson SM. 2008. Disruption of growth hormone signaling retards prostate carcinogenesis in the probasin/TAg rat. Endocrinology 149:1366–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang X, Mehta RG, Lantvit DD, Coschigano KT, Kopchick JJ, Green JE, Hedayat S, Christov KT, Ray VH, Unterman TG, Swanson SM. 2007. Inhibition of estrogen-independent mammary carcinogenesis by disruption of growth hormone signaling. Carcinogenesis 28:143–150 [DOI] [PubMed] [Google Scholar]
- 30. Schmitz KR, Ferguson KM. 2009. Interaction of antibodies with ErbB receptor extracellular regions. Exp Cell Res 315:659–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tvorogov D, Anisimov A, Zheng W, Leppänen VM, Tammela T, Laurinavicius S, Holnthoner W, Heloterä H, Holopainen T, Jeltsch M, Kalkkinen N, Lankinen H, Ojala PM, Alitalo K. 2010. Effective suppression of vascular network formation by combination of antibodies blocking VEGFR ligand binding and receptor dimerization. Cancer Cell 18:630–640 [DOI] [PubMed] [Google Scholar]
- 32. Zhang Y, Guan R, Jiang J, Kopchick JJ, Black RA, Baumann G, Frank SJ. 2001. Growth hormone (GH)-induced dimerization inhibits phorbol ester-stimulated GH receptor proteolysis. J Biol Chem 276:24565–24573 [DOI] [PubMed] [Google Scholar]
- 33. Jiang J, Liang L, Kim SO, Zhang Y, Mandler R, Frank SJ. 1998. Growth hormone-dependent tyrosine phosphorylation of a GH receptor-associated high molecular weight protein immunologically related to JAK2. Biochem Biophys Res Commun 253:774–779 [DOI] [PubMed] [Google Scholar]
- 34. Kim SO, Jiang J, Yi W, Feng GS, Frank SJ. 1998. Involvement of the Src homology 2-containing tyrosine phosphatase SHP-2 in growth hormone signaling. J Biol Chem 273:2344–2354 [DOI] [PubMed] [Google Scholar]
- 35. Alele J, Jiang J, Goldsmith JF, Yang X, Maheshwari HG, Black RA, Baumann G, Frank SJ. 1998. Blockade of growth hormone receptor shedding by a metalloprotease inhibitor. Endocrinology 139:1927–1935 [DOI] [PubMed] [Google Scholar]
- 36. Xu J, Zhang Y, Berry PA, Jiang J, Lobie PE, Langenheim JF, Chen WY, Frank SJ. 2011. Growth hormone signaling in human T47D breast cancer cells: potential role for a growth hormone receptor-prolactin receptor complex. Mol Endocrinol 25:597–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aalbers AM, Chin D, Pratt KL, Little BM, Frank SJ, Hwa V, Rosenfeld RG. 2009. Extreme elevation of serum growth hormone-binding protein concentrations resulting from a novel heterozygous splice site mutation of the growth hormone receptor gene. Horm Res 71:276–284 [DOI] [PubMed] [Google Scholar]
- 38. Liang L, Zhou T, Jiang J, Pierce JH, Gustafson TA, Frank SJ. 1999. Insulin receptor substrate-1 enhances growth hormone-induced proliferation. Endocrinology 140:1972–1983 [DOI] [PubMed] [Google Scholar]
- 39. Yang N, Langenheim JF, Wang X, Jiang J, Chen WY, Frank SJ. 2008. Activation of growth hormone receptors by growth hormone and growth hormone antagonist dimers: insights into receptor triggering. Mol Endocrinol 22:978–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang N, Jiang J, Deng L, Waters MJ, Wang X, Frank SJ. 2010. Growth hormone receptor targeting to lipid rafts requires extracellular subdomain 2. Biochem Biophys Res Commun 391:414–418 [DOI] [PubMed] [Google Scholar]
- 41. Wang X, He K, Gerhart M, Huang Y, Jiang J, Paxton RJ, Yang S, Lu C, Menon RK, Black RA, Baumann G, Frank SJ. 2002. Metalloprotease-mediated GH receptor proteolysis and GHBP shedding. Determination of extracellular domain stem region cleavage site. J Biol Chem 277:50510–50519 [DOI] [PubMed] [Google Scholar]
- 42. He K, Wang X, Jiang J, Guan R, Bernstein KE, Sayeski PP, Frank SJ. 2003. Janus kinase 2 determinants for growth hormone receptor association, surface assembly, and signaling. Mol Endocrinol 17:2211–2227 [DOI] [PubMed] [Google Scholar]
- 43. Frank SJ, Wang X, He K, Yang N, Fang P, Rosenfeld RG, Hwa V, Chaudhuri TR, Deng L, Zinn KR. 2006. In vivo imaging of hepatic growth hormone signaling. Mol Endocrinol 20:2819–2830 [DOI] [PubMed] [Google Scholar]
- 44. Wang X, Jiang J, Warram J, Baumann G, Gan Y, Menon RK, Denson LA, Zinn KR, Frank SJ. 2008. Endotoxin-induced proteolytic reduction in hepatic growth hormone receptor: a novel mechanism for GH insensitivity. Mol Endocrinol 22:1427–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zinn KR, Chaudhuri TR, Szafran AA, O'Quinn D, Weaver C, Dugger K, Lamar D, Kesterson RA, Wang X, Frank SJ. 2008. Noninvasive bioluminescence imaging in small animals. ILAR J 49:103–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zinn KR, Szalai AJ, Stargel A, Krasnykh V, Chaudhuri TR. 2004. Bioluminescence imaging reveals a significant role for complement in liver transduction following intravenous delivery of adenovirus. Gene Ther 11:1482–1486 [DOI] [PubMed] [Google Scholar]
- 47. Wan Y, Zheng YZ, Harris JM, Brown R, Waters MJ. 2003. Epitope map for a growth hormone receptor agonist monoclonal antibody, MAb 263. Mol Endocrinol 17:2240–2250 [DOI] [PubMed] [Google Scholar]
- 48. Liang L, Jiang J, Frank SJ. 2000. Insulin receptor substrate-1-mediated enhancement of growth hormone-induced mitogen-activated protein kinase activation. Endocrinology 141:3328–3336 [DOI] [PubMed] [Google Scholar]
- 49. He K, Loesch K, Cowan JW, Li X, Deng L, Wang X, Jiang J, Frank SJ. 2005. JAK2 enhances the stability of the mature GH receptor. Endocrinology 145:4755–4765 [DOI] [PubMed] [Google Scholar]
- 50. Loesch K, Deng L, Cowan JW, Wang X, He K, Jiang J, Black RA, Frank SJ. 2006. JAK2 influences growth hormone receptor metalloproteolysis. Endocrinology 147:2839–2849 [DOI] [PubMed] [Google Scholar]
- 51. Zhang Y, Jiang J, Black RA, Baumann G, Frank SJ. 2000. TACE is a growth hormone binding protein sheddase: the metalloprotease TACE/ADAM-17 is critical for (PMA-induced) growth hormone receptor proteolysis and GHBP generation. Endocrinology 141:4324–4348 [DOI] [PubMed] [Google Scholar]
- 52. Guan R, Zhang Y, Jiang J, Baumann CA, Black RA, Baumann G, Frank SJ. 2001. Phorbol ester- and growth factor-induced growth hormone (GH) receptor proteolysis and GH-binding protein shedding: relationship to GH receptor down-regulation. Endocrinology 142:1137–1147 [DOI] [PubMed] [Google Scholar]
- 53. Rowlinson SW, Behncken SN, Rowland JE, Clarkson RW, Strasburger CJ, Wu Z, Baumbach W, Waters MJ. 1998. Activation of chimeric and full-length growth hormone receptors by growth hormone receptor monoclonal antibodies. A specific conformational change may be required for full-length receptor signaling. J Biol Chem 273:5307–5314 [DOI] [PubMed] [Google Scholar]
- 54. Barnard R, Bundesen PG, Rylatt DB, Waters MJ. 1985. Evidence from the use of monoclonal antibody probes for structural heterogeneity of the growth hormone receptor. Biochem J 231:459–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Baumgartner JW, Wells CA, Chen CM, Waters MJ. 1994. The role of the WSXWS equivalent motif in growth hormone receptor function. J Biol Chem 269:29094–29101 [PubMed] [Google Scholar]
- 56. Frank SJ. 2002. Receptor dimerization in GH and erythropoietin action—it takes two to tango, but how? Endocrinology 143:2–10 [DOI] [PubMed] [Google Scholar]
- 57. Cunningham BC, Wells JA. 1991. Rational design of receptor-specific variants of human growth hormone. Proc Natl Acad Sci USA 88:3407–3411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Goffin V, Bernichtein S, Carrière O, Bennett WF, Kopchick JJ, Kelly PA. 1999. The human growth hormone antagonist B2036 does not interact with the prolactin receptor. Endocrinology 140:3853–3856 [DOI] [PubMed] [Google Scholar]
- 59. Flyvbjerg A, Bennett WF, Rasch R, Kopchick JJ, Scarlett JA. 1999. Inhibitory effect of a growth hormone receptor antagonist (G120K-PEG) on renal enlargement, glomerular hypertrophy, and urinary albumin excretion in experimental diabetes in mice. Diabetes 48:377–382 [DOI] [PubMed] [Google Scholar]