Abstract
Cortistatin (CST) and somatostatin (SST) evolve from a common ancestral gene and share remarkable structural, pharmacological, and functional homologies. Although CST has been considered as a natural SST-analogue acting through their shared receptors (SST receptors 1–5), emerging evidence indicates that these peptides might in fact exert unique roles via selective receptors [e.g. CST, not SST, binds ghrelin receptor growth hormone secretagogue receptor type 1a (GHS-R1a)]. To determine whether the role of endogenous CST is different from SST, we characterized the endocrine-metabolic phenotype of male/female CST null mice (cort−/−) at hypothalamic-pituitary-systemic (pancreas-stomach-adrenal-liver) levels. Also, CST effects on hormone expression/secretion were evaluated in primary pituitary cell cultures from male/female mice and female primates (baboons). Specifically, CST exerted an unexpected stimulatory role on prolactin (PRL) secretion, because both male/female cort−/− mice had reduced PRL levels, and CST treatment (in vivo and in vitro) increased PRL secretion, which could be blocked by a GHS-R1a antagonist in vitro and likely relates to the decreased success of female cort−/− in first-litter pup care at weaning. In contrast, CST inhibited GH and adrenocorticotropin-hormone axes in a gender-dependent fashion. In addition, a rise in acylated ghrelin levels was observed in female cort−/− mice, which were associated with an increase in stomach ghrelin/ghrelin O-acyl transferase expression. Finally, CST deficit uncovered a gender-dependent role of this peptide in the regulation of glucose-insulin homeostasis, because male, but not female, cort−/− mice developed insulin resistance. The fact that these actions are not mimicked by SST and are strongly gender dependent offers new grounds to investigate the hitherto underestimated physiological relevance of CST in the regulation of physiological/metabolic processes.
Cortistatin (CST) is a neuropeptide that shares structural, pharmacological, and functional homology with somatostatin (SST), where these peptides have evolved from a common ancestral gene (1, 2) and bind with comparable affinity to SST receptors (sst1–sst5) (3). The actions of SST are well studied and diverse, including the (patho)physiological regulation of pituitary hormone release, locomotor activity, pancreatic function, gastrointestinal motility (1, 2, 4). Although not as extensively studied, exogenous CST, like SST, has been shown to inhibit pituitary GH and pancreatic insulin release (4–6). However, the functions of SST and CST may not be one and the same (6, 7), because the systemic and central expression of SST and CST do not precisely overlap (4, 8, 9), which may explain the ability of CST to exert unique immunomodulatory and antiinflammatory actions not shared by SST (10). Also, even when CST and SST are colocalized to the same neuron, their expression was shown to be differentially regulated (9), which may in part explain the fact that CST, but not SST, induces slow-wave sleep and hypomotility (11).
These and other results have led to the hypothesis that CST exerts its unique actions through specific receptors not affected comparably by SST, although the true nature of these putative receptors remains elusive (1, 7, 12). We do know that much of the functional versatility of SST is related to the complex set of widely distributed receptors (3), where each cell type expresses variable levels of sst, which can interact with each other or with other G protein-coupled receptors forming homo- and/or heterodimers to activate different signaling cascades and mediate multiple actions (3, 13). Therefore, molecular differences between CST and SST may cause a unique pattern of homo- and/or heterodimers after ligand binding, which could result in a differential activation of intracellular signal transduction pathways. Consistent with this hypothesis is a recent observation by our group showing novel truncated sst5 variants in human (14) and mice (15), which display differential ability to mediate CST- and SST-induced Ca2+ signaling. Finally, it remains a possibility that CST activates non-sst to exert its unique actions, and one study suggested that CST may interact with the Mas-related MrgX2 receptor; however, this receptor is not present in rodents and more likely functions as a proadrenomedulin receptor (16). Interestingly, CST, but not SST, was shown to displace acylated ghrelin from its binding sites in the pituitary [likely growth hormone secretagogue receptor type 1a (GHS-R1a)] (17), suggesting a unique functional interaction between CST and the ghrelin system (12), where this action of CST may provide a potential link between the antiinflammatory actions of CST and ghrelin (18).
Although studies examining the effects of exogenously administered CST indicate that some, but not all, actions mimic those of SST (4, 6), information is lacking regarding the physiologic role of endogenous CST in maintaining endocrine function. To address this issue, in the present study, we characterized the hypothalamic-pituitary-systemic phenotypes of male and female CST null (cort−/−) mice and compared them with endocrine phenotype of SST null (sst−/−) mice, which has been previously reported (19–24) or investigated in parallel with cort−/− in the current series of studies. In addition, primary pituitary cell cultures from normal male/female mice and female primates were used to further ascertain whether CST can directly regulate the expression and secretion of pituitary hormones in diverse species. Our results demonstrate that many of the actions of endogenous CST are distinct from those previously established for SST.
Materials and Methods
In vivo animal models
All experimental procedures were approved by the Animal Care Committees of the University of Córdoba and University of Illinois at Chicago. The development and validation of cort−/− mice were previously described (25); sst−/− mice were generously provided by Dr. Hochgeschwender (24). C57Bl/6J male and female mice, heterozygous for the cort null or sst null allele, were bred to C57Bl/6J mice purchased from Charles River (Barcelona, Spain) to generate cort+/+, cort+/−, and cort−/− or sst+/+, sst+/−, and sst−/− mice. Genotypes were determined by PCR of tail-snip DNA as previously reported (21). Specific primer sequences and genotyping protocols are provided in Supplemental Fig. 1 and Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org. All mice were housed under standard light and temperature conditions (12-h light, 12-h dark cycle, 22–24 C), with free access to food and tap water. A subset of mice was supplied standard rodent chow (SAFE-diets, Barcelona, Spain) and killed (∼11 wk old) by decapitation, without anesthesia, under fed conditions. Blood and tissues were snap frozen and stored at −80 C for further analysis. Other sets of mice were provided: 1) standard chow or a low-fat diet (starting at 4 wk of age; 10% kcal from fat; Research Diets, Gentofte, Denmark) and glucose tolerance test (GTT) and insulin tolerance test (ITT) performed at 15–17 wk of age, or 2) standard chow diet and treated with CST-14 at approximately 20 wk of age by continuous delivery via sc osmotic pumps (125 ng/h for 7d; Alzet, Cupertino, CA), as previously described (26).
Table 1.
Proportion of immuno-positive somatotroph, lactotroph, corticotroph, thyrotroph, and gonadotroph cells from male and female cort+/+ and cort−/− mice (n = 4 mice/genotype per gender)
| Males |
Females |
|||
|---|---|---|---|---|
| cort+/+ (mean ± sem) | cort−/− (mean ± sem) | cort+/+ (mean ± sem) | cort−/− (mean ± sem) | |
| Somatotrophs | 55.92 ± 2.01 | 56.70 ± 2.10 | 44.70 ± 1.82 | 44.57 ± 1.16 |
| Lactotrophs | 18.62 ± 0.92 | 20.76 ± 1.29 | 21.35 ± 2.33 | 23.01 ± 1.19 |
| Corticotrophs | 11.41 ± 1.34 | 14.11 ± 2.24 | 9.85 ± 1.16 | 11.86 ± 1.40 |
| Thyrotrophs | 6.78 ± 0.65 | 6.64 ± 0.57 | 5.71 ± 0.46 | 5.76 ± 0.80 |
| Gonadotrophs | 8.10 ± 0.76 | 8.69 ± 0.94 | 6.72 ± 0.51 | 5.69 ± 0.22 |
Values represent the mean ± sem (three to four pictures/immunohistochemistry per genotype/mice), where the proportions shown were derived from counting more than 1000 cells/slide for each pituitary hormone tested.
In vitro cell models (mouse and primate)
Experiment 1
For assessment and comparison of basal GH, prolactin (PRL), and ACTH release, pituitaries of adult male and female cort+/+ and cort−/− mice [n = 4 (three to five pituitaries pooled/experiment)] were dispersed into single cells and cultured in serum-containing α-medium as previously described (15, 21, 22).
Experiment 2
For studies examining the direct effects of CST on the expression of all mouse pituitary hormones [GH, PRL, proopiomelanocortin (POMC) (ACTH-precursor), β-subunits of LH, FSH, and TSH and the glycoprotein α-subunit (GAS)] and sst1–sst5, as well as on GH, PRL, and ACTH release, pituitaries of normal adult male and female C57Bl/6J mice [n = 4 experiments (three to five pituitaries pooled/experiment)] were also dispersed into single cells and cultured. In addition, pituitaries from female nonhuman primates [olive baboon (Papio anubis); 9–14 yr of age] were obtained after pentobarbital sodium overdose from control animals used on other studies and dispersed into single cells as previously described (21, 27). Doses of CST used have previously identified to exert a maximal effect on pituitary hormone synthesis and/or release in other models (23, 28). In addition, an antagonist for the GHS-R1a (BIM-28163) (28) was used to determine whether CST signals through this receptor to mediated PRL release.
Assessment of mRNA levels
Total RNA was extracted from tissues or primary pituitary cultures, reverse transcribed, and amplified by quantitative real-time RT-PCR as previously described (15, 21, 22, 26, 27). Information about primers is provided in Supplemental Table 1. mRNA copy numbers of all mouse transcripts were adjusted by a normalization factor (NF) calculated from the mRNA copy numbers of two to four separate housekeeping genes (glyceraldehyde-3-phosphate-dehydrogenase, hypoxanthine-ribosyltransferase, β-actin, and/or cyclophilin-A) using the GeNorm 3.3 application (29), whereas expression of the mouse and baboon transcripts analyzed in pituitary cultures were adjusted by cyclophilin-A expression, where mouse NF or mouse/baboon cyclophilin-A mRNA levels did not significantly vary between experimental groups, within tissue type (data not shown).
Analysis of fertility and pup survival in cort−/−, compared with cort+/+ mice
We compared the time with conception (by inspection of plugs), days of gestation, and litter size of female cort−/− and cort+/+ mice. In addition, we compared the percentage of cort−/− and cort+/+ female that raised a litter successfully until weaning (21 d). Female mice were mated twice [at 9–10 (first crossbred) and at 13–30 (second crossbred) wk of age].
For details of in vitro cell models, immunohistochemistry of pituitary cells, measurement of free cytosolic calcium concentration ([Ca2+]i), assessment of circulating hormones and metabolite, GTT and ITT, and statistical analysis, please see Supplemental Material and Methods.
Results
Confirmation of CST deficiency
Genotypes of all mice (Supplemental Fig. 1A) were determined by using a protocol previously reported for the genotyping and validation of the sst colony (21). CST mRNA was undetectable in cerebral cortex and hypothalamus of cort−/− mice (Supplemental Fig. 1B). However, CST expression in cort+/− compared with cort+/+ was 62.6 ± 13.7 and 29.9 ± 6.9% in males and females, respectively, indicating that there is limited or no compensation by the remaining CST allele. It should be noted that there were no significant differences between cort+/+ and cort+/− mice in all the endpoints analyzed (data not shown); and therefore, only comparisons between cort+/+ and cort−/− are shown and discussed below.
Impact of CST deficiency on somatotrope, lactotrope, corticotrope, gonadotrope, and thyrotrope axes
Somatotropes
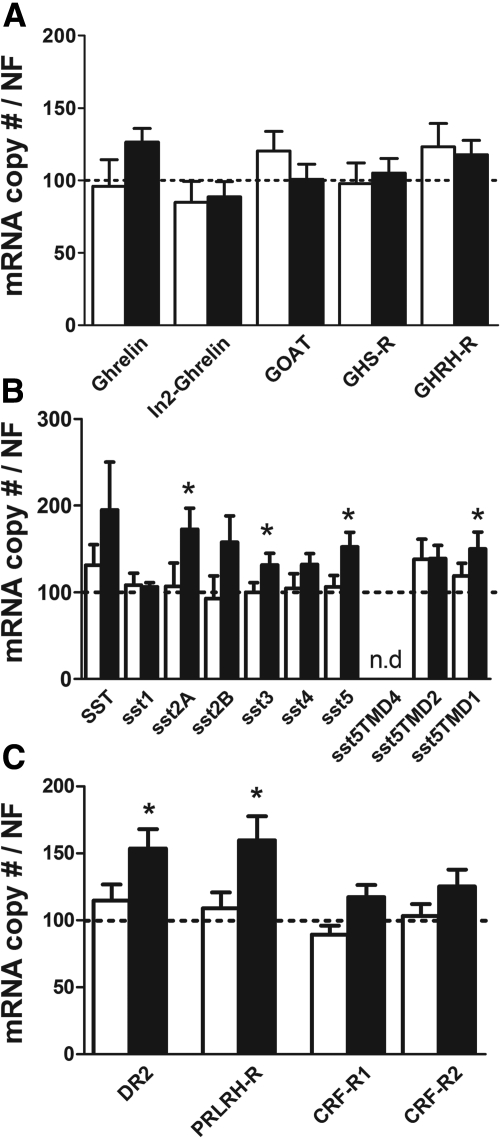
Circulating GH levels were significantly higher in male and female cort−/− mice, whereas GH mRNA levels where elevated in female but not male cort−/− pituitaries, compared with cort+/+ controls (Fig. 1A). Differences in GH output between genotypes were not associated with significant changes in expression of hypothalamic GHRH or SST (Supplemental Table 2), pituitary size (data not shown), or the appearance and relative proportion of somatotropes (Table 1 and Supplemental Fig. 2). Furthermore, elevation in GH output in cort−/− mice was not associated with changes in pituitary expression of key components of pathways, which would be predicted to enhance GH release [GHRH receptor (GHRH-R), growth hormone secretagogue receptor (GHS-R), ghrelin, In2-ghrelin, and ghrelin O-acyl transferase (GOAT)] (Fig. 2A), whereas paradoxically there was a female-specific increase in pituitary expression of sst2A/sst3/sst5/sst5TMD1 (Fig. 2B).
Fig. 1.
Regulation of pituitary somatotrope, lactotrope, and corticotrope cell axes in male and female wild-type (cort+/+, open columns) vs. cort−/− (black columns) mice. A, Basal GH release (left), GH expression (middle), and circulating IGF-I levels (right). B, Growth curves from males and females cort+/+ (solid lines) and cort−/− (dotted line) littermates from 4 to 13 wk of age. C, Basal PRL release (left), PRL expression (middle), and percentage of survival of the first litter of female cort+/+ (open column) vs. cort−/− (solid column) mice (right). D, Basal PRL release in a second group of male and female cort+/+ (open columns) and cort−/− (black columns) as compared with male and female sst+/+ (open columns) and sst−/− (gray columns). E, Basal PRL levels in 7 d vehicle-infused (Veh) and CST14-infused (CST) female cort+/+ and cort−/− mice via sc osmotic pumps. F, Basal ACTH hormone release (left), POMC expression (middle), and circulating corticosterone levels (right). Values represent mean ± sem (six to seven mice/genotype per gender) of hormonal circulating levels or absolute hormone mRNA copy numbers (adjusted by NF). Percentages of litter survival from female mice are expressed as mean ± sem (n = 11–37 breeders). Asterisks indicate values that significantly differ from their controls (cort+/+ or sst+/+) (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Fig. 2.
Expression of pituitary components involved in the regulation of somatotrope, lactotrope, and corticotrope axes in cort−/− and cort+/+ mice. A, Expression of ghrelin, In2-ghrelin, GOAT, ghrelin receptor (GHS-R), and GHRH-R; B, Expression of SST and SST receptors isoforms/variants (sst); C, Expression of DR2, PRL-releasing hormone receptor (PRLRH-R), and CRF-R1 and CRF-R2 in male (open columns) and female (solid columns) cort−/− mice. Values are showed as relative percentage of male or female cort−/− vs. control (cort+/+) mice (shown by the dotted line set at 100%) and represent the mean ± sem of five to eight mice/gender. Asterisk (*, P < 0.05) indicates values that significantly differ from cort+/+ within gender.
The elevated plasma GH levels found in cort−/− did not result in significant changes in circulating IGF-I levels (Fig. 1A) or hepatic expression of total IGF-I or IGF-I variants 1–5, acid-labile subunit, GH-R, PRL receptor, or MUP-3 (Supplemental Table 2). Pup weight at weaning (3–4 wk old) did not differ between cort−/− and controls (male cort+/+ 8.94 ± 0.34 vs. cort−/− 8.04 ± 0.38 g; female cort+/+ 8.50 ± 0.41 vs. cort−/− 8.70 ± 0.31 g) and growth rates were similar (Fig. 1B). Also, organ weights [fat depots (visceral/sc), liver, and pancreas] (Table 2) and food intake (male cort+/+ 3.88 ± 0.12 vs. cort−/− 4.11 ± 0.15 g/d; female cort+/+ 3.78 ± 0.11 vs. cort−/− 3.59 ± 0.11 g/d) were similar between cort−/− and cort+/+ mice.
Table 2.
Body weight, organs weight, and glucose levels in ad libitum-fed and fasting state and glucagon levels in male and female cort−/− mice vs. littermate controls (cort+/+)
| Males |
Females |
|||
|---|---|---|---|---|
| cort+/+ (mean ± sem) | cort−/− (mean ± sem) | cort+/+ (mean ± sem) | cort−/− (mean ± sem) | |
| Body weight (g) | 26.61 ± 0.57 | 25.87 ± 0.66 | 20.64 ± 0.44 | 20.01 ± 0.25 |
| Visceral fat (g) | 0.44 ± 0.03 | 0.43 ± 0.04 | 0.33 ± 0.04 | 0.25 ± 0.02 |
| Subcutaneous fat (g) | 0.29 ± 0.02 | 0.25 ± 0.02 | 0.20 ± 0.03 | 0.16 ± 0.03 |
| Liver (g) | 1.13 ± 0.05 | 1.08 ± 0.06 | 0.95 ± 0.04 | 0.88 ± 0.01 |
| Pancreas (g) | 0.12 ± 0.02 | 0.13 ± 0.02 | 0.10 ± 0.00 | 0.09 ± 0.00 |
| Glucose; fed (mg/dl) | 169.00 ± 10.04 | 146.25 ± 16.27 | 160.10 ± 9.86 | 156.37 ± 9.81 |
| Glucose; fasted (mg/dl) | 121.67 ± 9.67 | 137.75 ± 7.77 | 101.30 ± 8.61 | 118.13 ± 12.16 |
| Glucagon (ng/ml) | 2.79 ± 0.69 | 2.14 ± 0.21 | 2.69 ± 0.15 | 2.97 ± 0.45 |
Values represent the mean ± sem of five to eight mice/genotype per gender.
Lactotropes
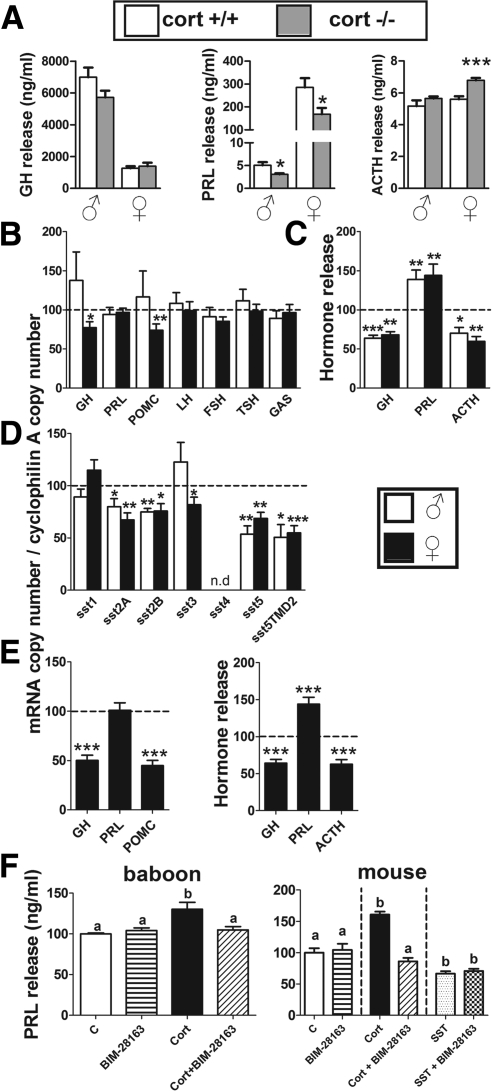
Circulating PRL levels were markedly decreased in both male/female cort−/− mice at 11 wk of age (Fig. 1C, left). A reduction in circulating PRL levels was also observed in another set of cort−/− mice, whereas PRL levels in sst−/− mice were elevated, compared with controls (16–17 wk old) (Fig. 1D). Plasma PRL levels were higher in female cort+/+, compared with male cort+/+ mice, and this gender-related divergence was maintained in cort−/− mice (Fig. 1, C left and D). Moreover, in vivo CST treatment increased basal circulating PRL levels in female cort+/+ and cort−/− mice as compared with their vehicle-treated control groups (Fig. 1E). Interestingly, PRL levels were rescued in CST-treated cort−/− to levels comparable with those observed in vehicle-treated cort+/+ mice (Fig. 1E). The reduction in PRL output observed in cort−/− mice was not associated with changes in pituitary PRL mRNA levels (Fig. 1C, middle) or the appearance and relative proportion of lactotropes (Table 1 and Supplemental Fig. 2). However, pituitary expression of dopamine receptor type-2 (DR2) and PRL-releasing hormone receptor were selectively elevated in female cort−/− mice, compared with controls (Fig. 2C). Days to conception, length of gestation, and litter size of cort−/− females did not differ from that of cort+/+ females (Supplemental Table 3). However, the percentage of cort−/− females that successfully cared for their first litter until weaning was drastically reduced compared with cort+/+ female mice (Fig. 1C, right), but these differences disappeared in the second litter (Supplemental Table 3).
Corticotropes
Circulating ACTH levels were significantly elevated in female but not male cort−/− mice, compared with controls, which was reflected by a significant increase in pituitary POMC expression only in female cort−/− mice (Fig. 1F), without changes in the appearance or proportion of corticotropes (Table 1 and Supplemental Fig. 2) or in the expression of pituitary corticotrope-releasing factor receptors (CRF-R)1/2 (Fig. 2C). Despite the sex-dependent differences in ACTH synthesis and release, both male/female cort−/− mice displayed elevations in circulating corticosterone levels (Fig. 1F), although these alterations were not associated with changes in the expression of hypothalamic factors known to regulate adrenal-axis function (CRF, urocortins 2 and 3, POMC, or neuropeptide Y) (Supplemental Table 2).
Gonadotropes and thyrotropes
CST deficiency did not alter pituitary expression of β-subunits of LH, FSH and TSH, or GAS (Supplemental Fig. 3A) and the appearance and proportion of gonadotropes and thyrotropes did not differ from controls (Table 1 and Supplemental Fig. 2). In addition, there was no change in the expression level of hypothalamic factors known to regulate gonadotrope (GnRH, kisspeptin-1) or thyrotrope (TRH) function (Supplemental Table 2). Functional integrity of the gonadotropes is further supported by the fact that circulating levels of LH, testosterone, and estradiol did not differ between cort−/− and cort+/+ mice (Supplemental Fig. 3B) and by the fact that female cort−/− mice were fertile (Supplemental Table 3).
In vitro pituitary hormone output in the presence and absence of endogenous CST and effect of CST on primary pituitary cell cultures of mouse and primate
Decreased levels of circulating PRL observed in vivo in cort−/− mice of both genders and the elevated ACTH levels observed only in female cort−/− mice were similarly maintained in vitro, under basal conditions (Fig. 3A). In contrast, basal GH release did not differ between primary pituitary cultures from cort−/− and cort+/+ mice (Fig. 3A).
Fig. 3.
Direct effect of CST or lack of endogenous CST on pituitary hormonal expression and secretion of male/female mice and female baboons. A, GH, PRL, and ACTH release levels (24-h culture; ng/ml) in primary pituitary cell cultures of male and female cort+/+ (white columns) and cort−/− (gray columns) mice under basal condition. B, Effect of CST-14 (100 nm, 24 h) on GH, PRL, POMC, LH, FSH, TSH, and GAS expression on primary pituitary cell cultures of male (white columns) and female (black columns) wild-type (cort+/+) mice. C, Effect of CST-14 (100 nm, 24 h) on GH, PRL, and ACTH release on primary pituitary cell cultures of male (white columns) and female (black columns) wild-type (cort+/+) mice. D, Effect of CST-14 (100 nm, 24 h) on SST receptors isoforms/variants (sst) on primary pituitary cell cultures of male (white columns) and female (black columns) wild-type (cort+/+) mice. E, Effect of CST-17 (100 nm, 24 h) on GH, PRL, and POMC expression (left) and on GH, PRL, and ACTH release (right) in primary pituitary cell cultures of female baboon. F, Effect of 100 nm CST-17 (in baboons; 100 nm), 1 μm CST-14, or 100 nm SST-14 (in mice) (24 h) alone or in combination with BIM-28163 (GHS-R1a antagonist; 10 nm) on PRL release in primary pituitary cell cultures of female baboon and mice [vehicle-treated control (C) was set at 100%]. Values are represented as the means ± sem of three to four independent experiments (three to five wells/treatment per genotype/gender) and in B–E are expressed as percentage of vehicle-treated controls (shown by the dotted line set at 100%). Asterisks (*, P < 0.05; **, P < 0.01; ***, P < 0.001) indicate values that differ from the corresponding controls within genders (from vehicle-treated controls for B–E and from cort+/+ within genders for A). In F, values that differ significantly (P < 0.05) are designated by different letters (a and b).
Consistent with the observations obtained in cort−/− mice in vivo, CST did not alter the expression of PRL, β-subunits of LH, FSH, and TSH, or GAS in pituitary cultures of normal male or female mice (Fig. 3B). However, CST inhibited GH and POMC mRNA levels in pituitary cells from normal female, but not male, mice (Fig. 3B). Moreover, CST significantly suppressed GH and ACTH release in vitro, whereas it increased PRL secretion in both normal male/female mice (Fig. 3C). In line with both, the stimulatory and inhibitory, effects of CST on pituitary hormone release, we observed that CST increased [Ca2+]i levels, a second messenger required for hormone secretory vesicle release (30), in a select population of mouse pituitary cells (Table 3), whereas in another population, CST decreased [Ca2+]i levels. Therefore, these results reinforce the idea of the existence of two populations of pituitary cells that differentially respond to CST, one negatively (likely somatotropes and/or corticotropes) and another positively (likely lactotropes).
Table 3.
Percentage of primary cultured pituitary cells showing positive or negative changes in [Ca2+]i in response to CST-14 (100 nm) in female mice
| cort+/+ (75 cells) |
|||
|---|---|---|---|
| Cells | %Max ± sem | Time ± sem | |
| ↑ [Ca2+]i | 12% | 140.47 ± 9.82 | 41.11 ± 7.49 |
| ↓ [Ca2+]i | 16% | 77.69 ± 3.72 | 40.00 ± 6.42 |
Percentage of maximum response (%Max) and time of response to CST administration are also indicated.
CST treatment did not alter sst1 expression in pituitary cultures, whereas it decreased sst2A/sst2B/sst5/sst5TMD2 in both male/female mice and decreased sst3 only in pituitary cultures from females (Fig. 3D).
As observed in mice, in vitro treatment with CST inhibited the spontaneous secretion of GH and ACTH and decreased GH and POMC expression in pituitary cultures from female baboons (Fig. 3E). Moreover, the same CST treatment stimulated baboon PRL release, whereas it did not alter PRL expression. Of note, CST-stimulated PRL release was completely blocked by an antagonist of GHS-R1a (Fig. 3F, left), suggesting that the stimulatory effect of CST on baboon PRL release was directly mediated via the ghrelin receptor. As observed with the baboon model, the stimulatory effect of CST on mouse PRL release may also be mediated via GHS-R1a, because CST-stimulated PRL release observed in mouse primary pituitary culture was completely blocked by the GHS-R1a antagonist, whereas coincubation of the antagonist with SST did not alter the inhibitory effect observed with SST alone on mouse basal PRL release (Fig. 3F, right).
Effect of genotype (cort−/− vs. sst−/−) on the ghrelin system
Because we have previously reported that sst−/− mice have elevated levels of total ghrelin, but not acylated ghrelin (21, 22, 26), and ghrelin can act at the pituitary level to regulate somatotrope, lactotrope, and corticotrope functions, we explored in detail the ghrelin system in cort−/− mice as well as expanded our investigations in sst−/− mice. Specifically, plasma levels of acylated and total (acylated + unacylated) ghrelin were clearly elevated in female but not in male cort−/− mice compared with cort+/+ controls (Fig. 4A, left). This pattern compared well with, and is likely supported by, the markedly increased levels of expression of ghrelin and GOAT in the stomach of female cort−/− mice (Fig. 4B, left). In contrast to the up-regulation of ghrelin and GOAT expression in the stomach of cort−/− mice, pituitary (Fig. 2A) and hypothalamic (Supplemental Table 2) expressions of the various components of the ghrelin system were similar to controls (i.e. ghrelin, In2-ghrelin variant, GHS-R, and GOAT).
Fig. 4.
Impact of lack of endogenous CST or SST in the regulation of stomach ghrelin and SST systems. A, Circulating levels of acylated and total (acylated plus nonacylated) ghrelin in male and female cort+/+ (white columns) vs. cort−/− (gray columns) mice (left) and sst+/+ (white columns) vs. sst−/− (gray columns) mice (right). B, Stomach expression of ghrelin and GOAT of male (open columns) and female (solid columns) cort+/+ vs. cort−/− mice (left panel) and sst+/+ vs. sst−/− mice (right panel). C, Stomach expression of SST and SST receptors isoforms/variants (sst) of male (open columns) and female (solid columns) cort+/+ vs. cort−/− mice. D, Circulating SST levels in male and female cort+/+ (white columns) vs. cort−/− (gray columns) mice. Circulating hormone levels (A and D) are represented as the mean ± sem of male and female cort−/− or sst−/− vs. littermate controls (cort+/+ or sst+/+, respectively; four to 10 mice/genotype per gender). Expression levels (B and C) are showed as relative percentage of male or female cort−/− vs. control (cort+/+) mice (shown by the dotted line set at 100%) and represent the mean ± sem of five to eight mice/genotype per gender. Asterisks (*, P < 0.05; **, P < 0.01; ***, P < 0.001) indicate differences between controls (+/+) and knockout (−/−) mice within gender. n.d., Not determined; n.m., not measured.
Of note, the rise in acylated ghrelin observed in female cort−/− mice was associated with an increase in stomach SST expression (a known inhibitor of gastrointestinal ghrelin release), without alterations in the expression of sst (Fig. 4C) or gastrin (Supplemental Table 2). The increase in stomach SST expression observed in female cort−/− was associated with an increase in circulating SST levels, which did not reach statistical significance (P = 0.07) (Fig. 4D).
As in cort−/− mice, the ghrelin system is also altered in sst−/− mice; however, the level of regulation differs. Specifically, total ghrelin levels were elevated in both male/female sst−/− mice, whereas acylated ghrelin levels were not altered (Fig. 4A, right). In addition, stomach expression of ghrelin was up-regulated in male, but not female, sst−/− mice, whereas GOAT mRNA levels remained unchanged (Fig. 4B, right).
Effect of genotype (cort−/− vs. sst−/−) on glucose homeostasis
Given that the alterations in the endocrine phenotype of cort−/− mice (elevated GH, corticosterone, acylated and nonacylated ghrelin, and a reduction in PRL) could all impact systemic insulin sensitivity, coupled with the fact that CST and SST have been shown to inhibit insulin release, we explored the effect of genotype on whole-body glucose homeostasis. Circulating insulin (Fig. 5A), glucagon or glucose (Table 2) levels, did not significantly differ between cort−/− and cort+/+ mice. Also, the response to GTT did not differ between cort−/− and cort+/+ mice (Fig. 5, B and C). Interestingly, cort−/− males, but not females, were insulin resistant as assessed by ITT, where glucose levels remained significantly higher in male cort−/− mice, compared with controls, at both 60 and 120 min after insulin injection (Fig. 5, B and C).
Fig. 5.
Impact of lack of endogenous CST or SST on glucose homeostasis. Circulating insulin levels in male and female cort+/+ (white columns) vs. cort−/− (gray columns) (A) and sst+/+ (white columns) vs. sst−/− (gray columns) (D) mice. GTT (top) and ITT (bottom) of male (right) and female (left) cort+/+ (solid lines) vs. cort−/− (dotted lines) mice (B) and of male (right) and female (left) sst+/+ (solid lines) vs. sst−/− (dotted lines) mice (E). Area under curve (AUC) of GTT (top) and ITT (bottom) conducted in male and female cort+/+ (white columns) vs. cort−/− (gray columns) mice (C) and of male and female sst+/+ (white columns) vs. sst−/− (gray columns) mice (F). GTT and ITT were performed as we previously reported (26). Values are represented as the means ± sem (n = 6–12 mice/genotype per gender). Asterisks (*, P < 0.05; **, P < 0.01; ***, P < 0.001) indicate values that significantly differ from cort+/+ (A–C) or sst+/+ (D and E) within gender.
Similar to cort−/− mice, it has been previously reported that insulin and glucagon levels are unaltered in sst−/− mice (22, 31), which was confirmed in this study for female sst−/−. However, we found that insulin levels were significantly increased in male sst−/− and slightly, but not significantly, elevated (P = 0.08) in male cort−/− (Fig. 5D). However, in contrast to the insulin resistance observed in male cort−/− mice, the response to ITT observed in male sst−/− mice did not differ from their respective controls (Fig. 5, E and F).
Discussion
Based on the remarkable structural similarity between CST and SST, their close sst-binding profiles and a limited set of in vivo and in vitro results, it has been implicitly assumed that CST is a mere SST analog regulating endocrine function. This overly simplistic assumption is challenged by the results obtained in the present study, where we have performed for the first time a thorough characterization of the endocrine-metabolic profile of cort−/− mice at the hypothalamic-pituitary and systemic levels. We report that endogenous CST serves to enhance pituitary PRL and suppress GH and ACTH output, in a gender-dependent fashion. The fact that these changes occur without alterations in the appearance and number of somatotropes, corticotropes, or lactotropes indicates that endogenous CST is critical for regulating pituitary hormone synthesis and/or release but does not play a major role in the development of these pituitary cell types. These observations, coupled with the fact that the normal mouse pituitary does not express high levels of CST (data not shown), suggest that the absence of CST from a nonpituitary source likely accounts for changes in GH, ACTH, and PRL release observed in cort−/− mice in vivo. The actions of CST on pituitary cell function may be in part direct, because CST application to primary pituitary cell cultures of normal mice and baboons resulted in stimulation of PRL and inhibition of GH and ACTH release. There is also a possibility that CST-mediated changes in hypothalamic and systemic inputs to the pituitary may contribute to changes in hormone release observed in cort−/− mice. However, the involvement of CST-mediated changes in hypothalamic input is lessened by the observation that expression of key hypothalamic factors known to regulate somatotrope, corticotrope, and lactotrope function did not differ between cort−/− and cort+/+ mice. However, we cannot discount the possibility that in the absence of CST, there could be changes in neuronal activity, without changes in neuropeptide synthesis, which may contribute to PRL, GH, and ACTH levels observed in cort−/− mice. In the following discussion, we will separately explore the potential direct and indirect mechanisms by which CST may mediate each pituitary axis (PRL, GH, and ACTH) and compare and contrast these effects with those of SST.
Previous studies have reported that CST, as well as SST, can inhibit PRL release from prolactinomas in vivo and in vitro (32, 33). However, exogenous administration of CST does not affect PRL output in healthy men (32, 34, 35) and actually stimulates PRL release in male rats (36) and female mice (present study). Results from the current study show for the first time that endogenous CST is indeed a physiological stimulator of lactotrope function, because circulating PRL levels are markedly suppressed in male/female cort−/− mice. In fact, reduced PRL output was maintained in vitro, suggesting the absence of endogenous CST input may have permanently changed the internal programming of lactotropes. The observed reduction in circulating PRL levels is indeed of physiologic importance, because cort−/− females, although fertile, did not adequately care for their first litters, whereas multiparous cort−/− females effectively maintained litters until weaning. It should be noted that a similar maternal phenotype was reported in mice heterozygote for the null PRL-receptor allele (37), indicating that appropriate PRL input is required to initiate optimum milk production and maternal behavior in first time mothers (38, 39).
These results are in sharp contrast to that observed in sst−/− mice, where circulating PRL levels are in fact elevated. These disparate effects of CST vs. SST on lactotrope function directed us to explore the potential mechanisms mediating CST regulation of PRL secretion. We observed that the stimulatory effect of CST on PRL output can might be direct, because CST clearly increased PRL release (but not expression) in primary pituitary cultures of mice and baboons. These results are in striking contrast with the inhibitory effect exerted by SST on PRL secretion in pituitary cultures of mouse (present study), baboon (40), rat, and fish (6), especially in light of the highly similar binding profile of SST and CST for the family of sst1–sst5 (41). Therefore, it is reasonable to think that the unique stimulatory effect of CST on PRL release is exerted through a receptor not shared by SST. Interestingly, it has been reported that CST, but not SST, binds with high affinity to GHS-R1a, the receptor for ghrelin (12, 17), which, in turn, is known to stimulate PRL secretion (34, 42, 43). Therefore, we sought to determine whether CST-induced PRL release could be mediated by GHS-R1a activation. Indeed, use of a specific GHS-R1a antagonist fully blocked CST-stimulated PRL release in mouse and baboon pituitary cultures, thereby indicating that CST may act via GHS-R1a to stimulate PRL release. Of note, there is circumstantial evidence suggesting that CST and ghrelin can work together to enhance PRL release, based on a report showing CST enhances ghrelin-mediated PRL release in humans (34). In addition to the direct stimulatory action of CST on PRL release, we cannot exclude the possibility that CST may also serve to reduce dopaminergic tone to the pituitary, where dopamine is a direct inhibitor of PRL synthesis and release (44). And indeed, pituitary expression of the DR2 was up-regulated in female cort−/− mice, which may have contributed to suppression of circulating PRL. CST may also serve to suppress hypothalamic dopamine production; however, this possibility is lessened by the observation that expression of hypothalamic tyrosine hydroxylase (the rate limiting enzyme in dopamine synthesis) was modestly down-regulated in cort−/− mice (Supplemental Table 2). Nonetheless, future in vivo studies, using dopamine receptor antagonist, will be required to unequivocally determine whether CST can also alter PRL output by influencing dopamine production.
It has been previously reported that CST treatment, like SST, inhibits GH release in vitro and in vivo (32–34, 45). However, this inhibitory action of CST is not consistently observed (33, 46, 47), and depending on the species studied and the dose used, CST can actually stimulate GH release (32, 46, 48). Results from the current study show for the first time that endogenous CST is indeed a physiological inhibitor of GH secretion (being markedly elevated in both male/female cort−/− mice), similar to that reported in sst−/− mice (20, 22, 24). Also, consistent with that previously reported for sst−/− mice (20, 22, 24), elevated plasma GH levels observed in cort−/− mice did not increase linear growth, suggesting that the absence of CST is not a critical factor in the control of GH-induced somatic growth, at least under the conditions examined here. However, in contrast to sst−/− mice, where elevated GH was associated with an increase in IGF-I in females and feminization of hepatic PRL receptor and MUP-3 expression in males (22), these endpoints were not altered in cort−/− mice. Therefore, CST and SST may differentially regulate the pattern of GH release, a parameter whose nature is critical in regulating GH receptor sensitivity and downstream signaling.
The inhibitory actions of endogenous CST on GH secretion may be in part direct, because we observed that CST can inhibit GH release from pituitary cultures of mice and baboons. Curiously, the regulation of GH release by CST was associated with parallel changes in GH mRNA levels only in females. In addition to the direct effects, CST-mediated changes in systemic factors may augment GH release in cort−/− mice, including elevated glucocorticoids, which have been previously shown to directly stimulate GH release in primary pituitary cultures from a number of species (23, 49). Also, in female cort−/− mice, in vivo GH release may be further enhanced by the increase in circulating acylated ghrelin levels (probably due to the up-regulation in stomach ghrelin and GOAT expression), which has been previously shown to be a direct stimulator of GH release (23, 49). Although both CST and SST ultimately suppress GH output, we believe that the mechanisms by which endogenous CST inhibits GH release and the systemic impact of these GH changes differ from that mediated by SST, because 1) elevated GH output was not maintained in primary pituitary cultures of cort−/− mice, whereas it was in sst−/− cultures (22); 2) only GH expression was elevated in the pituitaries of female cort−/− mice, whereas GH, GHRH-R, and GHS-R expression was enhanced in pituitaries of female sst−/− mice (22); and 3) an overall up-regulation in the expression of pituitary sst subtypes (sst2/sst3/sst5/sst5TMD1) was found in female cort−/− but not in sst−/− mice (19). Therefore, these results demonstrate that CST is not a mere natural analog of SST in regulating gender-dependent, metabolic/endocrine GH-related secretions.
Although studies examining the impact of CST on corticotrope function are limited, it has been reported that CST, like SST, reduces cortisol levels in patients with Cushing disease (50). Results from the current study show for the first time that endogenous CST is also a physiological inhibitor of adrenal-axis function, because circulating corticosterone levels are markedly elevated in both male and female cort−/− mice, compared with their cort+/+ controls, similar to that reported in sst−/− mice (21, 24). Intriguingly, one might expect that chronic elevation in circulating glucocorticoids would serve to feedback negatively at the hypothalamic and pituitary level to prevent excess ACTH and subsequent glucocorticoid overproduction. However, in cort−/− mice, ACTH levels and pituitary POMC expression remain elevated in female mice (and there was a trend for an increase in POMC mRNA in pituitaries of male cort−/− mice). Also, female-specific increases in ACTH release are maintained in vitro. The increase in ACTH secretion observed in vivo may be due to a direct pituitary effect, because CST inhibited male/female ACTH release and female POMC mRNA levels in primary pituitary cultures of mice and baboons. It is also possible, but not directly tested here, that central loss of CST disrupts central glucocorticoid feedback inhibition allowing for elevated ACTH production, a hypothesis that is supported by the fact that hypothalamic CRH mRNA levels were unaltered in cort−/− mice. In addition, the female-specific elevation of circulating ACTH levels in cort−/− may be in part related indirectly to the effects of CST on ghrelin system function, where elevated acylated ghrelin can augment ACTH production, as previously reported in humans (34, 35, 51), baboons (21), and other species (52). Although both CST and SST ultimately suppress ACTH output, we believe the mechanisms by which endogenous CST inhibits corticotrope function and the overall systemic impact of elevated glucocorticoids differ from that mediated by SST, because 1) a gender- and model-dependent regulation of pituitary POMC (up-regulated in female cort−/− and in male sst−/−), ghrelin, and GHS-R (elevated only in female sst−/−) was observed (22); 2) circulating total ghrelin levels were increased in male/female sst−/− and in female, but not male, cort−/− mice; and 3) an up-regulation in the expression of stomach ghrelin was found in female cort−/− and in male sst−/− mice (22).
It is clear that the lack of endogenous CST leads to gender-dependent enhancement of GH, corticosterone, nonacyl, and acylated ghrelin, whereas PRL output is impaired. Because each of these hormones has been shown to have profound effects on metabolic function, and the fact that CST and SST has been shown to suppress insulin release in vivo (4, 6, 32, 34), we performed preliminary experiments to evaluate whole-body metabolic function in cort−/− and sst−/− mice. Male cort−/− mice were insulin resistant but glucose tolerant, whereas the response of female cort−/− mice, or male and female sst−/− mice, to ITT and GTT did not differ from controls. A normal GTT response in male sst−/− has also been observed by others (31). Keeping in mind that male and female cort−/− and sst−/− mice all show elevations in circulating GH and corticosterone (present study and Refs. 21, 24), and that both, GH and glucocorticoids, are known to impair insulin signaling (53), the questions arises why do male cort−/− exclusively develop insulin resistance? One possibility is that the pattern and/or magnitude of GH release in male cort−/− mice is such that the well-characterized counterregulatory actions of GH on hepatic glucose production in response to insulin-driven hypoglycemia are stronger in males, compared with females. This is supported by the elevated levels of glucose at late time points (60 and 120 min) after insulin injection. In addition, the elevated levels of nonacylated ghrelin, shown to be up-regulated in female cort−/− mice and male and female sst−/− (21, 22, 54), but not male cort−/− mice, could also play a protective role in pancreatic function, because it has been previously shown that nonacylated ghrelin exerts a favorable influence on insulin sensitivity and glucose homeostasis (55, 56).
In summary, the present detailed evaluation of the endocrine axis of cort−/− mice has revealed for the first time that endogenous CST is required to maintain normal levels of PRL and directly enhance pituitary PRL release. Conversely, CST serves an inhibitory role for gastrointestinal acylated ghrelin production, as well as for GH and ACTH production, all of these actions being modulated in a gender-dependent fashion. Indeed, despite the striking structural similarities of CST and SST (1, 2), comparison of the endocrine phenotype of cort−/− and sst−/− mice clearly demonstrate that the mechanisms by which CST inhibits GH and ACTH release are unique and that CST, but not SST, augments PRL production. Hence, our results reveal that CST is not a simple natural alternate for SST but possesses unique endocrine-metabolic actions of its own, which may involve physiologically relevant functions, including PRL production and the linked maternal care, ghrelin, insulin, and glucose homeostasis and additional components of the somatotropic and the stress axes. These results also underscore the importance of identifying the selective mechanisms of action (tissue-specific source, cell-specific targets, receptors, intracellular signals) that confer the distinct functional abilities of these two peptides. In addition, we provide evidence that the CST, SST, and ghrelin systems are intricately interconnected. Further investigations into this relationship may provide important information into designing drugs to modulate both health and disease.
Supplementary Material
Acknowledgments
We thank the staff of the University of Córdoba, Servicio Centralizado de Animales de Experimentación, and the veterinarian staff of the University of Illinois at Chicago, Biological Resource Center, for its invaluable help; Rosario Moyano Salvago, Rocío Fdez.-Palacios O'Connor, and Lisa Halliday; and Dr. Manuel Tena-Sempere and Leonor Pinilla for their help with the circulating LH measurement. Sst−/− mice were generously provided by Dr. Ute Hochgeschwender (Oklahoma Medical Research Foundation, Oklahoma City, OK). We also thank Dr. Michael Culler (Ipsen, Milford, MA) for providing the BIM-28163 analog.
This work was supported by Ayudas Predoctorales de Formacion en Investigacion en Salud del Fondo de Investigación Sanitaria Grants ISCIII:FI06/00804 (to J.C.-C.) and FPU-AP20052473 (to M.D.G.); the Veterans Affairs Merit Award R01DK030677 (to R.D.K.); BFU2010-19300 and CTS-5051 (to J.P.C.); and Programa Ramón y Cajal del Ministerio de Educación y Ciencia Grants RYC-2007-00186 and JC2008-00220, BFU2008-01136/BFI (to R.M.L.). Cort−/− mice were generated by Ld.L., who was supported by grants from National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CRF-R
- Corticotrope-releasing factor receptor
- CST
- cortistatin
- DR2
- dopamine receptor type-2
- GAS
- glycoprotein α-subunit
- GHRH-R
- GHRH receptor
- GHS-R
- growth hormone secretagogue receptor
- GHS-R1a
- GHS-R type 1a
- GOAT
- ghrelin O-acyl transferase
- GTT
- glucose tolerance test
- ITT
- insulin tolerance test
- NF
- normalization factor
- POMC
- proopiomelanocortin
- PRL
- prolactin
- SST
- somatostatin.
References
- 1. Gahete MD, Cordoba-Chacón J, Duran-Prado M, Malagón MM, Martinez-Fuentes AJ, Gracia-Navarro F, Luque RM, Castaño JP. 2010. Somatostatin and its receptors from fish to mammals. Ann NY Acad Sci 1200:43–52 [DOI] [PubMed] [Google Scholar]
- 2. Tostivint H, Joly L, Lihrmann I, Parmentier C, Lebon A, Morisson M, Calas A, Ekker M, Vaudry H. 2006. Comparative genomics provides evidence for close evolutionary relationships between the urotensin II and somatostatin gene families. Proc Natl Acad Sci USA 103:2237–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Møller LN, Stidsen CE, Hartmann B, Holst JJ. 2003. Somatostatin receptors. Biochim Biophys Acta 1616:1–84 [DOI] [PubMed] [Google Scholar]
- 4. Broglio F, Grottoli S, Arvat E, Ghigo E. 2008. Endocrine actions of cortistatin: in vivo studies. Mol Cell Endocrinol 286:123–127 [DOI] [PubMed] [Google Scholar]
- 5. de Lecea L. 2008. Cortistatin—functions in the central nervous system. Mol Cell Endocrinol 286:88–95 [DOI] [PubMed] [Google Scholar]
- 6. Gahete MD, Durán-Prado M, Luque RM, Martínez-Fuentes AJ, Vázquez-Martínez R, Malagón MM, Castaño JP. 2008. Are somatostatin and cortistatin two siblings in regulating endocrine secretions? In vitro work ahead. Mol Cell Endocrinol 286:128–134 [DOI] [PubMed] [Google Scholar]
- 7. de Lecea L, Castaño JP. 2006. Cortistatin: not just another somatostatin analog. Nat Clin Pract Endocrinol Metab 2:356–357 [DOI] [PubMed] [Google Scholar]
- 8. Dalm VA, Van Hagen PM, de Krijger RR, Kros JM, Van Koetsveld PM, Van Der Lely AJ, Lamberts SW, Hofland LJ. 2004. Distribution pattern of somatostatin and cortistatin mRNA in human central and peripheral tissues. Clin Endocrinol 60:625–629 [DOI] [PubMed] [Google Scholar]
- 9. Calbet M, Guadaño-Ferraz A, Spier AD, Maj M, Sutcliffe JG, Przewłocki R, de Lecea L. 1999. Cortistatin and somatostatin mRNAs are differentially regulated in response to kainate. Brain Res Mol Brain Res 72:55–64 [DOI] [PubMed] [Google Scholar]
- 10. Gonzalez-Rey E, Delgado M. 2008. Emergence of cortistatin as a new immunomodulatory factor with therapeutic potential in immune disorders. Mol Cell Endocrinol 286:135–140 [DOI] [PubMed] [Google Scholar]
- 11. de Lecea L, Criado JR, Prospero-Garcia O, Gautvik KM, Schweitzer P, Danielson PE, Dunlop CL, Siggins GR, Henriksen SJ, Sutcliffe JG. 1996. A cortical neuropeptide with neuronal depressant and sleep-modulating properties. Nature 381:242–245 [DOI] [PubMed] [Google Scholar]
- 12. Broglio F, Papotti M, Muccioli G, Ghigo E. 2007. Brain-gut communication: cortistatin, somatostatin and ghrelin. Trends Endocrinol Metab 18:246–251 [DOI] [PubMed] [Google Scholar]
- 13. Durán-Prado M, Malagón MM, Gracia-Navarro F, Castaño JP. 2008. Dimerization of G protein-coupled receptors: new avenues for somatostatin receptor signalling, control and functioning. Mol Cell Endocrinol 286:63–68 [DOI] [PubMed] [Google Scholar]
- 14. Durán-Prado M, Gahete MD, Martínez-Fuentes AJ, Luque RM, Quintero A, Webb SM, Benito-López P, Leal A, Schulz S, Gracia-Navarro F, Malagón MM, Castaño JP. 2009. Identification and characterization of two novel truncated but functional isoforms of the somatostatin receptor subtype 5 differentially present in pituitary tumors. J Clin Endocrinol Metab 94:2634–2643 [DOI] [PubMed] [Google Scholar]
- 15. Córdoba-Chacón J, Gahete MD, Duran-Prado M, Pozo-Salas AI, Malagón MM, Gracia-Navarro F, Kineman RD, Luque RM, Castaño JP. 2010. Identification and characterization of new functional truncated variants of somatostatin receptor subtype 5 in rodents. Cell Mol Life Sci 67:1147–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Robas N, Mead E, Fidock M. 2003. MrgX2 is a high potency cortistatin receptor expressed in dorsal root ganglion. J Biol Chem 278:44400–44404 [DOI] [PubMed] [Google Scholar]
- 17. Deghenghi R, Papotti M, Ghigo E, Muccioli G. 2001. Cortistatin, but not somatostatin, binds to growth hormone secretagogue (GHS) receptors of human pituitary gland. J Endocrinol Invest 24:RC1-3. [DOI] [PubMed] [Google Scholar]
- 18. Gonzalez-Rey E, Delgado-Maroto V, Souza Moreira L, Delgado M. 2010. Neuropeptides as therapeutic approach to autoimmune diseases. Curr Pharm Des 16:3158–3172 [DOI] [PubMed] [Google Scholar]
- 19. Córdoba-Chacón J, Gahete MD, Castaño JP, Kineman RD, Luque RM. 2011. Somatostatin and its receptors contribute, in a tissue-specific manner, to the sex-dependent, metabolic (fed/fasting) control of growth hormone axis in mice. Am J Physiol Endocrinol Metab 300:E46–E54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Low MJ, Otero-Corchon V, Parlow AF, Ramirez JL, Kumar U, Patel YC, Rubinstein M. 2001. Somatostatin is required for masculinization of growth hormone-regulated hepatic gene expression but not of somatic growth. J Clin Invest 107:1571–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luque RM, Gahete MD, Hochgeschwender U, Kineman RD. 2006. Evidence that endogenous SST inhibits ACTH and ghrelin expression by independent pathways. Am J Physiol Endocrinol Metab 291:E395–E403 [DOI] [PubMed] [Google Scholar]
- 22. Luque RM, Kineman RD. 2007. Gender-dependent role of endogenous somatostatin in regulating growth hormone-axis function in mice. Endocrinology 148:5998–6006 [DOI] [PubMed] [Google Scholar]
- 23. Luque RM, Park S, Kineman RD. 2008. Role of endogenous somatostatin in regulating GH output under basal conditions and in response to metabolic extremes. Mol Cell Endocrinol 286:155–168 [DOI] [PubMed] [Google Scholar]
- 24. Zeyda T, Diehl N, Paylor R, Brennan MB, Hochgeschwender U. 2001. Impairment in motor learning of somatostatin null mutant mice. Brain Res 906:107–114 [DOI] [PubMed] [Google Scholar]
- 25. Tallent MK, Calbet M, Lamp T, de Lecea L. 2002. Physiological consequences of cortistatin deficiency. Program no. 134.5 Washington, DC: Society for Neuroscience [Google Scholar]
- 26. Luque RM, Lin Q, Córdoba-Chacón J, Subbaiah PV, Buch T, Waisman A, Vankelecom H, Kineman RD. 2011. Metabolic impact of adult-onset, isolated, growth hormone deficiency (AOiGHD) due to destruction of pituitary somatotropes. PLoS One 6:e15767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kineman RD, Luque RM. 2007. Evidence that ghrelin is as potent as growth hormone (GH)-releasing hormone (GHRH) in releasing GH from primary pituitary cell cultures of a nonhuman primate (Papio anubis), acting through intracellular signaling pathways distinct from GHRH. Endocrinology 148:4440–4449 [DOI] [PubMed] [Google Scholar]
- 28. Halem HA, Taylor JE, Dong JZ, Shen Y, Datta R, Abizaid A, Diano S, Horvath T, Zizzari P, Bluet-Pajot MT, Epelbaum J, Culler MD. 2004. Novel analogs of ghrelin: physiological and clinical implications. Eur J Endocrinol 151(Suppl 1):S71–S75 [DOI] [PubMed] [Google Scholar]
- 29. Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stojilković SS, Izumi S, Catt KJ. 1988. Participation of voltage-sensitive calcium channels in pituitary hormone release. J Biol Chem 263:13054–13061 [PubMed] [Google Scholar]
- 31. Hauge-Evans AC, King AJ, Carmignac D, Richardson CC, Robinson IC, Low MJ, Christie MR, Persaud SJ, Jones PM. 2009. Somatostatin secreted by islet δ-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes 58:403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grottoli S, Gasco V, Broglio F, Baldelli R, Ragazzoni F, Gallenca F, Mainolfi A, Prodam F, Muccioli G, Ghigo E. 2006. Cortistatin-17 and somatostatin-14 display the same effects on growth hormone, prolactin, and insulin secretion in patients with acromegaly or prolactinoma. J Clin Endocrinol Metab 91:1595–1599 [DOI] [PubMed] [Google Scholar]
- 33. Rubinfeld H, Hadani M, Barkai G, Taylor JE, Culler MD, Shimon I. 2006. Cortistatin inhibits growth hormone release from human fetal and adenoma pituitary cells and prolactin secretion from cultured prolactinomas. J Clin Endocrinol Metab 91:2257–2263 [DOI] [PubMed] [Google Scholar]
- 34. Broglio F, Arvat E, Benso A, Gottero C, Prodam F, Grottoli S, Papotti M, Muccioli G, van der Lely AJ, Deghenghi R, Ghigo E. 2002. Endocrine activities of cortistatin-14 and its interaction with GHRH and ghrelin in humans. J Clin Endocrinol Metab 87:3783–3790 [DOI] [PubMed] [Google Scholar]
- 35. Gottero C, Prodam F, Destefanis S, Benso A, Gauna C, Me E, Filtri L, Riganti F, Van Der Lely AJ, Ghigo E, Broglio F. 2004. Cortistatin-17 and -14 exert the same endocrine activities as somatostatin in humans. Growth Horm IGF Res 14:382–387 [DOI] [PubMed] [Google Scholar]
- 36. Baranowska B, Bik W, Baranowska-Bik A, Wolinska-Witort E, Chmielowska M, Martynska L. 2009. Cortistatin and pituitary hormone secretion in rat. J Physiol Pharmacol 60:151–156 [PubMed] [Google Scholar]
- 37. Lucas BK, Ormandy CJ, Binart N, Bridges RS, Kelly PA. 1998. Null mutation of the prolactin receptor gene produces a defect in maternal behavior. Endocrinology 139:4102–4107 [DOI] [PubMed] [Google Scholar]
- 38. Bartke A. 1999. Role of growth hormone and prolactin in the control of reproduction: what are we learning from transgenic and knock-out animals? Steroids 64:598–604 [DOI] [PubMed] [Google Scholar]
- 39. Mann PE, Bridges RS. 2001. Lactogenic hormone regulation of maternal behavior. Prog Brain Res 133:251–262 [DOI] [PubMed] [Google Scholar]
- 40. Kineman RD, Luque RM. Low doses of somatostatin signal through AC/cAMP to dramatically increase GH release in primary pituitary cell cultures from a non-human primate (Papio anubis). Program of the 89th Annual Meeting of The Endocrine Society, Toronto, ON, Canada, 2007 (Abstract P2-391) [Google Scholar]
- 41. Siehler S, Nunn C, Hannon J, Feuerbach D, Hoyer D. 2008. Pharmacological profile of somatostatin and cortistatin receptors. Mol Cell Endocrinol 286:26–34 [DOI] [PubMed] [Google Scholar]
- 42. Petersenn S. 2002. Growth hormone secretagogues and ghrelin: an update on physiology and clinical relevance. Horm Res 58(Suppl 3):56–61 [DOI] [PubMed] [Google Scholar]
- 43. Rubinfeld H, Hadani M, Taylor JE, Dong JZ, Comstock J, Shen Y, DeOliveira D, Datta R, Culler MD, Shimon I. 2004. Novel ghrelin analogs with improved affinity for the GH secretagogue receptor stimulate GH and prolactin release from human pituitary cells. Eur J Endocrinol 151:787–795 [DOI] [PubMed] [Google Scholar]
- 44. Ben-Jonathan N, LaPensee CR, LaPensee EW. 2008. What can we learn from rodents about prolactin in humans? Endocr Rev 29:1–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Deghenghi R, Avallone R, Torsello A, Muccioli G, Ghigo E, Locatelli V. 2001. Growth hormone-inhibiting activity of cortistatin in the rat. J Endocrinol Invest 24:RC31–RC33 [DOI] [PubMed] [Google Scholar]
- 46. Luque RM, Peinado JR, Gracia-Navarro F, Broglio F, Ghigo E, Kineman RD, Malagón MM, Castaño JP. 2006. Cortistatin mimics somatostatin by inducing a dual, dose-dependent stimulatory and inhibitory effect on growth hormone secretion in somatotropes. J Mol Endocrinol 36:547–556 [DOI] [PubMed] [Google Scholar]
- 47. Jeandel L, Okuno A, Kobayashi T, Kikuyama S, Tostivint H, Lihrmann I, Chartrel N, Conlon JM, Fournier A, Tonon MC, Vaudry H. 1998. Effects of the two somatostatin variants somatostatin-14 and [Pro2, Met13]somatostatin-14 on receptor binding, adenylyl cyclase activity and growth hormone release from the frog pituitary. J Neuroendocrinol 10:187–192 [DOI] [PubMed] [Google Scholar]
- 48. Baranowska B, Chmielowska M, Wolinska-Witort E, Bik W, Baranowska-Bik A, Martynska L. 2006. Direct effect of cortistatin on GH release from cultured pituitary cells in the rat. Neuro Endocrinol Lett 27:153–156 [PubMed] [Google Scholar]
- 49. Luque RM, Gahete MD, Cordoba-Chacon J, Childs GV, Kineman RD. 2011. Does the pituitary somatotrope play a primary role in regulating GH output in metabolic extremes? Ann NY Acad Sci 1220:82–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Giordano R, Picu A, Bonelli L, Broglio F, Prodam F, Grottoli S, Muccioli G, Ghigo E, Arvat E. 2007. The activation of somatostatinergic receptors by either somatostatin-14 or cortistatin-17 often inhibits ACTH hypersecretion in patients with Cushing's disease. Eur J Endocrinol 157:393–398 [DOI] [PubMed] [Google Scholar]
- 51. Martínez-Fuentes AJ, Moreno-Fernández J, Vázquez-Martínez R, Durán-Prado M, de la Riva A, Tena-Sempere M, Diéguez C, Jiménez-Reina L, Webb SM, Pumar A, Leal-Cerro A, Benito-López P, Malagón MM, Castaño JP. 2006. Ghrelin is produced by and directly activates corticotrope cells from adrenocorticotropin-secreting adenomas. J Clin Endocrinol Metab 91:2225–2231 [DOI] [PubMed] [Google Scholar]
- 52. van der Lely AJ, Tschöp M, Heiman ML, Ghigo E. 2004. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev 25:426–457 [DOI] [PubMed] [Google Scholar]
- 53. Byrne CD. 2001. Programming other hormones that affect insulin. Br Med Bull 60:153–171 [DOI] [PubMed] [Google Scholar]
- 54. Gahete MD, Córdoba-Chacón J, Salvatori R, Castaño JP, Kineman RD, Luque RM. 2010. Metabolic regulation of ghrelin O-acyl transferase (GOAT) expression in the mouse hypothalamus, pituitary, and stomach. Mol Cell Endocrinol 317:154–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gauna C, Meyler FM, Janssen JA, Delhanty PJ, Abribat T, van Koetsveld P, Hofland LJ, Broglio F, Ghigo E, van der Lely AJ. 2004. Administration of acylated ghrelin reduces insulin sensitivity, whereas the combination of acylated plus unacylated ghrelin strongly improves insulin sensitivity. J Clin Endocrinol Metab 89:5035–5042 [DOI] [PubMed] [Google Scholar]
- 56. Granata R, Baragli A, Settanni F, Scarlatti F, Ghigo E. 2010. Unraveling the role of the ghrelin gene peptides in the endocrine pancreas. J Mol Endocrinol 45:107–118 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.