Abstract
A eubacterial ribosome stalled on a defective mRNA can be released through a quality control mechanism referred to as trans-translation, which depends on the coordinating binding actions of transfer-messenger RNA, Small protein B and ribosome protein S1. By means of cryo-electron microscopy, we obtained a map of the complex composed of a stalled ribosome and small protein B, which appears near the decoding center. This result suggests that, when lacking a codon, the A site on the small subunit is a target for small protein B. To investigate the role of S1 played in trans-translation, we obtained a cryo-electron microscopic map including a stalled ribosome, transfer-messenger RNA, and small protein Bs, but in the absence of S1. In this complex, several connections between the 30S subunit and transfer-messenger RNA that appear in the +S1 complex are no longer found. We propose the unifying concept of scaffolding for the roles of small protein B and S1 in binding of transfer-messenger RNA to the ribosome during trans-translation, and infer a pathway of sequential binding events in the initial phase of trans-translation.
Keywords: cryo-electron microscopy, defective mRNA, bacterial ribosome, elongation factor Tu
Trans-translation is a bacterial quality control mechanism that allows the rescue of stalled ribosomes by transfer-messenger RNA (tmRNA), a unique chimeric molecule acting as both tRNA and mRNA. tmRNA is ∼230-400 nucleotides (nts) long, depending on the bacterial species, and its consensus secondary structure encompasses a tRNA-like domain (TLD), a small internal open-reading frame (ORF, sometimes referred to as mRNA-like domain, MLD), and a string of pseudoknots (1). Although the secondary structure of the TLD is very similar to that of a canonical tRNA, it lacks an anticodon loop, possesses a reduced D-domain, and the X-ray structure shows an increased interstem angle at the elbow level (120° versus 90°) (2,3). Small protein B (SmpB) is a basic protein interacting with tmRNA during trans-translation (4). Its gene is conserved among prokaryotes, and its deletion causes phenotypes similar to those observed when tmRNA is inactivated.
The process of trans-translation has been described as follows (5,6): the TLD in tmRNA is aminoacylated with an alanine, then the complex of tmRNA, SmpB, and EF-Tu binds to the stalled ribosome, characterized by the absence of a stop codon in the A site due to a defective mRNA. Subsequent insertion of the ORF into the ribosome and its complete translation, which requires the presence of protein S1 in solution, leads to the release of both the tagged polypeptide to be degraded and the stalled ribosome to be recycled.
An earlier cryo-EM study (7) identified the position of the pseudoknots and the TLD in tmRNA, as well as a single copy of SmpB Some portions of the density map were less well resolved, leaving ambiguities in the path of ORF and the number of SmpBs. Both ambiguities were recently resolved by an improved cryo-EM map (8), whose interpretation could take advantage of new X-ray data on a complex between SmpB and TLD (3). Accordingly, two SmpB molecules were identified, one (SmpB-2) bound in the decoding site and the other (SmpB-1) perched at the GTP-associated center. This result is in line with the earlier suggestion by Wower et al. (9) that several SmpB molecules might interact with tmRNA, both on its CCA stem and its pseudo-anticodon stem. Indeed, a recent biochemical study demonstrates that during trans-translation in vivo two SmpBs interact with the stalled ribosome, one with the small and one with the large subunit of a stalled ribosome (10).
According to the biochemical data (11,12), SmpB primarily has three functions: (i) facilitation of the binding of tmRNA to the ribosomes, (ii) enhancement of tmRNA alanylation by the alanyl-tRNA synthetase (AlaRS), and (iii) protection of tmRNA from degradation. Essentially, no trans-translation can be triggered in the absence of SmpB. The finding of two copies of SmpB bound to the stalled ribosome raised question on functional identity of the two SmpBs, and the sequence in which the binding occurs in the initiation of trans-translation. Our present study takes a cue from the in vivo study by Hallier et al. (13), who found that SmpB can bind to the stalled ribosome independently from the presence of tmRNA, suggesting that the stalled ribosome is first targeted by SmpB, and that this might be the first step of trans-translation. However, the questions that remained to be addressed are: (1) When SmpB binds to the stalled ribosome independently, then where precisely is its binding position? (2) Given the binding position of the SmpB on the stalled ribosome, what is the likely mechanism of the targeting of the ribosome by tmRNA? (3) Which of the two molecules of SmpB that are associated with the ribosome-bound tmRNA according to Kaur et al. (8) is used to protect free tmRNA from degradation?
In effort to address these questions, we used cryo-EM to study two complexes, one obtained by incubating SmpB with stalled ribosomes, the other (the control) with non-stalled ribosomes that had a sense codon in the A site. Comparison of these maps revealed the presence of SmpB at the decoding site of the stalled ribosome, roughly in the position of SmpB-2 as defined by Kaur and coworkers (8). The study demonstrates that the presence of a codon in the A site prevents the binding of SmpB. This result gives a rationale as to why SmpB does not interfere with canonical translation. Furthermore, in order to elucidate the role played by the ribosomal protein S1 in solution during this early step of trans-translation, we also obtained a cryo-EM reconstruction (at a resolution of 13 Å) of a stalled ribosome bound with tmRNA, EF-Tu-GTP, SmpB in a pre-accommodated state, but in the absence of S1. In this map, the ORF in the tmRNA is well-structured and continuous outside the decoding center, unlike the discontinuous, disrupted structure seen in the presence of S1.
Following the study by Kaur and coworkers (8), the present study aimed at determining the roles of both SmpB and S1 in the assembly and function of the trans-translation complex. Our findings can be summarized by one unifying concept: the concept of scaffolding. Trans-translation, specifically the formation of a productive tmRNA structure, ORF insertion into the mRNA channel, and TLD insertion into the A site all appear to be mediated and controlled by these structural proteins.
Experimental Procedures
Preparation of components and formation of SmpB-ribosome complexes
Thermus thermophilus ribosomes were prepared as previously described (14). C-terminal His-tagged SmpB was overexpressed in E. coli using the T7 expression system and isolated by Ni2+ precharged Hi-Trap chelating column, according to the manufacturer's procedures (Amersham). Ribosomal P-site tRNA complexes were obtained by incubating 70S ribosomes (1μM) with 2μM of mRNA consisting of the sequence GGCAAGGAGGUAAAAAUG for the stalled one, and CGAGAAGGAGAAGCUUAUGGUGACCUUUUUUUUUUUUUUUCCCCCC for the non-stalled, and E. coli fMet-tRNAfMet (2μM) for 30 minutes at 37°C in a 48 μl medium containing 5 mM Hepes-KOH pH 7.5, 50 mM KCl, 10 mM NH4Cl, 10 mM MgOAc, 6 mM β-Mercaptoethanol. An initiation complex including an SD sequence, an AUG on the mRNA, and an initiator tRNA was used to simulate a rescue complex because it gives a stable complex that mimics a stalling situation in the most simple case. Complexes were then obtained by adding SmpB (4μM) for 15 minutes at 37°C. No S1 was used in the complexes. Pre-accommodated complexes in the absence of the ribosomal protein S1 were prepared as described earlier (7).
Equilibrium binding measurement by filter-binding
Ribosome-SmpB complexes (100μl) were applied to a Superdex 200 HR 10/30 column (GE Healthcare, Orsay, France) equilibrated with 5 mM Hepes-KOH pH 7.5, 10 mM NH4Cl, 10mM MgOAc, 50mM KCl, at 4°C. The fractions containing the purified 70S ribosome were pooled and concentrated. In vitro transcribed tmRNA was end-labeled with [γ-32P]ATP and phage T4 polynucleotide kinase after dephosphorylation with alkaline phosphatase. Following purification (Megaclear, Ambion) and ethanol precipitation, tmRNA was denatured for 2 min at 80°C in a folding buffer (5mM MgCl2, 20 mM NH4Cl, 10 mM Hepes-KOH pH 7.5) and slowly cooled down to room temperature for 30 min before being applied to the SmpB-ribosome complexes described above. 1 μl labelled tmRNA was added to the ribosomal complexes for either 2 or 5 min (similar results) at 20°C, in a total volume of 15 μl in a buffer containing (5 mM Hepes-KOH pH 7.5, 50 mM KCl, 10 mM NH4Cl, 10 mM MgOAc, 6 mM β-Mercaptoethanol). 13 μl were then filtered under vacuum suction over nitrocellulose filters (Schleicher & Schuell), followed by rapid washing with 3 × 1ml of the same cold buffer. Membranes were dried and the holding radioactivity measured by scintillation counting (WALLAC 1409).
Sucrose density gradient centrifugation and analysis
Crude ribosomes from E. call were obtained as previously described (13). 70S ribosomes were dissociated into 50S and 30S subunits by diluting the crude ribosome with 10 fold volume of Mg2+- free lysis buffer. Ribosome subunits were fractionated on a 10-30% sucrose gradient in 25 mM Tris-HCl pH 7.5, 100 mM ammonium acetate, 1 mM magnesium acetate for 15 h at 27 000 r.p.m and 4°C. RNAs were isolated from one half of each fraction by phenol extraction followed by ethanol precipitation. For Northern hybridization, RNAs were separated by electrophoresis on a 1% (w/v) agarose gel containing 6.5% (v/v) formaldehyde and transferred in 10 × SSC to nylon membrane by the capillary method. Pre-hybridization and hybridization with 32P-labeled DNA oligonucleotides complementary to Ssra RNA (5′ –CGG GTA CGG GTA GGA TCG CAC ACC-3′) were carried out in ExpressHyb according to the manufacturer's protocol (Clontech). For Western-Blot proteins were extracted by TCA precipitation, electrophoresed on a 15% acrylamide Tris-glycine gel and transferred to PVDF membranes. SmpB was then detected by using a rabbit polyclonal antibody as previously described (13).
Cryo-electron microscopy and image processing
Ribosomal samples were diluted to a final concentration of 32 nM and used directly for cryo-EM grid preparation following standard procedures. Micrographs at 50,000× (± 2%) magnification were taken on a Philips FEI (Eindhoven, The Netherlands) Tecnai F20 with field emission gun operated at 200 KV and with low electron dose (∼15e Å−2). The micrographs were scanned with a pixel size corresponding to 2.82 Å on the object scale with a Zeiss Imaging scanner (Z/I Imaging Corporation, Huntsville, Alabama, USA). The image processing was carried out with the SPIDER package (15), including a reference-guided projection classification and alignment, contrast transfer function correction of segregated defocus groups, and correction of the high-frequency amplitudes using low-angle X-ray scattering data (16). The numbers of particles used in the 3D reconstructions were 48,138, 37,000, and 19,472 for the 70S·tRNA with a sense codon in the A site, the 70S·tRNA·SmpB lacking a codon in the A site, and the 70S tRNA·tmRNA·EF-Tu·SmpB·kir lacking a sense codon in the A site and in the absence of S1 for a pre-accommodated complex, respectively. The map resolutions were 10 Å, 11.8 Å, and 13.6 Å, respectively, using a 0.5 cutoff in the Fourier shell correlation. The docking of atomic model of tmRNA into the cryo-EM maps was done using O and the visualization was performed using IRIS Explorer (Numerical Algorithm Group Ltd., Downers Grove, Illinois, USA), Ribbons (17), and INSIGHT II (Accelrys Inc., San Diego, USA).
Results
Binding of SmpB to the stalled ribosome
Our study was motivated by the finding, using biochemical methods, that SmpB binds the stalled 70S ribosome in the absence of tmRNA (13), suggesting that this binding may be an initial step of trans-translation. We set out to visualize this binding complex by means of cryo-EM and single-particle reconstruction (15). Two cryo-EM samples were made by incubating SmpB protein with the 70S ribosome, tRNA, and two different mRNAs, one bearing a stop codon at the A site, the other lacking any codon at the A site (see Experimental procedures). Both SmpB and the ribosome were from Thermus thermophilus. Using these two samples, two cryo-EM maps were produced, at resolutions of 10 Ǻ and 11.8 Ǻ, respectively (Figs. 1A and B). These two maps promised to provide a comparison for binding of SmpB under the two conditions.
Figure 1.
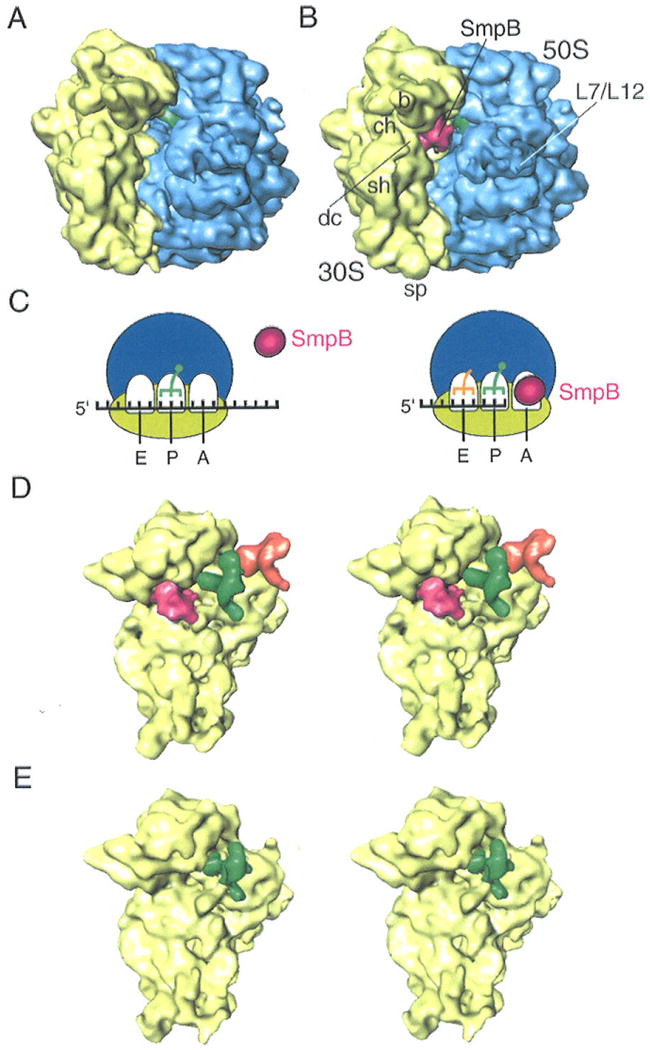
Cryo-EM maps of SmpB-ribosome complexes.
(A) Cryo-EM map obtained for 70S-tRNA ribosome complex in the presence of an mRNA bearing a sense codon at the A site (sec Experimental Procedures). No density attributable to SmpB appears in the map even though SmpB is in the solution. (B) Cryo-EM map obtained for 70S-tRNA ribosome stalled with a truncated mRNA in the presence of SmpB (see Experimental Procedures). The density attributable to SmpB is shown in red, while the 50S subunit is shown in blue, the 30S subunit in yellow, and the P-site tRNA in green. Landmarks on the 50S subunit: L7/L12, stalk formed by proteins L7/L12. Landmarks on 30S subunit: sh, shoulder; b, beak; dc, decoding center; ch, entrance of mRNA channel; and sp, spur. (C) Schematic recapitulation of the results shown in (A) and (B): SmpB is excluded when a sense codon is in the A site (left panel), while absence of any codon “invites” SmpB to occupy this site (right panel). (D) Stereo view of map computationally separated from the map shown in (B). The 30S subunit is shown in yellow, SmpB in pink, P-site tRNA in green, and E-site tRNA in orange. (E) Stereo view of the 30S subunit computationally separated map from (A). Only the P site is occupied.
The difference between the two maps proved to be an extra piece of density located at the decoding center of the ribosome (Fig. 1). This density indicates high (close to 100%) occupancy of a protein ligand. To ascertain SmpB as origin, its atomic structure (PDB 1P6V) was fitted into the cryo-EM map by visual criteria, leading to a good match with the map in both size and shape (Fig. 2). We regard this result as a positive identification of SmpB, which was confirmed by Western Blot analyses using anti-SmpB antibodies (not shown). The X-ray structure shows long random coils in both the N- and C-termini. In addition, residues 73-76 are missing in the X-ray structure, which leave the two disconnected ends less structured. In addition to the overall match between the density of the current cryo-EM map and the X-ray structure, the best candidate orientation of this SmpB can be inferred by comparing it with the SmpB bound to the A site (i.e., SmpB-2) in the complex with tmRNA (8). Placing the SmpB at the position and orientation of SmpB-2 in the tmRNA-SmpBs model leads to a fairly good match with the current cryo-EM map. Thus, our cryo-EM results indicate that SmpB is recognized by the stalled ribosome, but not by the ribosome encountering a stop codon in the A site in the process of normal translation.
Figure 2.
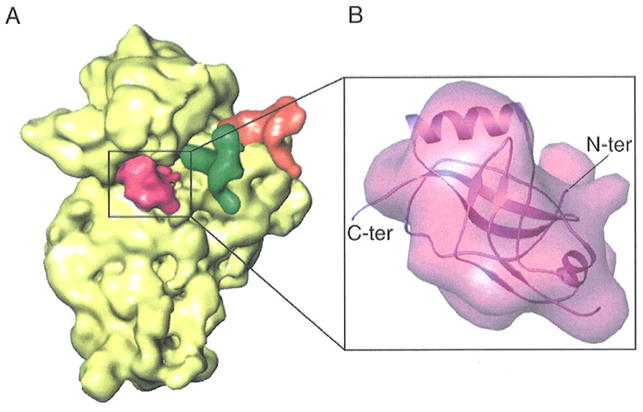
Location and interpretation of density attributed to SmpB. (A) Cryo-EM map of SmpB bound with the 30S subunit, obtained by computational separation from the map shown in fig. 1B. SmpB is shown in red, and P- and E- site tRNAs are in green and orange, respectively. The 30S subunit is depicted in yellow. (B) Density attributed to SmpB, shown in the same orientation as in (A), and docked atomic coordinates shown in Ribbons representation. Coordinates are from PDB code 1P6V. The N- and C-terminal ends of SmpB are indicated.
In the fitting position, SmpB fills the space near the decoding center. Based on this fitting position, the potential binding sites are on different sides of the molecule and include the position for normal A-site codon, helix 44 of the 30S subunit, which are known to participate actively in the decoding process during normal translation (18); the TLD domain of tmRNA in the pre-accommodation complex, as well as the other SmpB oriented toward the 50S subunit. As described by Dong et al (19), conserved amino acids in SmpB are distributed on different sides of the molecule, exposing more than one potential intermolecular binding site. This suggestion thus fits our current observation. The orientation of the SmpB seems plausible in facilitating the entry of tmRNA into the ribosome (see Discussion).
To check this hypothesis and to investigate farther the contribution by stalled ribosomes to SmpB recognition, measurements of the binding of tmRNA to the ribosomal complexes described above were carried out by filter-binding assays (20). The complexes were loaded on a gel filtration Superdex 200 column (GE Healthcare, Orsay, France) in order to eliminate the free fraction of SmpB that could interfere with tmRNA. Equilibrium dissociation experiments were then carried out between radiolabelled tmRNA and each of the purified complexes (see Experimental Procedures). According to the data, tmRNA binds to stalled ribosomes once the empty A site has been recognized and occupied by SmpB (Fig. 3; compare curves with squares to diamonds).
Figure 3.
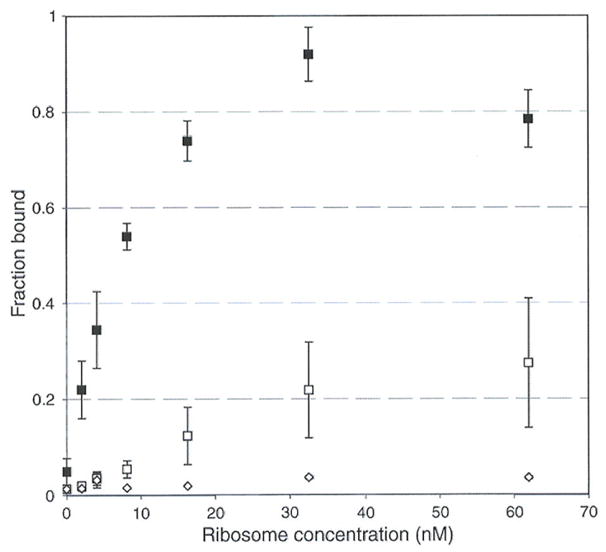
Equilibrium dissociation experiments of tmRNA on programmed ribosomes, measured by filter-binding assays. The maximum of binding that we could obtain, at the highest ribosome concentration, was set to 1, and the other values were measured on the 0 to 1 scale, expressed as “fraction bound”. ◆: 70S-tRNA stalled with a truncated mRNA leaving the A site empty, in the absence of SmpB. ▲: 70S-tRNA stalled with an mRNA bearing a sense codon at the A site, in the presence of SmpB. ■: 70S-tRNA stalled with a truncated mRNA leaving the A site empty, in the presence of SmpB.
Experiments related to the assembly pathway
In order to ascertain specificity of SmpB for an empty A site, a control experiment was carried out by preparing a sample in which all conditions are kept the same, except for placement of a sense codon at the A site (see Experimental procedures and Fig. 1C). Despite the presence of SmpB in the initial mix, tmRNA is apparently unable to target the stalled ribosome (Fig. 3, triangles). Altogether, these results confirm the cryo-EM data (Fig. 1) showing that SmpB binds exclusively to stalled ribosomes bearing an empty A site, thereby facilitating their subsequent interaction with tmRNA.
Our cryo-EM study and the finding by Hallier et al. (13) agree in the result that in the absence of tmRNA one SmpB molecule binds stably to the stalled 70S ribosome, and that this molecule is found on the 30S subunit. We designed biochemical experiments to investigate whether or not this 30S·mRNA·SmpB complex has any relevance in vivo, and whether under these conditions SmpB molecules strongly bind with tmRNA. Translating 70S ribosomes were purified in vivo and dissociated into their large and small subunits on a sucrose density gradient at low salt concentration (Fig. 4A). After gel separation, the 23S and 16S ribosomal RNA were detected by ethidium bromide staining, allowing a correct matching of the two subunits extracts on the gel (Fig. 4B). tmRNA and SmpB distribution were monitored in each fraction by Northern and Western Blot, respectively. Dissociation of the two subunits releases tmRNA from the ribosomes to the soluble fraction (Fig. 4C). tmRNA was detected neither in the 50S nor in the 30S subunits. Using an antibody raised against SmpB, immunoblots of these fractions indicate that, in the same way, the protein mostly co-sediments with tmRNA outside the separated ribosomal subunits. The SmpB protein coming from a mixture containing ribosomes in all stages of trans-translation is released with tmRNA, while a small portion of SmpB bound alone to the stalled ribosomes is found associated with the 30S fraction (Fig. 4D).
Figure 4.
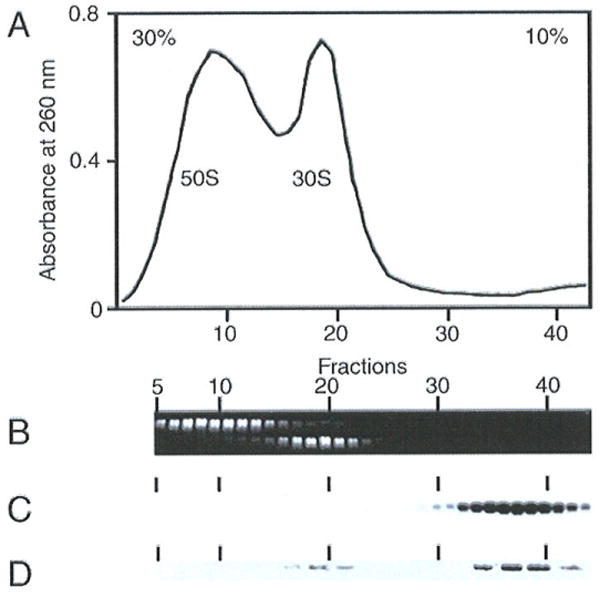
In vivo interaction of SmpB with the small subunit and tmRNA. Crude ribosomes (P100) from E. coli cells were fractionated by sucrose gradient centrifugation at low concentration of Mg2+ ions. (A) Absorbencies of each fraction. (B) 23S and 16S ribosomal RNAs were detected by ethidium bromide staining and were separated on a formaldehyde-agarose gel. (C) Detection of tmRNA by Northern hybridization. (D) Detection of the presence of SmpB protein in sucrose fractions by Western blotting.
These results confirm that in vivo one SmpB molecule binds to a fraction of the stalled 70S ribosomes on the small subunit, and further that, once the trans-translating complex is formed, tmRNA and two SmpB molecules form a strong association that even persists after forced subunit dissociation. Given the fact that two copies of SmpB are found in the stalled trans-translating 70S ribosome (8,10), the assembly of the trans-translating complex brings two SmpBs in close contact so that they form a structural scaffold.
SmpB forms a binding framework for tmRNA
The in vitro experiment demonstrates that when tmRNA is present, two molecules of SmpB on the 70S ribosome are required for trans-translation (13). One is tightly bound to the 30S subunit, while the other contacts the large subunit transiently (10). Accordingly, a recent cryo-EM reconstruction at higher resolution has identified density for two molecules of SmpB in a pre-accommodated state: the one depicted above (SmpB-2) and the other directed toward the large subunit (SmpB-1) (8).
On the side view depicted in Fig. 5, interacting regions of the two SmpB proteins with the tRNA portion of tmRNA are emphasized. Both SmpB proteins interact with tmRNA such that the two molecules come in close contact (Fig. 5). While SmpB-2 binds the elbow region of the TLD in tmRNA as shown in the X-ray structure (3), as well as the CCA arm as shown in our model, SmpB-1 interacts with the T stem-loop (Fig. 5). In addition, SmpB-1 contacts SmpB-2 through the unstructured C-terminal of SmpB-1. In the fitting position (3), the bi-lobed shape of the density for SmpB-1 near the 50S subunit was fitted quite well by placing β4-β5 and β7-α3 into the two lobes. However, this placement causes noticeable overlap between the C-terminal of SmpB-1 and the T stem-loop. In the model shown in Fig. 5, the X-ray position of the C-terminal (residues 125-130) is highlighted to indicate that we are potentially dealing with a highly flexible portion of the molecule, so that concerns about the overlap may be moot. In addition, we made a slight adjustment for the position of SmpB-1 to release some residual regional overlapping. Furthermore, we noticed that the bi-lobed shape of the density can also be fitted by a position of the X-ray structure related to the current position through rotation by 180° about an axis normal to the plane of the figure, but this would remove the potential dimeric contact via C-terminal binding. It is hoped that a future higher-resolution map will provide a decisive answer for the position of SmpB-1.
Figure 5.
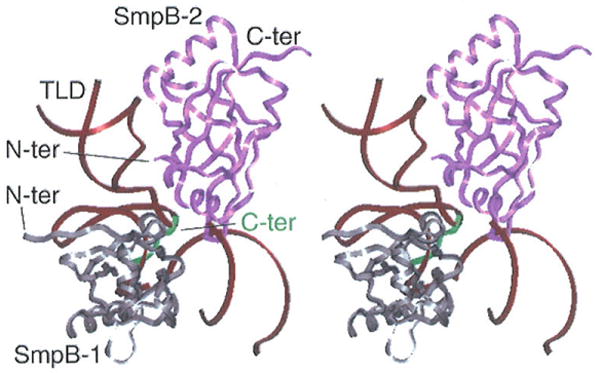
Concomitant interactions between SmpB-1, SmpB-2, and tmRNA's tRNA-like domain (TLD). SmpB directed toward the large subunit (SmpB-1) is in grey, with C-terminal indicated in green; SmpB directed toward the small subunit (SmpB-2) is in pink. The TLD (dark brown) is shown in dark brown.
Intriguingly, when monitoring the interactions of SmpB with a full-length thermophile tmRNA, not all the footprints identified on the TLD are clustered around the 3′ end of the D-loop (21), as expected from the X-ray crystal data of the complex between the tRNA-like domain of tmRNA and SmpB. Additional protections arc in the T stem and loop, reflecting either indirect changes due to a rearrangement of the binding to the elbow, or a direct interaction between the protein and the RNA, as suggested by the position of SmpB-1 in our model. In the same way, a combination of enzymatic probing and UV cross-linking experiments indicates that the acceptor and T-arms constitute a major binding site for SmpB (9). Thus the two SmpB proteins surrounding the TLD create a stable scaffold that allows the RNA to make indirect contacts with two essential structural elements of the ribosome: the decoding site on the small subunit and the GTPase-associated center on the large subunit. Both biochemical data and the structural evidence depicted here strengthen the idea of the SmpB molecules forming a protein scaffold that provides a stabilizing framework for the flexible tmRNA molecule on the ribosome, allowing its accurate positioning and tuning of its activity.
Regulation of tmRNA activity by ribosomal protein S1
The crucial role played by SmpB in scaffolding tmRNA into an active conformation for aminoacylation and recognition by the ribosome raises the question about the roles played by the other protein partners during the process. Among these, the role of ribosomal protein S1 is still not completely understood despite numerous studies. Two recent studies have suggested that S1 might play a role at a stage prior the recruitment of tmRNA to the ribosome (22,23).
To investigate the way in which ribosomal protein S1 regulates the activity of tmRNA in trans-translation, a higher-resolution, 13-Å map (as compared to the earlier 18Å map by Valle et al., ref.7) of the pre-accommodated complex in the absence of S1 was obtained (Fig. 6A). Assignment of the atomic coordinates for each portion of the map was done as described in our previous cryo-EM study (8), allowing us to refine the atomic model of tmRNA and SmpB in this complex that was presented in the previous publication (7). Though in agreement with the previous map in all essential features, the new map shows the complex with much higher definition. We see the 70S ribosome and a density attributed to the complex of tmRNA, SmpB, and EF-Tu. This map must be compared with the map showing the complex in the presence of S1, to clarify whether or not this complex is associated with the functionally related structural feature. Evidently, the absence of S1 produces a complex that differs from the pre-accommodated complex in the presence of S1 in four major features (for the placement of structural elements in the tmRNA map, see Fig. 6):
Figure 6.
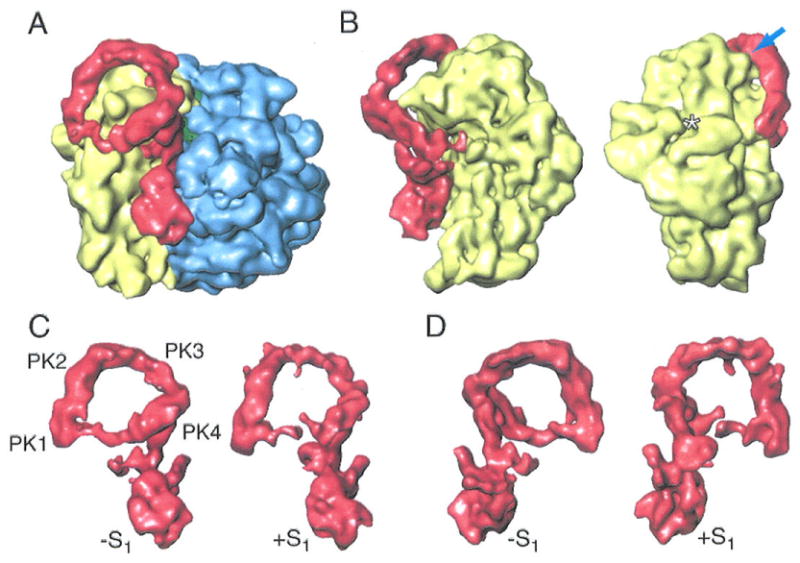
Cryo-EM map showing the binding between the ribosome and tmRNA in the absence of S1. (A) Cryo-EM map for the EF-Tu·tmRNA·SmpBs complex bound to the ribosome in the absence of S1. The density attributable to the EF-Tu·tmRNA·SmpBs complex is in red. 50S subunit is in blue, the 30S subunit is in yellow. (B) Two views of the 30S subunit bound with EF-Tu·tmRNA·SmpBs in the absence of S1. The arrow points to the contact between PK2 and the head of the 30S subunit. Asterisk: position where ribosomal protein S1 would appear in the 30S subunit. (C) Side view of the tmRNA·SmpBs·EF-Tu·GDP complexes in the absence and presence of S1. (D) View of complexes in (c), after rotation by 180°.
the formation of a closed ring by the tmRNA elements ORF, PK1, PK2, PK3, PK4, and helix 5. Presumably related to the stabilized structure of the ORF region, tmRNA in the absence of S1 forms a closed loop rather than an open one-turn spiral (7). The closed loop places the ORF outside the decoding center so that trans-translation cannot start, as this process relies on the recognition of codons included in the ORF. The presence of S1 influences the position and structure of the ORF, implying S1 as a decisive factor instrumental for sending the ORF into the decoding site. The density appearing between PK1 and helix 5 is strong enough to account for a single-stranded RNA in a slightly curved path, reflecting the fact that the ORF is highly structured in the absence of S1. These data are consistent with the prediction for the secondary structure of tmRNA, according to which the ORF contains structured single-stranded domains (24);
the absence of a direct contact between the ORF and the beak of the 30S subunit;
the appearance of an enlarged area of contact between helix 5 of tmRNA and the head of the 30S subunit, as well as an extra contact between PK2 and the head of the 30S subunit (fig. 6B);
the weakening of the connection between the loop linking H2a and H2b of tmRNA and the head of 30S subunit.
Discussion
Trans-translation requires a complex assembly of several elements in the correct order and correct spatial arrangement. Our study, in conjunction with the study presented by Kaur and coworkers (8) indicates that this process can be understood as the result of the interaction of the main players (tmRNA and the 70S ribosome) with two proteins, SmpB and S1. Both interactions can be subsumed under the single perspective of scaffolding. In the following, the separate roles of these proteins, in assembling an A/T-like pre-trans-translation complex and in guiding the ORF into the mRNA entrance channel, will be illuminated.
Scaffolding of tmRNA by S1
The new cryo-EM data show that the presence of S1 directly affects the conformation of tmRNA even though S1 appears neither at the canonical ribosomal binding site (16) (indicated by asterisk in fig. 6B) nor around tmRNA. Our previous observations indicated that S1 binds to the ribosome rather weakly (16). The fact that S1 interacts with tmRNA in solution has been supported by biochemical data (22,23).
Going by its size (25), S1 could approximately match the inner diameter of the circle, about 80 Å, of the one-turn spiral formed by tmRNA, which is composed of the sequence of pseudoknots from PK1 to PK4. Biochemical studies revealed that the S1-binding sites along tmRNA include PK2, PK3 and MLD (26,27). Our cryo-EM maps show that these tmRNA domains interact with the 30S subunit after the tmRNA complex enters the ribosome. Our interpretation is that these binding elements could be actively involved in the binding with S1 in the pre-binding stage. In view of the normal role of S1 as a ribosomal protein, and since it is known to bind loosely, it is interesting to ask if the absence of S1 in solution might have an indirect effect, in depleting ribosome-bound S1 and thereby affecting the conformation of the ribosome adversely, such that the formation of the productive spiral conformation of tmRNA is disfavored. One observation is relevant here, although it falls short of answering this question: we see some differences in the conformation of the 30S subunit head between the +S1 and −S1 complexes in the region of the PK2 contact point (arrow in Fig. 6).
Taking into account the available data, we can postulate the following pathway: first, S1 binds with tmRNA in solution and thereby activates tmRNA into a functional conformation. (We cannot exclude at the present time that S1 might play an additional role in binding to the 30S subunit and effecting a change in its conformation.) Then, upon tmRNA's initial, SmpB-mediated binding to the stalled ribosome, S1 is released from the pre-binding complex. The 30S subunit beak replaces S1 in its stabilizing function and positions tmRNA such that the ORF is placed in the decoding site, as required for trans-translation. Accordingly, the S1 protein is dispensable for the tRNA-like function of tmRNA but essential for its mRNA function in vitro and in vivo (28).
The present cryo-EM map shows that while the absence of S1 does not prevent tmRNA recruitment, and the ORF in such a complex is highly stable and structured, the position of this structured ORF is remote from the decoding site, so the complex must be in a non-functional state. In fact, the effect of S1 in destabilizing the ORF region was already seen for the tmRNA ribosome complex at lower resolution (7).
Scaffolding of the tmRNA-ribosome complex by SmpB
Both biochemical results and cryo-EM studies have demonstrated that two molecules of SmpB bind to the complex of the stalled 70S ribosome with tmRNA when trans-translation is initiated. The positions of these two SmpB molecules have been identified in the cryo-EM maps: SmpB-2 in the decoding site on the small subunit and SmpB-1 toward the GTPase-associated center on the large subunit (Fig. 5). Both SmpB-1 and SmpB-2 interact with the TLD in tmRNA such that a stable scaffold is created, allowing tmRNA to be correctly positioned into the decoding site. Although not being present in our map in the absence of tmRNA, the SmpB protein directed towards the large subunit (SmpB-1) probably plays a major role in the early events of trans-translation since it makes close interactions with helix 69 in the 23S RNA, which is involved in the ribosomal subunit association as well as in the GTPase activation of EF-Tu (29). This is in agreement with biochemical data showing that SmpB binds the large subunit transiently, whereas it binds the small subunit stably (10). Transient binding to the large subunit can be rationalized by the fact that SmpB in this binding position would clash with the P-site tRNA upon accommodation of the TLD.
Along the same lines, in a recent work Ivanova et al. (30) monitored the interactions of SmpB with empty ribosomes by footprinting in vitro. As demonstrated there, two binding sites for SmpB were detected per ribosome, one toward the large subunit, close to the GAC, and the second toward the small one, but in the vicinity of the P site. It should be noted here that the ribosomes used in their study were vacant, leaving all three tRNA sites (A, P and E) empty and available for interacting with the protein. In our study the ribosomes were in a functionally relevant, stalled state, with mRNA present but truncated, and the P and E sites filled by tRNAs, leaving only the A site empty, precisely corresponding to the situation triggering trans-translation in vivo (for a review, see ref.6).
Pathway of initiation of trans-translation
One of the most intriguing questions about trans-translation is how tmRNA recognizes a stalled ribosome. From the pioneering work by Sauer and collaborators in the late 90s (31,4) it has been known that SmpB is essential during trans-translation. It was not clear until recently (13) that trans-translation might be triggered by the pre-loading of SmpB into the stalled ribosome. The present cryo-EM data directly show the binding of SmpB to the stalled ribosome as an early step, in the absence of any other components associated with tmRNA entry into the ribosome. However, the presence of a codon in the decoding site of the small ribosomal subunit prevents this binding from occurring. Thus, the SmpB at the decoding site acts as a sentinel, able to discriminate stalled ribosomes from the active ones that are translating intact messages. As another cryo-EM study shows (8), this SmpB position at the decoding center is retained in the accommodated state of tmRNA. The fact that the two copies of SmpB tightly interact with each other in the pre-accommodated state suggests that the association of these molecules is instrumental in placing the TLD correctly, in line with the central theme of scaffolding in trans-translation.
Acknowledgments
We would like to thank Richard Gursky for collecting the images on the electron microscope. We would like to thank Michael Waiters for assistance with the preparation of the illustrations. We are grateful to V. Ramakrishnan (MRC-LMB, NIH grant no. GM 67624) for providing the T. thermophilus ribosomes used in this study.
The abbreviations used are
- TLD
tRNA-like domain
- tmRNA
transfer-messager RNA
- ORF
open reading frame
- MLD
mRNA-like domain
- SmpB
small protein S
- cryo-EM
cryo electron microscopic
- EF-Tu
elongation factor Tu
- PDB
Protein Data Bank
Footnotes
The atomic coordinates of tmRNA, SmpB-1, SmpB-2, and (for reference) helix 44 of the 30S subunit (1N34 for Thermus thermophilus) (code XXXX) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/). The cryo-EM density maps of the complexes (1) 70S·SmpB; (2) 70S· SmpBs·tmRNA·EF-Tu+S1; and (3) 70S· SmpBs·tmRNA·EF-Tu-S1 have been deposited in the EBI three-dimensional EM database (EMDB) (http://www.eblac.ak/msd/Services.html) under accession codes: EMD-XXXX, EMD-YYYY, and EMD-ZZZZ.
This work was supported in part by grants from from Région Bretagne (PRIR Grant n°691 and CRB 2004-1483), ACI BCMS 136 and ANR programme MIME (to R.G. and B.F.) and by grants from HHMI, NIH R01 GM55440 and R37 GM29169 (to J.F.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Gueneau de Novoa P, Williams KP. Nucleic Acids Res. 2004;32(Database issue):D104–108. doi: 10.1093/nar/gkh102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stagg SM, Frazer-Abel AA, Hagerman PJ, Harvey SC. J Mol Biol. 2001;309:727–735. doi: 10.1006/jmbi.2001.4632. [DOI] [PubMed] [Google Scholar]
- 3.Gutmann S, Haebel PW, Metzinger L, Sutter M, Felden B, Ban N. Nature. 2003;424:699–703. doi: 10.1038/nature01831. [DOI] [PubMed] [Google Scholar]
- 4.Karzai AW, Susskind MM, Sauer RT. EMBO J. 1999;18:3793–3799. doi: 10.1093/emboj/18.13.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karzai AW, Roche ED, Sauer RT. Nat Struct Biol. 2000;6:449–455. doi: 10.1038/75843. [DOI] [PubMed] [Google Scholar]
- 6.Saguy M, Gillet R, Metzinger L, Felden B. Biochimie. 2005;87:897–903. doi: 10.1016/j.biochi.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Valle M, Gillet R, Kaur S, Henne A, Ramakrishnan V, Frank J. Science. 2003;300:127–130. doi: 10.1126/science.1081798. [DOI] [PubMed] [Google Scholar]
- 8.Kaur S, Gillet R, Li W, Gursky R, Frank J. Proc, Natl Acad Sci (USA) 2006;103:16484–16489. doi: 10.1073/pnas.0607438103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wower J, Zwieb CW, Hoffman DW, Wower IK. Biochemistry. 2002;41:8826–8836. doi: 10.1021/bi0201365. [DOI] [PubMed] [Google Scholar]
- 10.Hallier M, Desreac J, Felden B. Nucleic Acids Res. 2006;34:1935–1943. doi: 10.1093/nar/gkl097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanawa-Suetsugu K, Takagi M, Inokuchi H, Himeno H, Muto A. Nucleic Acids Res. 2002;30:1620–1629. doi: 10.1093/nar/30.7.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimizu Y, Ueda T. FEBS Lett. 2002;514:74–77. doi: 10.1016/s0014-5793(02)02333-5. [DOI] [PubMed] [Google Scholar]
- 13.Hallier M, Ivanova N, Rametti A, Pavlov M, Ehrenberg M, Felden B. J Biol Chem. 2004;279:25978–25985. doi: 10.1074/jbc.M314086200. [DOI] [PubMed] [Google Scholar]
- 14.Clemons WM, Jr, Brodersen DE, McCutcheon JP, May JL, Carter AP, Morgan-Warren RJ, Wimberly BT, Ramakrishnan V. J Mol Biol. 2001;310:827–843. doi: 10.1006/jmbi.2001.4778. [DOI] [PubMed] [Google Scholar]
- 15.Frank J. Three-dimensional Electron Microscopy of Macromolecular Assemblies. Oxford University Press; New York: 2006. [Google Scholar]
- 16.Gabashvili IS, Agrawal RK, Spahn CM, Grassucci RA, Svergun DI, Frank J, Penczek P. Cell. 2000;100:537–549. doi: 10.1016/s0092-8674(00)80690-x. [DOI] [PubMed] [Google Scholar]
- 17.Carson M. J Appl Cryst. 1991;24:103–106. [Google Scholar]
- 18.Ogle JM, Murphy FV, Tarry MJ, Ramakrishnan V. Cell. 2002;111:721–732. doi: 10.1016/s0092-8674(02)01086-3. [DOI] [PubMed] [Google Scholar]
- 19.Dong G, Nowakowski J, Hoffman DW. EMBO J. 2002;21:1845–1854. doi: 10.1093/emboj/21.7.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cochella L, Green R. Science. 2005;308:1178–1180. doi: 10.1126/science.1111408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Metzinger L, Hallier M, Felden B. Nucleic Acids Res. 2005;33:2384–2394. doi: 10.1093/nar/gki534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGinness KE, Sauer RT. Proc Natl Acad Sci USA. 2004;101:13454–13459. doi: 10.1073/pnas.0405521101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okada T, Wower IK, Wower J, Zwieb CW, Kimura M. Biosci Biotechnol Biochem. 2004;68:2319–2325. doi: 10.1271/bbb.68.2319. [DOI] [PubMed] [Google Scholar]
- 24.Felden B, Himeno H, Muto A, McCutcheon JP, Atkins JF, Gesteland RF. RNA. 1997;3:89–103. [PMC free article] [PubMed] [Google Scholar]
- 25.Sengupta J, Agrawal RK, Frank J. Proc Natl Acad Sci USA. 2001;98:11991–11996. doi: 10.1073/pnas.211266898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wower IK, Zwieb CW, Guven SA, Wower J. EMBO J. 2000;19:6612–6621. doi: 10.1093/emboj/19.23.6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bordeau V, Felden B. Biochimie. 2002;84:723–729. doi: 10.1016/s0300-9084(02)01442-6. [DOI] [PubMed] [Google Scholar]
- 28.Saguy M, Gillet R, Skorski P, Himeno H, Felden B. Nucleic Acids Research. 2006 doi: 10.1093/nar/gkm100. submitted to. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maivali U, Remme J. RNA. 2004;10:600–604. doi: 10.1261/rna.5220504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ivanova N, Pavlov MY, Bouakaz E, Ehrenberg M, Schiavone LH. Nucleic Acids Res. 2005;33:3529–3539. doi: 10.1093/nar/gki666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keiler KC, Shapiro L, Williams KP. Proc Natl Acad Sci USA. 2000;97:7778–7783. doi: 10.1073/pnas.97.14.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]


