Abstract
Transforming growth factor beta-1 (TGF-β1) plays a critical role in progression of cardiac fibrosis, which may involve intracellular calcium change. We examined effects of efonidipine, a dual T-type and L-type calcium channel blocker (CCB), on TGF-β1–induced fibrotic changes in neonatal rat cardiac fibroblast. T-type and L-type calcium channel mRNAs were highly expressed in cultured cardiac fibroblasts. TGF-β1 (5 ng/mL) significantly increased Smad2 phosphorylation and [3H]-leucine incorporation, which were attenuated by pretreatment with efonidipine (10 μM). Neither R(−)efonidipine (10 μM), selective T-type CCB, nor nifedipine (10 μM), selective L-type CCB, efficaciously inhibited both TGF-β1–induced Smad2 phosphorylation and [3H]-leucine incorporation. However, both were markedly attenuated by combination of R(−)efonidipine and nifedipine, EDTA, or calcium-free medium. Pretreatment with Smad2 siRNA significantly attenuated [3H]-leucine incorporation induced by TGF-β1. These data suggest that efonidipine elicits inhibitory effects on TGF-β1– and Smad2-dependent protein synthesis through both T-type and L-type calcium channel–blocking actions in cardiac fibroblasts.
Keywords: transforming growth factor beta-1, Smad2, T-type calcium channel, cardiac fibrosis, efonidipine
Introduction
Cardiac fibrosis is induced by concomitant increased biosynthesis of proteins, such as extracellular matrix components (1). Cardiac fibroblasts, the most numerous cell type in the heart (2), are a major factor in the pathogenesis of cardiac fibrosis (3). Several lines of evidence pointed to transforming growth factor beta-1 (TGF-β1) having a crucial role in the myocardial remodeling process, particularly in cardiac fibrosis (4 – 6). In addition, TGF-β1, as a multifunctional peptide, plays an important role in regulation of numerous physiological and pathophysiological processes, including cell proliferation, differentiation, apoptosis, early embryonic development, and extracellular matrix protein synthesis in cardiac fibroblasts (7). The best-defined signaling pathways of TGF-β1 are through Smad family members, including Smad2 and Smad3 (8). However, little is known about how TGF-β1 induces fibrotic change via Smad2 in cardiac fibroblasts.
Calcium channel blockers (CCBs) are widely used for treatment of hypertension. Cardioprotective effects of CCBs are mainly explained by a hemodynamic mechanism, while their non-hemodynamic cardioprotective effects remain controversial. In vitro studies have indicated that intracellular Ca2+ signaling is an important second messenger of the TGF-β1 signal transduction pathway (9). Akiyama-Uchida et al. (1) showed that L-type CCBs partially inhibited TGF-β1–induced cardiac fibrosis; this beneficial effect may mediated by suppression of cardiac hypertrophy through non-hemodynamic mechanisms. Several classes of voltage-gated calcium channels, including L-type, N-type, and T-type calcium channels have been identified in the heart (10). However, the roles of these calcium channels in TGF-β1–induced cardiac fibrosis are as yet unclear.
During past years, L-type CCBs were the most commonly used type of CCB. Less attention had been paid to the other subtypes of calcium channels such as the T-type calcium channel. In contrast to the L-type calcium channel, the T-type calcium channel has been previously described in embryonic ventricular cells (11) and down-regulated in normal adult cardiac ventricles (12). Under pathological conditions such as pressure-overload cardiac hypertrophy, myocardial infarction, and heart failure, T-type calcium channels have been reported to be re-expressed in the ventricle (8, 13, 14). Furthermore, some evidence of cardioprotective effects of efonidipine, a dual L-type and T-type CCB, has also been seen in patients with essential hypertension (15). Therefore, we examined the effect of efonidipine, R(−)efonidipine, a selective T-type CCB, and nifedipine, a selective L-type CCB, on the TGF-β1–Smad signaling pathway in cardiac fibroblasts.
Materials and Methods
Cell culture
Cardiac fibroblasts were isolated from left ventricles of 5-day-old Sprague-Dawley rats, as previously described (16). In brief, hearts were harvested and minced in ADS buffer (116 mM NaCl, 20 mM HEPES, 9.4 mM NaH2PO4, 5.5 mM glucose, 5.4 mM KCl, 0.4 mM MgSO4, pH 7.4). The left ventricular tissue was digested at 37°C with collagenase type II in sterile ADS buffer for 10 min. The digestion was repeated five times. Then cardiac fibroblasts were purified from contaminating cardiac myocytes using a Percoll gradient centrifugation (GE Healthcare, Buckinghamshire, UK). Finally, cardiac fibroblasts were resuspended and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich, St. Louis, MO, USA) containing 10% fetal bovine serum (FBS; HyClone, Logan, UT, USA) and penicillin (100 U/mL; Life Technologies, Carlsbad, CA, USA) maintained at 37°C under 5% CO2 in a humidified incubator. Passages 2 – 4 were used for the experiment.
Western blot analysis
Whole-cell lysates were collected in lysis buffer, and total protein content was determined by Bradford protein assay, as previously described (17). Equal amounts of protein samples were separated by SDS-PAGE and immunoblotted with appropriate anti-phospho–Smad2, anti-Smad2 antibodies (Cell Signaling Technology, Danvers, MA, USA). Protein bands were transferred to a PVDF membrane using standard techniques and visualized with an ECL plus system (GE Healthcare). Band intensities were quantified by densitometry of the immunoblots using NIH ImageJ software.
RT-PCR
For real-time RT-PCR analysis, total RNAs were extracted using ISOGEN (Nippon Gene, Tokyo); cDNA (from 1 μg RNA) was synthesized as described previously (18). The mRNA expression was analyzed using a Light Cycler Fast Start DNA Master SYBR Green I kit (Applied Biosystems, Foster City, CA, USA). The primer sequences of the calcium channels were as follows: L-type forward: 5′-GATGCGGGTGCTGAAGCTAG-3′, reverse: 5′-TGACAGGCAGCTGAATACAG-3′; and T-type forward: 5′-AGACGTGCTGTACTGGATGC-3′, reverse: 5′-CACTTCTGTGAGCCAGTGAG-3′.
[3H]-Leucine incorporation
Total protein synthesis was assessed by measurement of [3H]-leucine incorporation (17). Quiescent cardiac fibroblasts cultured in 6-well plates were treated with or without TGF-β1 containing 1 μCi/mL [3H]-leucine for 24 h. The radioactivity of the cell lysates was determined using a liquid scintillation counter.
Rat Smad2 siRNA
Transfection of cardiac fibroblasts with siRNA was performed as previously described (18). The silencer pre-designed siRNAs against Smad2 (Life Technologies) were synthesized according to rat-specific sequences. As a negative control, a non-targeting scrambled siRNA (control siRNA) was used (Life Technologies). Cardiac fibroblasts were transfected with Lipofectamine 2000 (Life Technologies) in suspension with 50 nM Smad2 siRNA for 8 h.
Statistical analyses
Results are expressed as the mean ± S.E.M. Statistical significance was assessed using one way analysis of variance (ANOVA) followed by Tukey’s test. Student’s t-tests were performed to compare the means when the experimental design contained two individual groups. A value of P < 0.05 was considered to be statistically significant.
Results
Effect of TGF-β1 on Smad2 phosphorylation
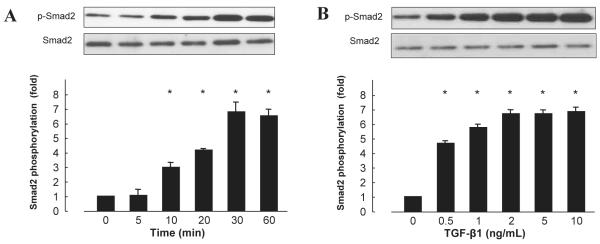
We first investigated TGF-β1–induced Smad2 phosphorylation. The TGF-β1 (5 ng/mL) significantly induced Smad2 phosphorylation in a time-dependent manner in cardiac fibroblasts, peaking 30 min after adding TGF-β1 and remaining phosphorylated for at least 60 min (Fig. 1A, n = 6, P < 0.05). To determine the optimal dose for TGF-β1–induced Smad2 phosphorylation, cardiac fibroblasts were treated with increasing concentrations of TGF-β1 for 30 min. TGF-β1 significantly stimulated Smad2 phosphorylation in a concentration-dependent manner (Fig. 1B, n = 6, P < 0.05).
Fig. 1.
Time-course and dose-dependent effect of TGF-β1 on Smad2 phosphorylation in cardiac fibroblasts. A: TGF-β1 (5 ng/mL) used to stimulate cells. B: Cells stimulated with TGF-β1 for 30 min. Bar graphs represent the mean ± S.E.M. (n = 6), expressed as the fold change in phosphorylation compared with unstimulated cells. *P < 0.05 vs. control cardiac fibroblasts.
Expression of T-type and L-type calcium channels in cardiac fibroblasts
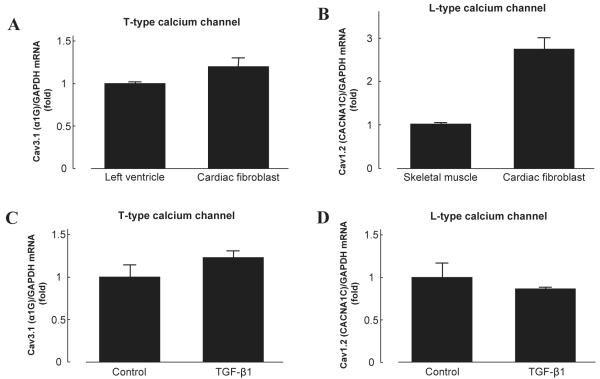
Skeletal muscle and left ventricles were used as positive controls separately for T-type and L-type calcium channels. T-type calcium channels reportedly contribute to electrical activity in the early-stage embryonic heart and reappear in the ventricle under pathological conditions (19). Expression of L-type calcium channels in the ventricle are well established in many species (10). RT-PCR demonstrated that mRNA from both T-type and L-type calcium channels were highly expressed in cultured cardiac fibroblasts compared to positive controls (Fig. 2: A and B). In addition, incubation with TGF-β1 (5 ng/mL) for 24 h did not cause any significant changes in mRNA expression of T-type or L-type calcium channel in cardiac fibroblasts (Fig. 2: C and D).
Fig. 2.
Expression of T-type and L-type calcium channel mRNA in cardiac fibroblasts. A: A subunit of the T-type calcium channel, Cav3.1 (α1G), was highly expressed in cardiac fibroblasts. B: A subunit of the L-type calcium channel, Cav1.2 (CACNA1C), was highly expressed in cardiac fibroblasts. C and D: TGF-β1 (5 ng/mL, 24 h) did not increase T-type or L-type calcium channel in cardiac fibroblasts. Bar graphs represent the mean ± S.E.M. (n = 6), expressed as the fold change in mRNA expression compared to positive controls; mRNA levels are normalized to GAPDH mRNA levels.
Effect of CCBs on TGF-β1–induced Smad2 phosphorylation
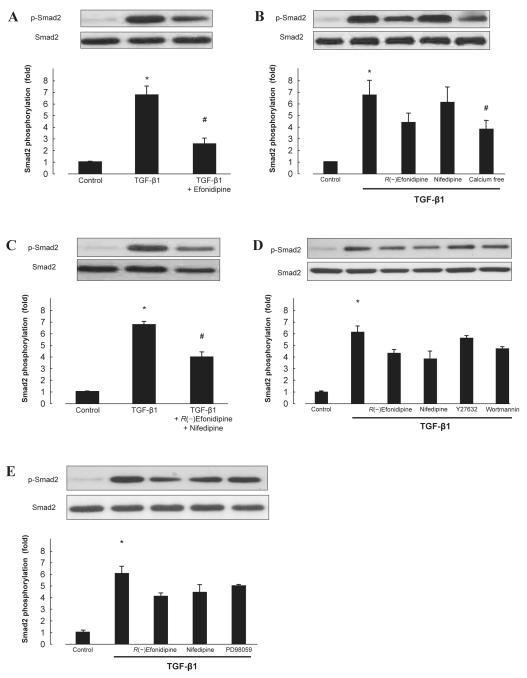
We determined the roles of calcium channel in TGF-β1–induced Smad2 phosphorylation. Pretreatment with efonidipine (10 μM, 30 min) significantly suppressed the TGF-β1 (5 ng/mL, 30 min)-induced Smad2 phosphorylation (Fig. 3A, n = 6, P < 0.05). R(−)efonidipine (10 μM), an isomer of efonidipine that inhibits only the T-type calcium channel (20), partially inhibited TGF-β1–induced Smad2 phosphorylation (Fig. 3B, n = 6). Similar results were observed in pretreatment with nifedipine (10 μM), a selective L-type CCB (Fig. 3B, n = 6). Meanwhile, combination treatment with R(−)efonidipine (10 μM) and nifedipine (10 μM) exerted significant inhibitory effect on TGF-β1–induced Smad2 phosphorylation in cardiac fibroblasts (Fig. 3C, n = 6, P < 0.05). In addition, TGF-β1 also failed to phosphorylate Smad2 in calcium-free medium (DMEM, Life Technologies) (Fig. 3B, n = 6). However, TGF-β1–induced Smad2 phosphorylation was not inhibited by Y27632 (1 μM), a Rho kinase inhibitor; wortmannin (100 nM), a phosphatidylinositol-3-kinase (PI3K) inhibitor (Fig. 3D, n = 6); or PD98059 (30 μM), a mitogen-activated protein kinase (MAPK) kinase inhibitor (Fig. 3E, n = 6).
Fig. 3.
Effect of CCBs on TGF-β1–induced Smad2 phosphorylation in cardiac fibroblasts. Smad2 phosphorylation induced by TGF-β1 was measured in the absence or presence of efonidipine (10 μM) (A); R(−)efonidipine (10 μM), nifedipine (10 μM), calcium-free medium (B); combination of R(−)efonidipine and nifedipine (C); Y27632 (1 μM), wortmannin (100 nM) (D); or PD98059 (30 μM) (E). Bar graphs represent the mean ± S.E.M. (n = 6), expressed as the fold change in phosphorylation compared with unstimulated cells. *P < 0.05, control cardiac fibroblasts vs. TGF-β1 alone; #P < 0.05, TGF-β1 alone vs. TGF-β1 with treatment.
Effect of TGF-β1, R(−)efonidipine, and nifedipine on protein synthesis
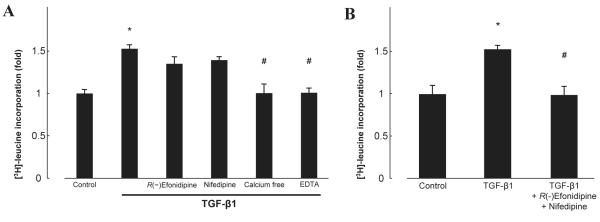
TGF-β1 is involved in collagen synthesis via Smad phosphorylation in various cell types (21, 22). We therefore measured [3H]-leucine incorporation as an index of protein synthesis. Treatment with TGF-β1 (5 ng/mL, 24 h) significantly increased [3H]-leucine incorporation in cardiac fibroblasts (Fig. 4A, n = 6, P < 0.05). This effect was abolished in calcium-free medium or EDTA and reversed by neither R(−)efonidipine (10 μM) nor nifedipine (10 μM) (Fig. 4A, n = 6). Combination treatment with R(−)efonidipine and nifedipine exerted complete attenuation of TGF-β1–induced leucine incorporation (Fig. 4B, n = 6).
Fig. 4.
Effect of TGF-β1 on [3H]-leucine incorporation in the absence or presence of R(−)efonidipine, nifedipine, calcium-free medium, EDTA (A) or the combination of R(−)efonidipine and nifedipine (B). Bar graphs represent the mean ± S.E.M. (n = 6), expressed as the fold change compared with unstimulated cells. *P < 0.05, control cardiac fibroblasts vs. TGF-β1 alone; #P < 0.05, TGF-β1 alone vs. TGF-β1 with treatment.
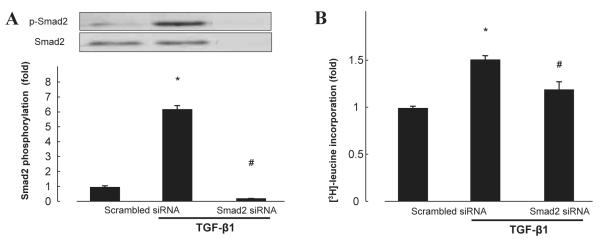
Involvement of Smad2 in TGF-β1–induced protein synthesis
To elucidate the possible role of Smad2 in TGF-β1–induced protein synthesis, we knocked down Smad2 using siRNA (Fig. 5A). Reduction in Smad2 protein levels significantly abolished TGF-β1–induced [3H]-leucine incorporation in cardiac fibroblasts (Fig. 5B, n = 6, P < 0.05). These results suggest that TGF-β1–induced protein synthesis is dependent on activation of Smad2.
Fig. 5.
Effect of Smad2 siRNA on protein expression (A) and TGF-β1–induced [3H]-leucine incorporation (B) in cardiac fibroblasts. Bar graphs represent the mean ± S.E.M. (n = 6), expressed as the fold change compared with unstimulated cells. *P < 0.05, control cardiac fibroblasts vs. TGF-β1 alone; #P < 0.05, TGF-β1 alone vs. TGF-β1 with treatment.
Discussion
Calcium channel blockers are indicated to have cardioprotective effects. In the present study, TGF-β1 significantly increased protein synthesis partially through the Smad2-dependent pathway in cardiac fibroblasts. This signaling pathway was inhibited by efonidipine, and by the combination of R(−)efonidipine and nifedipine. However, R(−)efonidipine or nifedipine monotherapy is not efficacious to abolish Smad2 phosphorylation as well as protein synthesis induced by TGF-β1. We also found that TGF-β1 failed to increase Smad2 phosphorylation and [3H]-leucine incorporation in calcium-free medium or EDTA. In addition, Smad2 siRNA prevented TGF-β1–induced protein synthesis, suggesting that TGF-β1–induced cellular protein synthesis is dependent on activation of Smad2 in cardiac fibroblasts, which may lead to cardiac fibrosis. These data indicate that the combined effects of T-type and L-type calcium channels play an essential role in TGF-β1– and Smad2-dependent cardiac fibrosis. Thus, our data also suggest a novel therapeutic benefit of efonidipine on cardiac fibrosis during the development of heart failure.
Smad proteins are thought to play crucial roles in mediating intracellular responses to TGF-β1 and related factors. The canonical pathway can be summarized as follows: Smad proteins, such as Smad2 and Smad3, are activated by TGF-β1 receptors and then translocated to the nucleus, where they regulate transcription, further modifying multiple cell functions, including hypertrophy and proliferation. Indeed, our data showed TGF-β1 to induce Smad2 phosphorylation and subsequent protein synthesis in cardiac fibroblasts. The TGF-β1 receptor also activates Smad-independent pathways (23). These non-canonical pathways include branches of MAPK, Rho GTPase, and PI3K/Akt pathways (24, 25). However, the present results showed that inhibitors of extracellular-signal regulated kinase, Rho kinase, and PI3K failed to attenuate TGF-β1–induced Smad2 phosphorylation, indicating that TGF-β1–induced activation of Smad2 in cardiac fibroblasts is independent of MAPK-, Rhokinase–, and PI3K-dependent pathways. Although efonidipine partially but significant inhibited Smad phosphorylation, leucine incorporation was completely inhibited. This discrepancy suggests that TGF-β1–induced leucine incorporation was largely, but not totally, dependent on Smad2 activation. Other calcium-dependent signaling pathways may be involved in this process. However, the precise mechanism needs to be further elucidated.
T-type calcium channels have been identified as three different subtypes of genes encoding α1 subunits: Cav3.1 (α1G), Cav3.2 (α1H), and Cav3.3 (α1I) (20, 26, 27). These subunits are expressed in hearts of adult humans (20, 27), mice (28), and rats (29). Accumulating evidence indicated that T-type calcium channel could be a novel therapeutic target for the treatment of various cardiovascular disorders such as heart failure, arrhythmia, and hypertension (30, 31). In this study, we found that Cav3.1 was highly expressed in cardiac fibroblasts, indicating that the T-type calcium channel was expressed in cultured cardiac fibroblasts. Efonidipine, a derivative of dihydropyridine, has a unique pharmacological profile, characterized by blockade of both T-type and L-type calcium channels. Efonidipine is a mixture of R(−) and S(+) isomers, whereas R(−)efonidipine only preferentially blocks T-type calcium channels (32). Our results suggest that activation of both T-type and L-type calcium channels are needed for TGF-β1–induced protein synthesis in cardiac fibroblasts through the Smad2-dependent pathway. We also observed similar inhibitory effects in calcium-free medium. These results imply that this inhibitory effect is due to reduction of calcium influx, which is thought to trigger increasing intracellular calcium. This intracellular calcium change may affect some calcium-dependent protein kinase, which in turn modulates activity of Smad protein, as well as downstream factors leading to protein synthesis.
Cardioprotective effects of efonidipine were clarified by several in vivo experiments. Suzuki et al. have clearly demonstrated that efonidipine brought significant improvement of cardiac function in cardiomyopathic hamsters (33). The strong cardioprotective effect of efonidipine in rats has also been reported by Morimoto et al. (34). In addition, inhibition on TGF-β1 associated fibrotic change by efonidipine has been reported in another study (35). These cardioprotective effects were thought largely attributed to the T-type calcium channel–blocking action for heart rate control and anti-arrhythmia. Although numerous in vivo and in vitro studies have clarified the cardioprotective effect of efonidipine, only sparse support from clinical research has been reported. Efonidipine shows some additional cardioprotective effect compared with amoldipine, an L-type CCB (36). Almendral et al. reported a possible role of TGF-β1 in the process of cardiac remodeling in hypertensive patients (37). In addition, efonidipine was suggested to improve cardiac function and prevent heart failure (38). Long-term treatment with efonidipine significant decreased left ventricular mass index in essential hypertensive patients (15). However, clinical trials, which focus on cardiac fibrosis and heart failure, are needed to reveal the beneficial effects of T-type and L-type calcium channel inhibition in hypertensive patients with heart failure.
In conclusion, the present findings show that TGF-β1 induces protein synthesis in cardiac fibroblasts via a Smad2-dependent pathway. Our data also indicate that activation of both T-type and L-type calcium channel are needed to activate these signaling pathways. These findings offer a strong basis for clarifying the molecular mechanisms of cardiac fibrosis in hypertensive patients who are treated with the T-type and L-type dual CCB efonidipine.
Acknowledgments
We are grateful to Shionogi Co., Ltd. for supplying efonidipine. This work was supported in part by grants-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (20590253 and 22790792).
References
- 1.Akiyama-Uchida Y, Ashizawa N, Ohtsuru A, Seto S, Tsukazaki T, Kikuchi H, et al. Norepinephrine enhances fibrosis mediated by TGF-beta in cardiac fibroblasts. Hypertension. 2002;40:148–154. doi: 10.1161/01.hyp.0000025443.61926.12. [DOI] [PubMed] [Google Scholar]
- 2.Takeda N, Manabe I, Uchino Y, Eguchi K, Matsumoto S, Nishimura S, et al. Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload. J Clin Invest. 2010;120:254–265. doi: 10.1172/JCI40295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagamatsu Y, Nishida M, Onohara N, Fukutomi M, Maruyama Y, Kobayashi H, et al. Heterotrimeric G protein G alpha13-induced induction of cytokine mrnas through two distinct pathways in cardiac fibroblasts. J Pharmacol Sci. 2006;101:144–150. doi: 10.1254/jphs.fp0051036. [DOI] [PubMed] [Google Scholar]
- 4.Eghbali M, Tomek R, Sukhatme VP, Woods C, Bhambi B. Differential effects of transforming growth factor-beta 1 and phorbol myristate acetate on cardiac fibroblasts. Regulation of fibrillar collagen mrnas and expression of early transcription factors. Circ Res. 1991;69:483–490. doi: 10.1161/01.res.69.2.483. [DOI] [PubMed] [Google Scholar]
- 5.Sigel AV, Centrella M, Eghbali-Webb M. Regulation of proliferative response of cardiac fibroblasts by transforming growth factor-beta 1. J Mol Cell Cardiol. 1996;28:1921–1929. doi: 10.1006/jmcc.1996.0185. [DOI] [PubMed] [Google Scholar]
- 6.Agocha A, Lee HW, Eghbali-Webb M. Hypoxia regulates basal and induced DNA synthesis and collagen type I production in human cardiac fibroblasts: effects of transforming growth factor-beta1, thyroid hormone, angiotensin II and basic fibroblast growth factor. J Mol Cell Cardiol. 1997;29:2233–2244. doi: 10.1006/jmcc.1997.0462. [DOI] [PubMed] [Google Scholar]
- 7.Huang Y, Hutter D, Liu Y, Wang X, Sheikh MS, Chan AM, et al. Transforming growth factor-beta 1 suppresses serum deprivation-induced death of A549 cells through differential effects on c-Jun and JNK activities. J Biol Chem. 2000;275:18234–18242. doi: 10.1074/jbc.M909431199. [DOI] [PubMed] [Google Scholar]
- 8.Huang B, Qin D, Deng L, Boutjdir M, Nabil E-S. Reexpression of T-type Ca2+ channel gene and current in post-infarction remodeled rat left ventricle. Cardiovasc Res. 2000;46:442–449. doi: 10.1016/s0008-6363(00)00017-1. [DOI] [PubMed] [Google Scholar]
- 9.Nesti LJ, Caterson EJ, Li WJ, Chang R, McCann TD, Hoek JB, et al. TGF-beta1 calcium signaling in osteoblasts. J Cell Biochem. 2007;101:348–359. doi: 10.1002/jcb.21180. [DOI] [PubMed] [Google Scholar]
- 10.Katz AM. Calcium channel diversity in the cardiovascular system. J Am Coll Cardiol. 1996;28:522–529. doi: 10.1016/0735-1097(96)00170-2. [DOI] [PubMed] [Google Scholar]
- 11.Kawano S, DeHaan RL. Low-threshold current is major calcium current in chick ventricle cells. Am J Physiol. 1989;256:H1505–H1508. doi: 10.1152/ajpheart.1989.256.5.H1505. [DOI] [PubMed] [Google Scholar]
- 12.Niwa N, Yasui K, Opthof T, Takemura H, Shimizu A, Horiba M, et al. Cav3.2 subunit underlies the functional T-type Ca2+ channel in murine hearts during the embryonic period. Am J Physiol Heart Circ Physiol. 2004;286:H2257–H2263. doi: 10.1152/ajpheart.01043.2003. [DOI] [PubMed] [Google Scholar]
- 13.Nuss HB, Houser SR. T-type Ca2+ current is expressed in hypertrophied adult feline left ventricular myocytes. Circ Res. 1993;73:777–782. doi: 10.1161/01.res.73.4.777. [DOI] [PubMed] [Google Scholar]
- 14.Martinez ML, Heredia MP, Delgado C. Expression of T-type Ca2+ channels in ventricular cells from hypertrophied rat hearts. J Mol Cell Cardiol. 1999;31:1617–1625. doi: 10.1006/jmcc.1999.0998. [DOI] [PubMed] [Google Scholar]
- 15.Tsutamoto T, Tanaka T, Nishiyama K, Yamaji M, Kawahara C, Fujii M, et al. Long-term effect of efonidipine therapy on plasma aldosterone and left ventricular mass index in patients with essential hypertension. Hypertens Res. 2009;32:670–674. doi: 10.1038/hr.2009.78. [DOI] [PubMed] [Google Scholar]
- 16.Frank D, Kuhn C, Brors B, Hanselmann C, Ludde M, Katus HA, et al. Gene expression pattern in biomechanically stretched cardiomyocytes: evidence for a stretch-specific gene program. Hypertension. 2008;51:309–318. doi: 10.1161/HYPERTENSIONAHA.107.098046. [DOI] [PubMed] [Google Scholar]
- 17.Nishiyama A, Yao L, Fan Y, Kyaw M, Kataoka N, Hashimoto K, et al. Involvement of aldosterone and mineralocorticoid receptors in rat mesangial cell proliferation and deformability. Hypertension. 2005;45:710–716. doi: 10.1161/01.HYP.0000154681.38944.9a. [DOI] [PubMed] [Google Scholar]
- 18.Moriwaki K, Kiyomoto H, Hitomi H, Ihara G, Kaifu K, Matsubara K, et al. Interferon-gamma enhances superoxide production in human mesangial cells via the JAK-STAT pathway. Kidney Int. 2006;70:788–793. doi: 10.1038/sj.ki.5001639. [DOI] [PubMed] [Google Scholar]
- 19.Yasui K, Niwa N, Takemura H, Opthof T, Muto T, Horiba M, et al. Pathophysiological significance of T-type Ca2+ channels: expression of T-type Ca2+ channels in fetal and diseased heart. J Pharmacol Sci. 2005;99:205–210. doi: 10.1254/jphs.fmj05002x3. [DOI] [PubMed] [Google Scholar]
- 20.Furukawa T, Miura R, Honda M, Kamiya N, Mori Y, Takeshita S, et al. Identification of R(-)-isomer of efonidipine as a selective blocker of T-type Ca2+ channels. Br J Pharmacol. 2004;143:1050–1057. doi: 10.1038/sj.bjp.0705944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sysa P, Potter JJ, Liu X, Mezey E. Transforming growth factor-beta1 up-regulation of human alpha(1)(I) collagen is mediated by Sp1 and Smad2 transacting factors. DNA Cell Biol. 2009;28:425–434. doi: 10.1089/dna.2009.0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao X, He X, Luo B, Peng L, Lin J, Zuo Z. Angiotensin II increases collagen I expression via transforming growth factor-beta1 and extracellular signal-regulated kinase in cardiac fibroblasts. Eur J Pharmacol. 2009;606:115–120. doi: 10.1016/j.ejphar.2008.12.049. [DOI] [PubMed] [Google Scholar]
- 23.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 24.Moustakas A, Heldin CH. Non-Smad TGF-beta signals. J Cell Sci. 2005;118:3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- 25.Liu P, Zhang C, Feng JB, Zhao YX, Wang XP, Yang JM, et al. Cross talk among Smad, MAPK, and integrin signaling pathways enhances adventitial fibroblast functions activated by transforming growth factor-beta1 and inhibited by Gax. Arterioscler Thromb Vasc Biol. 2008;28:725–731. doi: 10.1161/ATVBAHA.107.159889. [DOI] [PubMed] [Google Scholar]
- 26.Cribbs LL, Lee JH, Yang J, Satin J, Zhang Y, Daud A, et al. Cloning and characterization of alpha1H from human heart, a member of the T-type Ca2+ channel gene family. Circ Res. 1998;83:103–109. doi: 10.1161/01.res.83.1.103. [DOI] [PubMed] [Google Scholar]
- 27.Lee JH, Daud AN, Cribbs LL, Lacerda AE, Pereverzev A, Klockner U, et al. Cloning and expression of a novel member of the low voltage-activated T-type calcium channel family. J Neurosci. 1999;19:1912–1921. doi: 10.1523/JNEUROSCI.19-06-01912.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez-Reyes E, Cribbs LL, Daud A, Lacerda AE, Barclay J, Williamson MP, et al. Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature. 1998;391:896–900. doi: 10.1038/36110. [DOI] [PubMed] [Google Scholar]
- 29.Cribbs LL, Martin BL, Schroder EA, Keller BB, Delisle BP, Satin J. Identification of the T-type calcium channel (Ca(v)3.1d) in developing mouse heart. Circ Res. 2001;88:403–407. doi: 10.1161/01.res.88.4.403. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka H, Shigenobu K. Pathophysiological significance of T-type Ca2+ channels: T-type Ca2+ channels and drug development. J Pharmacol Sci. 2005;99:214–220. doi: 10.1254/jphs.fmj05002x5. [DOI] [PubMed] [Google Scholar]
- 31.Hayashi K, Wakino S, Homma K, Sugano N, Saruta T. Pathophysiological significance of T-type Ca2+ channels: role of T-type Ca2+ channels in renal microcirculation. J Pharmacol Sci. 2005;99:221–227. doi: 10.1254/jphs.fmj05002x6. [DOI] [PubMed] [Google Scholar]
- 32.Ferron L, Capuano V, Deroubaix E, Coulombe A, Renaud JF. Functional and molecular characterization of a T-type Ca2+ channel during fetal and postnatal rat heart development. J Mol Cell Cardiol. 2002;34:533–546. doi: 10.1006/jmcc.2002.1535. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki S, Ohkusa T, Ono K, Sato T, Yoshida MA, Yano M, et al. Beneficial effects of the dual l- and T-type Ca2+ channel blocker efonidipine on cardiomyopathic hamsters. Circ J. 2007;71:1970–1976. doi: 10.1253/circj.71.1970. [DOI] [PubMed] [Google Scholar]
- 34.Morimoto S, Jo F, Maki K, Iwasaka T. Effects of efonidipine hydrochloride on heart rate and circulatory changes due to stress. Clin Exp Hypertens. 2009;31:83–91. doi: 10.1080/10641960802627363. [DOI] [PubMed] [Google Scholar]
- 35.Matsuda H, Mori T, Kurumazuka D, Kitada K, Hayashi T, Nagatoya K, et al. Inhibitory effects of T/L-type calcium channel blockers on tubulointerstitial fibrosis in obstructed kidneys in rats. Urology. 2011;77:249, e9–e15. doi: 10.1016/j.urology.2010.07.496. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka T, Tsutamoto T, Sakai H, Fujii M, Yamamoto T, Horie M. Comparison of the effects of efonidipine and amlodipine on aldosterone in patients with hypertension. Hypertens Res. 2007;30:691–697. doi: 10.1291/hypres.30.691. [DOI] [PubMed] [Google Scholar]
- 37.Almendral JL, Shick V, Rosendorff C, Atlas SA. Association between transforming growth factor-beta(1) and left ventricular mass and diameter in hypertensive patients. J Am Soc Hypertens. 2010;4:135–141. doi: 10.1016/j.jash.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 38.Kinoshita H, Kuwahara K, Takano M, Arai Y, Kuwabara Y, Yasuno S, et al. T-type Ca2+ channel blockade prevents sudden death in mice with heart failure. Circulation. 2009;120:743–752. doi: 10.1161/CIRCULATIONAHA.109.857011. [DOI] [PubMed] [Google Scholar]