Abstract
Hypertension occurs twice as commonly in diabetics than in comparable nondiabetics. Patients with both disorders have a markedly higher risk for premature microvascular and macrovascular complications. Aggressive control of blood pressure (BP) reduces both micro- and macrovascular complications. In diabetic hypertensives, angiotensin converting enzyme inhibitors (ACEIs) are the first line in management of hypertension, and can be replaced by angiotensin II receptor blockers (ARBs) if patients are intolerant of them. Recent studies suggest ARBs to be on par with ACEI in reducing both macro- and microvascular risks. Adding both these agents may have a beneficial effect on proteinuria, but no extra macrovascular risk reduction. Thiazides can also be used as first line drugs, but are better used along with ACEI/ARBs. Beta-blockers [especially if the patient has coronary artery disease] and calcium channel blockers are used as second line add-on drugs. Multidrug regimens are commonly needed in diabetic hypertensives. Achieving the target BP of <130/80 is the priority rather than the drug combination used in order to arrest and prevent the progression of macro- and microvascular complications in diabetic hypertensives.
Keywords: Angiotensin converting enzyme inhibitor, angiotensin II receptor blockers, diabetes mellitus, hypertension, life-style modification
INTRODUCTION
Hypertension and diabetes are becoming increasingly common. Hypertension occurs more commonly in diabetics than in comparable nondiabetics. Hypertension (defined as a blood pressure [BP] ≥140/90 mmHg) affects 20 to 60% of patients with diabetes, depending on obesity, ethnicity, and age.[1–3] Overall, hypertension is disproportionately higher in diabetics,[4] while persons with elevated BP are two and a half times more likely to develop diabetes within 5 years.[5,6] In India, about 50% of diabetics have hypertension.[7,8]
Most patients with both disorders have a markedly worsened risk for premature microvascular and macrovascular complications. The presence of hypertension causes a 7.2-fold increase and a 37-fold increase in mortality in diabetic patients.[9–11]
In the U.K. Prospective Diabetes Study (UKPDS) epidemiological study, each 10-mmHg decrease in mean systolic BP was associated with reductions in risk of 12% for any complication related to diabetes, 15% for deaths related to diabetes, 11% for myocardial infarction, and 13% for microvascular complications.[12]
There is no threshold value for BP, and risk continues to decrease well into the normal range. Achieving lower levels, however, would increase the cost of care as well as drug side effects and is often difficult in practice. Therefore, a target BP goal of <130/80 mmHg is reasonable if it can be safely achieved.
Hence, aggressive BP control becomes imperative in diabetic patients.
ADVANTAGES OF TREATING HYPERTENSION IN DIABETICS
UKPDS and Hypertension Optimum Trial (HOT) showed early treatment of BP and tight BP control lead to significant reduction in microvascular complications (retinopathy, nephropathy, neuropathy) and macrovascular complications [coronary artery disease (CAD)/stroke/peripheral vascular disease].[12–15]
The UKPD study and other UK study groups have shown that the long-term tight BP control in hypertensive patients with type 2 diabetes mellitus results in a significant reduction in all diabetes-related end points.[12,16–18]
Tight control of blood glucose only decreases the risk of microvascular complications,[19] whereas tight control of BP reduces both micro- and macrovascular complications. Also, the beneficial results also come instantaneously with the later than with the former. Tight BP control is more cost effective and easier for clinicians and patients than tight blood glucose control.
SHEP (Systolic hypertension in elderly patients), SYST-EUR (systolic hypertension Europe trial), and HOT have confirmed that reduction in cardiovascular risk was achieved with tight BP control, and, the beneficial effect was twice or thrice when the patient is a diabetic hypertensive.[20–24]
The International Diabetic Federation Consensus Guidelines have shown reduction in stroke morbidity and mortality, heart failure morbidity and mortality, reduced left ventricular hypertrophy, decrease in CAD events, and reduction in progression of renal disease including diabetic nephropathy, by tight control of hypertension in diabetics.[25]
MANAGEMENT OF HYPERTENSION IN DIABETICS
Management of diabetic hypertensives starts with lifestyle changes (weight reduction; regular exercise; and moderation of sodium, protein, and alcohol), as well as control of hyperglycemia, dyslipidemia, and proteinuria apart from management hypertension per se. A comprehensive algorithm encompassing all the armamentarium of management is provided in Figure 1.
Figure 1.
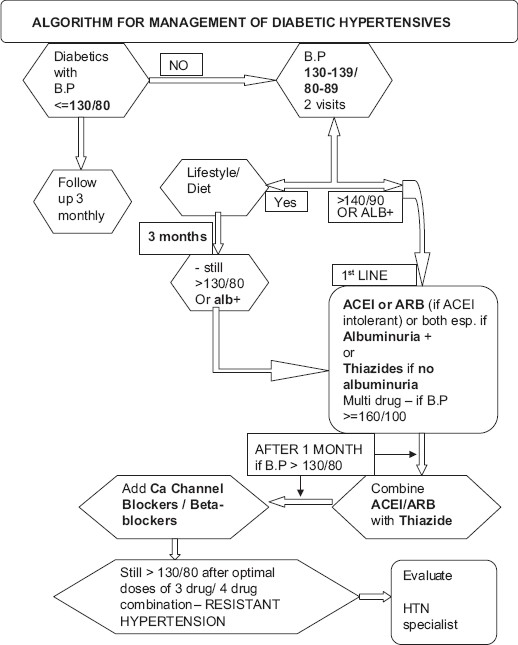
Algorithm for management of hypertension in diabetes
In the Dietary Approaches to Stop Hypertension trial (DASH), lifestyle modifications such as exercise, a diet low in sodium, saturated fat, cholesterol, and high in potassium, calcium, fiber, fruits have clearly been shown to decrease BP.[26] The DASH diet recommends keeping salt intake to less than 2 300 mg (1 500 mg a day – elderly).[27] Excessive sodium intake is particularly deleterious in patients with diabetes because it may decrease the antihypertensive effects of medications and their beneficial effects on proteinuria.[28] Also, DASH diet has beneficial effects for diabetes control and prevention of complications apart from pressure control.
The DASH study compared three eating plans: A plan that includes foods people regularly eat without intervention; a plan that includes regular food plus more fruits and vegetables alone; and the DASH eating plan, i.e., diet more in potassium, fruits, fiber, calcium and less in sodium, saturated fat, and cholesterol. All three plans included about 3 000 mg of sodium daily. Participants who followed both the plan that included more fruits and vegetables and the DASH eating plan had reduced BP, but the DASH eating plan had better control.[26]
The second DASH involved 412 participants who were randomly assigned to one of the two eating plans (DASH and REGULAR) and subdivided into three sodium intake levels (3.3 g, 2.3 g, and 1.5 g/day) and then followed for a month. Results showed that reducing dietary sodium lowered BP for both eating plans. At each sodium level, BP was lower on the DASH eating plan than on the regular plan. The greatest BP reductions were for the DASH eating plan at the sodium intake of 1 500 mg per day.[26]
A duration of 20 to 40 minutes of aerobic exercise performed five times a week has significantly lowered BP levels.[29] It is also noted that the results of low to moderate training are just as efficient in lowering BP compared to that with high-intensity cardiovascular exercise.[30] Studies show exercise and weight reduction helps independently in reducing BP, and combining both have additive benefits in diabetic hypertensives.[31]
DRUG MANAGEMENT OF HYPERTENSION
The choice of perfect antihypertensive remains elusive and dictated by patient's age, associated comorbidities such as chronic kidney disease (CKD), CAD, state of diabetes and hypertension, and other factors. Clinical trials with diuretics, angiotensin converting enzyme inhibitors (ACEIs), beta blockers, angiotensin II receptor blockers (ARBs), and calcium antagonists have demonstrated benefit in the treatment of diabetic hypertensives.[24,32–34]
ACEI are the first line in management of diabetic hypertensives. ACEIs may be used alone for BP lowering but are much more effective when combined with a thiazide-type diuretic or other antihypertensive drug.[35–38] They reduce the macrovascular and microvascular risks associated with diabetic hypertensives.
Macrovascular risks: In the subanalysis of the HOPE Study, which included both hypertensive and normotensive individuals, high-risk diabetic patients treated with ACEI added on to conventional therapy showed a reduction in all macrovascular complications (combined myoocardial infarction, stroke, and cardiovascular disease (CVD) death reduction of about 25% and of stroke by about 33%) compared with placebo plus conventional therapy.[32,39–41] Earlier studies did not prove the effectiveness of ARBs in cardiovascular risk reduction, as a replacement to ACEI intolerance. ONTARGET (largest and the first ARB-based outcome study) conducted in a broad cross-section of patients at high risk of cardiovascular disease has recently showed the cardiovascular benefit of ARB be on par with ACEI. ONTARGET showed that the ARB telmisartan was as effective as the reference standard ACE inhibitor ramipril in reducing macrovascular complications (death from cardiovascular causes, myocardial infarction, stroke, and hospitalization for heart failure). ONTARGET also showed that the combination of telmisartan with ramipril did not have additional cardiac risk reduction benefit.[39,42,43]
The American Diabetes Association (ADA) has recommended both ACEIs and ARBs for use in type 2 diabetic patients with CKD and other microvascular complications, because these agents delay the deterioration in glomerular filtration rate (GFR) and the worsening of albuminuria.[32,40,44] Unlike macrovascular risk reduction, microvascular risks reduction is found to be more on combining ACEI and ARBs, rather than using them alone. CALM and LORD study explains the benefit of combination of these two agents in reducing microalbuminuria.[45,46]
The ADA has recommended ACEIs for diabetic patients >55 years of age at high risk for CVD and Beta-blockers for those with known CAD as first-line agents.[32]
Beta 1 selective beta-blockers are beneficial to diabetics as part of multidrug therapy, have little adverse effects such as hypoglycemic unawareness and decreased sensitivity than the nonselective counterparts. Beta-blockers value as monotherapy is less clear. A beta blocker indicated in a diabetic with ischemic heart disease is less effective in preventing stroke than an ARB, as was found in the LIFE study.[47]
In diuretic-based therapy, a low-dose thiazide diuretic, has been shown to reduce the cardiovascular event rate 34% compared with placebo; the absolute risk reduction was twice as great for diabetic subjects vs nondiabetic subjects.[48]
Calcium channel blockers may be useful to diabetics, particularly as part of combination therapy, to control BP. They were shown to reduce CVD events in diabetics compared with placebo in several clinical outcome trials.[22,27,48]
The Appropriate Blood Pressure Control in Diabetes (ABCD) Trial in diabetics found that the nitrendipine was inferior to lisinopril in reducing the incidence of ischemic cardiac events.[50] However, in normotensive diabetics in the ABCD2 Trial, nitrendipine was equivalent to lisinopril in stroke prevention and in retardation of the development of albuminuria.[51] This explains the superiority of ACEI over the calcium channel blockers in diabetic hypertensives.
In patients with renal insufficiency, no creatinine level is an absolute contraindication to angiotensin blockade therapy (ACEI/ARB). ACE inhibitors are not nephrotoxic. ACE inhibitors are renoprotective even for levels of renal function between 10 and 30 ml/min, indicating the need not to withhold ACE inhibitors, even when GFR approximates levels requiring replacement therapy.[52] Nevertheless, careful monitoring is needed while administering ACE inhibitors/ARB when serum creatinine levels are above 3.0 mg/dl due to fear of hyperkalemia and rapid decline in renal function in patients with advanced renal insufficiency.[53]
Treatment of hypertension in both type 1 and 2 diabetics does not vary much. Nevertheless, few preferences can be mentioned from various studies. In hypertensive type 1 diabetic patients with any degree of albuminuria (micro- and macroalbuminuria), ACE inhibitors have been preferably shown to delay the progression of nephropathy. In hypertensive type 2 diabetic patients with microalbuminuria, ACE inhibitors and ARBs have been shown equally to delay the progression to macroalbuminuria. In patients with type 2 diabetes, hypertension, macroalbuminuria, and renal insufficiency (serum creatinine >1.5 mg/dl), ARBs have been preferably shown to delay the progression of nephropathy.[54–57]
Hence, while treating diabetic hypertensives, first-line agents used must be an ACEI or ARB (if intolerant to ACEI) or a combination of both or a thiazide diuretic. If the target BP goal is not obtained with the initial doses of first-line drugs, increases in doses are recommended, or the addition of a second-line drug must be considered. Regardless of the initial treatment, it must be emphasized that most patients will require more than one drug to achieve the recommended target of ≤130/80 mmHg, and many will require three or more. Add-on drugs can be calcium channel blockers (preferably dihydropyridine calcium channel blockers [DCCB group], B1 Selective beta-blockers, or alpha-blockers. Achievement of the target BP may be more important than the particular drug regimen used.
From various studies and guidelines, the following are observed:[58,59]
In diabetic hypertensives
Goal (mmHg) for BP - <130<80 mmHg
Behavioral therapy alone (maximum 3 months), then add pharmacologic treatment – if – 130–139/80–89 mmHg
Behavioral therapy + pharmacologic treatment – if- ≥140/≥90 mmHg
GUIDELINES FOR MANAGING DIABETIC HYPERTENSIVES
The target BP should be below 130/80 mm Hg.
All routinely used antihypertensive drugs have been shown to be beneficial compared with placebo.
More than one drug will usually be required to achieve the target BP.
Patients with prehypertension (130-139/80-89 mmHg) should be given lifestyle/behavioral therapy alone for a maximum of 3 months and then, if targets are not achieved, should also be treate d pharmacologically. Attention should be paid to lifestyle changes (weight reduction; regular exercise; and moderation of sodium, protein, and alcohol), as well as control of hyperglycemia, dyslipidemia, and proteinuria, for all the patients.
The choice of drugs should always include an ACE inhibitor (or an angiotensin II receptor blocker, if ACE inhibitors cannot be tolerated) and should usually include a diuretic. If additional therapy is needed, a calcium-channel blocker, β-blocker, or α-blocker may be used [Figure 1].
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Paul B, Sapra B, Maheswari S, Goyal RK. Role of losartan therapy in the management of diabetic hypertension. J Assoc Physicians India. 2000;48:514–7. [PubMed] [Google Scholar]
- 2.Sowers JR, Haffner S. Treatment of CV and renal risk factors in the diabetic hypertensive. Hypertension. 2002;40:781–8. doi: 10.1161/01.hyp.0000042097.39655.b7. [DOI] [PubMed] [Google Scholar]
- 3.Tarnow L, Rossing P, Gall MA, Nielsen FS, Parving HH. Prevalence of arterial hypertension in diabetic patients before and after the JNC-V. Diabetes Care. 1994;17:1247–51. doi: 10.2337/diacare.17.11.1247. [DOI] [PubMed] [Google Scholar]
- 4.Sowers JR, Haffner S. Treatment of cardiovascular and renal risk factors in the diabetic hypertensive. Hypertension. 2002;40:781–8. doi: 10.1161/01.hyp.0000042097.39655.b7. [DOI] [PubMed] [Google Scholar]
- 5.Gress TW, Nieto FJ, Shahar E, Wofford MR, Brancati FL. Hypertension and antihypertensive therapy as risk factors for type 2 diabetes mellitus.Atherosclerosis Risk in Communities Study. N Engl J Med. 2000;342:905–12. doi: 10.1056/NEJM200003303421301. [DOI] [PubMed] [Google Scholar]
- 6.Sowers JR, Bakris GL. Antihypertensive therapy and the risk of type 2 diabetes mellitus. N Engl J Med. 2000;342:969–70. doi: 10.1056/NEJM200003303421310. [DOI] [PubMed] [Google Scholar]
- 7.Singh RB, Beegom R, Rastogi V, Rastogi SS, Madhu V. Clinical characteristics and hypertension among known patients of non-insulin dependent diabetes mellitus in North and South Indians. J Diab Assoc India. 1996;36:45–50. [Google Scholar]
- 8.Jain S, Patel JC. Diabetes and hypertension. J Diab Assoc India. 1983;23:83–6. [Google Scholar]
- 9.Prevalence of Diabetes and Impaired Fasting Glucose in Adults—United States, 1999-2000. MMWR Morb Mortal Wkly Rep. 2003;52:833–7. [PubMed] [Google Scholar]
- 10.Collins AJ, Kasiske B, Herzog C, Chen SC, Everson S, Constantini E, et al. Excerpts from the United States Renal Data System 2001 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Am J Kidney Dis. 2001;38:S7–247. [PubMed] [Google Scholar]
- 11.Molitch Mark E, Defronzo Ralph A, Franz Marion J, Keane William F, Mogensen Carl Erik. American Diabetes Association. Diabetic nephropathy. Diabetes Care. 2002;25:S85–9. doi: 10.2337/diacare.26.2007.s94. PR. [DOI] [PubMed] [Google Scholar]
- 12.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703–13. [PMC free article] [PubMed] [Google Scholar]
- 13.Safar ME, Blacher J, Staessen JA. Hypertension Optimal Treatment (HOT) trial. Lancet. 1998;352:573. doi: 10.1016/S0140-6736(05)79281-3. author reply 574-5. [DOI] [PubMed] [Google Scholar]
- 14.Hansson L, Zanchetti A, Carruthers SG, Dahlöf B, Elmfeldt D, Julius S, et al. For the HOTStudy Group, effect of intensive blood pressure lowering and low dose asprin in patients with hypertension: Principal results of the Hypertension Optimal Treatment (HOT) randomized trial. Lancet. 1998;351:1755–62. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 15.Bakris GL. The importance of blood pressure control in the patients with diabetes. Am J Med. 2004;116(Suppl 5A):30S–8S. doi: 10.1016/j.amjmed.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 16.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 17.Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow- up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22:99–111. doi: 10.2337/diacare.22.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mokdad AH, Ford ES, Bowman BA, Nelson DE, Engelgau MM, Vinicor F, et al. Diabetes trends in the U.S.: 1990-1998. Diabetes Care. 2000;23:1278–83. doi: 10.2337/diacare.23.9.1278. [DOI] [PubMed] [Google Scholar]
- 19.Retinopathy and nephropathy in patients with Type 1 diabetes -four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med. 2000;342:381–9. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansson L, Zanchetti A, Carruthers SG, Dahlöf B, Elmfeldt D, Julius S, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: Principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group Lancet. 1998;351:1755–62. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 21.Fagard R. Long-term outcome results of the Systolic Hypertension in Europe trial (Syst-Eur). Presented at the ESC Congress 2003; August 30-September 3, 2003; Vienna, Austria. Clinical Trial Update III Hypertension/Prevention, Presentation #3701 [Google Scholar]
- 22.Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhäger WH, et al. for the Systolic Hypertension in Europe (Syst-Eur) Trial investigators.Randomised double-blind comparison of placebo and active treatment or older patients with isolated systolic hypertension. Lancet. 1997;350:757–64. doi: 10.1016/s0140-6736(97)05381-6. [DOI] [PubMed] [Google Scholar]
- 23.Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. 1991;265:3255–64. [PubMed] [Google Scholar]
- 24.Hansson L, Zanchetti A, Carruthers SG, Dahlof B, Elmfeldt D, Julius S, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: Principal results of the Hypertension Optimal Treatment (HOT) randomised trial.HOT Study Group. Lancet. 1998;351:1755–62. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 25.Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N Engl J Med. 1987;316:1429–35. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 26.Moore TJ, Conlin PR, Ard J, Svetkey LP. DASH (Dietary Approaches to Stop Hypertension) diet is effective treatment for stage 1 isolated systolic hypertension. Hypertension. 2001;38:155–8. doi: 10.1161/01.hyp.38.2.155. [DOI] [PubMed] [Google Scholar]
- 27.Black HR, Elliott WJ, Grandits G, Grambsch P, Lucente T, White WB, et al. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) Trial. JAMA. 2003;289:2073–82. doi: 10.1001/jama.289.16.2073. [DOI] [PubMed] [Google Scholar]
- 28.Bakris GL, Smith A. Effects of sodium intake on albumin excretion in patients with diabetic nephropathy treated with long-acting calcium antagonists. Ann Intern Med. 1996;125:201–4. doi: 10.7326/0003-4819-125-3-199608010-00007. [DOI] [PubMed] [Google Scholar]
- 29.Wallace JP. Exercise in hypertension. Sports Med. 2003;33:585–98. doi: 10.2165/00007256-200333080-00004. [DOI] [PubMed] [Google Scholar]
- 30.Halbert JA, Silagy CA, Finucane P, Withers RT, Hamdorf PA, Andrews GR. The effectiveness of exercise training in lowering blood pressure: A meta-analysis of randomized controlled trials of 4 weeks or longer. J Hum Hypertens. 1997;11:641–9. doi: 10.1038/sj.jhh.1000509. [DOI] [PubMed] [Google Scholar]
- 31.Hagberg JM, Park JJ, Brown MD. The role of exercise training in the treatment of hypertension.An update. Sports Med. 2000;30:193–206. doi: 10.2165/00007256-200030030-00004. [DOI] [PubMed] [Google Scholar]
- 32.Arauz-Pacheco C, Parrott MA, Raskin P. Treatment of hypertension in adults with diabetes. Diabetes Care. 2003;26(Suppl 1):S80–2. doi: 10.2337/diacare.26.2007.s80. [DOI] [PubMed] [Google Scholar]
- 33.Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–97. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 34.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703–13. [PMC free article] [PubMed] [Google Scholar]
- 35.Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: The HOPE randomized trial. Ann Intern Med. 2001;134:629–36. doi: 10.7326/0003-4819-134-8-200104170-00007. [DOI] [PubMed] [Google Scholar]
- 36.Gress TW, Nieto FJ, Shahar E, Wofford MR, Brancati FL. Hypertension and antihypertensive therapy as risk factors for type 2 diabetes mellitus.Atherosclerosis Risk in Communities Study. N Engl J Med. 2000;342:905–12. doi: 10.1056/NEJM200003303421301. [DOI] [PubMed] [Google Scholar]
- 37.Sowers JR, Bakris GL. Antihypertensive therapy and the risk of type 2 diabetes mellitus. N Engl J Med. 2000;342:969–70. doi: 10.1056/NEJM200003303421310. [DOI] [PubMed] [Google Scholar]
- 38.Kohner EM, Aldington SJ, Stratton IM, Manley SE, Holman RR, Matthews DR, et al. United Kingdom Prospective Diabetes Study, 30: Diabetic retinopathy at diagnosis of non-insulin-dependent diabetes mellitus and associated risk factors. Arch Ophthalmol. 1998;116:297–303. doi: 10.1001/archopht.116.3.297. [DOI] [PubMed] [Google Scholar]
- 39.Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: Results of the HOPE Study and MICRO-HOPE Substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet. 2000;355:253–9. [PubMed] [Google Scholar]
- 40.Remuzzi G, Schieppati A, Ruggenenti P. Clinical practice.Nephropathy in patients with type 2 diabetes. N Engl J Med. 2002;346:1145–51. doi: 10.1056/NEJMcp011773. [DOI] [PubMed] [Google Scholar]
- 41.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients.The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–53. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 42.Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–59. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 43.The ONTARGET/TRANSCEND Investigators. Rationale, design, and baseline characteristics of 2 large, simple, randomized trials evaluating telmisartan, ramipril, and their combination in high-risk patients; The Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial/Telmisartan Randomized Assessment study In ACE Intolerant Subjects with Cardiovascular disease. [DOI] [PubMed]
- 44.Lewis EJ, Lewis JB. ACE Inhibitors versus Angiotensin Receptor Blockers in Diabetic Nephropathy: Is There a Winner? J Am Soc Nephrol. 2004;15:1358–60. [PubMed] [Google Scholar]
- 45.Mogensen CE, Neldam S, Tikkane I, Oren S, Viskoper R, Watts RW, et al. Randomised controlled trial of dual blockade of renin-angiotension system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: The candesartan and lisinopril microalbuminuria (CALM) study. BMJ. 2000;321:1440–4. doi: 10.1136/bmj.321.7274.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joshi SR, Yeolekar ME, Tripathi KK, Giri J, Maity AK, Chopda M, et al. Evaluation of efficacy and tolerability of Losartan and Ramipril combination in the management of hypertensive patients with associated diabetes mellitus in India (LORD Trial) J Assoc Physicians India. 2004;52:189–95. [PubMed] [Google Scholar]
- 47.Lindholm LH, Ibsen H, Dahlof B, Devereux RB, Beevers G, de Faire U, et al. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint Reduction in Hypertension Study (LIFE): A randomised trial against atenolol. Lancet. 2002;359:1004–10. doi: 10.1016/S0140-6736(02)08090-X. [DOI] [PubMed] [Google Scholar]
- 48.Curb JD, Pressel SL, Cutler JA, Savage PJ, Applegate WB, Black H, et al. Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension: Systolic Hypertension in the Elderly Program Cooperative Research Group. JAMA. 1996;276:1886–92. [PubMed] [Google Scholar]
- 49.Brown MJ, Palmer CR, Castaigne A, de Leeuw PW, Mancia G, Rosenthal T, et al. Morbidity and mortality in patients randomised to double-blind treatment with a long-acting calcium-channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT) Lancet. 2000;356:366–72. doi: 10.1016/S0140-6736(00)02527-7. [DOI] [PubMed] [Google Scholar]
- 50.Estacio RO, Jeffers BW, Hiatt WR, Biggerstaff SL, Gifford N, Schrier RW. The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non-insulin-dependent diabetes and hypertension. N Engl J Med. 1998;338:645–52. doi: 10.1056/NEJM199803053381003. [DOI] [PubMed] [Google Scholar]
- 51.Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int. 2002;61:1086–97. doi: 10.1046/j.1523-1755.2002.00213.x. [DOI] [PubMed] [Google Scholar]
- 52.Kamper AL, Strandgaard S, Leyssac PP. Effect of enalapril on the progression of chronic renal failure.A randomized controlled trial. Am J Hypertens. 1992;5:423–30. doi: 10.1093/ajh/5.7.423. [DOI] [PubMed] [Google Scholar]
- 53.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy.The Collaborative Study Group. N Engl J Med. 1993;329:1456–62. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 54.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Eng J Med. 2001;345:851–60. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 55.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy.The Collaborative Study Group. N Engl J Med. 1993;329:1456–62. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 56.Mogensen CE, Keane WF, Bennett PH, Jerums G, Parving HH, Passa P, et al. Prevention of diabetic renal disease with special reference to microalbuminuria. Lancet. 1995;346:1080–4. doi: 10.1016/s0140-6736(95)91747-0. [DOI] [PubMed] [Google Scholar]
- 57.The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329(1993):1456–62. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 58.Arauz-Pacheco C, Parrott MA, Raskin P. The treatment of hypertension in adult patients with diabetes (Technical Review) Diabetes Care. 2002;25:134–47. doi: 10.2337/diacare.25.1.134. [DOI] [PubMed] [Google Scholar]
- 59.Stults B, Jones RE. Management of hypertension in diabetes. Diabetes Spectr. 2006;19:25–31. [Google Scholar]


