Abstract
Natriuretic peptides (NPs) are hormones which are mainly secreted from heart and have important natriuretic and kaliuretic properties. There are four different groups NPs identified till date [atrial natriuretic peptide (ANP), B-type natriuretic peptide (BNP), C-type natriuretic peptide (CNP) and dendroaspis natriuretic peptide, a D-type natriuretic peptide (DNP)], each with its own characteristic functions. The N-terminal part of the prohormone of BNP, NT-proBNP, is secreted alongside BNP and has been documented to have important diagnostic value in heart failure. NPs or their fragments have been subjected to scientific observation for their diagnostic value and this has yielded important epidemiological data for interpretation. However, little progress has been made in harnessing the therapeutic potential of these cardiac hormones.
Keywords: Atrial natriuretic peptide, B-type natriuretic peptide, heart failure, natriuretic peptides, NT-proBNP
INTRODUCTION
Secretory granules were first identified in the atria of guinea pig in 1956.[1] However, it is de Bold, who is credited with the discovery and isolation, in 1979, of atrial natriuretic peptide (ANP), a polypeptide hormone secreted by heart muscle cells. This established the heart as an endocrine gland.[2,3] The family of natriuretic peptides (NPs) comprises at least eight structurally related amino acid peptides stored as three different prohormones: 126 amino acid atrial natriuretic peptide (ANP) prohormone, 108 amino acid B-type natriuretic peptide (BNP) prohormone, and 126 amino acid C-type natriuretic peptide (CNP) prohormone.[4] [Figure 1]. The function of dendroaspis natriuretic peptide, a D-type natriuretic peptide (DNP), the most recent addition to the family of NP, first isolated from the venom of the green mamba, in humans still remains unclear.[5]
Figure 1.
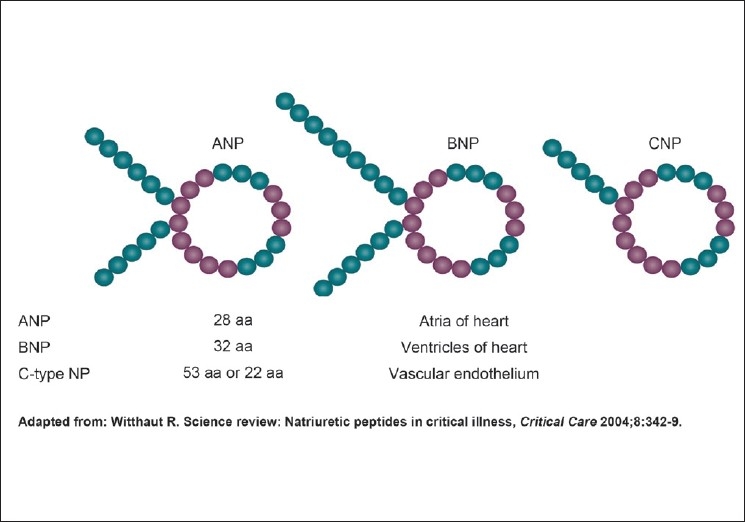
Structure of natriuretic peptides
The ANP prohormone is synthesized mainly within the atrial myocytes and in a variety of other tissues.[6] The prohormone consists of 126 amino acids which give rise to several peptides with blood pressure lowering properties, natriuretic properties, diuretic properties and/or kaliuretic properties.[7] These peptide hormones, numbered by their amino acid sequences beginning at the N-terminal end of the ANP prohormone, consist of the first 30 amino acids of the prohormone (i.e. proANP 1–30; long-acting NP), amino acids 31–67 (i.e. proANP 31–67; vessel dilator), amino acids 79–98 (proANP 79–98; kaliuretic peptide) and amino acids 99–126 (ANP).[7] The ANP prohormone processing is different within the kidney, resulting in an additional four amino acids being added to the N-terminus of ANP (i.e. proANP 95–126; urodilatin).[8] This was initially purified from human urine and is presumed to be the only one synthesized within the kidney. Urodilatin is not present in the circulation and appears to be a unique intrarenal NP with unexplored physiological significance.[9]
BNP was originally discovered in porcine brain, where it was thought to be a neurotransmitter,[10] hence its original name, brain natriuretic peptide. Subsequently, it was shown to be 10-fold more abundant in the heart than in the brain,[11] hence the current term, B-type natriuretic peptide. There appears to be little storage of BNP in the ventricle, which incidentally is the main source. ProBNP is processed within the human heart to form BNP (consisting of 32 amino acids) with amino acids 77–108 of its 108 amino acid prohormone, and an N-terminal proBNP peptide (amino acids 1–76; NT-proBNP), both of which circulate in humans[12] [Figure 2]. BNP is produced by direct synthesis in response to the degree of ventricular stretch, and also upregulated in failing ventricular myocardium. The messenger RNA for proBNP is unstable, so there is active regulation of BNP levels according to ventricular wall tension. Hence, it acts as a reliable biomarker of ventricular dilatation.[9]
Figure 2.
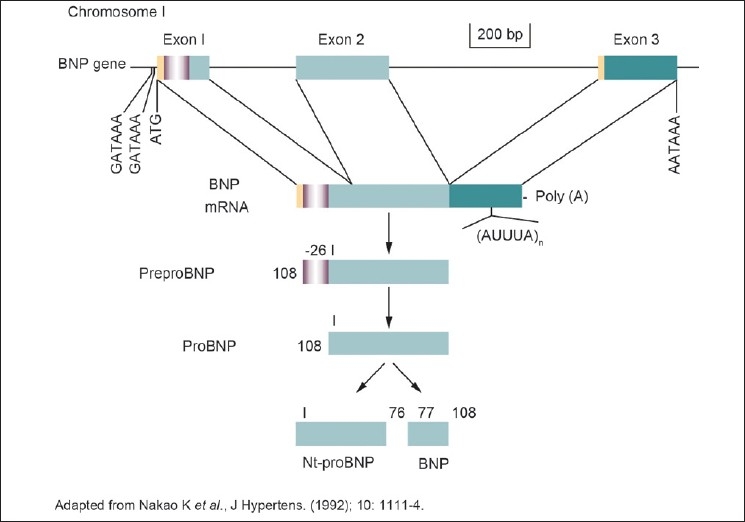
B-type natriuretic peptide transcription and translation
CNP was originally also found in the brain[13] and subsequently was suggested to be present also within the heart.[14] CNP has also been detected in human coronary arteries[15] and in the peripheral circulation in endothelial cells of human veins and arteries at various sites.[16] Two CNP molecules, of 22 and 53 amino acids in length, have been identified within the circulation.[13,14] The 22 amino acid form predominates in plasma and is more potent than the 53 amino acid form.[11] CNP lacks a significant natriuretic function[17] and serves as a regulator of vascular tone[18,19] and growth in a paracrine or autocrine fashion.[20,21]
FUNCTIONS OF CIRCULATING NATRIURETIC PEPTIDE
Apart from blood pressure lowering properties, natriuretic, diuretic and/or kaliuretic properties of the NP originating from the ANP prohormone[7] and from BNP, inhibition of the renin–angiotensin system, sympathetic outflow, and vascular smooth muscle and endothelial cell proliferation have been attributed to NP.[22] Furthermore, a link of ANP to the immune system has been suggested,[23] and a receptor-mediated modulation of macrophage function[24] and priming of polymorphonuclear neutrophils[25] has been observed. Whether NT-proBNP has biological effects on its own is currently unknown. The function of CNP seems to be the regulation of regional blood flow. The net effects of actions of NPs are a decrease in cardiac preload and afterload.
BNP is eliminated by binding to the NP clearance receptor (NPR-C) or degradation by neutral endopeptidase on endothelial cells, smooth muscle cells, cardiac myocytes, renal epithelium, and fibroblasts. NT-proBNP is cleared mainly by the kidney.[26] BNP has a relatively shorter half-life of about 20 minutes; the half-life of NT-proBNP is about 60–90 minutes and would be expected to be longer in the setting of renal dysfunction. Obese patients (especially those who have body mass index greater than 30) tend to have lower BNP levels than others. Neutral endopeptidases that are secreted by adipose tissue may be related to increased BNP clearance in obese patients.[27]
RECEPTORS OF NATRIURETIC PEPTIDE
Most biological effects of ANP and BNP are mediated by a guanylate cyclase coupled cell surface receptor, the A-receptor (NPR-A).[28] Long-acting NP and vessel dilator have distinct receptors separate from the ANP receptors.[29] The natriuretic effects of the long-acting NP and the vessel dilator have a different mechanism of action from ANP in that they inhibit renal Na+–K+-ATPase secondary to their ability to enhance the synthesis of prostaglandin E2, which ANP does not do.[30] CNP is a specific ligand for the B-receptor (NPR-B), another guanylate cyclase coupled NP receptor.[31] The third NP receptor, the so-called NP clearance receptor (NPR-C), binds ANP, BNP and CNP. Apart from a major role in the clearance of NP from the whole body,[31] an increasing number of reports describe that several effects of ANP are mediated via the NPR-C receptor.[32] Stimulation of the NPR-C seems to be related to a G-protein coupled inhibition of adenylate cyclase.[33] Apart from binding to the NPRs, NPs are also cleared through proteolysis by peptidases, the most closely studied being neutral endopeptidase 24.11. Renal excretion is currently regarded as the main clearance mechanism for NT-proBNP.[33]
NATRIURETIC PEPTIDES: DIAGNOSTIC USE
Natriuretic peptide and left ventricular dysfunction
Increased plasma levels of circulating NP have been described in patients with congestive heart failure and are directly proportional to the severity of congestive heart failure as classified by the New York Heart Association criteria. This rise is seen consistently and has been reported for long-acting NP, BNP and NT-proBNP.[34,35] N-terminal proANP and BNP have been reported to be more sensitive indicators of systolic left ventricular (LV) dysfunction.[36,37] N-terminal proANP has been reported to identify patients with asymptomatic LV dysfunction, with a sensitivity and specificity of more than 90%.[37] For vessel dilator as the only peptide (including ANP, BNP, NT-proBNP, etc.), 100% sensitivity and 100% specificity have been reported in differentiating persons with mild congestive heart failure from healthy individuals.[38] N-terminal proANP has also been reported to be an independent predictor of the development of congestive heart failure and cardiovascular mortality.[39] BNP and NT-proBNP have been shown to be useful markers for prognosis in patients with asymptomatic LV dysfunction and different degrees of congestive heart failure.[40,41] The major site of synthesis and release of BNP, the cardiac ventricles, and BNP's rapid upregulation by gene expression followed by a remarkably augmented plasma concentration exceeding that of ANP in severe cases[42] not only make this peptide useful in estimating the severity of disease in patients with LV dysfunction,[34] but may also help guide the treatment of systolic LV impairment in the future.[43]
In the urgent care setting, it is often difficult to distinguish between cardiac and pulmonary causes of dyspnea. Physical signs, routine laboratory tests, electrocardiograms and chest films are not diagnostically consistent in differentiating heart failure from other diseases, such as pulmonary disease. Rapid testing of BNP and NT-proBNP has been reported to differentiate pulmonary etiologies from cardiac etiologies of dyspnea.[44] However, some types of pulmonary disease, such as cor pulmonale, pulmonary embolism and lung cancer, are also associated with elevated NP levels.[45]
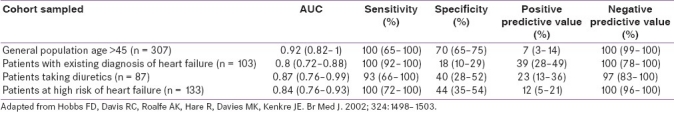
Measurement of NT-proBNP by enzyme-linked immunosorbent assay (ELISA) method compared with a clinical diagnosis was evaluated by receiver operator characteristic (ROC) curve analysis in different populations in primary care [Table 1].[46] The area under the curve (AUC) was consistently greater than 0.85, and confirmed the excellent negative predictive value of the test. This result has been confirmed subsequently in a study in 672 subjects in primary care in Copenhagen where again the AUC was 0.94. The role of BNP as an outcome predictor was explored in a Framingham offspring cohort study.[47] Here, a BNP in the upper tertile was one of the most powerful predictors of cardiovascular events, death and heart failure. A number of observational studies have examined the role of BNP measurement in monitoring treatment in congestive cardiac failure (CCF). The responses to the beta-blocker carvedilol[48] and to the angiotensin 2 antagonist valsartan have been shown to be predicated by BNP levels.[49]
Table 1.
NT-proBNP for diagnosis of cardiac failure in primary care
CLINICAL TRIALS OF NATRIURETIC PEPTIDE AS A GUIDE FOR HEART FAILURE MANAGEMENT
Over the last decade, several randomized-controlled trials (RCTs) have investigated the NP-guided approach in HF patients. Of these, Strategies for Tailoring Advanced Heart Failure Regimens in the Outpatient Setting: Brain Natriuretic Peptide Versus the Clinical Congestion Score (STARBRITE)[50] and Signal-HF[51] failed to show any benefit of the NP-guided approach, while NT-proBNP–guided management of chronic heart failure, based on an individual target value (PRIMA)[52] showed a significant decrease in mortality and hospitalization in patients who remained on target NP levels. In addition, the Christchurch, New Zealand,[53] Systolic Heart Failure Treatment supported by the BNP (STARS-BNP)[54] and BNP-Guided Care in Addition to Multidisciplinary Care[55] trials showed a decrease in hospitalizations as well as mortality in the NP-guided group compared with usual clinical care. The Placebo-Controlled Randomized Study of the Selective A(1) Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function (PROTECT) was terminated early after 1 year due to a significant drop in hospitalizations and cardiovascular-related deaths in the NP-guided group.[56]
Very recently, two large-scale trials, an NT-proBNP–assisted treatment to lessen serial cardiac readmission and death (BATTLESCARRED)[57] and Trial of Intensified versus Standard Medical Therapy in Elderly Patients With Congestive Heart Failure (TIME-CHF),[58] showed better outcomes with NP-guided uptitration of medications only in patients aged under 75 years. There are two studies still recruiting patients: The Improvement of Patients With Chronic Heart Failure Using NT-proBNP (EX-IMPROVE-CHF), which is double-blinded aiming to recruit 400 patients, and an NT-proBNP stratified follow-up in outpatient heart failure clinics (NORTHSTAR),[59] which will involve 1250 patients.
Meta-analysis of these trials would have been heterogeneous owing to differences in study populations, cut-off NP targets and inclusion levels, interventions, duration of follow-ups, monitoring algorithms, difficulties with double blinding and different end points. Nevertheless, two groups of investigators, one from Duke (NC, USA)[60] and the other from Australia,[61] performed a meta-analysis of NP-guided therapy trials. The former included all major trials except the Signal-HF (six trials with total n = 1627), while the latter meta-analysis added two more small trials (eight trials with total n = 1726). Neither included the BNP-Guided Care in Addition to Multidisciplinary Care trial,[35] as this was more recently published. Both meta-analyses concluded that NP-guided therapy could reduce all-cause mortality in patients with chronic HF, particularly in those aged less than 75 years. Investigators acknowledged the need for additional well-powered studies with a larger number of patients to provide robust evidence of the benefit of NP-guided therapy.
NATRIURETIC PEPTIDE AS A CARDIAC RISK FACTOR
Serial measurements of NT-proBNP in community-dwelling elderly people had been shown to provide additional prognostic information to that from traditional risk factors. NT-proBNP levels independently predict heart failure and cardiovascular death in older adults. NT-proBNP levels frequently change over time, and these fluctuations reflect dynamic changes in cardiovascular risk.[62]
Recent studies have also convincingly documented that both BNP and NT-proBNP are powerful, independent prognostic indicators in patients with stable coronary artery disease. The associations are strongest for the end points of death and heart failure, whereas the association with cardiac ischemic events is weaker or nonexistent, after adjustment for confounding factors. Importantly, BNP and NT-proBNP appear to provide incremental prognostic information to conventional risk factors, including markers of ventricular function and ischemia. Data documenting that BNP or NT-proBNP measurements can be used to guide treatment decisions in patients with stable coronary artery disease, however, is still lacking.[63]
NT-proBNP has also been documented to be an important biomarker for risk prediction in patients with ongoing non–ST-segment elevation acute coronary syndrome and after clinical stabilization. The C-reactive protein (CRP) exhibits increasing predictive value at later measurements. However, only NT-proBNP provided incremental prognostic value and was an independent risk predictor, and might therefore be considered as a complement for early follow-up controls after non–ST-segment elevation acute coronary syndrome.[64]
Cardiac interventions like balloon angioplasty can also increase the BNP, as the release of BNP may be triggered by tissue hypoxia. In a recent study from India, it was documented that BNP levels rose following percutaneous coronary angioplasty not only in patients with acute coronary syndrome but also in patients with chronic stable angina.[65]
NT-proBNP has also been found to be a powerful independent predictor of adverse cardiovascular outcomes following noncardiac surgery. In another study from India, elevated pre-operative BNP or NT-proBNP measurement is documented to be a powerful, independent predictor of cardiovascular events in the first 30 days after noncardiac surgery.[66]
NT-proBNP levels are elevated in patients with rheumatic mitral stenosis and decrease after a successful percutaneous transvenous mitral commissurotomy(PTMC). In yet another Indian study it was shown that NT-pro-BNP levels fall significantly after a successful PTMC, and a significant decrease in levels is a good marker of success of PTMC.[67]
NATRIURETIC PEPTIDE IN NONCARDIAC DISEASES
Natriuretic peptide in pulmonary disease
Cardiac and pulmonary causes of dyspnea often masquerade each other. Sometimes, physical signs, routine laboratory tests, electrocardiograms and chest films are not diagnostically consistent in differentiating heart failure from other diseases, such as pulmonary disease.[68] Rapid testing of BNP and NT-proBNP has been reported to differentiate pulmonary etiologies from cardiac etiologies of dyspnea.[69,70] Some types of pulmonary disease, such as cor pulmonale, pulmonary embolism and lung cancer, however, are also associated with elevated NP levels, but generally not to the same extent as those in patients with acute LV dysfunction. BNP levels in the intermediate range (100–500 pg/ml) have been reported to be attributable to causes other than congestive heart failure.[71]
Increased plasma levels of NP (i.e. ANP,[72] N-terminal proANP[73] and BNP[74] ) have also been found in patients with acute respiratory distress syndrome (ARDS). Acute cor pulmonale as a consequence of increased pulmonary vascular resistance occurs in up to 60% of patients with ARDS submitted to conventional mechanical ventilation.[75] An increase of pulmonary vascular resistance observed in ARDS may lead to right ventricular overload and decreased right ventricular output in the presence of impaired right ventricular contractility.[76] BNP levels secreted by the right ventricular myocardium are said not to exceed 300–600 pg/ml.[69] However, there might be a considerable overlap of patients with increased BNP due to ARDS and patients with primary symptomatic congestive heart failure, where BNP levels have to reach more than 500 pg/ml to ensure the diagnosis with a probability greater than 95%.[77] However, in contrast, a BNP cutoff value of 100 pg/ml measured at admission of patients presenting in the emergency department has been reported to have a strong negative predictive value for congestive heart failure in acute dyspneic patients.[35]
Pleural effusions arising from heart failure are usually discriminated from other causes based on clinical criteria in association with biochemical analysis, particularly the discrimination of transudates versus exudates, most commonly using Light's criteria. The sensitivity of Light's criteria for identifying exudative pleural effusions is very high (98%).[78] However, the criteria fare poorly in its ability to exclude transudative effusions.[79] As a result, heart failure associated with pleural effusions can be misclassified as exudates using Light's criteria, particularly after diuretics have been used. Pleural fluid NT-pro-BNP is a very useful biomarker for diagnosing pleural effusions of cardiac origin. A meta-analysis has shown that the pooled sensitivity and specificity of all studies combined was 94% [95% confidence interval (CI): 90–97] and 94% (95% CI: 89–97), respectively. The pooled positive likelihood ratio was 15.2 (95% CI: 8.1–28.7) and the pooled negative likelihood ratio was 0.06 (95% CI: 0.03–0.11). The area under the ROC curve was 0.98 (95% CI: 0.96–0.99) and the diagnostic odds ratio was 246 (95% CI: 81–745).[80]
Natriuretic peptide in renal disease
Renal function clearly influences the diagnostic performance of NT-proBNP; Goei et al. showed that NT-proBNP had more favorable discriminative value in patients with glomerular filtration rate (GFR) more than 90 ml/min/1.73 m2 and lost its prognostic value in patients with GFR less than 30 ml/min/1.73 m2.[81] Unlike NT-proBNP, BNP levels are relatively independent of GFR. BNP may, therefore, be the more appropriate biomarker to screen for cardiac dysfunction in patients with renal failure.[82] BNP levels could be a valuable tool for risk stratification of hemodialysis patients by confining echocardiographic studies to only patients with BNP levels above the established cutoff values.[83]
Natriuretic peptide in cirrhosis of liver
In patients with cirrhosis of the liver, elevated proBNP and BNP levels reflect increased cardiac ventricular generation of NPs and thus indicate the presence of cardiac dysfunction. Hyperdynamic systemic circulation could also be contributed to elevated NP levels in patients with cirrhosis of liver. In a study evaluating 52 non-alcoholic cirrhotic patients, BNP levels were significantly higher in cirrhotic patients, and BNP levels significantly correlated with Child score, interventricular septal thickness, and LV posterior wall thickness.[84] In another study of 51 cirrhotic patients, hemodynamic investigation revealed that circulating proBNP and BNP levels were related to the severity of liver decompensation (Child score, serum albumin, coagulation factors, and hepatic venous pressure gradient) and to markers of cardiac dysfunction (QT interval, heart rate, plasma volume).[85]
Natriuretic peptide in hyperthyroidism
Serum NT-proBNP levels are affected by thyroid functions and seem to be a direct stimulatory effect of thyroid hormones.[86] In a comparison between 67 patients with clinical hyperthyroidism and normal subjects, elevated BNP levels were mainly found in hyperthyroid patients who had clinical and echocardiographic evidence of LV dysfunction [increased left atrial (LA) diameter and decreased left ventricular ejection fraction (LVEF)], but not in those with normal LV function and normal subjects.[87] Multiple linear regression analysis demonstrated that free T4 and free T3 were independently associated with a high serum NT-proBNP, whereas cardiac output and resting pulse rate were not. Both NT-proBNP and BNP levels were higher in 21 patients with hyperthyroidism than in hypothyroid patients and normal controls, and treatment of thyroid dysfunctions could result in normalization of NT-proBNP levels in both hypothyroid and hyperthyroid groups.[88]
Natriuretic peptide in subarachnoid hemorrhage
Patients with subarachnoid hemorrhage (SAH) show an increased urine output and urinary excretion of sodium as well as higher BNP levels than the controls.[89] In a study involving 50 patients with traumatic SAH, early rise in BNP levels were associated with myocardial necrosis, pulmonary edema, and LV dysfunction. BNP levels may be elevated in patients with head injuries without echocardiographic evidence of HF.[90] In the absence of evidence for activation of NPs within the brain, prompt and consistent increase in both ANP and BNP strongly supports the view that the heart is the source of increased release of NPs after acute SAH.[91] In a study involving 30 patients with severe isolated head injury, BNP levels were elevated shortly after head injury and progressively rose for 7–8 days after the event in patients with diffused SAH as compared to patients with mild or no SAH.; Similar elevation was noted in patients with elevated intracranial pressure (ICP) as compared to patients without elevated ICP who had a better outcome.[92]
Natriuretic peptide in carbon monoxide poisoning
The levels of NT-proBNP and carbon monoxide (CO) Hb were increased in patients with CO poisoning. In a study involving 15 patients with CO poisoning, there was a positive correlation between the levels of COHb and NTproBNP. Thus, determining plasma NT-proBNP levels may contribute to the early diagnosis of cardiotoxicity in patients with CO poisoning.[93]
NATRIURETIC PEPTIDES: THERAPEUTIC USES
Natriuretic peptides or congeners as therapeutic agents
It is conceptually viable that NPs per se or their agonists and antagonists would be a welcome addition to the armamentarium of the clinicians for the treatment of cardiac failure because of the obvious salutary effects of NPs on the cardiovascular system.
Increased levels of BNP in patients with congestive heart failure suggest a plausible beneficial effect of it in this condition. Hence, nesiritide, a recombinant human brain NP, has been tried as an infusion in patients with congestive heart failure, which resulted in beneficial hemodynamic effects, including arterial and venous dilatation, enhanced sodium excretion, and suppression of the renin–angiotensin–aldosterone and sympathetic nervous systems. However, pooled analysis from three trials showed that compared with non-inotrope–based control therapy, Nesiritide may be associated with an increased risk of death after treatment for acutely decompensated heart failure. Death within 30 days tended to occur more often among patients randomized to nesiritide therapy [35 (7.2%) of 485 vs. 15 (4.0%) of 377 patients; risk ratio from meta-analysis, 1.74; 95% CI, 0.97–3.12; P = 0.059; and hazard ratio after adjusting for study, 1.80; 95% CI, 0.98–3.31; P = 0.057].[94] Moreover, it has been noted that usage of nesiritide worsens of renal failure, though it is not clearly understood whether it occurs due to the hemodynamic effect or renal injury. The prognostic importance of worsening renal function demands a reevaluation of nesiritide as a useful adjunct in the treatment of heart failure.[95]
Nesiritide use has been limited in India. However, the successful first usage has been documented in a recent publication.[96]
Omapatrilat, an orally active vasopeptidase inhibitor, is a molecule with potent, long-acting and selective inhibitory activities against neutral endopeptidase and angiotensin converting enzyme (ACE). As a result, this dual inhibitor, omapatrilat increases multiple endogenous vasodilatory peptides including ANP, BNP, bradykinin and adrenomedullin, while it simultaneously inhibits the generation of the vasoconstrictive peptide, angiotensin II. Merely inhibiting the neutral endopeptidase does not really lead to decrease in blood pressure, as unopposed angiotensin II annihilates the effect of increased level of NPs. Omapatrilat's effect on ACE inhibitions adds to the antihypertensive effects and is currently under review by Food and Drug Administration (FDA) for usage as a new group of antihypertensives.[97,98]
SUMMARY
NT-proBNP measurement is a powerful diagnostic and prognostic tool for detection of ventricular dysfunction. It is an ideal test for detection of cardiac failure in primary care, allowing cardiac failure to be definitively ruled out as a cause of dyspnea. Elevated NP levels have shown predictive value in various diseases that have direct or indirect influences on the heart functions in many non-heart failure circumstances, even in the absence of depressed cardiac function. It should be noted that NPs should never be interpreted without a thorough clinical history. Potential clinical applications of NP are expanding. Reports are emerging regarding its role for screening of the presence of secondary cardiac dysfunction, monitoring the therapeutic responses, risk stratifications, or providing prognostic values in many settings. It should form part of the repertoire of all laboratories. Newer vistas of treatment are being designed based on this important physiological pathway.
ACKNOWLEDGMENTS
The contribution of Mr. Pradip Mondal, Department of Endocrinology, IPGME & R and SSKM Hospital, Kolkata, gratefully acknowledged.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28:89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 2.Kisch B. Electron microscopy of the atrium of the heart. I. Guinea pig. Exp Med Surg. 1956;14:99–112. [PubMed] [Google Scholar]
- 3.De Bold AJ. Heart atria granularity effects of changes in water electrolyte balance. Proc Soc Exp Biol Med. 1979;161:508–11. doi: 10.3181/00379727-161-40584. [DOI] [PubMed] [Google Scholar]
- 4.Vesely DL. Englewood Cliffs, NJ: Prentice Hall; 1992. Atrial Natriuretic Hormones; pp. 1–256. [Google Scholar]
- 5.Schirger JA, Heublein DM, Chen HH, Lisy O, Jougasaki M, Wennberg PW, et al. Presence of Dendrosaspis natriuretic peptide-like immunoreactivity in human plasma and its increase during human heart failure. Mayo Clin Proc. 1999;74:126–30. doi: 10.4065/74.2.126. [DOI] [PubMed] [Google Scholar]
- 6.Gardner DG, Deschepper CF, Ganong WF, Hane S, Fiddes J, Baxter JD, et al. Extra atrial expression of the gene for atrial natriuretic factor. Proc Natl Acad Sci USA. 1986;83:6697–701. doi: 10.1073/pnas.83.18.6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vesely DL, Douglass MA, Dietz JR, Gower WR, Jr, McDormick MT, Rodriguez-Paz G, et al. Three peptides form the atrial natriuretic factor prohormone amino terminus lower blood pressure and produce a diuresis, natriuresis, and/or kaliuresis in humans. Circulation. 1994;90:1129–40. doi: 10.1161/01.cir.90.3.1129. [DOI] [PubMed] [Google Scholar]
- 8.Schulz-Knappe P, Forssmann K, Herbst F, Hock D, Pipkorn R, Forssmann WG. Isolation and structural analysis of “urodilatin”, a new peptide of the cardiodilatin-(ANP)-family, extracted from human urine. Klin Wochenschr. 1988;66:752–9. doi: 10.1007/BF01726570. [DOI] [PubMed] [Google Scholar]
- 9.Witthaut R. Science review: Natriuretic peptides in critical illness. Crit Care. 2004;8:342–9. doi: 10.1186/cc2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sudoh T, Kangawa K, Minamino W, Matsuo H. A new natriuretic peptide in porcine brain. Nature. 1988;332:78–81. doi: 10.1038/332078a0. [DOI] [PubMed] [Google Scholar]
- 11.Saito Y, Nakao K, Itoh H, Yamada T, Mukoyama M, Arai H, et al. Brain natriuretic peptide is a novel cardiac hormone. Biochem Biophys Res Commun. 1989;158:360–8. doi: 10.1016/s0006-291x(89)80056-7. [DOI] [PubMed] [Google Scholar]
- 12.Hunt PH, Yandle TG, Nicholls MG, Richards AM, Espiner EA. The amino-terminal portion of probrain natriuretic peptide (proBNP) circulates in human plasma. Biochem Biophys Res Commun. 1995;214:1175–83. doi: 10.1006/bbrc.1995.2410. [DOI] [PubMed] [Google Scholar]
- 13.Sudoh T, Minamino N, Kangawa K, Matsuo H. C-type natriuretic peptide (CNP).A new member of the natriuretic peptide family identified in porcine brain. Biochem Biophys Res Commun. 1990;168:863–70. doi: 10.1016/0006-291x(90)92401-k. [DOI] [PubMed] [Google Scholar]
- 14.Barr CS, Rhodes P, Struthers AD. C-type natriuretic peptides. Peptides. 1996;17:1243–51. doi: 10.1016/s0196-9781(96)00110-6. [DOI] [PubMed] [Google Scholar]
- 15.Naruko T, Ueda M, van der Wal AC, van der Loos CM, Itoh H, Nakao K, et al. C-type natriuretic peptide expression in human coronary atherosclerotic lesions. Circulation. 1996;94:3103–8. doi: 10.1161/01.cir.94.12.3103. [DOI] [PubMed] [Google Scholar]
- 16.Komatsu Y, Nakao K, Itoh H, Suga S, Ogawa Y, Imura H. Vascular natriuretic peptide. Lancet. 1992;340:622. doi: 10.1016/0140-6736(92)92167-e. [DOI] [PubMed] [Google Scholar]
- 17.Vesely DL. Which of the cardiac natriuretic peptides is most effective for the treatment of congestive heart failure, renal failure and cancer? Clin Exp Pharmacol Physiol. 2006;33:169–76. doi: 10.1111/j.1440-1681.2006.04344.x. [DOI] [PubMed] [Google Scholar]
- 18.Cargil R, Struthers A, Lipworth B. Human C-type natriuretic peptide: Effects on the hemodynamic and endocrine responses to angiotensin II. Cardiovasc Res. 1995;29:108–11. [PubMed] [Google Scholar]
- 19.Ikeda T, Itoh H, Komatsu Y, Hanyu M, Yoshimasa T, Matsuda K, et al. Natriuretic peptide receptors in human arterial and venous coronary bypass vessels and rabbit vein grafts. Hypertension. 1996;27:833–7. doi: 10.1161/01.hyp.27.3.833. [DOI] [PubMed] [Google Scholar]
- 20.Furuya M, Yoshida M, Hayashi Y, Ohnuma N, Minamino N, Kangawa K, et al. C-type natriuretic peptide is a growth inhibitor of rat vascular smooth muscle cells. Biochem Biophys Res Commun. 1991;177:927–31. doi: 10.1016/0006-291x(91)90627-j. [DOI] [PubMed] [Google Scholar]
- 21.Furuya M, Aisaka K, Miyazaki T, Honbou N, Kawashima K, Ohno T, et al. C-type natriuretic peptide inhibits intimal thickening after vascular injury. Biochem Biophys Res Commun. 1993;193:248–53. doi: 10.1006/bbrc.1993.1616. [DOI] [PubMed] [Google Scholar]
- 22.Nakao K, Ogawa Y, Suga S, Imura H. Molecular biology and biochemistry of the natriuretic system. I. Natriuretic peptides. J Hypertens. 1992;10:907–12. [PubMed] [Google Scholar]
- 23.Vollmar AM, Schmidt KN, Schulz R. Natriuretic peptide receptors on rat thymocytes: Inhibition of proliferation by atrial natriuretic peptide. Endocrinology. 1996;137:1706–13. doi: 10.1210/endo.137.5.8612505. [DOI] [PubMed] [Google Scholar]
- 24.Vollmar AM, Förster R, Schulz R. Effects of atrial natriuretic peptide on phagocytosis and respiratory burst in murine macrophages. Eur J Pharmacol. 1997;319:279–85. doi: 10.1016/s0014-2999(96)00859-x. [DOI] [PubMed] [Google Scholar]
- 25.Wiedemann CJ, Niedermühlbichler M, Braunsteiner H. Priming of polymorphonuclear neutrophils by atrial natriuretic peptide in vitro. J Clin Invest. 1992;89:1580–6. doi: 10.1172/JCI115752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med. 1999;341:577–85. doi: 10.1056/NEJM199908193410806. [DOI] [PubMed] [Google Scholar]
- 27.Young JB, Correia NG, Francis GS, Maisel A, Michota F. Testing for B-type natriuretic peptide in the diagnosis and assessment of heart failure: What are the nuances? Cleve Clin J Med. 2004;71(Suppl 5):S1–17. doi: 10.3949/ccjm.71.suppl_5.s1. [DOI] [PubMed] [Google Scholar]
- 28.Garbers DL. Guanylate cyclase receptors and their endocrine, paracrine, and autocrine ligands. Cell. 1992;71:1–4. doi: 10.1016/0092-8674(92)90258-e. [DOI] [PubMed] [Google Scholar]
- 29.Vesely DL, Cornett LE, MacLeod SL, Nash AA, Norris JS. Specific binding sites for prohormone atrial natriuretic peptides 1–30, 31–67, and 99–126. Peptides. 1990;11:193–7. doi: 10.1016/0196-9781(90)90070-l. [DOI] [PubMed] [Google Scholar]
- 30.Gunning ME, Brady HR, Otuechere G, Brenner BM, Zeidel ML. Atrial natriuretic peptide (31–67) inhibits Na+ transport in rabbit inner medullary collecting duct cells: Role of prostaglandin E2. J Clin Invest. 1992;89:1411–7. doi: 10.1172/JCI115730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maack T. Receptors of atrial natriuretic factor. Annu Rev Physiol. 1992;54:11–27. doi: 10.1146/annurev.ph.54.030192.000303. [DOI] [PubMed] [Google Scholar]
- 32.Maack T. Role of natriuretic factor in volume control. Kidney Int. 1996;49:1732–7. doi: 10.1038/ki.1996.257. [DOI] [PubMed] [Google Scholar]
- 33.Levine ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339:321–8. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 34.Mukoyama M, Nakao K, Saito Y, Saito Y, Yamada T, Shirakami G, et al. Increased human brain natriuretic peptide in congestive heart failure. N Engl J Med. 1990;313:757–8. doi: 10.1056/NEJM199009133231114. [DOI] [PubMed] [Google Scholar]
- 35.Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, et al. Breathing Not Properly Multinational Study Investigators.Bedside B-type natriuretic peptide in the emergency diagnosis of heart failure: Primary results from the Breathing Not Properly (BNP) Multinational Study. N Engl J Med. 2002;347:161–7. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 36.Muders F, Kromer E, Griese DP, Pfeifer M, Hense HW, Riegger GA, et al. Evaluation of plasma natriuretic peptides as markers for left ventricular dysfunction. Am Heart J. 1997;134:442–9. doi: 10.1016/s0002-8703(97)70079-6. [DOI] [PubMed] [Google Scholar]
- 37.Lerman A, Gibbons RJ, Rodeheffer RJ, Bailey KR, McKinley LJ, Heublein DM, et al. Circulating N-terminal atrial natriuretic peptide as a marker for symptomless left ventricular dysfunction. Lancet. 1993;341:1105–9. doi: 10.1016/0140-6736(93)93125-k. [DOI] [PubMed] [Google Scholar]
- 38.Daggubati S, Parks JR, Overton RM, Cintron G, Schocken DD, Vesely DL. Adrenomedullin, endothelin, neuropeptide Y, atrial, brain, and C-natriuretic prohormone peptides compared as early heart failure indicators. Cardivasc Res. 1997;36:246–55. doi: 10.1016/s0008-6363(97)00164-8. [DOI] [PubMed] [Google Scholar]
- 39.Hall C, Rouleau JL, Moye L, de Champlain J, Bichet D, Klein M, et al. N-terminal proatrial natriuretic factor.An independent predictor of long term prognosis after myocardial infarction. Circulation. 1994;89:1934–42. doi: 10.1161/01.cir.89.5.1934. [DOI] [PubMed] [Google Scholar]
- 40.Tsutamoto T, Wada A, Maeda K, Fukai D, Ohnishi M, Sugimoto Y, et al. Attenuation of compensation of endogenous cardiac natriuretic peptide system in chronic heart failure.Prognostic role of plasma brain natriuretic peptide concentration in patients with chronic symptomatic left ventricular dysfunction. Circulation. 1997;96:509–16. doi: 10.1161/01.cir.96.2.509. [DOI] [PubMed] [Google Scholar]
- 41.Gardner RS, Ozalp F, Murday AJ, Robb SD, McDonagh TA. N-terminal pro-brain natriuretic peptide.A new gold standard in predicting mortality in patients with advanced heart failure. Eur Heart J. 2003;24:1735–43. doi: 10.1016/j.ehj.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Mukoyama M, Nakao K, Hosoda K, Suga S, Saito Y, Ogawa Y, et al. Brain natriuretic peptide (BNP) as a novel cardiac hormone in humans — evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest. 1991;87:1402–12. doi: 10.1172/JCI115146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng V, Kazanagra R, Cheng V, Kazanagra R, Garcia A, Lenert L, et al. A rapid bedside test for B-type natriuretic peptide predicts treatment outcomes in patients admitted with decompensated heart failure. J Am Coll Cardiol. 2001;37:386–91. doi: 10.1016/s0735-1097(00)01157-8. [DOI] [PubMed] [Google Scholar]
- 44.Nielsen LS, Svanegaard J, Klitgaard NA, Egeblad H. N-terminal pro-brain natriuretic peptide for discriminating between cardiac and non-cardiac dyspnoe. Eur J Heart Failure. 2004;6:63–70. doi: 10.1016/j.ejheart.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 45.Tsai S, Lin Y, Chu S, Hsu C, Cheng S. Interpretation and use of Natriuretic Peptides in non-congestive heart failure settings. Yonsei Med J. 2010;51:151–63. doi: 10.3349/ymj.2010.51.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hobbs FD, Davis RC, Roalfe AK, Hare R, Davies MK, Kenkre JE. Reliability of N-terminal pro-brain natriuretic peptide assay in diagnosis of heart failure: Cohort study in representative and high risk community populations. Br Med J. 2002;324:1498–502. doi: 10.1136/bmj.324.7352.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–63. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 48.Richards AM, Doughty R, Nicholls MG, Macmahon S, Ikram H, Sharpe N, et al. Neurohumoral prediction of benefit from carvedilol in ischemic left ventricular dysfunction.Australia-New Zealand Heart Failure Group. Circulation. 1999;99:786–92. doi: 10.1161/01.cir.99.6.786. [DOI] [PubMed] [Google Scholar]
- 49.Anand IS, Fisher LD, Chiang YT, Latini R, Masson S, Maggioni AP, et al. Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val-HeFT) Circulation. 2003;107:1278–83. doi: 10.1161/01.cir.0000054164.99881.00. [DOI] [PubMed] [Google Scholar]
- 50.Shah MR, Claise KA, Bowers MT, Bhapkar M, Little J, Nohria A, et al. Testing new targets of therapy in advanced heart failure: The design and rationale of the Strategies for Tailoring Advanced Heart Failure Regimens in the Outpatient Setting: BRain NatrIuretic Peptide Versus the Clinical CongesTion ScorE (STARBRITE) trial. Am Heart J. 2005;150:893–8. doi: 10.1016/j.ahj.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 51.Cleland JG, Coletta AP, Yassin A, Buga L, Torabi A, Clark AL. Clinical trials update from the European Society of Cardiology heart failure meeting 2009: CHANCE, B-Convinced, CHAT, CIBIS-ELD, and Signal-HF. Eur J Heart Fail. 2009;11:802–5. doi: 10.1093/eurjhf/hfp102. [DOI] [PubMed] [Google Scholar]
- 52.Eurlings LW, van Pol PE, Kok WE, van Wijk S, Lodewijks-van der Bolt C, Balk AH, et al. Management of chronic heart failure guided by individual N-terminal pro-B-type natriuretic peptide targets: Results of the PRIMA (Can PRo-brain-natriuretic peptide guided therapy of chronic heart failure IMprove heart fAilure morbidity and mortality.) study? J Am Coll Cardiol. 2010;56:2090–100. doi: 10.1016/j.jacc.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 53.Troughton RW, Frampton CM, Yandle TG, Espiner EA, Nicholls MG, Richards AM. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N-BNP) concentrations. Lancet. 2000;355:1126–30. doi: 10.1016/s0140-6736(00)02060-2. [DOI] [PubMed] [Google Scholar]
- 54.Jourdain P, Jondeau G, Funck F, Gueffet P, Le Helloco A, Donal E, et al. Plasma brain natriuretic peptide-guided therapy to improve outcome in heart failure: The STARS-BNP Multicenter Study. J Am Coll Cardiol. 2007;49:1733–9. doi: 10.1016/j.jacc.2006.10.081. [DOI] [PubMed] [Google Scholar]
- 55.Berger R, Moertl D, Peter S, Ahmadi R, Huelsmann M, Yamuti S, et al. N-terminal pro-B-type natriuretic peptide-guided, intensive patient management in addition to multidisciplinary care in chronic heart failure a 3-arm, prospective, randomized pilot study. J Am Coll Cardiol. 2010;55:645–53. doi: 10.1016/j.jacc.2009.08.078. [DOI] [PubMed] [Google Scholar]
- 56.Davis ME, Richards AM, Nicholls MG, Yandle TG, Frampton CM, Troughton RW. Introduction of metoprolol increases plasma B-type cardiac natriuretic peptides in mild, stable heart failure. Circulation. 2006;113:977–85. doi: 10.1161/CIRCULATIONAHA.105.567727. [DOI] [PubMed] [Google Scholar]
- 57.Lainchbury JG, Troughton RW, Strangman KM, Frampton CM, Pilbrow A, Yandle TG, et al. N-terminal pro-B-type natriuretic peptide-guided treatment for chronic heart failure: Results from the BATTLESCARRED (NT-proBNP-Assisted Treatment To Lessen Serial Cardiac Readmissions and Death) trial. J Am Coll Cardiol. 2009;55:53–60. doi: 10.1016/j.jacc.2009.02.095. [DOI] [PubMed] [Google Scholar]
- 58.Pfisterer M, Buser P, Rickli H, Gutmann M, Erne P, Rickenbacher P, et al. TIME-CHF Investigators: BNP-guided vs symptom-guided heart failure therapy: The Trial of Intensified vs Standard Medical Therapy in Elderly Patients With Congestive Heart Failure (TIME-CHF) randomized trial. JAMA. 2009;301:383–92. doi: 10.1001/jama.2009.2. [DOI] [PubMed] [Google Scholar]
- 59.Schou M, Gustafsson F, Videbaek L, Markenvard J, Ulriksen H, Ryde H, et al. Design and methodology of the NorthStar Study: NT-proBNP stratified follow-up in outpatient heart failure clinics – a randomized Danish multicenter study. Am Heart J. 2008;156:649–55. doi: 10.1016/j.ahj.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 60.Felker GM, Hasselblad V, Hernandez AF, O’Connor CM. Biomarker-guided therapy in chronic heart failure: A meta-analysis of randomized controlled trials. Am Heart J. 2009;158:422–30. doi: 10.1016/j.ahj.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 61.Porapakkham P, Porapakkham P, Zimmet H, Billah B, Krum H. B-type natriuretic peptide-guided heart failure therapy: A meta-analysis. Arch Intern Med. 2010;170:507–14. doi: 10.1001/archinternmed.2010.35. [DOI] [PubMed] [Google Scholar]
- 62.deFilippi CR, Christenson RH, Gottdiener JS, Kop WJ, Seliger SL. Dynamic Cardiovascular Risk Assessment in Elderly People.The Role of Repeated N-Terminal Pro–B-Type Natriuretic Peptide Testing. J Am Coll Cardiol. 2010;55:441–50. doi: 10.1016/j.jacc.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Omland T. B-type natriuretic peptides: Prognostic markers in stable coronary artery disease. Expert Rev Mol Diagn. 2008;8:217–25. doi: 10.1586/14737159.8.2.217. [DOI] [PubMed] [Google Scholar]
- 64.Eggers KM, Lagerqvist B, Venge P, Wallentin L, Lindahl B. Prognostic value of biomarkers during and after non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol. 2009;21(54):357–64. doi: 10.1016/j.jacc.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 65.Krishna A, Kapoor A, Kumar S, Tewari S, Garg N, Goel P. Elevated B-type natriuretic peptide levels in patients undergoing coronary stenting. J Invasive Cardiol. 2011;23:240–5. [PubMed] [Google Scholar]
- 66.Karthikeyan G, Moncur RA, Levine O, Heels-Ansdell D, Chan MT, Alonso-Coello P, et al. Is a pre-operative brain natriuretic peptide or N-terminal pro-B-type natriuretic peptide measurement an independent predictor of adverse cardiovascular outcomes within 30 days of noncardiac surgery? A systematic review and meta-analysis of observational studies. J Am Coll Cardiol. 2009;54:1599–606. doi: 10.1016/j.jacc.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 67.Ramakrishnan S, Agarwal A, Singh S, Karthikeyan G, Seth S, Narang R, et al. NT-pro-BNP levels as a marker of success of percutaneous transvenous mitral commissurotomy. Indian Heart J. 2010;62:35–8. [PubMed] [Google Scholar]
- 68.McCullough PA, Philbin EF, Spertus JA, Sandberg KR, Sullivan RA, Kaatz S. Opportunities for improvement in the diagnosis and treatment of heart failure. Clin Cardiol. 2003;26:231–7. doi: 10.1002/clc.4960260507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morrison LK, Harrison A, Krishnaswamy P, Kazanegra R, Clopton P, Maisel A. Utility of a rapid B-natriuretic peptide (BNP) assay in differentiating CHF from lung disease in patients presenting with dyspnoe. J Am Coll Cardiol. 2002;39:202–9. doi: 10.1016/s0735-1097(01)01744-2. [DOI] [PubMed] [Google Scholar]
- 70.Nielsen LS, Svanegaard J, Klitgaard NA, Egeblad H. N-terminal pro-brain natriuretic peptide for discriminating between cardiac and non-cardiac dyspnoe. Eur J Heart Failure. 2004;6:63–70. doi: 10.1016/j.ejheart.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 71.Knudsen CW, Clopton P, Westheim A, Klemsdal TO, Wu AH, Duc P, et al. Predictors of elevated B-type natriuretic peptide concentrations in dyspneic patients without heart failure: An analysis from the breathing not properly multinational study. Ann Emerg Med. 2005;45:573–80. doi: 10.1016/j.annemergmed.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 72.Tanabe M, Ueda M, Endo M, Kitajima M. Effect of acute lung injury and coexisting disorders on plasma concentrations of atrial natriuretic peptide. Crit Care Med. 1994;22:1762–8. [PubMed] [Google Scholar]
- 73.Mazul-Sunko B, Zarkovic N, Vrkic N, Klinger R, Peric M, Bekavac-Beslin M, et al. Proatrial natriuretic peptide hormone from right atria is correlated with cardiac depression in septic patients. J Endocrinol Invest. 2001;24:RC22–4. doi: 10.1007/BF03343878. [DOI] [PubMed] [Google Scholar]
- 74.Mitaka C, Hirata Y, Nagura T, Tsunoda Y, Itoh M, Amaha K. Increased plasma concentrations of brain natriuretic peptide in patients with acute lung injury. J Crit Care. 1997;12:66–71. doi: 10.1016/s0883-9441(97)90003-4. [DOI] [PubMed] [Google Scholar]
- 75.Viellard-Baron A, Schmitt JM, Augarde R, Prin S, Qanadli S, Beauchet A, et al. Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: Incidence, clinical implications, and prognosis. Crit Care Med. 2001;29:1551–5. doi: 10.1097/00003246-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 76.Vlahakes GJ, Turley K, Hoffman JL. The pathophysiology of failure in right ventricular hypertension: Hemodynamic and biochemical correlates. Circulation. 1981;63:87–95. doi: 10.1161/01.cir.63.1.87. [DOI] [PubMed] [Google Scholar]
- 77.Maisel AS, McCullough PA. Cardiac natriuretic peptides: A proteomic window to cardiac function and clinical management. Rev Cardiovasc Med. 2003;4(Suppl 4):3–12. [PubMed] [Google Scholar]
- 78.Light RW. Clinical practice: Pleural effusion. N Engl J Med. 2002;346:1971–7. doi: 10.1056/NEJMcp010731. [DOI] [PubMed] [Google Scholar]
- 79.Romero S, Candela A, Martin C, Hernandez L, Trigo C, Gil J. Evaluation of different criteria for the separation of pleural transudates from exudates. Chest. 1993;104:399–404. doi: 10.1378/chest.104.2.399. [DOI] [PubMed] [Google Scholar]
- 80.Janda S, Swiston J. Diagnostic accuracy of pleural fluid NT-pro-BNP for pleural effusions of cardiac origin: A systematic review and metaanalysis. BMC Pulm Med. 2010;10:58–68. doi: 10.1186/1471-2466-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goei D, Schouten O, Boersma E, Welten GM, Dunkelgrun M, Lindemans J, et al. Influence of renal function on the usefulness of N-terminal pro-B-type natriuretic peptide as a prognostic cardiac risk marker in patients undergoing noncardiac vascular surgery. Am J Cardiol. 2008;101:122–6. doi: 10.1016/j.amjcard.2007.07.058. [DOI] [PubMed] [Google Scholar]
- 82.Tagore R, Ling LH, Yang H, Daw HY, Chan YH, Sethi SK. Natriuretic peptides in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1644–51. doi: 10.2215/CJN.00850208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Biasioli S, Zamperetti M, Borin D, Guidi G, De Fanti E, Schiavon R. Significance of plasma B-type atriuretic peptide in hemodialysis patients: Blood sample timing and comorbidity burden. ASAIO J. 2007;53:587–91. doi: 10.1097/MAT.0b013e31814a57c3. [DOI] [PubMed] [Google Scholar]
- 84.Yildiz R, Yildirim B, Karincaoglu M, Harputluoglu M, Hilmioglu F. Brain natriuretic peptide and severity of disease in nonalcoholic cirrhotic patients. J Gastroenterol Hepatol. 2005;20:1115–20. doi: 10.1111/j.1440-1746.2005.03906.x. [DOI] [PubMed] [Google Scholar]
- 85.Henriksen JH, GØtze JP, Fuglsang S, Christensen E, Bendtsen F, MØller S. Increased circulating pro-brain natriuretic peptide (proBNP) and brain natriuretic peptide (BNP) in patients with cirrhosis: Relation to cardiovascular dysfunction and severity of disease. Gut. 2003;52:1511–7. doi: 10.1136/gut.52.10.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schultz M, Faber J, Kistorp C, JarlØv A, Pedersen F, Wiinberg N, et al. N-terminal-pro-B-type natriuretic peptide (NT-pro-BNP) in different thyroid function states. Clin Endocrinol (Oxf) 2004;60:54–9. doi: 10.1111/j.1365-2265.2004.01941.x. [DOI] [PubMed] [Google Scholar]
- 87.Wei T, Zeng C, Tian Y, Chen Q, Wang L. B-type natriuretic peptide in patients with clinical hyperthyroidism. J Endocrinol Invest. 2005;28:8–11. doi: 10.1007/BF03345522. [DOI] [PubMed] [Google Scholar]
- 88.Ozmen B, Ozmen D, Parildar Z, Mutaf I, Bayindir O. Serum N-terminal-pro-B-type natriuretic peptide (NT-pro-BNP) levels in hyperthyroidism and hypothyroidism. Endocr Res. 2007;32:1–8. doi: 10.1080/07435800701670047. [DOI] [PubMed] [Google Scholar]
- 89.Berendes E, Walter M, Cullen P, Prien T, Van Aken H, Horsthemke J, et al. Secretion of brain natriuretic peptide in patients with aneurysmal subarachnoid haemorrhage. Lancet. 1997;349:245–9. doi: 10.1016/s0140-6736(96)08093-2. [DOI] [PubMed] [Google Scholar]
- 90.Stewart D, Waxman K, Brown CA, Schuster R, Schuster L, Hvingelby EM, et al. B-type natriuretic peptide levels may be elevated in the critically injured trauma patient without congestive heart failure. J Trauma. 2007;63:747–50. doi: 10.1097/01.ta.0000240458.46050.38. [DOI] [PubMed] [Google Scholar]
- 91.Tung PP, Olmsted E, Kopelnik A, Banki NM, Drew BJ, Ko N, et al. Plasma B-type natriuretic peptide levels are associated with early cardiac dysfunction after subarachnoid hemorrhage. Stroke. 2005;36:1567–9. doi: 10.1161/01.STR.0000170699.59783.d6. [DOI] [PubMed] [Google Scholar]
- 92.Espiner EA, Leikis R, Ferch RD, MacFarlane MR, Bonkowski JA, Frampton CM, et al. The neuro-cardio-endocrine response to acute subarachnoid haemorrhage. Clin Endocrinol (Oxf) 2002;56:629–35. doi: 10.1046/j.1365-2265.2002.01285.x. [DOI] [PubMed] [Google Scholar]
- 93.Davutoglu V, Gunay N, Kocoglu H, Gunay NE, Yildirim C, Cavdar M, et al. Serum levels of NT-ProBNP as an early cardiac marker of carbon monoxide poisoning. Inhal Toxicol. 2006;18:155–8. doi: 10.1080/08958370500305885. [DOI] [PubMed] [Google Scholar]
- 94.Sackner-Bernstein JD, Kowalski M, Fox M, Aaronson K. Short-term risk of death after treatment with nesiritide for decompensated heart failure: A pooled analysis of randomized controlled trials. JAMA. 2005;293:1900–5. doi: 10.1001/jama.293.15.1900. [DOI] [PubMed] [Google Scholar]
- 95.Sackner-Bernstein JD, Skopicki HA, Aaronson KD. Risk of worsening renal function with Nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111:1487–91. doi: 10.1161/01.CIR.0000159340.93220.E4. [DOI] [PubMed] [Google Scholar]
- 96.Manchanda A, Kohli V. Nesiritide: First use in India. Indian Heart J. 2009;61:371–2. [PubMed] [Google Scholar]
- 97.Cuculi F, Erne P. Combined neutral endopeptidase inhibitors. Expert Opin Investig Drugs. 2011;20:457–63. doi: 10.1517/13543784.2011.556617. [DOI] [PubMed] [Google Scholar]
- 98.Neal B, MacMahon S, Ohkubo T, Brnabic A, Tonkin A. Pacific Study Group. Effects of the vasopeptidase inhibitor, omapatrilat, in 723 patients with coronary heart disease. J Renin-Angiotensin Aldosterone Syst. 2002;3:270–6. doi: 10.3317/jraas.2002.049. [DOI] [PubMed] [Google Scholar]


