Abstract
Within the past decade, many oncolytic viruses (OVs) have been studied as potential treatments for pancreatic cancer and some of these are currently under clinical trials. The applicability of certain OVs, such as adenoviruses, herpesviruses and reoviruses, for the treatment of pancreatic cancer has been intensively studied for several years, whereas the applicability of other more recently investigated OVs, such as poxviruses and parvoviruses, is only starting to be determined. At the same time, studies have identified key characteristics of pancreatic cancer biology that provide a better understanding of the important factors or pathways involved in this disease. This review aims to summarise the different replication-competent OVs proposed as therapeutics for pancreatic cancer. It also focuses on the unique biology of these viruses that makes them exciting candidate virotherapies for pancreatic cancer and discusses how they could be genetically manipulated or combined with other drugs to improve their efficacy based on what is currently known about the molecular biology of pancreatic cancer.
Pancreatic cancer is the fourth leading cause of cancer-related deaths in the USA and prognosis for patients is still poor, regardless of treatment. After initial diagnosis, only 6% of patients survive past 5 years and most die within the first year following diagnosis. At the time of diagnosis, most patients have locally advanced or metastatic disease and only 20% are diagnosed at a stage for which the tumour is resectable. Even in cases where the cancer is diagnosed at an early resectable stage, the survival after 5 years is still only 22% (Ref. 1). In addition, pancreatic cancer is notorious for its resistance to currently available chemotherapy agents, and this is another key factor that contributes to the poor prognosis of these patients. Oncolytic virotherapy offers a novel treatment for this disease, which can be used in combination with currently available therapies. This review will summarise the different candidate oncolytic viruses (OVs) currently under development that possess promising oncolytic activities for pancreatic cancer. Insights into their selectivity and potential clinical use in combination with other therapies are also discussed.
Molecular biology of pancreatic cancer cells and their tumour microenvironment
Approximately 90% of pancreatic cancers are exocrine adenocarcinomas and are believed to arise most often from ductal cells in the pancreas (Ref. 2). It is well established that several key genetic alterations are linked to the development of pancreatic ductal adenocarcinoma (PDAC) (Ref. 2). Almost 100% of PDACs contain a mutated, activated KRAS oncogene (Refs 3, 4). KRAS belongs to the family of Ras GTPases that control important downstream signalling pathways involved in tumourigenesis, such as the Raf/MAPK1 (mitogen-activated protein kinase 1) and the phosphoinositide 3-kinase (PI3K) pathways (reviewed in Ref. 5). Transgenic mice with Kras mutations have been shown to develop preinvasive pancreatic neoplastic lesions (Ref. 6). Furthermore, in these mice, the progression to metastatic PDAC appears to require both the mutation of Kras oncogene and the loss of tumour suppressor genes, such as cyclin-dependent kinase inhibitor 2A (Cdkn2a), tumour protein p53 (Tp53) and SMAD family member 4 (Smad4) (Refs 6, 7, 8). These results suggest that the onset of pancreatic cancer involves at least two events: (1) mutation of the KRAS oncogene and (2) genetic alterations that lead to the inactivation of several tumour suppressor genes. In addition, global genomic analysis of pancreatic cancers has led to the identification of several core signalling pathways that are commonly dysregulated in this type of cancer. In this study, 12 signalling pathways or processes were found to be altered in 67–100% of the cancers tested. Importantly, specific genes or components that are altered within these commonly dysregulated pathways are not necessarily the same, and these varied greatly between the cancer samples analysed (Ref. 9). The signalling pathways commonly associated with pancreatic cancer are listed in Table 1
Table 1.
Altered signalling pathways in pancreatic cancera
| Altered signaling pathway |
Example of mutated geneb |
|---|---|
| K-Ras | KRAS |
| TGF-β | SMAD4 |
| c-Jun N-terminal kinase | MAP4K3 |
| Integrin | LAMA1 |
| Wnt/Notch | MYC |
| Hedgehog | SOX3 |
| Control of G1–S phase | CDKN2A |
| Apoptosis | CASP10 |
| DNA damage | TP53 |
| Small GTPase | ARHGEF7 |
| Invasion | ADAM11 |
| Homophilic cell addition | CDH1 |
Adapted from Ref. 9
MAP4K3, mitogen-activated protein kinase kinase kinase kinase 3; LAMA1, laminin α1; MYC, myelocytomatosis viral oncogene homologue; SOX3, SRY (sex-determining region Y)-box 3; CASP10, caspase 10; ARHGEF7, rho guanine nucleotide exchange factor (GEF) 7; ADAM11, ADAM metallopeptidase domain 11; CDH1, E-cadherin.
Recent studies have shown that the effectiveness of current pancreatic cancer treatments depends not only on the specific genetic alterations of pancreatic cancer cells, but also on the tumour microenvironment. Pancreatic cancer is usually hypoxic, with poor vasculature and a dense stromal matrix, which makes up the majority of the tumour mass (Ref. 10). In addition, the poor vasculature and the dense stroma of pancreatic cancers are determinants that influence the lack of outcome of systemically delivered therapies, such as systemic chemotherapy. Inhibition of Hedgehog signalling improves pancreatic tumour vasculature concomitant with an improvement in the delivery of chemotherapy drugs, suggesting that the tumour microenvironment can be directly linked to chemoresistance (Ref. 11).
It has also become evident that pancreatic cancers can undergo an epithelial to mesenchymal transition (EMT) (Refs 12, 13). EMT is considered an initial step towards metastasis and invasion and is usually characterised by a ‘cadherin switch’ in which epithelial cell–cell junction markers such as E-cadherin are lost and the expression of mesenchymal markers such as N-cadherin, vimentin and the zinc finger E-box-binding homeobox 1, ZEB1 (among other changes) is acquired (Ref. 14). In fact, the loss or reduction in E-cadherin expression has been associated with late disseminated stages of the disease and with the invasion of lymph nodes (Ref. 15), and the expression of N-cadherin has been associated with aggressive and metastatic phenotypes (Ref. 16). EMT has also been associated with resistance to chemotherapy (Refs 13, 17).
Oncolytic virotherapy as a treatment for pancreatic cancer
Surgery, radiation therapy and chemotherapy are the current treatments for pancreatic cancer patients. For the past 10 years, the standard of care for patients with pancreatic cancer is chemotherapy with gemcitabine (2′,2′-difluorodeoxycytidine, Gemzar®) (Ref. 18). Gemcitabine is a nucleoside analogue that incorporates into the DNA of actively dividing cells to stop DNA replication and ultimately leads to cell death (Refs 19, 20). In the past decade, in an effort to improve the effectiveness of chemotherapy, other agents have been tested in combination with gemcitabine. These agents include platinum-based, DNA crosslinking or alkylating chemotherapy agents such as cisplatin or oxaliplatin, respectively (Refs 21, 22), topoisomerase 1 inhibitors such as irinotecan (Ref. 23) and mitotic inhibitors such as docetaxel (Ref. 24). However, these combination therapies rarely improved survival when compared with gemcitabine alone. Recently erlotinib (Tarceva®), a new targeted therapy agent, has been approved by the US Food and Drug Administration (FDA) for use in combination with gemcitabine. Erlotinib acts as an inhibitor of the epidermal growth factor receptor, and when used in combination with gemcitabine, slightly increases the survival in some patients when compared with treatment with gemcitabine alone (Refs 25, 26). The addition of drugs as adjuvants to gemcitabine chemotherapy is currently decided on a case-by-case basis. Other potential treatment options for pancreatic cancer, such as the FOLFIRINOX regimen, involve the use of four chemotherapy agents: 5-fluorouracil (5-FU, another nucleoside analogue), leucovorin (a folic acid analogue that enhances the effects of 5-FU), irinotecan and oxaliplatin. In a recent study, this chemotherapy regimen was shown to increase survival from 6.8 months (with gemcitabine) to 11.1 months (Refs 27, 28). However, these advances in treatment are small and prognosis for pancreatic cancer patients still remains poor. Thus pancreatic cancer patients are in need of new, more effective treatments. A myriad of targeted agents have been proposed for pancreatic cancer and are currently under intense study, as reviewed in Refs 29, 30.
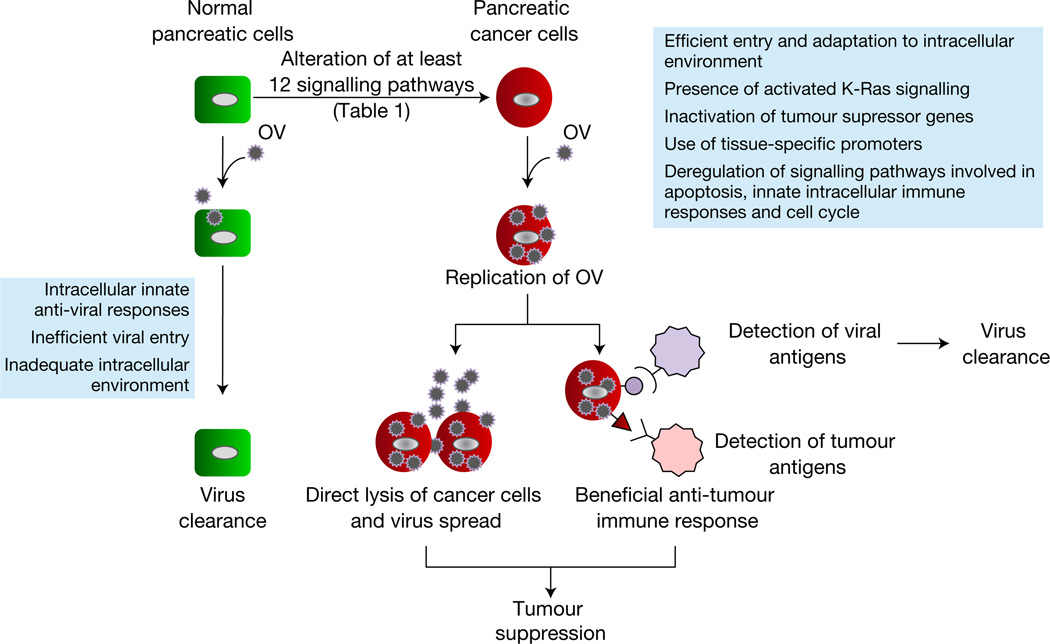
Oncolytic virotherapy uses actively replicating OVs as agents against cancer (Refs 31, 32). An ideal OV must (1) have a select tropism for cancer cells and not normal cells, determined either at entry by the overexpression of specific receptors or later by the dysregulated intracellular environment of cancer cells; (2) cause the death of the cancer cell upon infection (oncolysis), prevent the further participation of the cell in disease progression or induce an antitumour immune response that aids in tumour clearance; and (3) spread intratumourally within the complex tumour microenvironment as well as intertumourally (Fig. 1). One clear advantage of OVs as treatment for cancer is the fact that they can be genetically manipulated to assume greater potency against the cancer in question. Genetic manipulation of OVs might be done to increase the specificity and also the safety of the OVs or to introduce transgenes that can increase the therapeutic potential of the virus.
Figure 1. Specificity of oncolytic viruses for pancreatic cancer.
Pancreatic cancer cells arise from normal ductal cells upon genetic alterations that lead to the dysregulation of specific signalling pathways. Oncolytic viruses (OVs) cannot replicate in normal cells owing to inefficient viral entry, induction of innate intracellular antiviral responses or an inadequate intracellular signalling environment. The specificity of OVs can be dictated at the step of viral entry or later, within the specific intracellular environment of the pancreatic cancer cell. Successful replication of an OV in the cancer cell should lead to oncolysis. Oncolysis can be achieved through several methods, which include, among others, the direct lysis of cancer cells as a product of lytic viral replication as well as the induction of a beneficial antitumour immune response triggered as a consequence of the activation of the immune reponse by the virus. Ultimately, virus is cleared by a protective antiviral immune response and tumour clearance can proceed by the establishment of the antitumour response.
The ideal OV for pancreatic cancer should be able to selectively replicate in the intracellular environment specific for pancreatic cancers, which normally involves the aberrant signalling pathways listed in Table 1. Ideally, the ability of a given OV to replicate in pancreatic cancer cells must not be compromised by intracellular changes associated with transitions such as EMT that result in more aggressive, metastatic phenotypes. The OV must also be able to spread in the hypoxic and densely stromal-rich microenvironment of pancreatic tumours. Finally, the ability of the OV to be combined with novel targeted drugs or currently approved drugs, such as gemcitabine, is highly desirable.
The selectivity of OVs for pancreatic cancer frequently relies on particular signalling pathways commonly dysregulated in this cancer, such as the Ras signalling pathway and the loss of tumour suppressor gene functions (TP53, SMAD4, etc.). Other OVs whose specificity does not rely on these intracellular signalling pathways might be particularly targeted to pancreatic cancer cells through the use of tissue-specific promoters or through receptor retargeting techniques. Table 2 summarises what is currently known about the cancer selectivity of different OVs with activity against pancreatic cancer. Second- or third-generation OVs have now been engineered to express particular transgenes with therapeutic or cytotoxic effects, and they show promise as pancreatic cancer therapies in animal models.
Table 2.
Selectivity of replication-competent oncolytic viruses for pancreatic cancer
| Oncolytic virus | Basis of cancer selectivity | Refs | |
|---|---|---|---|
| Adenoviruses | Lacking E1B 55 kDa |
Loss of TP53 function or altered nuclear mRNA transport |
33, 34 |
| Lacking E1ACR2 | Aberrant pRB pathway | 35, 36 | |
| Lacking E1B 19 kDa |
Aberrant apoptotic pathways | 37 | |
| Herpesviruses | Lacking ICP6 | Upregulated ribonucleotide reductase activity | 38 |
| Lacking ICP34.5 | Aberrant PKR signalling or PI3K pathway | 39, 40 | |
| Lacking ICP10 | Upregulated Ras signalling pathway | 41 | |
| Parvoviruses | H-1PV | Altered Ras–Raf signalling pathway, SMAD4 mutations and IFN signalling |
42, 43, 44 |
| Reoviruses | Reovirus serotype 3 |
Altered Ras signalling pathway | 45 |
| Poxviruses | Vaccinia virus | Several pathways depending on engineered or naturally occurring mutations in the attenuated virus |
46, 47 |
| Myxoma virus | Akt signalling; loss of tumour suppressor functions |
48, 49 | |
| Paramyxoviruses | Measles virus | Receptor retargeting | 50, 51 |
| Sendai virus | Receptor retargeting | 52 |
As a prerequisite for clinical trials, OVs must first be tested for efficacy in preclinical models of pancreatic cancer. Immunodeficient xenograft murine models and immunocompetent syngeneic murine or Syrian hamster models are commonly used to investigate the efficacy of OV therapies for pancreatic cancer. The efficacy of OVs, including adenoviruses, herpes simplex viruses (HSVs), poxviruses, parvoviruses (PVs), reoviruses and paramyxoviruses, has been tested in these models. Each has its advantages and disadvantages. Xenograft models allow the investigation of OV efficacy directly in pancreatic cancer cells of human origin, but are used in immunodeficient animals and do not allow the study of OV therapy in the context of an intact immune system. However, syngeneic animal models for pancreatic cancer are useful to study the effects of immune responses on the efficacy of oncolytic virotherapy. The Syrian hamster model of pancreatic cancer is particularly useful to study oncolytic adenovirus therapies owing to its permissiveness to adenovirus infection and replication, in contrast to murine models, which do not support adenovirus replication (Ref. 53). Until the development of this hamster model, testing of replication-competent oncolytic adenoviruses had been limited to human xenografts in immunodeficient murine models.
Aside from the animal background used, preclinical pancreatic cancer models can also differ in the site of tumour engraftment. In orthotopic models, pancreatic cancer cells are surgically engrafted into the pancreas, which closely mimics the tumour microenviroment during early stages of the disease when the tumour has not metastasised. In other models, pancreatic cancer cells are injected into the intraperitoneal (IP) cavity to resemble a late-stage disseminated disease, which might be clinically relevant because most pancreatic cancer patients are diagnosed at this stage of the disease. In yet other models, subcutaneous pancreatic tumours can be established, which are convenient for intratumoural injection studies of OVs and for monitoring of tumour burden, but do not properly resemble the tumour environment of pancreatic cancers or disseminated disease. The most recent preclinical and clinical studies involving OV therapy for the treatment of pancreatic cancer are summarised in this review.
Replication-competent oncolytic adenoviruses
For cancer therapy applications, adenoviruses can be genetically modified into conditionally replicating adenoviruses (CRAds) that preferentially replicate in cancer cells and not normal somatic cells. Several CRAds have been developed whose specificity for cancer cells depends on the dysregulation of particular signalling pathways, some of which might be applicable to pancreatic cancer treatment. ONXY-015 (dl1520) was the first replication-competent oncolytic adenovirus used in clinical trials for pancreatic cancer. ONYX-015 has been engineered to lack expression of the E1B 55 kDa protein. The E1B 55 kDa protein binds to and inhibits the function of the p53 protein, thereby promoting cell cycle progression and replication of wild-type (WT) adenoviruses in normal cells (Ref. 33). In contrast to WT adenoviruses, the replication of adenoviruses lacking the E1B 55 kDa protein was originally thought to be restricted to cells with an aberrant p53 pathway. Later, it was discovered that the E1B 55 kDa protein is also involved in the nuclear export of the late viral mRNA encoding the 100 kDa protein (a viral protein that inhibits host protein synthesis) and showed that altered nuclear mRNA transport in cancer cells is a major determinant of tumour-selective replication for ONX-015 (Ref. 34). In addition, loss of E1B-55-kDa-mediated mRNA export could be rescued by heat shock responses. These recent findings suggest that heat shock agents could be used to sensitise tumour cells to ONYX-015 oncolysis (Ref. 54). This strategy could be explored for future oncolytic virotherapy for pancreatic cancer. Even though issues remain about which cellular pathway defects best support the replication of this virus, about 50% of human pancreatic cancers contain a mutated or inactive p53 (Ref. 3), and at least some of these are presumably susceptible to productive ONYX-015 infection. Using xenograft animal models, early studies showed that intratumoural injection of ONYX-015 results in viral replication and a reduction in tumour size (Ref. 55).
Other oncolytic adenoviruses have been generated to selectively replicate in cancer cells with a dysregulated retinoblastoma protein 1 (pRB) and E2F transcription factor 1 (E2F1) pathway. E1A mutant adenoviruses preferentially replicate in cancer cells with an aberrant pRb pathway. Ad dl922–947 and Delta24 are adenoviruses with deletions in the pRb binding site of the E1A protein (E1ACR2) (Refs 35, 36). The binding of E1A to pRb and the subsequent release of E2F1 are required for effective virus replication in normal cells. In most tumour cells, the pRb pathway is deregulated, and E2F1 is free to support the replication of adenoviruses lacking the E1ACR2 region (Ref. 56). Even though pancreatic cancers are not reported to have mutations in the retinoblastoma 1 (RB1) gene, they frequently contain mutations in genes involved in G1–S regulation, particularly CDKN2A (Ref. 9), which, in turn, can lead to a disruption of the pRB–E2F1 pathway. Hence, adenoviruses with E1ACR2 deletions have a reasonable potential as virotherapy candidates for pancreatic cancers.
Adenoviruses lacking the E1B 19 kDa protein are also candidates for pancreatic cancer treatments because E1B-19k is an antiapoptotic B-cell lymphoma 2 (Bcl-2) homologue and it is known that pancreatic cancer cells have aberrant apoptotic pathways (Ref. 9). Lack of E1B 19 kDa expression has also been shown to significantly enhance adenovirus and gemcitabine combined therapy for pancreatic cancer (Ref. 37). A double-deleted adenovirus lacking both E1ACR2 and the E1B 19 kDa protein was shown to be more attenuated in normal cells than the single-deleted E1ACR2 virus and still retained potent oncolytic activity. This virus was also shown to synergise with docetaxel and mitoxantrone (Ref. 57). With continuing advances in the understanding of adenovirus replication as it relates to dysregulation of cell signalling, as well as the growing appreciation of the genetic basis for pancreatic cancers, most current studies attempt to increase the specificity and efficacy of engineered adenoviruses for pancreatic cancer therapy.
Armed oncolytic CRAds
Several therapeutic genes have been examined to determine whether they can enhance the potency of oncolytic adenoviruses. Angiogenesis is a crucial step for the growth and survival of many tumours, including pancreatic carcinomas. Canstatin is a matrix-derived protein that can inhibit angiogenesis and tumour growth (Ref. 58). CRAd-Cans, a canstatin-expressing adenovirus, induced stronger tumour suppression effects than the parental ONYX-015, and markedly prolonged animal survival. The loss of somatostatin receptor 2 (SSTR2) gene expression has been reported in most pancreatic cancers (Ref. 59) and restoration of its expression in pancreatic cancer cells reversed their tumourigenicity in vivo (Ref. 59). ZD55-SSTR2, an oncolytic adenovirus strain lacking E1B 19 kDa and armed with SSTR2, showed only minor antipancreatic carcinoma effects. However, this recombinant adenovirus greatly sensitised xenografted pancreatic cancer cells to treatment with another recombinant adenovirus, ZD55-TRAIL, a ZD55 virus expressing the tumour necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) (Ref. 60). Most pancreatic cancer cell lines are refractory to TRAIL treatment. In this study, complete eradication of pancreatic cancer cells was observed in nine out of ten mice receiving combined treatment. TRAIL is a TNF family member that induces apoptosis in a variety of cancers by binding to the TRAIL receptors TRAILR1 (TNFRSF10A) and TRAILR2 (TNFRSF10B). The study suggested that SSTR2 expression induces upregulation of TRAILR1, thereby enabling TRAIL-mediated apoptosis (Ref. 60).
Suicide gene therapy has also been combined with oncolytic adenoviruses for the development of pancreatic cancer treatments. Freytag and colleagues constructed a suicide adenovirus by replacing the E1B (19 kDa) gene with a fusion gene of yeast cytosine deaminase (yCD) and a mutant HSV thymidine kinase (TK) with enhanced enzyme activity (mutTKsr39) (Ref. 61). In addition, the 11.6 kDa adenovirus death protein (ADP) gene, which has been shown to enhance adenovirus oncolysis, was inserted within the E3 region of the same virus. The resulting virus, Ad5-yCD/mutTKSR39rep-ADP, in combination with the prodrugs 5-fluorocytosine and ganciclovir (GCV) showed minor antitumour effects in a pancreatic cancer animal model. However, when this virus was combined with prodrug treatment and radiation therapy, animal survival rate was greatly increased compared with virus plus prodrug treatment alone, or with radiation treatment alone (Ref. 61). These results suggested that combination of oncolytic adenoviruses and suicide gene prodrug therapy sensitises cancer cells to radiotherapy.
Enhanced tumour targeting of CRAds
In addition to arming the oncolytic adenoviruses with therapeutic genes, many approaches are also being developed to increase the tumour specificity of these viruses, including transductional retargeting and use of tumour-specific promoters (TSPs). Tumour tropism of adenoviruses can be increased by switching the fibre knob between serotypes that display differences in tissue tropism. Adenovirus serotype 5 (Ad5) uses its fibre protein to interact with the Coxsackie adenovirus receptor (CAR) for initial cell binding, followed by a second interaction between its RGD motif on the penton base protein and a cellular integrin, primarily αvβ3 and αvβ5 integrins, which leads to virus internalisation. However, many cancers, including pancreatic cancers, do not express CAR or express the receptor at low levels. Despite this, adenovirus infectivity for pancreatic cancer cells was enhanced both by the insertion of an RGD motif in the fibre knob and by substituting the Ad5 fibre knob with that of adenovirus serotype 3 (Ad3), which binds to a different receptor for cell attachment, allowing CAR-independent virus infection (Ref. 62). The attachment receptor for Ad3 and other adenovirus serotypes has recently been identified as the Desmoglein-2 protein, and the implications of this finding for adenovirus biology and cancer therapy are only starting to be understood (Ref. 63). Furthermore, modified adenoviruses that specifically recognise a panel of pancreatic cancer cells have been selected from an adenovirus library displaying random peptides on the fibre knob (Ref. 64).
At least four TSPs have been used to construct CRAds for pancreatic cancer therapy, including the cyclooxygenase 2 (COX2) promoter (Refs 62, 65), urokinase-type plasminogen activator receptor (UPAR) promoter (Ref. 66), telomerase reverse transcriptase (TERT) promoter (Ref. 67) and a hypoxia-responsive promoter (Ref. 68). Ramirez and colleagues studied the replication of a panel of CRAds that contained the E1A region of the genome under the control of different promoters. These were evaluated for both specificity and oncolytic potency in pancreatic cancer cells. Of these CRAds, CRAdCox2F, which uses the COX2 promoter, showed the best results (Ref. 62). COX2 catalyses the conversion of arachidonic acid to prostaglandins and is frequently overexpressed in pancreatic cancer, contributing to cancer progression (Ref. 69). Subsequently, 5/3Cox2CRAdF, a CRAdCox2F virus containing a chimeric Ad5/Ad3 fibre knob capsid and an RGD motif in the fibre region, was analysed for its potential in combination with chemotherapy agents. Treatment of pancreatic cancer in an immunocompetent Syrian hamster model with 5/3Cox2CRAdF and subsequent gemcitabine treatment induced a much greater antitumour response, compared with single virus or gemcitabine treatment (Ref. 65). UPAR is a glycosylphosphatidylinositol-anchored protein that has a role in cell migration, adhesion, metastasis and tumour growth, and is highly active in pancreatic tumours when compared with normal tissues. AduPARE1A, a CRAd containing the E1A region of the viral genome under the control of the UPAR promoter, showed remarkable ability to eradicate tumours in a pancreatic xenograft model (25%), and in a model of pancreatic liver metastasis when delivered intravascularly (33%) (Ref. 66). TERT is a catalytic subunit of the telomerase enzyme that can immortalise cancer cells. Pancreatic cancer lesions are known to have increased telomerase activity (Ref. 70). Ad5/3hTERTE1, a CRAd containing the E1 region under the control of the human TERT promoter and a chimeric Ad5/3 fibre knob, in combination with gemcitabine, has shown enhanced oncolytic effects for pancreatic cancer as a result of the chemosensitising effects of E1 expression and the gemcitabine-driven enhancement of TERT promoter activity (Ref. 71). In addition, Ad5/3hTERTE1 has been shown to synergistically improve the oncolytic activity of a replication-defective adenovirus expressing a therapeutic transgene in pancreatic cancer models (Ref. 67). Pancreatic tumours are typically hypoxic with the concomitant upregulation of hypoxia-inducible transcription factors (HIFs). The CRAd Ad-DHscIL12, which contains the E1A region under the control of a hypoxia-responsive promoter and expresses interleukin-12 (IL12) in place of the E1B (19 kDa) gene, showed robust control of viral replication and oncolysis in response to HIF-1α preferentially in cancer cells. This armed CRAd, expressing IL12, a cytokine that can stimulate innate and adaptive immunity against tumours, was significantly more effective than a control virus expressing luciferase in a Syrian hamster model of pancreatic cancer (Ref. 68).
In summary, many approaches have been exploited to improve the oncolytic effects and cancer specificity of adenoviruses since the original development of first-generation oncolytic adenoviruses such as ONYX-015. However, the specificity and efficacy of these new oncolytic adenoviruses in clinical applications remain to be determined.
Oncolytic herpesviruses
Herpes simplex viruses HSV-1 and HSV-2 are large enveloped viruses with a linear double-stranded DNA. HSV infection often causes sores in the mouth, lips or genitals and can establish latent infections in neural ganglia. HSV has been genetically engineered to gain selective lytic replication in tumour cells, but not in normal cells, and has shown promise as an oncolytic agent for cancer therapy in preclinical and clinical trials.
Oncolytic HSVs
Several HSV mutants have been generated to preferentially restrict HSV replication to tumour cells, including first-generation HSV-1 vectors hrR3, R3616 and 1716, as well as later HSV-1 vectors G207, NV1020, OncoVex, L1BR1, HF10 and HSV-2 vector FusOn-H2.
hrR3 lacks the UL39 gene, which encodes the ICP6 protein, a viral homologue of the cellular ribonucleotide reductase that is commonly upregulated in tumour cells and is involved in the biosynthesis of DNA. Therefore, lack of this protein causes the virus to preferentially infect cancer cells and be defective in normal cells (Ref. 38).
R3616, derived from temperature-sensitive HSV-1 F strain, has a deletion in the γ34.5 gene encoding the ICP34.5 virulence factor. ICP34.5 recruits the host cellular protein phosphatase-1α to dephosphorylate the eukaryotic translation initiation factor eIF2α, thus counteracting the activity of the double-stranded RNA-activated protein kinase R (PKR) and the subsequent inhibition of protein synthesis (Ref. 72). Cancer cells with higher MAPK kinase (MAP2K) activity are highly susceptible to γ34.5-deleted HSVs because of the inhibitory effects of this signalling pathway on the PKR–eIF2α pathway (Ref. 39). It has been shown that mutation and activation of the K-Ras pathway can lead to inhibition of PKR activity (Ref. 73). Interestingly, in pancreatic cancer cells, oncolysis by γ34.5 deletion mutants appears to be independent of K-Ras activation and PKR activity, but is dependent on dysregulation of the PI3K pathway (Ref. 40).
G207 is derived from R3616 and contains deletions of γ34.5 and the UL39 genes and shows more tumour specificity than the single-deletion R3616 virus (Ref. 74).
NV1020 is a clone derived from R7020, an attenuated, replication-competent virus based on the HSV-1 F strain (Ref. 75). It has a 15 kb deletion between the unique short and unique long regions and thus lacks the UL56 gene, a copy of the γ34.5, ICP0 and ICP4 genes, as well as the latency-associated transcripts. In addition, an insertion is found in place of this deleted region containing several HSV-2 genes. NV1020 also contains a 700 bp deletion in the endogenous TK locus, which blocks the overlapping transcription of the UL24 gene. A copy of the TK gene was inserted back under the control of α4 promoter to make the virus TK positive (Ref. 76).
OncoVex, generated by BioVex Inc., contains a complete deletion of the γ34.5 and tegument ICP47 genes. The deletion of the ICP47 gene places the US11 gene under the control of an immediate-early promoter, leading to the upregulation of the RNA-binding protein US11, which inhibits PKR and thus enhances viral replication (Ref. 77).
HF10 is a naturally mutated virus derived from an HF strain of HSV-1 (Ref. 78). HF10 is highly attenuated and less toxic than the parental HSV-1, but still retains high oncolytic activity.
L1BR1 is a US3-locus-deleted HSV-2 mutant (Ref. 79). The US3 locus encodes a multifunctional protein kinase that inhibits HSV-induced cell apoptosis. L1BR1 is 10 000 times less virulent than the parental virus in mice.
FusOn-H2, which is derived from HSV-2, has recently been examined for its oncolytic activity in pancreatic cancers (Ref. 80). FusOn-H2 contains a deletion of the ICP10 gene expressing a serine/threonine protein kinase, which activates the Ras/MAPK mitogenic pathway through the phosphorylation of the Ras GTPase-activating protein, thus making its oncolytic activity dependent on an activated Ras signalling pathway (Ref. 41).
Oncolytic HSV for pancreatic cancer
In an IP dissemination model of pancreatic cancer, IP injection of hrR3 followed by GCV treatment led to long-term survival (LTS) in 70% of treated mice, whereas LTS was seen in 40% of mice treated with hrR3 alone, and in 0% of untreated mice (Ref. 81). In a similar animal model, R3616 plus gemcitabine double treatment showed a higher percentage of LTS (60%) when compared with double treatment with hrR3 and gemcitabine (20%). Combination treatment with gemcitabine was advantageous only for the R3616 virus, not for hrR3 (Ref. 82), suggesting that oncolytic HSVs with γ34.5 deletions would be more advantageous over oncolytic HSVs with UL39 deletions in combination with gemcitabine, and that drug combinations should be tested individually for each OV strain. Second-generation G207 and NV1020 viruses have also been studied for their effects on pancreatic cancer. Compared with G207, NV1020 produced higher viral titres of viral progeny in tumour tissues in mice, and a greater tumour suppressive effect (Ref. 83). Considering the neurotropism of HSV, NV1023 (derived from NV1020 and expressing a lacZ reporter gene) was investigated for its ability to treat pancreatic cancer nerve invasion (Ref. 84). In this mouse model of neural invasion, mice treated with NV1023 maintained normal nerve function and showed significant tumour suppression.
Similarly to approaches used for the development of oncolytic adenoviruses, oncolytic HSV could also be armed with therapeutic genes to further increase tumour control. In one study, the yCD/uracil phospho-ribosyltransferase fusion gene (Fcy::Fur) and the fusogenic glycoprotein from gibbon ape leukaemia virus (GALV) were placed into OncoVex, generating the armed vector OncoVexGALV/CD. This virus showed increased killing of pancreatic cancer cells (Ref. 85).
Two HSV-2 mutants, L1BR1 and FusOn-H2, have been studied for pancreatic cancer treatment. L1BR1 was able to replicate in cultured pancreatic cancer cells, but not in normal human hepatocytes, and had the highest tumour-reducing effect in mouse xenograft models when compared with oncolytic HSV-1 R3616 and hrR3. In addition, the virus was reported to synergise with 5-FU and cisplatin (Ref. 79). The FusOn-H2 virus, which targets the Ras pathway, also showed striking oncolytic activity against pancreatic cancer in animal models (Ref. 80). These results suggest that oncolytic HSV-2 viruses are promising new agents against pancreatic cancer.
Oncolytic PVs
PVs are small, nonenveloped icosahedral viruses with a single-stranded DNA genome of about 5 kb. The most notable PVs that have been developed as OVs are rat H-1 PV and its close relative, minute virus of mice (MVM). H-1 PV and MVM cause no pathogenicity in animals and humans (Ref. 86), but can productively replicate in cancer cells and cause strong tumour suppressive effects in many animal models (Ref. 42). The cancer selectivity of PVs is not fully understood, but it has been suggested that their selectivity is dependent on, among other factors, the deregulation of Ras–Raf and SMAD4 signalling, as well as defective type-I interferon (IFN) pathways (Refs 42, 43, 44).
Oncolytic PVs could be suitable oncolytic agents for pancreatic malignancies because the majority of pancreatic cancers have mutations in the Ras–Raf signalling pathways. Preclinical studies have shown promising results. H-1 PV treatment suppressed tumour growth and increased animal survival in both immunodeficient and immunocompetent animal models (Ref. 87). Interestingly, H-1 PV treatment following gemcitabine administration significantly enhanced the antitumour effects in an immunocompetent model, but not in an immunodeficient model (Ref. 87), suggesting that the immune response of the host has a role in the outcome of virus and drug combination therapy. Consistently, H-1 PV infection of pancreatic cancer cells enhanced natural killer-cell-mediated tumour killing of the infected cells (Ref. 88). It has also been demonstrated that transfer of splenocytes of donor rats with H-1-PV-treated PDACs could significantly improve the survival of naive tumour-bearing recipients, suggesting that immune cells contribute to the oncolytic effects of H-1 PV (Ref. 89). Although H-1 PV has been shown to be effective for pancreatic cancer in animal models, not all pancreatic cancer cells are permissive to H-1 PV infection and resistance has been correlated with SMAD4 mutations (Ref. 43). Therefore, for potential clinical application of H-1 PV for pancreatic cancer, patients should be screened to determine whether they are suitable for H-1 PV therapy, possibly through the identification of SMAD4 mutations.
Reoviruses
Reoviruses are nonenveloped viruses with a segmented dsRNA genome commonly found in asymptomatic infections of the respiratory and gastrointestinal tract in humans. The ability of reoviruses to infect cancer cells was originally reported to depend on an activated Ras signalling pathway (Ref. 45); however, the link between permissive viral replication and the cellular signalling circuitry is likely to be more complex. Nevertheless, because pancreatic cancers are notorious for having activated Ras signalling pathways, reoviruses are suitable OVs for the treatment of this particular cancer.
In 2003, Etoh and co-workers proposed the use of reoviruses as a candidate OV for pancreatic cancer. In this study, reovirus serotype 3 was able to infect human pancreatic cancer cell lines, and their susceptibility to reovirus was dependent on the Ras signalling pathway. In subcutaneous tumour models, reovirus injected intratumourally was able to suppress tumour growth and was also able to produce a systemic antitumour effect in which virus was detected in uninjected contralateral tumours (Ref. 90). In a second study, reovirus serotype 3 was evaluated in a syngeneic hamster model of liver metastasis (Ref. 91). Here, reovirus was again able to infect the hamster cell lines tested in a Ras-dependent manner. Additionally, intraportal administration of reovirus in this model reduced the number and size of the liver metastasis. Importantly, this study showed that reovirus was effective in immunocompetent animal models (Ref. 91). IP administration of reovirus has also been shown to be effective in IP dissemination models using immunocompetent animal models (Ref. 92). All these studies also reported that reovirus was specifically detected only in tumours and not in normal tissues, and no adverse effects were detected, suggesting that reovirus is a safe candidate OV that selectively replicates in cancer cells (Refs 90, 91, 92). These reports also suggest that reovirus as a single-agent monotherapy has promise for pancreatic cancer. These positive results in animal models have recently led to clinical trials to test Reolysin, a formulation of WT reovirus produced by Oncolytics Biotech Inc., in patients with advanced malignancies, including pancreatic cancer.
Poxviruses
Poxviruses are enveloped viruses with a large dsDNA genome. Orthopoxviruses, which include several vaccinia virus (VACV) strains and raccoonpox virus (RCNV) (Ref. 93), as well as the Leporipoxvirus myxoma virus (MYXV), are the only replication-competent oncolytic poxviruses reported to date. Currently, VACV is by far the most extensively studied of the three. For pancreatic cancer in particular, several attenuated VACV strains, including common smallpox vaccine strains, have been used as replicating OV agents and some have been exploited as poxvirus-based vaccines. In addition, other strains have been engineered for cancer selectivity, by the deletion of genes that attenuate the virus and make it depend on aberrant signalling pathways in cancer cells for replication. Some of these genes include the TK gene and VACV growth factor genes (Refs 46, 47).
VACV has been tested for use in anticancer immunotherapies focusing on the delivery and expression of tumour-associated antigens commonly overexpressed in pancreatic cancer and other gastrointestinal cancer, such as mucin 1 (MUC-1) and carcinoembryonic antigen (CEA). To further enhance the immune response towards these antigens, VACV vectors expressing costimulatory genes in addition to cancer antigens have been developed. These VACV constructs expressing both cancer antigens and coimmunostimulatory genes have also been combined in regimens involving replication-incompetent poxvirus vectors such as fowlpox and canarypox, which express these transgenes, and the use of granulocyte macrophage colony-stimulating factor GM-CSF (Refs 94, 95, 96). Thus, these regimens use up to four different strategies to induce antitumour effects: (1) vaccination with several tumour antigens; (2) enhancement of antitumour immune responses with T-cell costimulatory molecules; (3) use of different vaccine vectors in prime–boost regimens in order to enhance the effects of booster vaccinations; and (4) use of a local vaccine adjuvant. In these studies, 60% of the patients developed CEA antibodies that correlated with longer survival rates (Ref. 94).
In addition to these genetically modified VACV strains for vaccination regimens, replication-competent VACV strains as OVs for pancreatic cancer have also been reported in recent years. With the attenuated VACV strain GLV-1h68, a single intravenous dose of the virus caused tumour regression with minimal toxicities and preferential replication in tumour tissues. In addition, combination treatment using GLV-1h68 with either gemcitabine or cisplatin led to enhanced therapeutic effects when compared with virus therapy alone (Ref. 97). Other studies have used the Lister strain of VACV, a common smallpox vaccination strain. In a recent study, the ability of the Lister strain of VACV to replicate in pancreatic cancer cells under hypoxic conditions showed that, under these conditions, viral protein production was unaltered and viral cytotoxicity was increased in some cell lines. Thus, this study proposed the use of Lister VACV as a potential new OV for the treatment of cancer with hypoxic phenotypes, such as pancreatic cancer (Ref. 98). The Lister strain of VACV armed with an endostatin–angiostatin fusion gene was also reported to have therapeutic effects for pancreatic cancer. In this report, the Lister strain was reported to be more potent than adenovirus in promoting cytotoxicity and was able to infect Ad5-insensitive cells. In vivo, the Lister VACV showed selectivity for tumour tissues and prolonged the survival of mice with subcutaneous human pancreatic xenografts. Expression of the transgene and therapeutic effects of the transgene were also reported. However, at high viral doses, toxicities were observed (Ref. 99).
MYXV is a rabbit-specific poxvirus that has been reported to replicate in a wide range of human cancer cells (Refs 48, 100). The tropism of MYXV was reported to depend in part on dysregulated signalling pathways in cancer cells, particularly the hyperactivation of Akt signalling (Ref. 48). Furthermore, MYXV, along with reovirus, has been reported to preferentially infect cells with dysfunctional or deleted TP53, ataxia telangiectasia mutated (ATM) and RB1 tumour suppressor genes (Ref. 49). In addition, MYXV has been shown to have oncolytic activity in animal models of human cancer, including gliomas, medulloblastomas, melanomas and rhabdoid tumours (Refs 101, 102, 103, 104). Recently, MYXV was also reported to infect human pancreatic cancer cells in culture, resulting in a reduction in cell viability (Ref. 105). Given these reports, MYXV is a promising OV that might be applicable to pancreatic cancers containing dysregulated Akt signalling or tumour suppressor functions.
In summary, poxviruses have potential as vaccine-based therapies and as replication-competent OVs for pancreatic cancer. For oncolytic poxviruses, their applicability remains to be determined in clinically relevant models involving immunocompetent hosts, as well as in models of late-stage metastatic disease.
Paramyxoviruses
Paramyxoviruses with reported oncolytic activity for pancreatic cancer include measles virus (MV) and Sendai virus (SV). MV strains commonly used for measles vaccination have been shown to have oncolytic activity, and their cancer selectivity depends on the overexpression of the viral entry receptor CD46 in many cancer types (Ref. 50). The applicability of MV as an OV is greatly enhanced by the ability to engineer recombinant viruses by reverse genetic manipulation (Ref. 106). These recombinant viruses are usually made to express therapeutic transgenes or to retarget the virus to a particular receptor that is overexpressed on a specific cancer type (Ref. 51). Recently, MV-NIS, an MV expressing the sodium iodide symporter (NIS) gene, was able to infect pancreatic cancer cells, as shown by syncytia formation and increased iodide uptake, resulting in cell death. Intratumoural injection of the virus also resulted in reduced tumour volume and prolonged survival of mice with human pancreatic xenografts. The expression of the NIS transgene proved useful as a means of monitoring and quantifying virus delivery, spread and viral gene expression (Ref. 107). Contrary to MV, SV is not a human pathogen. A genetically engineered oncolytic SV targeted by the presence of the cleavage site for urokinase-type plasminogen activator was effective in reducing the tumour growth of pancreatic cancer cells that express urokinase (Ref. 52).
Clinical applications
Even though there is a growing array of publications reporting promising results for many OVs in pancreatic cancer animal models, relatively few of these viruses have reached clinical trials. ONXY-015, HF10, OncoVexGM–CSF and Reolysin are examples of OVs that are currently in or that have undergone clinical trials. Trials involving these viruses are Phase I and Phase II clinical trials, which focus mainly on determining safety and maximum tolerated levels.
Even though ONYX-015 has proven to be successful in clinical trials for other cancers (Ref. 108), early clinical trials in pancreatic cancer patients have been disappointing and have shown limited efficacy, even in combination with gemcitabine. In a Phase I clinical trial, ONYX-015 was injected directly into pancreatic primary tumours. Viral doses were administered every 4 weeks until tumour regression was observed. Injection of up to 1 × 1011 p.f.u. of virus was well tolerated. After treatment, an increase in neutralising antibodies was observed in all patients. However, no objective responses were observed, with six out of 22 patients showing regression, 11 presenting stable disease and five showing disease progression. In addition, no viral replication was detected in tumour biopsies of treated patients (Ref. 109). In another Phase I/II clinical trial, ONYX-015 was evaluated again by intratumoural injection of the virus in combination with systemic gemcitabine treatment. The patients received eight doses of 1010–1011 p.f.u. of virus, with the last four doses given in combination with gemcitabine. In this trial, only two patients showed tumour regression out of 21, whereas approximately 50% of the patients showed tumour progression (Ref. 110).
Currently, only two oncolytic HSV-1 mutants are reported in clinical trials for pancreatic cancer: HF10 and OncoVexGM–CSF (OncoVex expressing GM-CSF, from BioVex). OncoVexGM-CSF is an OV currently in two Phase III trials for patients with metastatic melanoma or squamous cell carcinoma. A Phase I clinical trial of this virus for patients with unresectable pancreatic cancer is currently underway. According to the National Cancer Institute (NCI), this trial will administer the virus directly to the pancreas by endoscopic ultrasound-guided fine-needle injection, and its primary goal is to assess the safety and tolerability of the treatment. The results of a Phase I clinical trial involving HF10 in patients with advanced pancreatic cancer have recently been published. This trial recruited six cancer patients that were treated with three doses of HF10 and monitored for 30 days for adverse effects. No adverse side effects were observed. Three patients were classified with stable disease, one with partial response and two with disease progression (Ref. 111). However, more clinical data are needed to determine the efficacy of the HF10 treatment.
Phase I clinical trials for patients with advanced cancers or recurrent gliomas in which Reolysin was given both intratumourally and intravenously showed that reovirus is safe and well tolerated (Refs 112, 113). These clinical trials also demonstrated that virus is able to reach the tumour sites after systemic administration and gave insights into the nature and magnitude of the antiviral immune responses in patients receiving treatment with this OV (Refs 113, 114). Phase I and Phase II clinical trials are now underway to study the efficacy of reovirus in combination with chemotherapy agents (Ref. 115). Currently, the NCI lists several clinical trials studying the use of Reolysin as an oncolytic agent, and of these, one is a Phase II clinical trial to investigate the efficacy and safety of intravenously administered Reolysin in combination with gemcitabine in patients with advanced pancreatic cancer.
The efficacy of OVs for the treatment of advanced pancreatic cancer in clinical trials has only just started to be investigated. Thus far, all the OVs mentioned above have been reported to have minimal, if any, adverse effects and appear to be safe for use in a clinical setting with some encouraging results in terms of efficacy. Clearly, the next step towards the identification of effective oncolytic virotherapies for pancreatic cancer involves larger clinical trials to focus on efficacy and response for the most promising and potent OV strains determined from preclinical models of this disease.
Outstanding research questions
The use of replication-competent OVs for pancreatic cancer is an area that is beginning to be explored for true clinical applicability. It is already known that several OVs have oncolytic potential as agents against pancreatic cancer in animal models of the disease. However, to improve on the oncolytic efficacy of these OVs, a thorough understanding of the biology of these viruses and their behaviour within pancreatic cancer cells remains essential. For several of these OVs, it is still not clear what determines their ability to replicate within the specific intracellular environment of pancreatic cancer cells and what inhibits or enhances their oncolysis in vivo. It is also not known what local factors influence OV spread within the tumour microenvironment typical of pancreatic cancers and how these viruses interact with the noncancerous stroma that support pancreatic tumours. In addition, the effects that OVs have on particular transformation processes such as EMT have not been explored. It is still not known how OVs are affected by the EMT status of pancreatic cancer cells and, more importantly, whether infection of these cells with OVs alters their phenotype or sensitivity to chemotherapy agents.
Finally, the mechanisms by which candidate OVs exert their oncolytic effects on pancreatic cancer cells are not completely understood in vivo. OVs might cause oncolysis in several ways: (1) lysis of the OV-infected cells as a direct consequence of viral replication; (2) cell death due to the expression of viral or exogenous cytotoxic genes; and (3) indirect cell death through the induction of immune responses triggered as a consequence of viral infection (Refs 31, 32). In fact, the induction of beneficial antitumour immune responses has been reported as an important factor influencing the effectiveness of OVs (reviewed in Refs 116, 117). It is clear that the oncolytic effects of OVs are not only due to the direct lysis of cancer cells as a result of infection, but are also due to other indirect mechanisms of tumour destruction that might be therapeutically engaged during OV therapies.
The interactions of many candidate OVs with common chemotherapy agents have been studied. Efforts have mainly focused on the combination of these OVs with gemcitabine-based chemotherapy. This approach could be considered the most clinically relevant, because gemcitabine is the standard of care for pancreatic cancer patients; however, the potential of these OVs to synergise with other FDA-approved or experimental drugs and agents should not be overlooked. Pancreatic cancer patients are in dire need of more effective treatments than those currently available. Thus, the focus should be on the development of novel therapies regardless of whether gemcitabine is involved in these regimens. Once synergistic interactions are observed between an OV and a drug, the mechanisms behind this synergism must be explored in detail. Results from these studies may help identify ways in which the OVs might be manipulated to sensitise the cells to a second therapy, or vice versa.
Consideration must also be given to the limitations of OV therapy. The major limitations involve acquired antiviral immune responses against OVs, inefficient delivery of the virus to all the cells within tumour beds, and poor virus spread within tumour tissues or to metastatic sites. Strategies or methods to overcome these limitations must be designed to enhance the applicability of OVs, in particular for disseminated late-stage cancer, but it seems reasonable to predict that many, if not all, of these limitations could be overcome with further research and development.
Acknowledgments
Acknowledgements and funding
The authors thank the peer reviewers for their helpful comments. G.M.’s lab is supported by start-up funding from the University of Florida College of Medicine, NIH R01 CA138541 and R21 CA149869 from NCI. S.T.W. is supported by a postdoctoral fellowship from the American Cancer Society.
References
- 1.Jemal A, et al. Cancer statistics, 2010. CA: A Cancer Journal for Clinicians. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Basturk O, Coban I, Adsay NV. Biological classification and biological behavior of pancreatic neoplasia. In: Neoptolemos JP, Urrutia R, Abbruzzese J, Buchler M, editors. Pancreatic Cancer. New York: Springer Science and Business Media; 2010. pp. 40–70. [Google Scholar]
- 3.Rozenblum E, et al. Tumor-suppressive pathways in pancreatic carcinoma. Cancer Research. 1997;57:1731–1734. [PubMed] [Google Scholar]
- 4.Almoguera Cetal. Mosthuman carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- 5.Adjei AA. Blocking oncogenic Ras signaling for cancer therapy. Journal of the National Cancer Institute. 2001;93:1062–1074. doi: 10.1093/jnci/93.14.1062. [DOI] [PubMed] [Google Scholar]
- 6.Grippo PJ, et al. Preinvasive pancreatic neoplasia of ductal phenotype induced by acinar cell targeting of mutant Kras in transgenic mice. Cancer Research. 2003;63:2016–2019. [PubMed] [Google Scholar]
- 7.Aguirre AJ, et al. Activated Kras and Ink4a/ Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes and Development. 2003;17:3112–3126. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bardeesy N, et al. Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5947–5952. doi: 10.1073/pnas.0601273103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones S, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu GC, et al. Stromal biology of pancreatic cancer. Journal of Cellular Biochemistry. 2007;101:887–907. doi: 10.1002/jcb.21209. [DOI] [PubMed] [Google Scholar]
- 11.Olive KP, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah AN, et al. Development and characterization of gemcitabine-resistant pancreatic tumor cells. Annals of the Surgical Oncology. 2007;14:3629–3637. doi: 10.1245/s10434-007-9583-5. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, et al. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Research. 2009;69:2400–2407. doi: 10.1158/0008-5472.CAN-08-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thiery JP, et al. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Joo YE, et al. Expression of E-cadherin, alpha- and beta-catenins inpatients with pancreatic adenocarcinoma. Pancreatology. 2002;2:129–137. doi: 10.1159/000055903. [DOI] [PubMed] [Google Scholar]
- 16.Nakajima S, et al. N-cadherin expression and epithelial-mesenchymal transition in pancreatic carcinoma. Clinical Cancer Research. 2004;10(12 Pt 1):4125–4133. doi: 10.1158/1078-0432.CCR-0578-03. [DOI] [PubMed] [Google Scholar]
- 17.Arumugam T, et al. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Research. 2009;69:5820–5828. doi: 10.1158/0008-5472.CAN-08-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burris HA, III, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. Journal of Clinical Oncologys. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 19.Hertel LW, et al. Evaluation of the antitumor activity of gemcitabine 2′,2′-difluoro-2′-deoxycytidine. Cancer Research. 1990;50:4417–4422. [PubMed] [Google Scholar]
- 20.Huang P, et al. Action of 2′,2′-difluorodeoxycytidine on DNA synthesis. Cancer Researchs. 1991;51:6110–6117. [PubMed] [Google Scholar]
- 21.Colucci G, et al. Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma: a prospective, randomized phase III study of the Gruppo Oncologia dell’Italia Meridionale. Cancer. 2002;94:902–910. [PubMed] [Google Scholar]
- 22.Louvet C, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. Journal of Clinical Oncology. 2005;23:3509–3516. doi: 10.1200/JCO.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 23.Rocha Lima CM, et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. Journal of Clinical Oncology. 2004;22:3776–3783. doi: 10.1200/JCO.2004.12.082. [DOI] [PubMed] [Google Scholar]
- 24.Kulke MH, et al. Randomized phase II study of gemcitabine administered at a fixed dose rate or in combination with cisplatin, docetaxel, or irinotecan in patients with metastatic pancreatic cancer: CALGB 89904. Journal of Clinical Oncology. 2009;27:5506–5512. doi: 10.1200/JCO.2009.22.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rocha-Lima CM, Raez LE. Erlotinib (tarceva) for the treatment of non-small-cell lung cancer and pancreatic cancer. Pharmacy and Therapeutics. 2009;34:554–564. [PMC free article] [PubMed] [Google Scholar]
- 26.Moore MJ, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. Journal of Clinical Oncology. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 27.Conroy T, et al. Irinotecan plus oxaliplatin and leucovorin-modulated fluorouracil in advanced pancreatic cancer - a Groupe Tumeurs Digestives of the Federation Nationale des Centres de Lutte Contre le Cancer study. Journal of Clinical Oncology. 2005;23:1228–1236. doi: 10.1200/JCO.2005.06.050. [DOI] [PubMed] [Google Scholar]
- 28.Kim R. FOLFIRINOX: a new standard treatment for advanced pancreatic cancer? Lancet Oncology. 2010;12:8–9. doi: 10.1016/S1470-2045(10)70237-0. [DOI] [PubMed] [Google Scholar]
- 29.Plentz RR, Manns MP, Greten TF. Molecular therapy of pancreatic cancer. Minerva Endocrinologica. 2010;35:27–33. [PubMed] [Google Scholar]
- 30.Mackenzie RP, McCollum AD. Novel agents for the treatment of adenocarcinoma of the pancreas. Expert Review of Anticancer Therapy. 2009;9:1473–1485. doi: 10.1586/era.09.109. [DOI] [PubMed] [Google Scholar]
- 31.Davis JJ, Fang B. Oncolytic virotherapy for cancer treatment: challenges and solutions. Journal of Gene Medicine. 2005;7:1380–1389. doi: 10.1002/jgm.800. [DOI] [PubMed] [Google Scholar]
- 32.Vaha-Koskela MJ, Heikkila JE, Hinkkanen AE. Oncolytic viruses in cancer therapy. Cancer Letters. 2007;254:178–216. doi: 10.1016/j.canlet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bischoff JR, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 34.O’Shea CC, et al. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell. 2004;6:611–623. doi: 10.1016/j.ccr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 35.Heise C, et al. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nature Medicine6. 2000:1134–1139. doi: 10.1038/80474. [DOI] [PubMed] [Google Scholar]
- 36.Fueyo Jetal. Amutantoncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 37.Leitner S, et al. Oncolytic adenoviral mutants with E1B19K gene deletions enhance gemcitabine-induced apoptosis in pancreatic carcinoma cells and anti-tumor efficacy in vivo. Clinical Cancer Research. 2009;15:1730–1740. doi: 10.1158/1078-0432.CCR-08-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoon SS, et al. Cancer gene therapy using a replication-competent herpes simplex virus type 1 vector. Annals of Surgery. 1998;228:366–374. doi: 10.1097/00000658-199809000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith KD, et al. Activated MEK suppresses activation of PKR and enables efficient replication and in vivo oncolysis by Deltagamma(1)34.5 mutants of herpes simplex virus 1. Journal of Virology. 2006;80:1110–1120. doi: 10.1128/JVI.80.3.1110-1120.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarinella F, et al. Oncolysis of pancreatic tumour cells by a gamma34.5-deleted HSV-1 does not rely upon Ras-activation, but on the PI 3-kinase pathway. Gene Therapy. 2006;13:1080–1087. doi: 10.1038/sj.gt.3302770. [DOI] [PubMed] [Google Scholar]
- 41.Smith CC, et al. Ras-GAP binding and phosphorylation by herpes simplex virus type 2 RR1 PK (ICP10) and activation of the Ras/MEK/ MAPK mitogenic pathway are required for timely onset of virus growth. Journal of Virology. 2000;74:10417–10429. doi: 10.1128/jvi.74.22.10417-10429.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rommelaere J, et al. Oncolytic parvoviruses as cancer therapeutics. Cytokine and Growth Factor Reviews. 2010;21:185–195. doi: 10.1016/j.cytogfr.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 43.Dempe S, et al. SMAD4: a predictive marker of PDAC cell permissiveness for oncolytic infection with parvovirus H-1PV. International Journal of Cancer. 2010;126:2914–2927. doi: 10.1002/ijc.24992. [DOI] [PubMed] [Google Scholar]
- 44.Riolobos L, et al. Viral oncolysis that targets Raf-1 signaling control of nuclear transport. Journal of Virology. 2010;84:2090–2099. doi: 10.1128/JVI.01550-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strong JE, et al. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO Journal. 1998;17:3351–3362. doi: 10.1093/emboj/17.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thorne SH, Hwang TH, Kirn DH. Vaccinia virus and oncolytic virotherapy of cancer. Current Opinion in Molecular Therapeutics. 2005;7:359–365. [PubMed] [Google Scholar]
- 47.Kirn DH, Thorne SH. Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nature Reviews. Cancer. 2009;9:64–71. doi: 10.1038/nrc2545. [DOI] [PubMed] [Google Scholar]
- 48.Wang G, et al. Infection of human cancer cells with myxoma virus requires Akt activation via interaction with a viral ankyrin-repeat host range factor. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:4640–4645. doi: 10.1073/pnas.0509341103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim M, et al. The viral tropism of two distinct oncolytic viruses, reovirus and myxoma virus, is modulated by cellular tumor suppressor gene status. Oncogene. 2010;29:3990–3996. doi: 10.1038/onc.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galanis E. Therapeutic potential of oncolytic measles virus: promises and challenges. Clinical Pharmacology and Therapeutics. 2010;88:620–625. doi: 10.1038/clpt.2010.211. [DOI] [PubMed] [Google Scholar]
- 51.Paraskevakou G, et al. Epidermal growth factor receptor (EGFR)-retargeted measles virus strains effectively target EGFR- or EGFRvIII expressing gliomas. Molecular Therapy. 2007;15:677–686. doi: 10.1038/sj.mt.6300105. [DOI] [PubMed] [Google Scholar]
- 52.Kinoh H, et al. Generation of optimized and urokinase-targeted oncolytic Sendai virus vectors applicable for various human malignancies. Gene Therapy. 2009;16:392–403. doi: 10.1038/gt.2008.167. [DOI] [PubMed] [Google Scholar]
- 53.Spencer JF, et al. New pancreatic carcinoma model for studying oncolytic adenoviruses in the permissive Syrian hamster. Cancer Gene Therapy. 2009;16:912–922. doi: 10.1038/cgt.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Shea CC, et al. Heat shock phenocopies E1B-55K late functions and selectively sensitizes refractory tumor cells to ONYX-015 oncolytic viral therapy. Cancer Cell. 2005;8:61–74. doi: 10.1016/j.ccr.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 55.Heise C, et al. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nature Medicine. 1997;3:639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 56.Mymryk JS. Tumour suppressive properties of the adenovirus 5 E1A oncogene. Oncogene. 1996;13:1581–1589. [PubMed] [Google Scholar]
- 57.Oberg D, et al. Improved potency and selectivity of an oncolytic E1ACR2 and E1B19K deleted adenoviral mutant in prostate and pancreatic cancers. Clinical Cancer Research. 2010;16:541–553. doi: 10.1158/1078-0432.CCR-09-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kamphaus GD, et al. Canstatin, a novel matrix-derived inhibitor of angiogenesis and tumor growth. Journal of Biological Chemistry. 2000;275:1209–1215. doi: 10.1074/jbc.275.2.1209. [DOI] [PubMed] [Google Scholar]
- 59.Delesque N, et al. sst2 somatostatin receptor expression reverses tumorigenicity of human pancreatic cancer cells. Cancer Research. 1997;57:956–962. [PubMed] [Google Scholar]
- 60.Zhang Z, et al. Reexpression of human somatostatin receptor gene 2 gene mediated by oncolytic adenovirus increases antitumor activity of tumor necrosis factor-related apoptosis-inducing ligand against pancreatic cancer. Clinical Cancer Research. 2009;15:5154–5160. doi: 10.1158/1078-0432.CCR-09-0025. [DOI] [PubMed] [Google Scholar]
- 61.Freytag SO, et al. Replication-competent adenovirus-mediated suicide gene therapy with radiation in a preclinical model of pancreatic cancer. Molecular Therapy. 2007;15:1600–1606. doi: 10.1038/sj.mt.6300212. [DOI] [PubMed] [Google Scholar]
- 62.Ramirez PJ, et al. Optimization of conditionally replicative adenovirus for pancreatic cancer and its evaluation in an orthotopic murine xenograft model. American Journal of Surgery. 2008;195:481–490. doi: 10.1016/j.amjsurg.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 63.Wang H, et al. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nature Medicine. 2011;17:96–104. doi: 10.1038/nm.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nishimoto T, et al. Oncolytic virus therapy for pancreatic cancer using the adenovirus library displaying random peptides on the fiber knob. Gene Therapy. 2009;16:669–680. doi: 10.1038/gt.2009.1. [DOI] [PubMed] [Google Scholar]
- 65.Nelson AR, et al. Combination of conditionally replicative adenovirus and standard chemotherapies shows synergistic antitumor effect in pancreatic cancer. Cancer Science. 2009;100:2181–2187. doi: 10.1111/j.1349-7006.2009.01289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huch M, et al. Urokinase-type plasminogen activator receptor transcriptionally controlled adenoviruses eradicate pancreatic tumors and liver metastasis in mouse models. Neoplasia. 2009;11:518–528. doi: 10.1593/neo.81674. 4 p following 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Onimaru M, et al. hTERT-promoter-dependent oncolytic adenovirus enhances the transduction and therapeutic efficacy of replication-defective adenovirus vectors in pancreatic cancer cells. Cancer Science. 2010;101:735–742. doi: 10.1111/j.1349-7006.2009.01445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bortolanza S, et al. Treatment of pancreatic cancer with an oncolytic adenovirus expressing interleukin-12 in Syrian hamsters. Molecular Therapy. 2009;17:614–622. doi: 10.1038/mt.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Molina MA, et al. Increased cyclooxygenase-2 expression in human pancreatic carcinomas and cell lines: growth inhibition by nonsteroidal anti-inflammatory drugs. Cancer Research. 1999;59:4356–4362. [PubMed] [Google Scholar]
- 70.Mihaljevic AL, et al. Molecular mechanism of pancreatic cancer --understanding proliferation, invasion, and metastasis. Langenbecks Arch Surg. 2010;395:295–308. doi: 10.1007/s00423-010-0622-5. [DOI] [PubMed] [Google Scholar]
- 71.Onimaru M, et al. Combination with low-dose gemcitabine and hTERT-promoter-dependent conditionally replicative adenovirus enhances cytotoxicity through their crosstalk mechanisms in pancreatic cancer. Cancer Letters. 2010;294:178–186. doi: 10.1016/j.canlet.2010.01.034. [DOI] [PubMed] [Google Scholar]
- 72.He B, Gross M, Roizman B. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:843–848. doi: 10.1073/pnas.94.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mundschau LJ, Faller DV. Endogenous inhibitors of the dsRNA-dependent eIF-2 alpha protein kinase PKR in normal and ras-transformed cells. Biochimie. 1994;76:792–800. doi: 10.1016/0300-9084(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 74.Mineta T, et al. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nature Medicine. 1995;1:938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 75.Meignier B, Longnecker R, Rand Roizman B. In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020: construction and evaluation in rodents. Journal of Infectious Diseases. 1988;158:602–614. doi: 10.1093/infdis/158.3.602. [DOI] [PubMed] [Google Scholar]
- 76.Varghese S, Rabkin SD. Oncolytic herpes simplex virus vectors for cancer virotherapy. Cancer Gene Therapy. 2002;9:967–978. doi: 10.1038/sj.cgt.7700537. [DOI] [PubMed] [Google Scholar]
- 77.Liu BL, et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Therapy. 2003;10:292–303. doi: 10.1038/sj.gt.3301885. [DOI] [PubMed] [Google Scholar]
- 78.Kimata H, et al. Effective treatment of disseminated peritoneal colon cancer with new replication-competent herpes simplex viruses. Hepatogastroenterology. 2003;50:961–966. [PubMed] [Google Scholar]
- 79.Kasuya H, et al. Suitability of a US3-inactivated HSV mutant (L1BR1) as an oncolytic virus for pancreatic cancer therapy. Cancer Gene Therapy. 2007;14:533–542. doi: 10.1038/sj.cgt.7701049. [DOI] [PubMed] [Google Scholar]
- 80.Fu X, et al. Effective treatment of pancreatic cancer xenografts with a conditionally replicating virus derived from type 2 herpes simplex virus. Clinical Cancer Research. 2006;12:3152–3157. doi: 10.1158/1078-0432.CCR-06-0045. [DOI] [PubMed] [Google Scholar]
- 81.Kasuya H, et al. Intraperitoneal delivery of hrR3 and ganciclovir prolongs survival in mice with disseminated pancreatic cancer. Journal of Surgical Oncology. 1999;72:136–141. doi: 10.1002/(sici)1096-9098(199911)72:3<136::aid-jso5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 82.Watanabe I, et al. Effects of tumor selective replication-competent herpes viruses in combination with gemcitabine on pancreatic cancer. Cancer Chemotherapy and Pharmacology. 2008;61:875–882. doi: 10.1007/s00280-007-0567-8. [DOI] [PubMed] [Google Scholar]
- 83.McAuliffe PF, et al. Effective treatment of pancreatic tumors with two multimutated herpes simplex oncolytic viruses. Journal of Gastrointestinal Surgery. 2000;4:580–588. doi: 10.1016/s1091-255x(00)80106-7. [DOI] [PubMed] [Google Scholar]
- 84.Gil Z, et al. Nerve-sparing therapy with oncolytic herpes virus for cancers with neural invasion. Clinical Cancer Research. 2007;13:6479–6485. doi: 10.1158/1078-0432.CCR-07-1639. [DOI] [PubMed] [Google Scholar]
- 85.Simpson GR, et al. Combination of a fusogenic glycoprotein, prodrug activation, and oncolytic herpes simplex virus for enhanced local tumor control. Cancer Research. 2006;66:4835–4842. doi: 10.1158/0008-5472.CAN-05-4352. [DOI] [PubMed] [Google Scholar]
- 86.Shaughnessy E, et al. Parvoviral vectors for the gene therapy of cancer. Seminars in Oncology. 1996;23:159–171. [PubMed] [Google Scholar]
- 87.Angelova AL, et al. Improvement of gemcitabine-based therapy of pancreatic carcinoma by means of oncolytic parvovirus H-1PV. Clinical Cancer Research. 2009;15:511–519. doi: 10.1158/1078-0432.CCR-08-1088. [DOI] [PubMed] [Google Scholar]
- 88.Bhat R, et al. Enhancement of NK cell anti-tumour responses using an oncolytic parvovirus. International Journal of Cancer. 2011;128:908–919. doi: 10.1002/ijc.25415. [DOI] [PubMed] [Google Scholar]
- 89.Grekova S, et al. Immune cells participate in the oncosuppressive activity of parvovirus H-1PV and are activated as a result of their abortive infection with this agent. Cancer Biology and Therapy. 2010;10:52–61. doi: 10.4161/cbt.10.12.13455. [DOI] [PubMed] [Google Scholar]
- 90.Etoh T, et al. Oncolytic viral therapy for human pancreatic cancer cells by reovirus. Clinical Cancer Research. 2003;9:1218–1223. [PubMed] [Google Scholar]
- 91.Himeno Y, et al. Efficacy of oncolytic reovirus against liver metastasis from pancreatic cancer in immunocompetent models. International Journal of Oncology. 2005;27:901–906. [PubMed] [Google Scholar]
- 92.Hirano S, et al. Reovirus inhibits the peritoneal dissemination of pancreatic cancer cells in an immunocompetent animal model. Oncology Reportss. 2009;21:1381–1384. doi: 10.3892/or_00000364. [DOI] [PubMed] [Google Scholar]
- 93.Evgin L, et al. Potent oncolytic activity of raccoonpox virus in the absence of natural pathogenicity. Molecular Therapy. 2010;18:896–902. doi: 10.1038/mt.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kaufman HL, et al. Poxvirus-based vaccine therapy for patients with advanced pancreatic cancer. Journal of Translational Medicine. 2007;5:60. doi: 10.1186/1479-5876-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Petrulio CA, Kaufman HL. Development of the PANVAC-VF vaccine for pancreatic cancer. Expert Reviews of Vaccines. 2006;5:9–19. doi: 10.1586/14760584.5.1.9. [DOI] [PubMed] [Google Scholar]
- 96.Kantor J, et al. Antitumor activity and immune responses induced by a recombinant carcinoembryonic antigen-vaccinia virus vaccine. Journal of the National Cancer Institute. 1992;84:1084–1091. doi: 10.1093/jnci/84.14.1084. [DOI] [PubMed] [Google Scholar]
- 97.Yu YA, et al. Regression of human pancreatic tumor xenografts in mice after a single systemic injection of recombinant vaccinia virus GLV-1h68. Molecular Cancer Therapeutics. 2009;8:141–151. doi: 10.1158/1535-7163.MCT-08-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hiley CT, et al. Lister strain vaccinia virus, a potential therapeutic vector targeting hypoxic tumours. Gene Therapy. 2010;17:281–287. doi: 10.1038/gt.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tysome JR, et al. Lister strain of vaccinia virus armed with endostatin-angiostatin fusion gene as a novel therapeutic agent for human pancreatic cancer. Gene Therapy. 2009;16:1223–1233. doi: 10.1038/gt.2009.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sypula J, et al. Myxoma virus tropism in human tumor cells. Gene Therapy and Molecular Biology. 2004;8:103–114. [Google Scholar]
- 101.Lun XQ, et al. Targeting human medulloblastoma: oncolytic virotherapy with myxoma virus is enhanced by rapamycin. Cancer Research. 2007;67:8818–8827. doi: 10.1158/0008-5472.CAN-07-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stanford MM, et al. Myxoma virus oncolysis of primary and metastatic B16F10 mouse tumors in vivo. Molecular Therapy. 2008;16:52–59. doi: 10.1038/sj.mt.6300348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu Y, et al. Oncolytic efficacy of recombinant vesicular stomatitis virus and myxoma virus in experimental models of rhabdoid tumors. Clinical Cancer Research. 2008;14:1218–1227. doi: 10.1158/1078-0432.CCR-07-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lun X, et al. Myxoma virus is a novel oncolytic virus with significant antitumor activity against experimental human gliomas. Cancer Research. 2005;65:9982–9990. doi: 10.1158/0008-5472.CAN-05-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Woo Y, et al. Myxoma virus is oncolytic for human pancreatic adenocarcinoma cells. Annals of the Surgical Oncology. 2008;15:2329–2335. doi: 10.1245/s10434-008-9924-z. [DOI] [PubMed] [Google Scholar]
- 106.Radecke F, et al. Rescue of measles viruses from cloned DNA. EMBO Journal. 1995;14:5773–5784. doi: 10.1002/j.1460-2075.1995.tb00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carlson SK, et al. Quantitative molecular imaging of viral therapy for pancreatic cancer using an engineered measles virus expressing the sodium-iodide symporter reporter gene. AJR. American Journal of Roentgenology. 2009;192:279–287. doi: 10.2214/AJR.08.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yu W, Fang H. Clinical trials with oncolytic adenovirus in China. Current Cancer Drug Targets. 2007;7:141–148. doi: 10.2174/156800907780058817. [DOI] [PubMed] [Google Scholar]
- 109.Mulvihill S, et al. Safety and feasibility of injection with an E1B-55 kDa gene-deleted, replication-selective adenovirus (ONYX-015) into primary carcinomas of the pancreas: a phase I trial. Gene Therapy. 2001;8:308–315. doi: 10.1038/sj.gt.3301398. [DOI] [PubMed] [Google Scholar]
- 110.Hecht JR, et al. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clinical Cancer Researchs. 2003;9:555–561. [PubMed] [Google Scholar]
- 111.Nakao A, et al. A phase I dose-escalation clinical trial of intraoperative direct intratumoral injection of HF10 oncolytic virus in non-resectable patients with advanced pancreatic cancer. Cancer Gene Therapy. 2011;18:167–175. doi: 10.1038/cgt.2010.65. [DOI] [PubMed] [Google Scholar]
- 112.Forsyth P, et al. A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Molecular Therapy. 2008;16:627–632. doi: 10.1038/sj.mt.6300403. [DOI] [PubMed] [Google Scholar]
- 113.Vidal L, et al. A phase I study of intravenous oncolytic reovirus type 3 Dearing in patients with advanced cancer. Clinical Cancer Research. 2008;14:7127–7137. doi: 10.1158/1078-0432.CCR-08-0524. [DOI] [PubMed] [Google Scholar]
- 114.White CL, et al. Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing type 3) during a phase I clinical trial. Gene Therapy. 2008;15:911–920. doi: 10.1038/gt.2008.21. [DOI] [PubMed] [Google Scholar]
- 115.Harrington KJ, et al. Clinical trials with oncolytic reovirus: moving beyond phase I into combinations with standard therapeutics. Cytokine and Growth Factor Reviews. 2010;21:91–98. doi: 10.1016/j.cytogfr.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Worschech A, et al. The immunologic aspects of poxvirus oncolytic therapy. Cancer Immunology and Immunotherapy. 2009;58:1355–1362. doi: 10.1007/s00262-009-0686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Boisgerault N, Tangy F, Gregoire M. New perspectives in cancer virotherapy: bringing the immune system into play. Immunotherapy. 2010;2:185–199. doi: 10.2217/imt.10.6. [DOI] [PubMed] [Google Scholar]
Further reading, resources and contacts
- Ottolino-Perry K, et al. Intelligent design: combination therapy with oncolytic viruses. Molecular Therapy. 2009;18:251–263. doi: 10.1038/mt.2009.283. This review details combination therapies with OVs.
- Power AT, Bell JC. Taming the Trojan horse: optimizing dynamic carrier cell/oncolytic virus systems for cancer biotherapy. Gene Therapy. 2008;15:772–799. doi: 10.1038/gt.2008.40. [DOI] [PubMed] [Google Scholar]
- Cattaneo R, et al. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nature Reviews. Microbiology. 2008;6:529–540. doi: 10.1038/nrmicro1927. These articles review cell-based delivery of OVs.
- Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nature Clinical Practice. Oncology. 2007;4:101–117. doi: 10.1038/ncponc0736. These authors review clinical trials with OVs.For an overview of the frequent mutations found in the KRAS gene and other genes such as DCP4, P16 and P53 in pancreatic cancer, including cell line mutation profiles, see: http://pathology2.jhu.edu/pancreas/geneticsweb/discover.htm.