Abstract
A new mechanism of ischemia/reperfusion (I/R) injury is discovered recently operating through innate autoimmunity. Studies of different animal I/R models showed that reperfusion of ischemic tissues elicits an acute inflammatory response involving complement system which is activated by autoreactive natural IgM. Whether similar mechanism operating in human is still unknown. We investigated this important question by testing if human natural IgM could induce I/R injury in an established murine intestinal model. RAG-1−/− mice (immunoglobulin deficient), which are protected from I/R injury, were reconstituted with purified normal human IgM and subjected in an intestinal injury model. Reconstituted RAG-1−/− mice that were underwent sham treatment did not show tissue injury in intestine. In contrast, reconstituted RAG-1−/− mice that underwent 40 minutes intestinal ischemia and 3 hours reperfusion showed significant injury in the local tissues. In addition, immunohistochemistry showed that complement C4 were deposited in intestinal villi of I/R but not sham treated mice. Therefore, our study is the first report describing that human natural IgM is capable to induce I/R injury in the intestinal model, and further suggests that innate autoimmunity may operate under pathogenic conditions in human.
Introduction
A novel mechanism of “innate autoimmunity” has recently been established in different animal I/R models (Zhang and Carroll, 2007). This new mechanism involves the innate immune system recognizing self targets and initiating inflammatory response in a similar way as with pathogens. Studies of intestinal, skeletal muscle, and heart I/R models showed that reperfusion of ischemic tissues elicits an acute inflammatory response involving circulating complement system which is activated by natural IgM (Fleming et al., 2002; Reid et al., 2002; Zhang et al., 2006a; Zhang et al., 2004; Zhang et al., 2006b; Zhang et al., 2006c). The recent identification of a monoclonal natural IgM that initiates I/R (Austen et al., 2004; Zhang et al., 2004) led to the identification of nonmuscle myosin heavy chain type II A and C as the self-targets in two different tissues (Zhang et al., 2006a). New evidence further suggests that IgM binds initially to ischemic antigen providing a binding site for mannan binding lectin (MBL), which subsequently leads to activation of complement and results in tissue injury (Hart et al., 2005; McMullen et al., 2006; Walsh et al., 2005; Zhang et al., 2006c). These studies opened an important question whether a similar mechanism operating in human.
Although the answer to this question is still unknown, results from some clinical studies indeed suggest a role of natural IgM in the pathogenesis of human ischemic diseases. For instance, Krijnen et al showed that IgM co-localized with complement and C-reactive protein in infarcted human myocardium, indicating that IgM targeted complement locally to the jeopardized cardiomyocytes after acute myocardial infarction (MI) (Krijnen et al., 2005). Interestingly, such IgM deposition patterns were present only in PMN phase infarcts, presumably as a result of compensatory reperfusion. Other evidence of complement activation in human MI includes consumption of classical complement components during the early phase of acute MI (Pinckard et al., 1975), significant changes in terminal complement components in MI patients diagnosed with reperfusion (but not those without reperfusion) (Oren et al., 1998), and deposition of terminal complement complex within reperfused heart tissues (Robert-Offerman et al., 2000; Schafer et al., 1986; Vakeva et al., 1993; Yasojima et al., 1998).
We investigated whether human natural IgM can directly induce I/R injury by using an established murine intestinal I/R model. Although the clinical cases of intestinal ischemia are much less than heart attack and stroke, its acute form of mesenteric ischemia is fatal with a mortality of 70–90% (Brandt, 2003). Our previous studies using the intestinal I/R model has proved that it is a fast and reliable way to validate fundamental hypotheses, and results from this model have been confirmed in heart and skeletal muscle models (Austen et al., 2004; Chan et al., 2006; Zhang et al., 2006a; Zhang et al., 2006b). In the current study, RAG-1−/− mice (immunoglobulin deficient), which are known to be protected from I/R injury, were reconstituted with purified normal human IgM and subjected to sham or I/R treatment. Histological analyses were carried out to evaluate the degree of injury, and immunohistochemistry were used to analyze the complement activation in local tissues.
Materials and Methods
Purification of normal human IgM
Plasma samples donated by normal human volunteers were collected through CBR Laboratory, Inc. (Boston, MA). Human IgM was purified by IgM affinity column (HiTrap IgM Purification HP, Amersham Pharmacia Biotech, NJ) and ÄKTA FPLC (Amersham Pharmacia Biotech, NJ). Procedure was carried out according to the manufacturer’s instructions. Briefly, plasma samples were first prepared with ammonium sulphate till the final concentration is 0.8 M. The prepared plasma was applied to the affinity column which has been pre-calibrated. IgM affinity binding buffer (20 mM sodium phosphate and 0.8 M (NH4)2SO4, pH 7.5.) was then applied to wash out unbound factors, and bound IgM was eluted by 20 mM sodium phosphate, pH 7.5. The flow rate of the overall purification procedure is 1 ml/min.
Animals and intestinal model of I/R injury
RAG-1−/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Affinity purified normal human IgM were reconstituted into RAG-1−/− mice (i.v. 50ug per animal) 30min prior to surgery. Surgical protocol for I/R was performed as previously described (Zhang et al., 2006a; Zhang et al., 2004). Briefly, after anesthesia, a laparotomy is performed, and the microclip (125g pressure, Roboz, MD) was applied to the superior mesenteric artery. After 40 minutes of ischemia, the microclip was removed, and all animals were kept warm during reperfusion. At the end of experiment, the ischemic segment of the jejunum was harvested and the central 4cm was excised for pathological analysis.
Histopathology and Immunohistochemistry Analysis
Cryostat sections of intestinal tissues were stained by hematoxylin and eosin (H&E), blind-coded by a different person and examined by light microscopy for mucosal damage. The pathology score was assessed based on a procedure modified from Chiu (Chiu et al., 1970a; Chiu et al., 1970b) that includes direct inspection of all microvilli over a 4 cm stretch of jejuneum thus more objective (Zhang et al., 2006a; Zhang et al., 2004). Our pathology scoring system includes following: (a) count the total number of villi in the entire section; (b) villi with following two morphological changes are considered pathologically damaged and counted separately: (score of 0.5) subepithelial space, defined as an acellular space under a continuous epithelial layer and milder form of damage, and (score of 1) epithelial disruption, defined as discontinuation of epithelial layer of villus and more sever form of damage; (c) The integrated pathology score = (number of villi with subepithelial space / number of total villi) × 50% + (number of villi with epithelial disruption / number of total villi) × 100%
Immunofluorescence staining C4 was achieved by staining with FITC labeled rabbit anti-huC4c (1:250 dilution, DAKO, CO), followed by anti-rabbit-Alexa 488 (1:250 dilution, Molecular Probes, OR) (Zhang et al., 2004). Sections were mounted in Antifade Mounting Medium with DAPI (Molecular Probes, OR). Fluorescent images were made with a Leica digital imaging system (Leica, NJ).
Results
Microvilli of small intestine are very sensitive to ischemia, and reperfusion is essential to salvage the ischemic tissue. Previous animal studies showed that reperfusion to the ischemic intestine can nevertheless cause acute damage to the microvilli by the influx of immune factors, particularly through natural IgM mediated complement system activation. Morphological damages of microvilli and deposition of complement in the local intestinal tissues have been established as the indicators for I/R injury (Zhang et al., 2006a; Zhang et al., 2004).
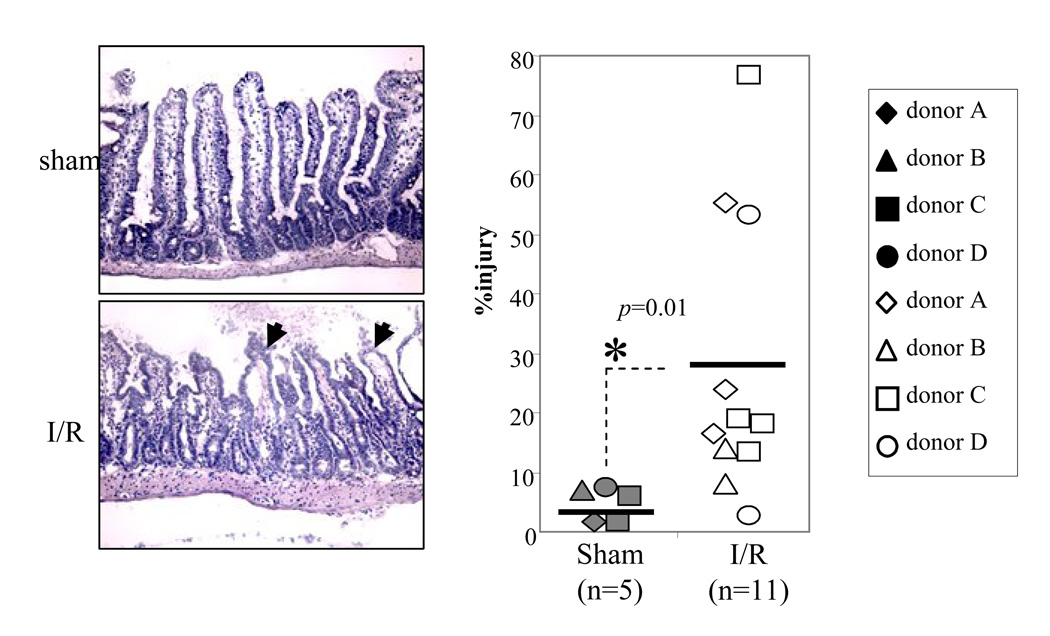
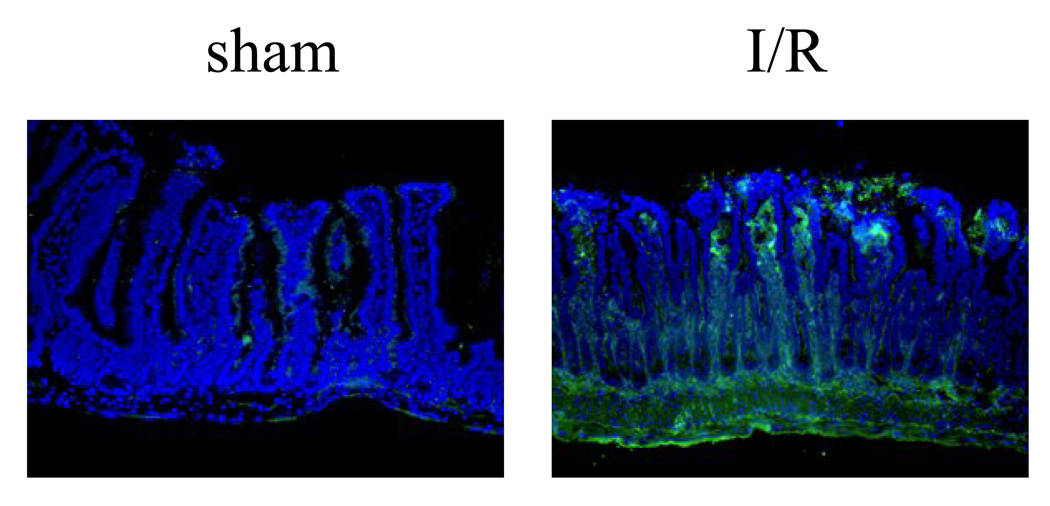
We investigated if human natural IgM could induce I/R injury in the mouse intestinal model. Normal human IgM were purified from healthy donors by IgM affinity column, and 50ug were reconstituted into each RAG-1−/− mouse 30 minutes prior to the ischemia. Intestinal ischemia was induced by blocking the superior mesentery artery for 40 minutes followed by reperfusion for 3 hours. Semi-quantitative morphological analysis of intestinal microvilli showed that human IgM reconstituted RAG-1−/− mice in sham surgical treatment group had no morphological damages (n=5, average pathology score=4 ± 4%) (Fig. 1). In addition, there is no complement C4 deposition in the microvilli of sham treated animals (Fig. 2). In contrast, I/R treatment induced significant injury in the microvilli of human IgM reconstituted RAG-1−/− mice (n=11, average pathology score=27 ± 23%, p<0.05) comparing to the sham group (Fig. 1). Immunohistochemistry staining also found C4 deposited in the microvilli of these mice, suggesting activation of complement (Fig. 2).
Figure 1.
Reconstitution of normal human IgM in RAG-1−/− mice induces local tissue injury in murine intestinal I/R model. Normal human IgM were purified by IgM affinity chromatography and reconstituted into RAG-1−/− mice (i.v. 50ug per animal) 30min prior to surgery. IgM reconstituted mice were divided into either sham or I/R treated groups. The I/R treated mice were undergone 40min intestinal ischemia followed by 3h reperfusion. Intestinal tissues were harvested and stained with H&E. The morphological injuries of intestine were quantified by an established pathology score system (Magnification=200×). Each symbol represents an individual mouse reconstituted with natural IgM from an individual human donor. The horizontal bars represent the arithmetic means of the injury scores. Asterisks indicate statistical significance (student t-test; p<0.05).
Figure 2.
Deposition of complement C4 in the intestine of I/R treated RAG-1−/− mice reconstituted with human IgM. Representative cryosections were stained with antibody specific for mouse C4 (Green). The left panel is sham treated mouse, and the right panel is I/R treated mouse (400× magnification). All sections were counterstained with DAPI (Violet).
Discussion
This study demonstrated that human natural IgM is capable to induce I/R injury in the intestinal model. RAG-1−/− mice reconstituted with normal human IgM in the I/R treated group showed significant damage in microvilli and complement C4 deposition comparing to those in the sham group (Fig. 1 and 2). Thus, our results suggested that human natural IgM functions similarly as murine natural IgM in activating complement and tissue damage under I/R condition.
Interestingly, when we tried to do immunoshistochemistry staining for human IgM on the intestinal tissues of I/R treated mice, we could not see strong deposition pattern (data not shown). One possibility is that 50ug of purified human IgM was enough to restore morphological injury in these mice, but this quantity may be below the detection sensitivity of immunohistochemistry on the local tissues. Our previous study of RAG-1−/− mice showed that 400ug murine IgM reconstitution was sufficient to be detectable by immunohistochemistry in the local I/R tissues (Zhang et al., 2004). We have attempted to administrate larger dose of normal human IgM (i.e. 100ug) to RAG-1−/− mice, but these reconstituted mice tended to die easily during the animal surgery, presumably by xeno-reaction of natural antibodies. With 50ug human IgM reconstitution, RAG-1−/− mice survived the entire period of the I/R surgery.
One potential question is whether human IgM specifically cause damage in this I/R model. Our data showed that RAG-1−/− mice in the sham group, which were also reconstituted with normal human IgM, had no damage in the microvilli (Fig. 1), thus proved that the local tissue injury induced human IgM is indeed specific to I/R.
The present study opens a new window for future research in the field. Future research may investigate whether human natural IgM recognizes similar ischemia specific self-antigen(s) as murine natural IgM does; whether ischemic specific human B cell clones are present as in mouse; and finally, if inhibitors of murine I/R injury can function in human pathological situations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Austen WG, Jr, Zhang M, Chan R, Friend D, Hechtman HB, Carroll MC, Moore FD., Jr Murine hindlimb reperfusion injury can be initiated by a self-reactive monoclonal IgM. Surgery. 2004;136:401–406. doi: 10.1016/j.surg.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Brandt JL. Mesenteric Vascular Disease. In: Friedman SL, editor. Current Diagnosis & Treatment in Gastroenterology. New York: The McGraw-Hill Companies, Inc.; 2003. p. Section I.9. [Google Scholar]
- Chan RK, Verna N, Afnan J, Zhang M, Ibrahim S, Carroll MC, Moore FD., Jr Attenuation of skeletal muscle reperfusion injury with intravenous 12 amino acid peptides that bind to pathogenic IgM. Surgery. 2006;139:236–243. doi: 10.1016/j.surg.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970a;101:478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- Chiu CJ, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. II. The protective effect of intraluminal glucose as energy substrate. Arch Surg. 1970b;101:484–488. doi: 10.1001/archsurg.1970.01340280036010. [DOI] [PubMed] [Google Scholar]
- Fleming SD, Shea-Donohue T, Guthridge JM, Kulik L, Waldschmidt TJ, Gipson MG, Tsokos GC, Holers VM. Mice deficient in complement receptors 1 and 2 lack a tissue injury- inducing subset of the natural antibody repertoire. J Immunol. 2002;169:2126–2133. doi: 10.4049/jimmunol.169.4.2126. [DOI] [PubMed] [Google Scholar]
- Hart ML, Ceonzo KA, Shaffer LA, Takahashi K, Rother RP, Reenstra WR, Buras JA, Stahl GL. Gastrointestinal Ischemia-Reperfusion Injury Is Lectin Complement Pathway Dependent without Involving C1q. J Immunol. 2005;174:6373–6380. doi: 10.4049/jimmunol.174.10.6373. [DOI] [PubMed] [Google Scholar]
- Krijnen PA, Ciurana C, Cramer T, Hazes T, Meijer CJ, Visser CA, Niessen HW, Hack CE. IgM colocalises with complement and C reactive protein in infarcted human myocardium. J Clin Pathol. 2005;58:382–388. doi: 10.1136/jcp.2004.022988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen ME, Hart ML, Walsh MC, Buras J, Takahashi K, Stahl GL. Mannose-binding lectin binds IgM to activate the lectin complement pathway in vitro and in vivo. Immunobiology. 2006;211:759–766. doi: 10.1016/j.imbio.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Oren S, Maslovsky I, Schlesinger M, Reisin L. Complement activation in patients with acute myocardial infarction treated with streptokinase. Am J Med Sci. 1998;315:24–29. doi: 10.1097/00000441-199801000-00005. [DOI] [PubMed] [Google Scholar]
- Pinckard RN, Olson MS, Giclas PC, Terry R, Boyer JT, O'Rourke RA. Consumption of classical complement components by heart subcellular membranes in vitro and in patients after acute myocardial infarction. J Clin Invest. 1975;56:740–750. doi: 10.1172/JCI108145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid RR, Woodcock S, Shimabukuro-Vornhagen A, Austen WG, Jr, Kobzik L, Zhang M, Hechtman HB, Moore FD, Jr, Carroll MC. Functional activity of natural antibody is altered in Cr2-deficient mice. J Immunol. 2002;169:5433–5440. doi: 10.4049/jimmunol.169.10.5433. [DOI] [PubMed] [Google Scholar]
- Robert-Offerman SR, Leers MP, van Suylen RJ, Nap M, Daemen MJ, Theunissen PH. Evaluation of the membrane attack complex of complement for the detection of a recent myocardial infarction in man. J Pathol. 2000;191:48–53. doi: 10.1002/(SICI)1096-9896(200005)191:1<48::AID-PATH583>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Schafer H, Mathey D, Hugo F, Bhakdi S. Deposition of the terminal C5b-9 complement complex in infarcted areas of human myocardium. J Immunol. 1986;137:1945–1949. [PubMed] [Google Scholar]
- Vakeva A, Laurila P, Meri S. Regulation of complement membrane attack complex formation in myocardial infarction. Am J Pathol. 1993;143:65–75. [PMC free article] [PubMed] [Google Scholar]
- Walsh MC, Bourcier T, Takahashi K, Shi L, Busche MN, Rother RP, Solomon SD, Ezekowitz RA, Stahl GL. Mannose-binding lectin is a regulator of inflammation that accompanies myocardial ischemia and reperfusion injury. J Immunol. 2005;175:541–546. doi: 10.4049/jimmunol.175.1.541. [DOI] [PubMed] [Google Scholar]
- Yasojima K, Schwab C, McGeer EG, McGeer PL. Human heart generates complement proteins that are upregulated and activated after myocardial infarction. Circ Res. 1998;83:860–869. doi: 10.1161/01.res.83.8.860. [DOI] [PubMed] [Google Scholar]
- Zhang M, Alicot EM, Chiu I, Li J, Verna N, Vorup-Jensen T, Kessler B, Shimaoka M, Chan R, Friend D, Mahmood U, Weissleder R, Moore FD, Carroll MC. Identification of the target self-antigens in reperfusion injury. J Exp Med. 2006a;203:141–152. doi: 10.1084/jem.20050390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Austen WG, Jr, Chiu I, Alicot EM, Hung R, Ma M, Verna N, Xu M, Hechtman HB, Moore FD, Jr, Carroll MC. Identification of a specific self-reactive IgM antibody that initiates intestinal ischemia/reperfusion injury. Proc Natl Acad Sci U S A. 2004;101:3886–3891. doi: 10.1073/pnas.0400347101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Carroll MC. Natural antibody mediated innate autoimmune response. Mol Immunol. 2007;44:103–110. doi: 10.1016/j.molimm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Zhang M, Michael LH, Grosjean SA, Kelly RA, Carroll MC, Entman ML. The role of natural IgM in myocardial ischemia-reperfusion injury. J Mol Cell Cardiol. 2006b;41:62–67. doi: 10.1016/j.yjmcc.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Zhang M, Takahashi K, Alicot EM, Vorup-Jensen T, Kessler B, Thiel S, Jensenius JC, Ezekowitz RA, Moore FD, Carroll MC. Activation of the Lectin Pathway by Natural IgM in a Model of Ischemia/Reperfusion Injury. J Immunol. 2006c;177:4727–4734. doi: 10.4049/jimmunol.177.7.4727. [DOI] [PubMed] [Google Scholar]