Abstract
Purpose/Background:
Historically, patellofemoral pain syndrome (PFPS) has been viewed exclusively as a knee problem. Recent findings have suggested an association between hip muscle weakness and PFPS. Altered neuromuscular activity about the hip also may contribute to PFPS; however, more limited data exist regarding this aspect. Most prior investigations also have not concurrently examined hip and knee strength and neuromuscular activity in this patient population. Additional knowledge regarding the interaction between hip and knee muscle function may enhance the current understanding of PFPS. The purpose of this study was to compare hip and knee strength and electromyographic (EMG) activity in subjects with and without PFPS.
Methods:
Eighteen females with PFPS and 18 matched controls participated in this study. First, surface EMG electrodes were donned on the gluteus medius, vastus medialis, and vastus lateralis. Strength measures then were taken for the hip abductors, hip external rotators, and knee extensors. Subjects completed a standardized stair-stepping task to quantify muscle activation amplitudes during the loading response, single leg stance, and preswing intervals of stair descent as well as to determine muscle onset timing differences between the gluteus medius and vastii muscles and between the vastus medialis and vastus lateralis at the beginning of stair descent.
Results:
Females with PFPS demonstrated less strength of the hip muscles. They also generated greater EMG activity of the gluteus medius and vastus medialis during the loading response and single leg stance intervals of stair descent. No differences existed with respect to onset activation of the vastus medialis and vastus lateralis. All subjects had a similar delay in gluteus medius onset activation relative to the vastii muscles.
Conclusion:
Rehabilitation should focus on quadriceps and hip strengthening. Although clinicians have incorporated gluteus medius exercise in rehabilitation programs, additional attention to the external rotators may be useful.
Level of Evidence: 4
Keywords: gluteus medius, knee, patella, surface electromyography
INTRODUCTION
Patellofemoral pain syndrome (PFPS) is a common problem experienced by active adults and adolescents;1 however, its etiology has remained vague and controversial.2 Often times, patients complain of diffuse peripatellar and retropatellar pain that may limit their ability to perform activities of daily living that require loading on a flexed knee. Such activities include descending stairs, squatting, and sitting for prolonged periods of time.3
PFPS is thought to result from abnormal patella tracking that causes excessive compression to the lateral patella facets.3 Possible reasons for faulty tracking have included quadriceps weakness,4,5 delayed activation (onset) of the vastus medialis (VM) relative to the vastus lateralis (VL),6,7 and decreased quadriceps electromyographic (EMG) amplitudes.8 However, conflicting results exist in the scientific literature. Some investigators9,10 have not found quadriceps onset timing differences while others11,12 have reported higher quadriceps EMG activity in subjects with PFPS. A possible reason for discrepancies might be the examination of these parameters during a variety of non-weight bearing and weight bearing activities.
More recent investigations have examined VM and VL amplitudes12 and onsets7,9,13 during a weight bearing activity like stair-stepping. Mohr et al12 reported higher VM and VL amplitudes for subjects with PFPS from patella instability and concluded that greater EMG activity reflected knee extensor weakness. A limitation of this study was that the investigators did not assess knee extensor strength. Regarding VM and VL onsets, Cowan et al13 and Boling et al7 found that subjects with PFPS demonstrated delayed VM onset whereas Brindle et al9 reported no onset differences. Lack of consensus between authors may reflect methodological differences. Cowan et al and Boling et al collected EMG activity as subjects completed the task at a standardized rate where as those in the Brindle et al study performed stair-stepping at a self-selected speed. In summary, most prior works have not concurrently examined knee extensor strength and VM and VL EMG activity. Simultaneous examination of these parameters may enhance the current understanding of quadriceps function in this patient population.
Faulty hip kinematics also may contribute to PFPS.14 Powers et al15 were the first to compare femoral and patellar movement during non-weight bearing and weight bearing knee extension using kinematic magnetic resonance imaging. They reported lateral patella movement on the femur during non-weight bearing exercise but increased femoral internal rotation, under a relatively stable patella, during weight bearing activity. Results from this and a subsequent study16 have demonstrated that excessive femoral internal rotation, not patella movement, may cause relative lateral patella tracking. Findings from both studies have suggested that hip muscle weakness, especially of the hip abductors and external rotators, may lead to altered lower extremity kinematics.
Conflicting data17–20 have existed regarding an absolute association between hip weakness and altered lower extremity kinematics. Furthermore, Willson and Davis18 reported weak correlations between hip abduction strength/hip adduction excursion (r=–.04) and hip external rotator strength/hip internal rotation excursion (r=–.12) during single-leg jumping. Regardless of different findings, a recent systematic review21 found hip weakness in this patient population, and data22–24 support hip exercise as a viable treatment.
Researchers7,9,19,25 also have examined hip muscle EMG data during weight bearing activities. Souza and Powers19 found increased gluteus maximus EMG activation during demanding activities (e.g., running, drop landings, and a step-down maneuver) in females with PFPS who demonstrated hip weakness. They concluded that these subjects required increased gluteus maximus neural drive to complete these tasks. Cowan et al25 and Brindle et al9 reported a delayed onset of the GM relative to the vastii muscles at the beginning of a stepping task. Together, these findings9,19,25 have highlighted altered hip neuromuscular factors that deserve additional investigation.
While clinicians historically have prescribed quadriceps exercise for treating PFPS, an emerging body of evidence26 supports the inclusion of hip exercise. Additional information regarding the interrelationship between knee and hip muscle function may enhance exercise prescription for this patient population. Therefore, the purpose of this study was to compare hip and knee strength and EMG activity during stair descent in subjects with and without PFPS. We hypothesized that subjects with PFPS would demonstrate 1) significantly less hip abductor, hip external rotator, and knee extensor strength; 2) greater EMG amplitudes of the gluteus medius (GM), VM, and VL during stair descent; 3) delayed activation of the VM and VL at the onset of stair descent; and 4) a greater delay in GM activation compared to the VM and VL at the onset of stair descent when compared to subjects in the control group.
METHODS
This study represents part of a larger investigation that compared hip strength and hip and knee kinematics during stair descent in females with and without PFPS.17 Results from the larger study agreed with prior works that females with PFPS exhibit hip weakness. However, no between-group hip and knee kinematic differences existed during stair descent. The authors concluded that subjects with PFPS may have used a compensatory stepping strategy, similar to controls, because of hip weakness. Another reason may have resulted from differences in hip and knee neuromuscular activity, which is the focus of this portion of the overall study.
Subjects
Recent studies27,28 have suggested gender differences associated with strength and EMG activity. Therefore, only female subjects were included in this investigation. Based on the works by Boling et al7 and Ireland et al,29 a total of thirty-six subjects was deemed sufficient to determine differences with respect to EMG and strength variables. Eighteen females with PFPS (age = 24.5 ± 3.2 years, height = 1.7 ± 0.1 m, mass = 63.1 ± 9.1 kg, pain = 4.4 ± 1.5 cm, duration of symptoms = 14.4 ± 12.8 months) and 18 asymptomatic females (age = 23.9 ± 2.8 years, height = 1.7 ± 0.1 m, mass = 62.1 ± 8.5 kg) participated in this study. Females with PFPS participated in this study if they complained of: 1) anterior knee pain during stair descent and 2) pain during at least two of the following provocative activities: a) stair ascent, b) squatting, c) kneeling, or d) excessive sitting.13 They also rated usual knee pain over the previous week at a minimum of 3 on a 10-cm visual analog scale (VAS).30 The most affected lower extremity was tested for subjects with PFPS.8 Six subjects reported bilateral symptoms. Control subjects participated in the study if they had 1) no history or diagnosis of knee pathology; 2) no pain with any of the above-named provocative activities; and 3) no history of hip pathology. The right lower extremity was tested for control subjects.12 This was done to make as consistent as possible the process of data collection for these subjects as they were matched to each subject with PFPS with respect to age, height, and weight.
Subjects were excluded if they had 1) previous knee surgery or significant injury; 2) traumatic patellar dislocation; 3) any neurologic involvement that would affect gait; or 4) previous hip surgery or significant injury.9 Prior to participation, all subjects signed an informed consent approved by the University of Kentucky Institutional Review Board.
Instrumentation
Subjects' pain was assessed using a 10-cm VAS. The extreme left side of the VAS stated “no pain” whereas the extreme right side stated “worse pain imaginable.” Subjects drew a perpendicular line on the scale at the position that most likely described their usual pain over the previous week.30
All isometric strength testing was performed using the Commander PowerTrack II™ (J Tech Medical, Salt Lake City, UT) hand-held dynamometer (HHD). The HHD's calibration was confirmed prior to the study by placing known weights on the HHD and comparing this to the HHD's reported weight. Accuracy was verified after every 10th testing session.
A 16-channel Myosystem 1400 EMG system (Noraxon USA, Inc, Scottsdale, AZ) was used to record muscle activity. EMG data were band pass filtered (10-1000 Hz) prior to sampling at 960 Hz. Video data were recorded using a seven camera video-based motion capture system (Motion Analysis Corporation, Santa Rosa, CA) operating at 60 Hz. EMG and video data were collected synchronously using EVaRT 4.2 software (Motion Analysis Corporation, Santa Rosa, CA), and stored on a personal computer for later analysis.
Procedures
First, subjects completed a 10-cm VAS reflecting the typical pain level during the past week.30 Next, they rode a stationary bike for 3 minutes in a pain-free range of motion at a submaximal speed. Subjects' skin was prepared for EMG instrumentation by shaving, abrading, and cleansing with isopropyl alcohol prior to application of surface electrodes. Bi-polar Ag-AgCl surface electrodes (Medicotest, Rolling Meadows, IL), measuring 5 mm in diameter with an interelectrode distance of approximately 20 mm, were placed in parallel arrangement over the muscle bellies of the GM,31 VM,32 and VL31 in a standardized manner. Electrodes were further secured to the skin with an adhesive tape to prevent slippage during testing. A ground electrode was placed on the ipsilateral clavicle. Electrode placements were visually confirmed on an oscilloscope using manual muscle testing techniques.33 A 3-second standing “quiet” file was also recorded to exclude ambient noise.
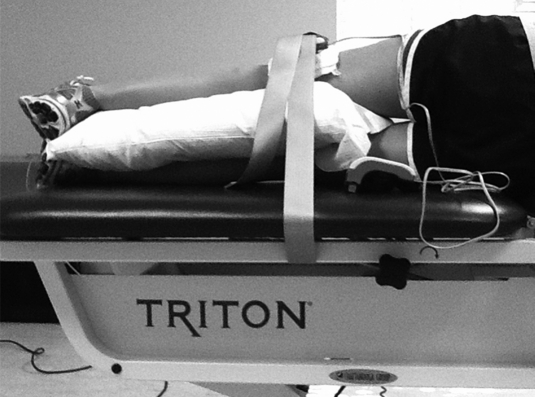
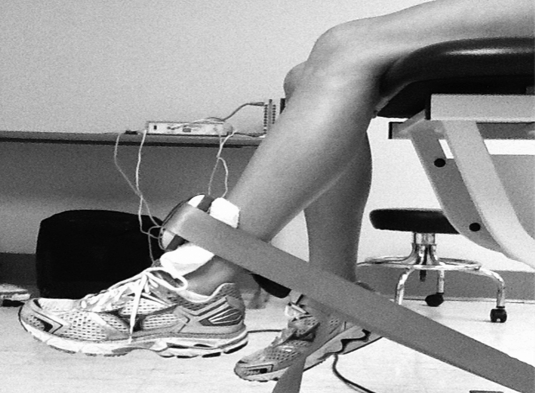
Following EMG electrode placement, isometric strength measures were taken for the hip abductors, hip external rotators, and knee extensors (Figures 1–3).29 For the hip abductors, subjects were positioned in sidelying (unaffected leg directly on the table) with the test leg in a neutral position by placing pillows between the lower extremities. The HHD was placed over the lateral femoral condyle and secured with an immovable strap. For the hip external rotators, subjects sat with the hip and knees in 90° of flexion. The HHD was placed just proximal to the medial malleolus and secured with an immovable strap. For the knee extensors, subjects were positioned with the hip in 90° flexion and the knee in 60° flexion. The HHD was placed just proximal to the malleoli and secured with an immovable strap.
Figure 1.
Test position for assessing hip abductor muscle strength.
Figure 2.
Test position for assessing hip external rotator muscle strength.
Figure 3.
Test position for assessing knee extensor muscle strength.
For testing, subjects produced a maximum voluntary isometric contraction (MVIC) using the “make” test 34 to the beat of a metronome set at 60 beats per minute.17 They generated maximum force over a 2-second period and maintained this force for an additional 5 seconds to the beat of the metronome. Subjects performed one practice34 and 3 test trials, with a 30-second rest period between trials. A coefficient of variation was calculated and an additional trial was taken, if necessary, to ensure that subjects had 3 peak force measures with variability less than 10%.35 The order of muscle testing was counterbalanced to account for any potential bias. The peak value from each trial was recorded in newtons and converted to kilograms. EMG activity was simultaneously collected for the GM, VM, and VL during strength testing to determine a MVIC for each muscle and enable normalization of EMG data.
Next, retroreflective markers, with a diameter of 20 mm, were placed on subjects using a standard Cleveland Clinic marker setup. After obtaining a static neutral file, subjects were shown the stair stepping task and allowed 5 practice trials. They were instructed to ascend and descend two 20-cm high steps, ensuring that the test extremity lifted and lowered the body on the first and third steps, respectively.32 Subjects also took a minimum of 3 strides prior to and immediately following stair stepping in order to maintain a continuous movement pattern. Because movement velocity may influence EMG activity, subjects performed the task at a standardized rate of 96 beats per minute.32
After demonstrating proficiency with the stair stepping task, subjects performed 10 test trials. Data from the last 5 trials were analyzed because of potential learning effects that might have been associated with earlier trials, even with subjects having performed 5 practice trials.13
Seven subjects returned to the laboratory within 5 to 7 days in order to determine measurement reliability. For this purpose, they completed all procedures in the identical manner as on the initial testing day. Data from these subjects suggested that procedures used in this study had acceptable reliability (ICC> 0.70).17,36
Data Processing
Strength
We expressed all peak force values recorded on the HHD as a percentage of each subject's body mass.29 The average of the normalized force values from 3 trials having a coefficient of variation less than 10% was used for statistical analysis.35
EMG Activation Amplitudes
Raw EMG signals were further band pass filtered at 20 to 480 Hz using Datapac Software (Run Technologies, Mission Viejo, CA). To determine muscle activation amplitudes, EMG data from the last 5 trials were root mean square (RMS) smoothed using a 55-ms time constant.10 These data were then normalized to 100% of the stair descent cycle, ensemble averaged, and expressed as a % MVIC.
Since varying amounts of muscle activation can occur throughout stair descent,12 we identified three intervals for the stance phase of stair descent. Loading response began at the initial point where any part of the ipsilateral foot contacted the step and ended as subjects lifted the contralateral foot off the previous step (0% to 7% of the stair descent cycle). Single leg stance occurred when the test extremity supported the entire body mass during the stair descent (8% to 46% of the stair descent cycle). Preswing began when any part of the contralateral foot contacted the ground and ended as subjects lifted the test extremity's foot off the stair (47% to 58% of the stair descent cycle). The remaining 42% of stair descent represented the swing phase; however, data during this phase were excluded from analysis since the purpose of this study was to compare EMG activity during a weight bearing task. Based on these time percentages, Datapac software (Run Technologies) then calculated the average % MVIC EMG amplitude for each muscle during each interval. Values from the 3 intervals of stance were used for statistical analysis.
Onset Timing Differences
Muscle activation onsets were determined at the beginning of stair descent using Datapac Software. For this purpose, data were band pass filtered as described above, full wave rectified, and low pass filtered at 50 Hz.32 A muscle onset was defined as the point in which the signal deviated by more than 3 standard deviations, for a minimum of 25 ms, over the baseline taken 200 ms before the trial began.32 All onsets were also visually confirmed since movement artifact could be incorrectly identified as the onset of muscle activity.37 After processing EMG signals and identifying muscle onsets, Datapac software calculated timing differences. GM onset was subtracted from the VM onset and VL onset, respectively, to quantify timing differences between the hip and knee musculature. A negative difference signified a delay in GM activation relative to the VM and VL where as a positive difference meant GM preactivation. VM onset also was subtracted from the VL onset to quantify quadriceps timing differences. A negative difference meant a delay in VM activation relative to the VL where as a positive difference signified VM preactivation. The average from the last 5 trials was used for statistical analysis.13
STATISTICAL ANALYSIS
Independent t-tests were used to determine group differences in age, height, and weight. Separate independent t-tests were used to determined differences in strength. Separate 2 by 3 (group X interval) analyses of variance (ANOVA) for repeated measures on stance interval were used to identify EMG amplitude differences for the GM, VM, and VL, respectively. A 2 by 3 (group X timing difference) ANOVA for repeated measures on muscle was used to determine EMG onset timing differences. An independent 1-group t-test was conducted to determine if timing differences varied significantly from 0 (meaning simultaneous VM and VL activation) for the PFPS and control groups.13 All statistical analyses were performed using SPSS version 17.0 (SPSS, Inc., Chicago, IL). Level of significance was established at the .05 level; the sequentially rejective Bonferroni (Bonferroni-Holm) post hoc test38 was used adjust the P-level to account for multiple pairwise comparisons of strength measures. The Bonferroni-Holm test also was used to determine the significance of interactions for the two-factor ANOVAs.
RESULTS
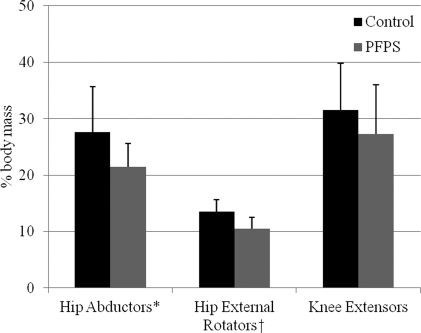
Independent t-tests for subject demographics revealed similar age, height, and weight (P > .44) characteristics for both groups. Subjects with PFPS generated 22% less hip abductor (P = .007) and 21% less hip external rotator (P = .001) force output on the HHD than controls. Although not significantly different (P = .148), subjects with PFPS exerted 13% less knee extensor force output than controls. Figure 4 displays these data.
Figure 4.
Descriptive statistics for force measures expressed as a percentage of body mass (% body mass).
PFPS: patellofemoral pain syndrome,*P = .007, †P = .001
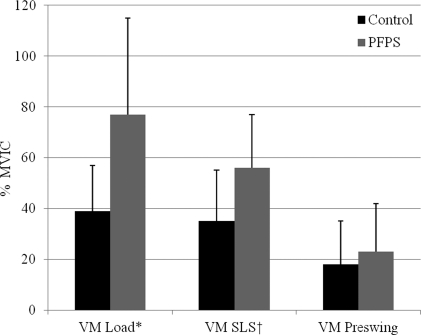
A group by interval interaction effect showed that subjects with PFPS generated greater GM and VM EMG amplitudes than controls. During loading response, subjects with PFPS generated 2.1 times more GM activity (P = .001) and 1.3 times more VM activity (P = .003) than controls. They also generated 2.4 and 1.2 times more activity during single leg stance for the GM (P = .002) and VM (P = .020), respectively. All subjects demonstrated similar GM (P = .602) and VM (P = .413) activity during preswing as well as similar VL activity (P ≥ .07) throughout all intervals of stance. Figures 5 through 7 summarize these data.
Figure 5.
Comparison of electromyographic amplitudes for the gluteus medius (GM) expressed as a percent maximum voluntary isometric contraction (% MVIC).
PFPS: patellofemoral pain syndrome, Load: loading response interval, SLS: single leg stance interval, *P = .001, †P = .002
Figure 6.
Comparison of electromyographic amplitudes for the vastus medialis (VM) expressed as a percent maximum voluntary isometric contraction (% MVIC).
PFPS: patellofemoral pain syndrome, Load: loading response interval, SLS: single leg stance interval, *P = .003, †P = .020
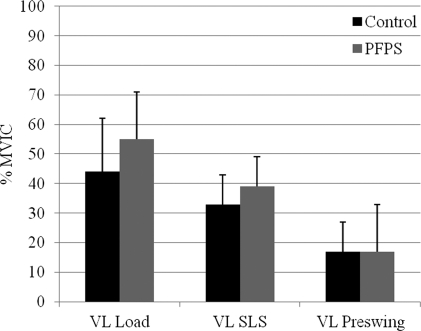
Figure 7.
Comparison of electromyographic amplitudes for the vastus lateralis (VL) expressed as a percent maximum voluntary isometric contraction (% MVIC).
PFPS: patellofemoral pain syndrome, Load: loading response interval, SLS: single leg stance interval
No differences were identified with respect to EMG timing parameters (P > .07). Results from independent 1-group t-test to determine if VL - VM onsets differed significantly from 0 were not significant (meaning both groups had simultaneous VM and VL activation). Table 1 summarizes descriptive data for the EMG onset timing differences.
Table 1.
Comparison of means (± standard deviation) for electromyographic onset timing differences expressed in milliseconds.
| Onset Timing Difference | Control | PFPS | P-value |
|---|---|---|---|
| VM – GM* | −79 ± 64 | −73 + 65 | .77 |
| VL – GM* | −83 ± 62 | −75 + 67 | .72 |
| VL – VM† | −1.28 ± 8‡ | −3.83 ± 9§ | .32 |
PFPS: patellofemoral pain syndrome
GM: gluteus medius
VM: vastus medialis
VL: vastus lateralis
A negative number represents a delay in GM activation
A negative number represents a delay in VM activation
Not significantly different from 0 (p = .07)
Not significantly different from 0 (p = .53)
DISCUSSION
This study compared hip and knee strength along with EMG activity during stair descent in females with and without PFPS. As originally hypothesized, subjects with PFPS demonstrated hip weakness compared to controls. They also generated greater GM and VM EMG activity during the loading response and single leg stance intervals of stair descent. No other between-group differences existed for the remaining dependent measures.
Together, these results suggested that females with PFPS have altered strength and neuromuscular activity of the hip and knee muscles during a simple functional activity like stair descent. These findings further support the importance of hip exercise as part of a comprehensive rehabilitation program for this patient population.26
Strength
Hip Abductors and External Rotators
Recently, many different groups of researchers21 have examined hip strength in females with PFPS and have consistently reported hip weakness. Moreover, many have assessed hip abductor18,29,39 and hip external rotator29,39–41 strength using similar subject position, HHD placement, and data normalization procedures as the current study. These similarities have enabled the ability to make meaningful comparison of our results to prior works.
Findings17,28,37 from prior investigations that used similar procedures reported hip abductor force values ranging from 21% to 29% body mass. Our results are in agreement as females with PFPS generated hip abductor force output equal to 22% of body mass. It is noteworthy that researchers40–43 that assessed hip abductor force values by placing the HHD proximal to the lateral malleolus reported relatively lower force values (range 9% to 18% body mass). Placing the HHD near the lateral malleolus provided the examiner a mechanical advantage (i.e., increased the externally applied moment arm) and would reduce the amount of force the subjects could place on the HHD.
Subjects in the current study generated hip external rotator force equal to 11% body mass, which also agrees with prior works18,29,39–41,43 that determined force output in a similar manner (range 9% to 17% body mass). These values were less than findings from Piva et al,42 who found that subjects with PFPS generated hip external rotator force output equal to 22% body mass. One reason for this difference may be the manner of testing since they placed the HHD proximal to the lateral malleolus with subjects positioned prone with the hip extended and the knee flexed to 90°.
Knee Extensors
Our results did not agree with previous works showing quadriceps weakness in this patient population.5,44–47 A reason for this finding may result from our subject sample. Subjects in the current study presented with a long-term history of PFPS and might not have experienced pain during strength testing. Since we did not assess pain during strength testing, we cannot conclusively make this determination. From a clinical standpoint, our subjects with PFPS did demonstrate a 13% strength deficit compared to controls. These findings may be clinically relevant because patients with PFPS have responded favorably to quadriceps strengthening programs.4,5
In summary, subjects with PFPS demonstrated significant hip abductor and external rotator weakness. Values from the current and prior works may serve as a benchmark that clinicians can use to identify females with PFPS who have hip weakness. However, knowledge of the assessment methods used are critical to ensure reliable use of the reported values.
EMG Activation Amplitudes
GM and VM Activity
Subjects with PFPS demonstrated significantly higher GM and VM EMG amplitudes during the loading response and single leg stance intervals of stair descent. Relatively higher GM activation may have reflected the need for increased neuromuscular activity to complete the task. Conversely, Souza and Powers19 found that females with PFPS had less hip abductor strength but similar GM activity as controls during a running, drop jump, and a step-down maneuver. They concluded that subjects could have compensated for hip abductor weakness through excessive trunk lean over the ipsilateral hip during these tasks. Excessive trunk lean would minimize the amount of required muscle force needed to stabilize the pelvis.48 Subjects in the current study completed a stair descent task, which was less demanding than tasks used by Souza and Powers. Therefore, subjects in the current study may have relied more on greater GM activity, and not a trunk lean strategy, to complete the task. The authors cannot conclusively make this determination as trunk kinematics were not assessed.
Regarding the VM activity, Sheehy et al10 identified two peaks of eccentric EMG activity for the VM and VL during stair descent. The first corresponded with the current study's loading response and single leg stance intervals. During these intervals, researchers have reported greater hip muscle activation in response to decelerating and controlling forward and downward motion of the body onto the step49,50 Higher VM amplitudes for PFPS subjects during these intervals also most likely reflected the need for greater activation when external knee flexion moments were greater.51 Sheehy et al also identified a second peak of activity, which corresponded to preswing in the current study (movement of the center of mass past the stance leg). During this interval, the body was likely positioned with the center of mass located more centrally over the foot, which would provide a stable base and require less muscle activation.49
VL Activity
Subjects with PFPS demonstrated similar VL amplitudes as controls throughout all intervals of stair descent, findings that agree with prior works.8,10,52 Compared to the VL, these subjects also generated greater VM activity. This difference may suggest relative VM insufficiency. Souza and Gross53 also reported relative differences in VM and VL activity for subjects with PFPS during stair-stepping. However, they reported decreased VM activity relative to the VL. It is unclear why Souza and Gross found less VM activity compared to the VL. It is noteworthy that they did not normalize the EMG data and had a smaller sample size. These methodological differences might account for the conflicting findings.8
EMG Onset Timing Differences
VM and VL Onset Timing Differences
Results from this study showed simultaneous activation of the VM and VL at the onset of stair descent, which agree with previous reports.8–10 However, findings from this study contradicted those reported by Cowan and colleagues6,25 and Boling et al.7 Conflicting results most likely reflected differences in methodology and subject variability. Future studies that use a standardized methodology and similar subject profile are needed to better understand the clinical importance, if any, of VM and VL onset timing differences.
GM and Vastii Muscle Onset Timing Differences
All subjects in the current study demonstrated delayed GM activation relative to the VM and VL; however, there were no significant between-group differences. Although Brindle et al.9 also reported a similar delay in GM activation, subjects with PFPS exhibited a significantly greater delay in GM activation than controls. Subjects in the Brindle et al study ascended 3 steps, stopped, turned around, and descended the steps. Subjects in the current study ascended and descended steps in a continuous manner. Variations in methodology compromised meaningful comparisons between studies; additional studies are needed to better understand timing characteristics between the GM, VM, and VL.
Clinical Implications
Findings from this study have provided additional insight regarding the interaction between hip and knee strength and EMG activity in females during stair descent. The authors' current data support that clinicians examine and address hip impairments for the treatment of PFPS. However, they also should not disregard knee function as patients who may not necessarily demonstrate marked knee weakness may have altered neuromuscular activity. It is noteworthy that programs designed to target the hip muscles7,22,24,54 also incorporated some weight bearing exercises that simultaneously engaged the hip and knee. Although patients in these investigations reported less pain and exhibited increased strength, it is unknown if changes occurred in neuromuscular recruitment. Directing more attention toward the effect that exercise has on hip and knee neuromuscular factors may provide invaluable information regarding future exercise prescription.
Limitations
This study had certain limitations that the authors would like to address. The first limitation was associated with the use of surface EMG with respect to signal crosstalk. Other muscles like the tensor fascia lata and gluteus minimus might have influenced EMG signals. We addressed this limitation by placing electrodes over the muscle belly of the gluteus medius in a standardized manner31,55 and confirmed EMG signals using manual muscle testing techniques. Future studies could address this issue by utilizing fine wire EMG techniques. A second limitation was that the primary examiner was not blinded to each subject's condition. Bias might have been introduced unintentionally during data collection and analysis. The authors did minimize potential bias by taking measures in accordance with a standardized protocol with proven reliability.17,36
Delimitations
First, the authors did not assess gluteus maximus function since prior studies specifically focused on the hip abductors and hip external rotators. However, more recent investigations19,39,40 have examined the gluteus maximus. Although considered the primary hip extensor, the gluteus maximus also functions as a strong hip external rotator. Emerging data19 have shown that subjects with PFPS exhibit gluteus maximus weakness and increased EMG activity during a running, drop jump, and step-down maneuver. Additional investigations are needed to better understand the influence of the gluteus maximus on patients with PFPS.
Second, the authors of the current study did not instrument any of the hip external rotators with EMG electrodes. These muscles would have required the use of fine wire techniques to record EMG activity, which presented concerns of possible wire breakage during testing. Furthermore, unlike the gluteus medius, the primary hip abductor, no single muscle within the hip external rotators would best represent the actions of this muscle group.
Finally, the muscles examined in this study produce rotatory joint movement, which should be measured as a unit of torque (force generated multiplied by the perpendicular distance of the applied resistance from the joint axis of rotation). We quantified strength as the amount of force applied to the HHD expressed as a % body mass to enable comparison of results to prior studies. Future studies should report data as a unit of torque to provide a more accurate reflection of strength.
CONCLUSION
The purpose of this study was to determine differences in hip and knee strength and EMG activity during stair descent in females with and without PFPS. Overall, subjects with PFPS exhibited hip weakness. Caution should be taken in interpreting this finding, since it is not known if hip weakness was the cause of or a result of PFPS. EMG data showed that subjects with PFPS generated greater GM and VM activity during the loading response and single leg stance intervals of stair descent. Overall, the findings of the current study concur with the emerging body of evidence regarding hip abductor and hip external rotator weakness21 in this patient population and support the need for further examination of neuromuscular factors.
REFERENCES
- 1.Heinjes E, Berger MY, Bierma-Zeinstra SMA, Bernsen RMD, Verhaar JAN, Koes BW. Exercise therapy for patellofemoral pain syndrome. The Cochrane Library. Vol 1 Chichester, UK: John Wiley & Sons, Ltd.; 2004 [Google Scholar]
- 2.Witvrouw E, Werner S, Mikkelsen C, Van Tiggelen D, Vanden Berghe L, Cerulli G. Clinical classification of patellofemoral pain syndrome: guidelines for non-operative treatment. Knee Surg Sports Traumatol Arthrosc. 2005;13:122–130 [DOI] [PubMed] [Google Scholar]
- 3.Fulkerson JP. Diagnosis and treatment of patients with patellofemoral pain. Am J Sports Med. 2002;30(3):447–456 [DOI] [PubMed] [Google Scholar]
- 4.Natri A, Kannus P, Jarvinen M. Which factors predict the long-term outcome in chronic patellofemoral pain syndrome? A 7-yr prospective follow-up study. Med Sci Sports Exer. 1998;30:1572–1577 [DOI] [PubMed] [Google Scholar]
- 5.Malone TR, Davies GJ, Walsh WM. Muscular control of the patella. Clin Sports Med. 2002;21(3):349–362 [DOI] [PubMed] [Google Scholar]
- 6.Cowan SM, Bennell KL, Crossley KM, Hodges PW, McConnell J. Physical therapy alters recruitment of the vasti in patellofemoral pain syndrome. Med Sci Sports Exer. 2002;34(12):1879–1885 [DOI] [PubMed] [Google Scholar]
- 7.Boling MC, Bolgla LA, Mattacola CG, Uhl TL, Hosey RG. Outcomes of a weight-bearing rehabilitation program for patients diagnosed with patellofemoral pain syndrome. Arch Phys Med Rehabil. 2006;87(11):1428–1435 [DOI] [PubMed] [Google Scholar]
- 8.Powers CM, Landel R, Perry J. Timing and intensity of vastus muscle activity during functional activities in subjects with and without patellofemoral pain. Phys Ther. 1996;76(9):946–955 [DOI] [PubMed] [Google Scholar]
- 9.Brindle TJ, Mattacola CG, McCrory JL. Electro–myographic changes in the gluteus medius during stair ascent and descent in subjects with anterior knee pain. Knee Surg Sports Traumatol Arthrosc. 2003;11:244–251 [DOI] [PubMed] [Google Scholar]
- 10.Sheehy P, Burdett RG, Irrgang JJ, Van Swearingen J. An electromyographic study of vastus medialis oblique and vastus lateralis activity while ascending and descending steps. J Orthop Sports Phys Ther. 1998;27(6):423–429 [DOI] [PubMed] [Google Scholar]
- 11.Powers CM. Patellar kinematics, part I: The influence of the vastus muscle activity in subjects with and without patellofemoral pain. Phys Ther. 2000;80(10):956–964 [PubMed] [Google Scholar]
- 12.Mohr KJ, Kvitne RS, Pink MM, Fideler B, Perry J. Electromyography of the quadriceps in patellofemoral pain with patellar subluxation. Clin Orthop. 2003;415:261–271 [DOI] [PubMed] [Google Scholar]
- 13.Cowan SM, Bennell KL, Hodges PW, Crossley KM, McConnell J. Delayed onset of electromyographic activity of vastus medialis obliquus relative to vastus lateralis in subjects with patellofemoral pain syndrome. Arch Phys Med Rehabil. 2001;82:183–189 [DOI] [PubMed] [Google Scholar]
- 14.Powers CM. The influence of abnormal hip mechanics on knee injury: a biomechanical perspective. J Orthop Sports Phys Ther. 2010;40(2):42–51 [DOI] [PubMed] [Google Scholar]
- 15.Powers CM, Ward SR, Fredericson M, Guillet M, Shellock FG. Patellofemoral kinematics during weight-bearing and non-weight-bearing knee extension in persons with lateral subluxation of the patella: A preliminary study. J Orthop Sports Phys Ther. 2003;33(11):677–685 [DOI] [PubMed] [Google Scholar]
- 16.Souza RB, Draper CE, Fredericson M, Powers CM. Femur rotation and patellofemoral joint kinematics: a weight-bearing magnetic resonance imaging analysis. J Orthop Sports Phys Ther. 2010;40(5):277–285 [DOI] [PubMed] [Google Scholar]
- 17.Bolgla LA, Malone TR, Umberger BR, Uhl TL. Hip strength and hip and knee kinematics during stair descent in females with and without patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2008;38(1):12–18 [DOI] [PubMed] [Google Scholar]
- 18.Willson JD, Davis I. Lower extremity strength and mechanics during jumping in women with patellofemoral pain. J Sport Rehabil. 2009;18(1):75–89 [DOI] [PubMed] [Google Scholar]
- 19.Souza RB, Powers CM. Differences in hip kinematics, muscle strength, and muscle activation between subjects with and without patellofemoral pain. J Orthop Sports Phys Ther. 2009;39(1):12–19 [DOI] [PubMed] [Google Scholar]
- 20.Dierks TA, Manal KT, Hamill J, Davis IS. Proximal and distal influences on hip and knee kinematics in runners with patellofemoral pain during a prolonged run. J Orthop Sports Phys Ther. 2008;38(8):448–456 [DOI] [PubMed] [Google Scholar]
- 21.Prins MR, van der Wurff P. Females with patellofemoral pain syndrome have weak hip muscles. a systematic review. Aust J Physiother. 2009;55:9–15 [DOI] [PubMed] [Google Scholar]
- 22.Earl JE, Hoch AZ. A proximal strengthening program improves pain, function, and biomechanics in women with patellofemoral pain syndrome. Am J Sports Med. 2011;39(1):154–163 [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa TH, Muniz TB, de Marche Baldon R, Marciel CD, de Menezes Reiff RB, Serrao FV. The effect of additional strengthening of hip abductor and lateral rotator muscles in patellofemoral pain syndrome: a randomized controlled pilot study. Clin Rehabil. 2008;22:1051–1060 [DOI] [PubMed] [Google Scholar]
- 24.Fukuda TY, Rossetto FM, Magalhães E, Bryk FF, Lucareli PR, de Almeida Aparecida Carvalho N. Short-term effects of hip abductors and lateral rotators strengthening in females with patellofemoral pain syndrome: a randomized controlled clinical trial. J Orthop Sports Phys Ther. 2010;40(11):736–742 [DOI] [PubMed] [Google Scholar]
- 25.Cowan SM, Crossley KM, Bennell KL. Altered hip and trunk muscle function in individuals with patellofemoral pain syndrome. Br J Sports Med. 2009;43:584–588 [DOI] [PubMed] [Google Scholar]
- 26.Bolgla LA, Boling MC. An update for the conservative management of patellofemoral pain syndrome: a systematic review of the literature from 2000 to 2010. Int J Sports Phys Ther. 2011;6(2):112–125 [PMC free article] [PubMed] [Google Scholar]
- 27.Zazulak BT, Ponce PL, Straub SJ, Medecky MJ, Avedisian L, Hewett TE. Gender comparison of hip muscle activity during single-leg landing. J Orthop Sports Phys Ther. 2005;35(5):292–299 [DOI] [PubMed] [Google Scholar]
- 28.Myer GD, Ford KR, Hewett TE. The effects of gender on quadriceps activation strategies during a maneuver that mimics a high ACL injury risk position. J Electromyogr Kinesiol. 2005;15:181–189 [DOI] [PubMed] [Google Scholar]
- 29.Ireland ML, Willson JD, Ballantyne BT, Davis IM. Hip strength in females with and without patellofemoral pain. J Orthop Sports Phys Ther. 2003;33(11):671–676 [DOI] [PubMed] [Google Scholar]
- 30.Crossley KM, Bennell KL, Cowan SM, Green S. Analysis of outcome measures for persons with patellofemoral pain: Which are reliable and valid? Arch Phys Med Rehabil. 2004;85:815–822 [DOI] [PubMed] [Google Scholar]
- 31.Cram JR, Kasman GS. Introduction to Surface Electromyography. Gaithersburg, MD: Aspen Publishers, Inc.; 1998 [Google Scholar]
- 32.Cowan SM, Bennell KL, Hodges PW. The test-retest reliability of the onset of concentric and eccentric vastus medialis obliquus and vastus lateralis electromyographic activity in a stair stepping task. Phys Ther Sport. 2000;1:129–136 [Google Scholar]
- 33.Hislop HJ, Montgomery J. Daniels & Worthingham's Muscle Testing. Techniques of manual examination. Vol 7 Philadelphia: W.B. Saunders; 2002 [Google Scholar]
- 34.Bohannon RW. Reference values for extremity muscle strength obtained by hand-held dynamometry from adults aged 20 to 79 years. Arch Phys Med Rehabil. 1997;78:26–32 [DOI] [PubMed] [Google Scholar]
- 35.Agre JC, Magness JL, Hull SZ, et al. Strength testing with a portable dynamometer: reliability for upper and lower extremities. Arch Phys Med Rehabil. 1987;68:454–458 [PubMed] [Google Scholar]
- 36.Bolgla LA, Malone TR, Umberger BR, Uhl TL. Reliability of electromyographic methods used for assessing hip and knee neuromuscular activity in females diagnosed with patellofemoral pain syndrome. J Electromyogr Kinesiol. 2010;20:142–147 [DOI] [PubMed] [Google Scholar]
- 37.Hodges PW, Bui BH. A comparison of computer-based methods for the determination of onset of muscle contraction using electromyography. Electroenceph Clin Neurophys. 1996;101:511–519 [DOI] [PubMed] [Google Scholar]
- 38.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70 [Google Scholar]
- 39.Cichanowski HR, Schmitt JS, Johnson RJ, Niemuth PE. Hip strength in collegiate female athletes with patellofemoral pain. Med Sci Sports Exer. 2007;39(8):1227–1232 [DOI] [PubMed] [Google Scholar]
- 40.Robinson RL, Nee RJ. Analysis of hip strength in females seeking physical therapy treatment for unilateral patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2007;37(5):232–238 [DOI] [PubMed] [Google Scholar]
- 41.Long-Rossi F, Salsich GB. Pain and hip lateral rotator muscle strength contribute to functional status in females with patellofemoral pain. Physiother Res Int. 2009;15(1):57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piva SR, Goodnite EA, Childs JD. Strength around the hip and flexibility of soft tissue in individuals with and without patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2005;35(12):793–801 [DOI] [PubMed] [Google Scholar]
- 43.Magalhães E, Fukuda TY, Sacramento SN, Forgas A, Cohen M, Abdalla RJ. A comparison of hip strength between sedentary females with and without patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2010;40(10):641–647 [DOI] [PubMed] [Google Scholar]
- 44.Powers CM, Perry J, Hsu A, Hislop HJ. Are patellofemoral pain and quadriceps strength associated with locomotor function? Phys Ther. 1997;77:1063–1074 [DOI] [PubMed] [Google Scholar]
- 45.Thomee R, Renstrom P, Karlsson J, Grimby G. Patellofemoral pain syndrome in young women, II: Muscle function in patients and healthy controls. Scand J Med Sci Sports. 1995;5:245–251 [PubMed] [Google Scholar]
- 46.Grabiner MD, Koh TJ, Draganich LF. Neuromechanics of the patellofemoral joint. Med Sci Sports Exer. 1994;26(1):10–21 [PubMed] [Google Scholar]
- 47.Stiene HA, Brosky T, Reinking MF, Nyland J, Mason MB. A comparison of closed kinetic chain and isokinetic joint isolation exercise in patients with patellofemoral dysfunction. J Orthop Sports Phys Ther. 1996;24(3):136–141 [DOI] [PubMed] [Google Scholar]
- 48.Bolgla LA, Uhl TL. Electromyographic analysis of hip rehabilitation exercises in a group of healthy subjects. J Orthop Sports Phys Ther. 2005;35(8):487–494 [DOI] [PubMed] [Google Scholar]
- 49.McFadyen BJ, Winter DA. An integrated biomechanical analysis of normal stair ascent and descent. J Biomech. 1988;21:733–744 [DOI] [PubMed] [Google Scholar]
- 50.Lyons K, Perry J, Gronley JK. Timing and relative intensity of hip extensors and abductor muscle action during level and stair ambulation. Phys Ther. 1983;63:1598–1605 [DOI] [PubMed] [Google Scholar]
- 51.Kadaba MP, Ramakrishnan ME, Wootten ME, Gainey J, Gorton G, Cochran GVB. Repeatability of kinematic, kinetic, and electromyographic data in normal adult gait. J Orthop Res. 1989;7:849–860 [DOI] [PubMed] [Google Scholar]
- 52.MacIntyre DL, Robertson GE. Quadriceps muscle activity in women runners with and without patellofemoral pain syndrome. Arch Phys Med Rehabil. 1992;73:10–14 [PubMed] [Google Scholar]
- 53.Souza DR, Gross MT. Comparison of vastus medialis oblique: vastus lateralis muscle integrated electromyographic ratios between healthy subjects and patients with patellofemoral pain. Phys Ther. 1991;71(4):310–320 [DOI] [PubMed] [Google Scholar]
- 54.Tyler TF, Nicholas SJ, Mullaney MJ, McHugh MP. The role of hip muscle function in the treatment of patellofemoral pain syndrome. Am J Sports Med. 2006;34(4):630–636 [DOI] [PubMed] [Google Scholar]
- 55.Basmajian JV, De Luca CJ. Apparatus, detection, and recording techniques. Muscles Alive, Their Functions Revealed by Electromyography. Baltimore, MD: Williams and Wilkins; 1985:19–64 [Google Scholar]