Abstract
The recruitment of circulating leukocytes from blood stream to the inflamed tissue is a crucial and complex process of inflammation1,2. In the postcapillary venules of inflamed tissue, leukocytes initially tether and roll on the luminal surface of venular wall. Rolling leukocytes arrest on endothelium and undergo firm adhesion in response to chemokine or other chemoattractants on the venular surface. Many adherent leukocytes relocate from the initial site of adhesion to the junctional extravasation site in endothelium, a process termed intraluminal crawling3. Following crawling, leukocytes move across endothelium (transmigration) and migrate in extravascular tissue toward the source of chemoattractant (chemotaxis)4. Intravital microscopy is a powerful tool for visualizing leukocyte-endothelial cell interactions in vivo and revealing cellular and molecular mechanisms of leukocyte recruitment2,5. In this report, we provide a comprehensive description of using brightfield intravital microscopy to visualize and determine the detailed processes of neutrophil recruitment in mouse cremaster muscle in response to the gradient of a neutrophil chemoattractant. To induce neutrophil recruitment, a small piece of agarose gel (~1-mm3 size) containing neutrophil chemoattractant MIP-2 (CXCL2, a CXC chemokine) or WKYMVm (Trp-Lys-Tyr-Val-D-Met, a synthetic analog of bacterial peptide) is placed on the muscle tissue adjacent to the observed postcapillary venule. With time-lapsed video photography and computer software ImageJ, neutrophil intraluminal crawling on endothelium, neutrophil transendothelial migration and the migration and chemotaxis in tissue are visualized and tracked. This protocol allows reliable and quantitative analysis of many neutrophil recruitment parameters such as intraluminal crawling velocity, transmigration time, detachment time, migration velocity, chemotaxis velocity and chemotaxis index in tissue. We demonstrate that using this protocol, these neutrophil recruitment parameters can be stably determined and the single cell locomotion conveniently tracked in vivo.
Protocol
1. Preparation of Chemoattractant in Agarose Gel
Pipette 10 mL of 2×PBS in a 50-mL conical tube, and warm up the tube by putting it into a beaker containing hot water.
Pipette 10 mL of distilled water and add 0.4 g agarose powder in another 50-mL conical tube (with its cap slightly loosened) and heat the mixture until just boiling in a microwave oven (for ~1 min in a 700-watt microwave oven).
Add the warmed 2×PBS to agarose solution tube, swirl the mixed solution and keep it warm in the hot water beaker.
Micropipette chemoattractant solution (e.g., 10 µl of 0.5 µg of CXC chemokine MIP-2 or 12 µl of 1 mM WKYMVm) into the lid of a 1.5-mL Eppendorf tube containing 3 µl India ink and mix well by aspiration using a micropipette (avoid air bubble).
Cut the tip end of a 200-µl pipet tip and micropipette 110 µl of agarose solution (42°C) into the lid and immediately mix well using another pipette tip (avoid air bubble).
Store the chemoattractant-containing gel at +4°C.
2. Preparation of Cremaster Muscle for Intravital Microscopy (Figure 1)
Anesthetize an adult male mouse by an i.p. injection of a mixture of 10 mg/kg xylazine and 200 mg/kg ketamine hydrochloride.
Shave the area over right external jugular vein and the anterior aspect of the scrotum with an electric razor. After anesthesia, it is important to give special attention and care to the anesthetized mouse. A heat lamp may be used to prevent the mouse from hypothermia. The mouse should be free from pain reflex.
Make a horizontal incision, find and catheterize the jugular vein using a PE-10 tubing filled with 100 U/mL heparin saline. Catheterization of the jugular vein is needed for the administration of additional anesthetics and drugs when required.
Fix mouse hind legs with umbilical tape with the mouse lying face up on a home-made cremaster muscle board (Figure 1).
Connect the board to a 37°C water circulator to keep the cremaster muscle and mouse body warm.
Note: All procedures from 2.6 to 2.14 must be performed very gently5.
Make an incision in the scrotal skin to expose the left cremaster muscle. Carefully dissect the muscle from the associated fascia.
Superfuse the cremaster muscle with 37°C-warmed bicarbonate-buffered saline (131.9 NaCl, 4.7 KCl, 1.2 MgSO4, 20 NaHCO3, in mM, pH 7.4) using a peristaltic pump.
Tie a 4−0 suture to the distal end of the cremaster muscle to hold it down on the clear viewing glass pedestal of the cremaster muscle board.
Cauterize the cremaster muscle longitudinally. With 4–0 suture, hold the muscle flat and secure it along the edges on the pedestal. Separate the testicle and the epididymis from the underlying muscle and move them into the abdominal cavity.
Superfuse the muscle at ~0.6 ml/min with the 37°C-warmed perfusion buffer and cover the exposed muscle with a 22×22 mm glass coverslip.
Place the cremaster muscle board on the microscope stage, examine the muscle under the microscope, find a suitable postcapillary venule (Select the venule that is straight and unbranched and has normal shear rate and a diameter in 25−40 µm) and adjust the video camera to allow the venule to be visualized in a vertical position on either left or right end of the TV monitor.
After 30 min's equilibrium, record the video images of the selected postcapillary venule for 5 min as baseline control data using a video recorder.
Stop the superfusion and remove the coverslip on the muscle.
Place an ~1-mm3 sized chemoattractant-containing gel on the surface of the cremaster muscle in a preselected area 350 µm from and parallel to the observed postcapillary venule, add coverslip to hold the gel in place and superfuse the muscle tissue at a very slow rate (≤10 µl/min) to allow the establishment of a gradient of chemoattractant that is slowly released and formed from the gel.
Record the video image for 90 min after the addition of the chemoattractant containing gel. During the recording, adjust and keep the microscope focus on the adhering, crawling, transmigrating and chemotaxing leukocytes inside the venule and in the muscle tissue.
After the experiment, import the video file to a computer for analysis.
3. Cell Tracking Using ImageJ
On a computer, extract and convert the video to AVI format (e.g., use free computer software bitRipper to convert DVD video to AVI file).
Use video editing software to generate the time-lapsed movie. For example, use Windows Movie Maker to make a time-lapsed movie (to 1/512 or 1/1024 time-lapse at 30-fps rate) from the original, real-time video. Convert and save the uncompressed time-lapsed movie to DV-AVI format.
Record the images of calibration micrometer under the same microscope setting, import images to the computer, open the images with ImageJ. In ImageJ, the total pixels appear on the top left of the screen (e.g., 720×480 pixels). Measure the size of the screen at both X and Y axes (e.g., 200×150 µm). From this, calculate the number of pixels per µm (e.g., x = 720/200 = 3.6 pixels/µm, and y = 480/150 = 3.2 pixels/µm).
To import the movie, open ImageJ again, click “File−Import−Using Quicktime Movies Plug-in”, select the movie to be analyzed and click “OK” at the interface of “QT Movie Opener”.
- Click “Plugins−Manual Tracking” to track cells. Fill in the relevant information into the fields at the bottom before start tracking. Briefly,
- Time interval (in sec) = fold-time-lapse/30 (For example, a 1020× time-lapse would be 1020/30 = 34 sec/frame interval).
- x/y calibration = the µm/pixel measurement using the calibration of the image of the micrometer.
- z calibration = 0 (as the mouse cremaster muscle is an extremely thin layer of tissue and cell crawling and migration are approximately 2D under bright-field transillumination).
- Search square size for centering = 1.
- Dot size/Line width/Font size: they can be adjusted if needed.
Select a stable and clear point as a reference point. This reference point can be any clear and small structural point that remains unchanged and stable throughout the whole experiment. Click “Add Track” to track the reference point from the first to the last frame and click “End Track”. The data appear on results table automatically.
Track crawling and migrating neutrophils one by one: click “Add Track” to track the cell from its appearance in tissue to its disappearance in each frame and click “End Track” to finish and save the results in Microsoft Excel.
4. Analysis of Neutrophil Recruitment Parameters
Open the results file in Microsoft Excel, and analyze the data (take the changes of reference point into analysis).
- Intraluminal crawling
- Crawling distance: the total distance the cell crawls in the lumen from the initial adherent site to the optimal transmigration site (µm).
- Crawling velocity: crawling distance/time (µm/min).
- Transendothelial migration
- Transmigration time: from the time the cell starts to transmigrate across endothelium to the time when the whole cell body is just outside the venule and no cell body can be seen in the lumen (min or sec).
- Detachment time: from the time the whole cell body is just outside the venule (immediately after transmigration) to the time point when the cell loses contact with the venule (the tail retracts) (min or sec).
- Chemotaxis in tissue
- Migration distance: the sum of the distance the cell moves from the start point to the end point of the migration in tissue (µm).
- Velocity of migration: migration distance in tissue/time (µm/min).
- Chemotaxis distance: the sum of distance the cell migrates in x-axis in tissue (µm).
- Velocity of chemotaxis: chemotaxis distance/time (µm/min).
- Chemotaxis index: the ratio of dividing the chemotaxis distance by the migration distance in tissue.
5. Representative Results:
Although bightfield intravital microscopy is used for the study of leukocyte-endothelial cell interactions and may not be necessarily for neutrophils, we confirmed that, by our histology studies, more than 95% of the recruited cells in neutrophil chemoattractant-treated cremaster muscles were indeed neutrophils. In this report, using neutrophil-selective chemoattractants, we present procedures of tracking the recruitment of neutrophils in vivo. Specifically, we describe a protocol of tracking neutrophil intraluminal crawling, transendothelial migration, and chemotaxis in cremaster muscle tissue in anesthetized mice using time-lapse intravital video microscopy and ImageJ. The chemoattractant-containing agarose gel on the cremaster muscle slowly releases chemoattractant and allows a chemoattractant gradient to be established in tissue. Neutrophil chemoattractant induces neutrophil-endothelial cell interactions in cremasteric postcapillary venules in mice. The whole experiment is visualized under an upright brightfield intravital microscope with video images projected by a color video camera to a TV monitor and recorded by a video recorder. We determined the neutrophil intraluminal crawling, transendothelial migration and migration and chemotaxis in muscle tissue in response to neutrophil chemoattractant MIP-2 and WKYMVm prepared in agarose gel (Figure 2). We found that MIP-2 (at 0.5 µM) and WKYMVm (at 0.1 mM) elicited neutrophil intraluminal crawling at similar velocity, neutrophil transendothelial migration and detachment from the venule for comparable length of time, neutrophil migration and chemotaxis in muscle tissue at nearly the same velocity and with similar neutrophil chemotaxis indexes (P > 0.05, Student t test).
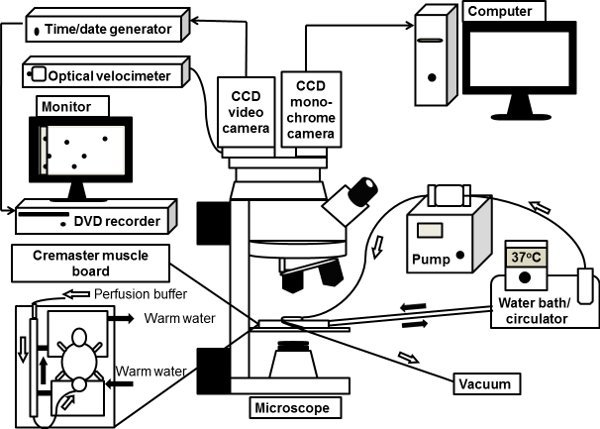 Figure 1. The schematic illustration of an intravital microscope system. The mouse
cremaster muscle is exteriorized on the clear viewing pedestal of cremaster muscle board
on microscope stage and superfused with 37°C-warmed bicarbonate-buffered saline. The
upright microscope is connected with a CCD color video camera for brightfield intravital
microscopy. A monochrome deep-cooled CCD digital camera is also connected to
microscope port for fluorescence intravital microscopy, the images from which are directly
processed by a computer.
Figure 1. The schematic illustration of an intravital microscope system. The mouse
cremaster muscle is exteriorized on the clear viewing pedestal of cremaster muscle board
on microscope stage and superfused with 37°C-warmed bicarbonate-buffered saline. The
upright microscope is connected with a CCD color video camera for brightfield intravital
microscopy. A monochrome deep-cooled CCD digital camera is also connected to
microscope port for fluorescence intravital microscopy, the images from which are directly
processed by a computer.
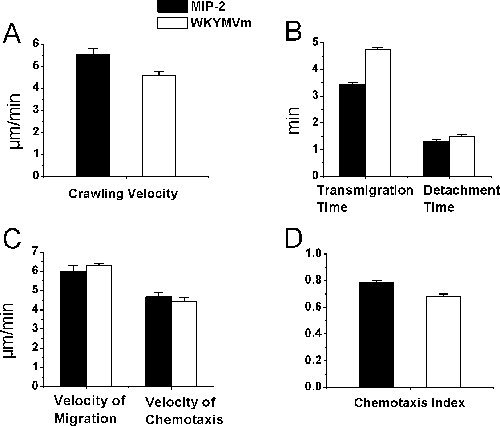 Figure 2. Neutrophil recruitment parameters of brightfield intravital microscopy.
Neutrophil recruitment was induced by the gradual release of neutrophil chemoattractant
MIP-2 or WKYMVm in the agarose gel preparation placed 350 µm adjacent to the
postcapillary venule. Time-lapsed video data were analyzed by ImageJ after processing
the real time video recording of the experiment. Neutrophil intraluminal crawling (A),
transmigration time and detachment time (B), migration velocity and chemotaxis velocity
in tissue (C), and chemotaxis index in cremaster muscle (D) were determined after the
administration of MIP-2 or WKYMVm agarose gel on cremaster muscle in C57BL/6 mice
(n = 3, # of tracked cells = 22 (in A and B) and 27 (in C and D) respectively for MIP-2, and
= 26 (in A and B) and 44 (in C and D) respectively for WKYMVm).
Figure 2. Neutrophil recruitment parameters of brightfield intravital microscopy.
Neutrophil recruitment was induced by the gradual release of neutrophil chemoattractant
MIP-2 or WKYMVm in the agarose gel preparation placed 350 µm adjacent to the
postcapillary venule. Time-lapsed video data were analyzed by ImageJ after processing
the real time video recording of the experiment. Neutrophil intraluminal crawling (A),
transmigration time and detachment time (B), migration velocity and chemotaxis velocity
in tissue (C), and chemotaxis index in cremaster muscle (D) were determined after the
administration of MIP-2 or WKYMVm agarose gel on cremaster muscle in C57BL/6 mice
(n = 3, # of tracked cells = 22 (in A and B) and 27 (in C and D) respectively for MIP-2, and
= 26 (in A and B) and 44 (in C and D) respectively for WKYMVm).
Discussion
Intravital microscopy is the essential tool for revealing the cellular and molecular mechanisms of leukocyte recruitment during inflammation. Quantitative visualization for determination of leukocyte-endothelial cell interactions in microvasculature of transluscent tissues such as cremaster muscle and mesentery remains the gold standard for the application of the technique1,5. The conventional brightfield intravital microscopy has many unique technical features and the recruitment mechanisms revealed in these tissues are applicable to most tissues in vivo1,2,6. However, the mechanisms of leukocyte recruitment in some other tissues such as the lung, liver and the brain have been found quite different from those revealed in cremaster muscle and mesentery1. In addition, fluorescence microscopy has to be used in some less transparent tissues.
Compared with the fluorescence-based microscopy which is known for photodamage to the functions of live cells, brightfield intravital microscopy is more physiological and less harmful to the cells and tissues (therefore with less artifacts) when long-time imaging for observing dynamic cellular behaviors in live animals is necessary7. It is also more convenient, less costly and no fluorescent labeling is needed. On the other hand, with transillumination imaging in intravital microscopy, automatic tracking cellular movement is impossible with currently available commercial imaging software on the market. However, it is very easy to set up a separate fluorescence intravital imaging system (e.g., by projecting the images to a fluorescence CCD camera and computer) on the same brightfield intravital microscope (Figure 1). This provides the convenience of switching between brightfield microscopy and fluorescence imaging on the same sample preparation in one single experiment. This also makes it possible to automatically track the movement of fluorescently labeled cells under fluorescence intravital microscopy when the contrast between the fluorescence intensity of labeled cells and the background is large enough and suitable imaging software is installed on the computer.
As depicted here in our presentation, we demonstrate the value of this in vivo technique for direct observation of the whole process of neutrophil recruitment and for the determination of the functions of cells and molecules in each recruitment step. With chemoattractant contained in agarose gel preparation and held on the tissue, a unidirectional chemotactic gradient can be established in the tissue that induces leukocyte recruitment responses resembling those naturally occurring during local inflammation8,9. The directional leukocyte movement from postcapillary venule toward the source of chemoattractant can be clearly visualized by brightfield intravital microscopy and video photography. With time-lapse video processing, cell movement can be tracked by ImageJ and a series of highly reproducible parameters can be measured. With specific transgenic mice, inhibitors and selective chemoattractants, the assay system helps us to reveal the functions of specific proteins in leukocyte recruitment. For example, this technique assisted us to identify the role for LSP1 in endothelial cells as the gatekeeper in the regulation of neutrophil transendothelial migration10, the role for Mac-1 (αMβ2 integrin) in neutrophil intraluminal crawling, an essential step for optimal transendothelial migration3, and the role for PI3Kγ in neutrophil chemotaxis in tissue11.
Disclosures
No conflicts of interest declared.
Acknowledgments
This work was supported by a research grant from Canadian Institutes of Health Research (CIHR, MOP-86749). L. Liu is a recipient of CIHR New Investigator Award (MSH-95374).
References
- Liu L, Kubes P. Molecular mechanisms of leukocyte recruitment: organ-specific mechanisms of action. Thromb. Haemost. 2003;89:213–220. [PubMed] [Google Scholar]
- Petri B, Phillipson M, Kubes P. The physiology of leukocyte recruitment: an in vivo perspective. J. Immunol. 2008;180:6439–6446. doi: 10.4049/jimmunol.180.10.6439. [DOI] [PubMed] [Google Scholar]
- Phillipson M. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J. Exp. Med. 2006;203:2569–2575. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CH, Heit B, Kubes P. Molecular regulators of leucocyte chemotaxis during inflammation. Cardiovasc. Res. 2010;86:183–191. doi: 10.1093/cvr/cvq040. [DOI] [PubMed] [Google Scholar]
- Gavins FN, Chatterjee BE. Intravital microscopy for the study of mouse microcirculation in anti-inflammatory drug research: focus on the mesentery and cremaster preparations. J. Pharmacol. Toxicol. Methods. 2004;49:1–14. doi: 10.1016/S1056-8719(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Bullen A. Microscopic imaging techniques for drug discovery. Nat. Rev. Drug Discov. 2008;7:54–67. doi: 10.1038/nrd2446. [DOI] [PubMed] [Google Scholar]
- Hazelwood KL. Entering the Portal: Understanding the Digital Image Recorded Through a Microscope. Imaging Cellular and Molecular Biological Functions. 2007:3–43. [Google Scholar]
- Hickey MJ. L-selectin facilitates emigration and extravascular locomotion of leukocytes during acute inflammatory responses in vivo. J. Immunol. 2000;165:7164–7170. doi: 10.4049/jimmunol.165.12.7164. [DOI] [PubMed] [Google Scholar]
- Cara DC, Kubes P. Intravital microscopy as a tool for studying recruitment and chemotaxis. Methods Mol. Biol. 2004;239:123–132. doi: 10.1385/1-59259-435-2:123. [DOI] [PubMed] [Google Scholar]
- Liu L. LSP1 is an endothelial gatekeeper of leukocyte transendothelial migration. J. Exp. Med. 2005;201:409–418. doi: 10.1084/jem.20040830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heit B. PI3K accelerates, but is not required for, neutrophil chemotaxis to fMLP. J. Cell Sci. 2008;121:205–214. doi: 10.1242/jcs.020412. [DOI] [PubMed] [Google Scholar]


