Abstract
The grafting of human tumor cells into the brain of immunosuppressed mice is an established method for the study of brain cancers including glioblastoma (glioma) and medulloblastoma. The widely used stereotactic approach only allows for the injection of a single animal at a time, is labor intensive and requires highly specialized equipment. The guide screw method, initially developed by Lal et al.,1 was developed to eliminate cumbersome stereotactic procedures. We now describe a modified guide screw approach that is rapid and exceptionally safe; both of which are critical ethical considerations. Notably, our procedure now incorporates an infusion pump that allows up to 10 animals to be simultaneously injected with tumor cells.
To demonstrate the utility of this procedure, we established human U87MG glioma cells as intracranial xenografts in mice, which were then treated with AMG102; a fully human antibody directed to HGF/scatter factor currently undergoing clinical evaluation2-5. Systemic injection of AMG102 significantly prolonged the survival of all mice with intracranial U87MG xenografts and resulted in a number of complete cures.
This study demonstrates that the guide screw method is an inexpensive, highly reproducible approach for establishing intracranial xenografts. Furthermore, it provides a relevant physiological model for validating novel therapeutic strategies for the treatment of brain cancers.
Keywords: Medicine, Issue 55, Neuroscience, Intracranial, Guide Screw, Xenografts, Glioma, Mouse
Protocol
1. Cell lines
U87MG glioma cells are cultured in large tissue culture flasks with DMEM-F12 supplemented with 5% fetal bovine serum (FBS).
Cells are harvested by washing flasks twice with warm Phosphate Buffered Saline (PBS) and incubating them at 37°C for 5 minutes with 10 ml of PBS containing 0.25% trypsin and 0.05% EDTA. Once cells are lifted, they are placed into a 50 ml tube containing 10 ml of culture media and centrifuged (300 X g for 4 min).
Following the wash, cells are resuspended at a concentration of 10 x 106/ml in culture media, which allowed for an inoculation of 50,000 cells/5 μl.
Cells are kept on ice until intracranial injection.
2. Guide screw intracranial bolting
This procedure can be carried out several days prior to the injection of cells. All procedures described here have been carried out under strict sterile conditions.
Mice (BALB/c nu/nu female; 5-6 weeks; approximately 18g) are numbered for identification purposes, weighed and anesthetised with an intraperitoneal (IP) injection of a mixture of ketamine (100 mg/kg) and xylazine (5 mg/kg).
The skin is wiped down with an iodine solution and a small incision (2-3 mm) is made along the right side of the midline and anterior to the interaural line. This exposes the coronal and sagittal sutures of the skull. The bregma is positioned at the junction of these two sutures.
The guide screw entry point is then marked at a point 2.5 mm lateral and 1 mm anterior to the bregma. This point is located directly above the caudate nucleus1.
A 1 x 1 mm deep hole is drilled with a sterile hand held twist drill through the skull to the dura.
A sterilised guide screw that will extend 1.6 mm below the skulls surface into the dura is then bolted into the hole with a screw driver until flush with the skull (Figure 1A).
A sterile stylet or screw dummy is then placed into the central hole of the guide screw to close the opening (Figure 1B).
The wound is closed with Vetbond Tissue Adhesive (n-butyl cyanoacrylate) and the mice are given an intraperitoneal injection of reversine (small animals) (0.1 ml/kg) and carprofen (5 mg/kg/100 μl) for analgesia. Mice are then allowed to recover on a warming mat (36°C) which can take up to 20 minutes. Mice are frequently monitored and observed during this recovery time until they are fully conscious.
3. Intracranial cellular engraftment
A sterile cuffed Hamilton syringe is prepared by applying a small plastic ring to the needle tip allowing only 2 mm of the needle to extend below the guide screw outlet (Figure 1B). The final inoculation point is therefore 3.5 mm below the skull surface.
Four days following the guide screw surgery, the mice are again anesthetized as above (2.1) and a small incision is made over the guide screw to remove the stylet.
The cuffed Hamilton syringe is then filled with 5 μl of well mixed cells taking precaution not to create or draw up any air bubbles.
The syringe is secured to the perfusion pump and the needle is inserted into the guide screw (Figure 1C). The cells are then infused at a rate of 30 μl per hour.
The automated apparatus (Figure 1D) allowed us to inject up to 10 animals at a time at a constant flow rate. Cells can also be injected manually by using the cuffed hamilton syringe or alternatively the syringe can be cuffed by a 200 μl pipette tip that has been trimmed by 3 mm to allow the needle tip to extend beyond the guide screw by 2 mm. The injection must then be performed at a constant steady pace.
When infusion is complete, the syringe is carefully removed and the stylet is replaced into the guide screw. The wound is glued closed and the mice are given recovery medications as before (2.7) and allowed to recover on a warming mat.
4. Therapeutic challenge
Four days after the U87MG cell inoculation, mice are weighed and randomly divided into control and treatment groups.
The control group is given an intra-peritoneal (IP) injection of PBS (100 μl), while the treatment group is given an IP injection of AMG102 (100 μg in 100 μl of PBS).
Injections are repeated every second day for 14 days, a total of 6 injections (Figure 2).
After the final injection, mice are monitored daily and weighed every second day.
When mice began to display significant neurological dysfunctions (balance disturbance, paralysis), dehydration, or more than 10% weight loss or moribund, they were humanely euthanised. These humane end-point days were then recorded on the Kaplan-Meier survival curve.
5. Evaluation of therapeutic efficiency
The Kaplan-Meier survival curve was generated using GraphPad Prism 4.03 for Windows; GraphPad Software, San Diego California USA, www.graphpad.com3. Deaths or euthanizations were marked as end-point events and scored as 1. Animals that remained alive at the end of the experiment were recorded as non-events and scored as 0.
6. Representative results:
Control animals began to show signs of neurological disturbance and weight loss 23 days after the initial inoculation of cells. This was significantly delayed in the AMG102 treated group to 35 days. Indeed by day 35, 77% (n = 7/9) of the control group had been euthanized. The Kaplan-Meier survival curve clearly demonstrates the significant increase in survival in response to AMG102 treatment (Figure 3). By day 70, 88% of control mice had been euthanized (n = 8/9) compared to 37% (n = 3/8) of AMG102 treated group. Histological analysis of the brains confirmed that all 9 animals in the control group developed tumors compared to only 3 of the 8 AMG102 treated mice (Figure 4).
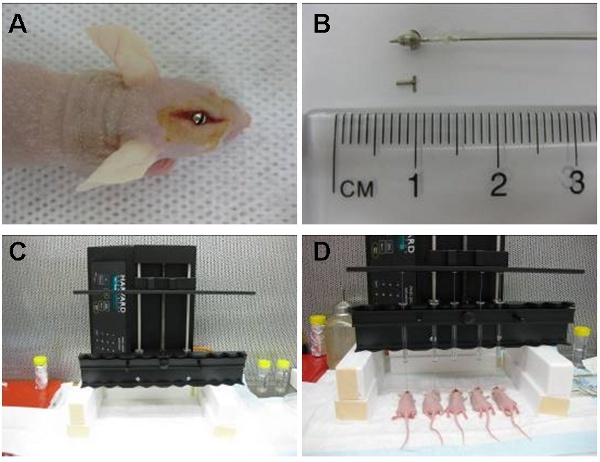 Figure 1: Image (A) shows intracranial guide screw positioned in an area located directly above the caudate nucleus. The cuffed hamilton syringe (pre-loaded with cells) is then placed inside the guide screw so that 2 mm of needle extends beyond the guide and the cells are injected 3.5 mm inside the caudate nucleus. The syringe is later removed and replaced by a stylet (B). The automatic infusion device (C) can inject up to 10 animals at a time. Shown here are five simultaneous injections (D).
Figure 1: Image (A) shows intracranial guide screw positioned in an area located directly above the caudate nucleus. The cuffed hamilton syringe (pre-loaded with cells) is then placed inside the guide screw so that 2 mm of needle extends beyond the guide and the cells are injected 3.5 mm inside the caudate nucleus. The syringe is later removed and replaced by a stylet (B). The automatic infusion device (C) can inject up to 10 animals at a time. Shown here are five simultaneous injections (D).
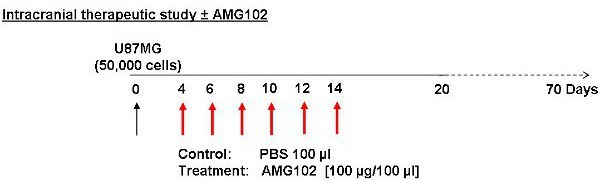 Figure 2: Schematic of study protocol. Cells were injected on day 0 with treatment commencing on day 4. Control animals received an injection of PBS (100 μl) IP while the treatment group received an injection of AMG102 (100 μg/100 μl) IP. These injections were given every second day for 10 days. A total of 6 injections were given. Animals were then monitored for 70 days for signs of neurological and physical disturbances.
Figure 2: Schematic of study protocol. Cells were injected on day 0 with treatment commencing on day 4. Control animals received an injection of PBS (100 μl) IP while the treatment group received an injection of AMG102 (100 μg/100 μl) IP. These injections were given every second day for 10 days. A total of 6 injections were given. Animals were then monitored for 70 days for signs of neurological and physical disturbances.
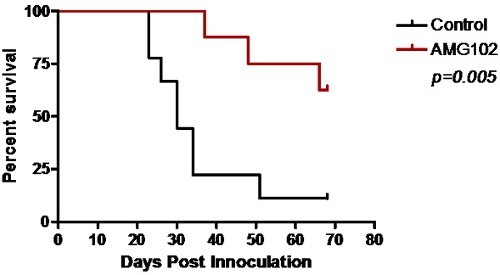 Figure 3: Kaplan-Meier survival curve comparing control and AMG102 treated mice. Significant survival was obtained following treatment with AMG102 (p=0.005).
Figure 3: Kaplan-Meier survival curve comparing control and AMG102 treated mice. Significant survival was obtained following treatment with AMG102 (p=0.005).
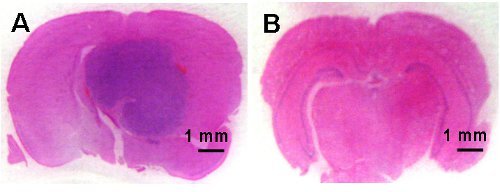 Figure 4: Microphotographs of coronal brain sections demonstrating tumor development in the control animals (A) and no brain tumor development in the AMG102 treated animals (B). Scale bar is 1 mm.
Figure 4: Microphotographs of coronal brain sections demonstrating tumor development in the control animals (A) and no brain tumor development in the AMG102 treated animals (B). Scale bar is 1 mm.
Discussion
The intracranial guide screw method presented here enables the rapid and reproducible establishment of intracranial xenografts and in the hands of a trained animal technician, this procedure is very easily performed without the need for a stereotactic device. Our method combines the use of a guide screw, to accurately locate the region of brain most suitable for in vivo tumor development, and an automated infusion pump that allows for the simultaneous inoculation of up to 10 animals at a time. The guide screw augmentation procedure takes less than 5 minutes to perform. The automated infusion of cells at 30 μl/hour takes just 10 minutes for a 5 μl volume. This ensures accurate dosing at a constant rate and reduces the risk of cell expulsion into the extra-cranial area due to increased intracranial pressure. We have also performed this procedure with 10 μl volumes and have not observed any extra-cranial tumor growth due to cellular reflux. As the automated infusion pump also allows several animals to be inoculated simultaneously we can inject up to 60 animals per hour compared to a stereotactic procedure which is limited to approximately 15 animals per hour.
The most critical step in this procedure is the location of guide screw in the skull as its position determines the ultimate location of the tumor bulk. If the guide screw is too central, the cells will be injected into a region between the two brain hemispheres whereas if the guide is placed to far forward, the cells will not be injected into the brain at all. It is therefore essential to be as accurate as possible in locating the guide 2.5 mm lateral and 1 mm anterior to the bregma1. In this study we used a steel guide screw, although plastic guide screws are also available for those studies that require brain imaging such as MRI or PET6. Even if the automated infusion pump is cost prohibitive for some groups, cells can still be injected manually more quickly than stereotactic approaches. Overall, this rapid, accurate and reproducible method can be used to study glioma, medulloblastoma and brain stem tumors7. This procedure has been performed on over 500 mice between our two laboratories with deaths due to unexpected anesthetic reactions (<1%). Mice that were bolted with the guide screw and subsequently infused with cells did not die or suffer any neurological complications as a result of the procedure. Animals have survived past 100 days without any complications. Although we preferred to use a hairless mouse, an immuno-compromised mouse with hair like the SCID would be just as appropriate provided the skull hair was removed prior to the surgery to keep the site clean and to prevent hair interfering with guide screw.
There are several novel therapeutics currently being developed for the treatment of glioma8-10. Recently we showed that subcutaneous U87MG glioma xenografts can be inhibited by treatment with AMG1023; a humanized antibody directed to HGF currently undergoing clinical evaluation in glioma5. We used the method describe here to establish intracranial U87MG tumors in the brains of nude mice. Our study clearly shows the utility of the method and demonstrates that AMG102 significantly inhibits the growth of orthotopic U87MG xenografts. We will continue to use this model to determine what other therapeutics can be used in combination with AMG102 to obtain more effective inhibition of tumor growth.
Disclosures
This work was supported by a Research Grant from Amgen Inc.
Acknowledgments
The authors would like to thank Verlene Henry and Lindsay Holmes for assistance in developing this model. This work was partially funded by the James S. McDonnell Foundation (#220020173).
References
- Lal S. An implantable guide-screw system for brain tumor studies in small animals. J Neurosurg. 2000;92:326–333. doi: 10.3171/jns.2000.92.2.0326. [DOI] [PubMed] [Google Scholar]
- Jun HT. AMG 102, a fully human anti-hepatocyte growth factor/scatter factor neutralizing antibody, enhances the efficacy of temozolomide or docetaxel in U-87 MG cells and xenografts. Clin Cancer Res. 2007;13:6735–6742. doi: 10.1158/1078-0432.CCR-06-2969. [DOI] [PubMed] [Google Scholar]
- Pillay V. The plasticity of oncogene addiction: implications for targeted therapies directed to receptor tyrosine kinases. Neoplasia. 2009;11:448–458. doi: 10.1593/neo.09230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan IM. Radiosensitization of glioma cells by modulation of Met signaling with the hepatocyte growth factor neutralizing antibody. AMG102. J Cell Mol Med. 2011;15:1999–2006. doi: 10.1111/j.1582-4934.2010.01122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen PY. A phase II study evaluating the efficacy and safety of AMG 102 (rilotumumab) in patients with recurrent glioblastoma. Neuro Oncol. 2011;13:437–446. doi: 10.1093/neuonc/noq198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caretti V. Monitoring of Tumor Growth and Post-Irradiation Recurrence in a Diffuse Intrinsic Pontine Glioma Mouse Model. Brain Pathol. 2011;4:441–451. doi: 10.1111/j.1750-3639.2010.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jallo GI, Volkov A, Wong C, Carson BS, Penno MB. A novel brainstem tumor model: functional and histopathological characterization. Childs Nerv. Syst. 2006;22:1519–1525. doi: 10.1007/s00381-006-0174-8. [DOI] [PubMed] [Google Scholar]
- Johns TG. The efficacy of epidermal growth factor receptor-specific antibodies against gliomaxenografts is influenced by receptor levels, activation status, and heterodimerization. Clin Cancer Res. 2007;13:1911–1925. doi: 10.1158/1078-0432.CCR-06-1453. [DOI] [PubMed] [Google Scholar]
- Scott AM. A phase I clinical trial with monoclonal antibody ch806 targeting transitional state and mutant epidermal growth factor receptors. Proc. Natl. Acad. Sci. U. S. A. 2007;104:4071–4076. doi: 10.1073/pnas.0611693104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyazi M. Therapeutic options for recurrent malignant glioma. Radiother. Oncol. 2011;98:1–14. doi: 10.1016/j.radonc.2010.11.006. [DOI] [PubMed] [Google Scholar]


