Abstract
The mouse heart is a popular model for cardiovascular studies due to the existence of low cost technology for genetic engineering in this species. Cardiovascular physiological phenotyping of the mouse heart can be easily done using fluorescence imaging employing various probes for transmembrane potential (Vm), calcium transients (CaT), and other parameters. Excitation-contraction coupling is characterized by action potential and intracellular calcium dynamics; therefore, it is critically important to map both Vm and CaT simultaneously from the same location on the heart1-4. Simultaneous optical mapping from Langendorff perfused mouse hearts has the potential to elucidate mechanisms underlying heart failure, arrhythmias, metabolic disease, and other heart diseases. Visualization of activation, conduction velocity, action potential duration, and other parameters at a myriad of sites cannot be achieved from cellular level investigation but is well solved by optical mapping1,5,6. In this paper we present the instrumentation setup and experimental conditions for simultaneous optical mapping of Vm and CaT in mouse hearts with high spatio-temporal resolution using state-of-the-art CMOS imaging technology. Consistent optical recordings obtained with this method illustrate that simultaneous optical mapping of Langendorff perfused mouse hearts is both feasible and reliable.
Keywords: Bioengineering, Issue 55, optical mapping, action potential, calcium transient, mouse, heart
Protocol
1. Advanced preparation of stock solutions
- Prepare two stock solutions of Tyrode's solution (16x) in advance in deionized water and store them at 4°C:
- Stock I (119.872 g/L NaCl, 3.056 g/L CaCl2 (2H2O), 5.6 g/L KCl, 2.6274 g/L NaH2PO4, 3.408 g/L MgCl2 (6H2O), (Fisher Scientific, Fair Lawn, NJ));
- Stock II (26.88 g/L NaHCO3, (Fisher Scientific, Fair Lawn, NJ)).
- Prepare stock solutions of fluorescent dyes. To avoid repeated freezing and thawing, we store 30 μl aliquots of both dyes at -20°C, which is sufficient for one experiment:
- Voltage-sensitive dye RH237 (Invitrogen, Carlsbad, CA) stock solution, 1.25 mg/mL solution in dimethyl sulfoxide (DMSO, Sigma, St. Louis, MO);
- Calcium indicator Rhod-2AM (Invitrogen, Carlsbad, CA) stock solution, 1 mg/mL solution in DMSO.
Prepare excitation-contraction uncoupler blebbistatin stock solution (Tocris Bioscience, St. Louis, MO, 2 mg/mL solution in DMSO) in advance and store the dissolved blebbistatin at 4°C.
2. Prepare perfusion solutions and experimental setup7
Freshly prepare 2L Tyrode's solution (128.2mM NaCl, 1.3mM CaCl2 (2H2O), 4.7mM KCl, 1.05mM MgCl2 (6H2O), 1.19mM NaH2PO4, 20mM NaHCO3, 11.1mM D-Glucose in deionized water, pH=7.35±0.05). If stock solution is being used to make 2L of Tyrode's solution (sufficient for one experiment) take 1750 mL of deionized water and mix in 125 mL of Stock I, 125 mL of Stock II, and 4g of glucose.
Turn on the two pumps of the perfusion systems. Set the peristaltic pump (Peri-Star, WPI, Sarasota, USA) that is used for retrograde perfusion to 40 mL/min. Set the other peristaltic pump (Cole-Parmer Masterflex Peristalic Pump L/S, Cole-Parmer Instrument Company, Vernon Hills, Illinois) that is used for superfusion and to return the perfusate back to the holding reservoir to 80 mL/min.
Wash the perfusion system with 70% ethanol for 30 min and then with 2L deionized water.
Once all the deionized water is evacuated from the chamber, circulate the Tyrode's solution and pass it through a 5-μm filter (Millipore, Billerica, MA, USA). Warm the perfusate to +37°C with a water jacket and circulator (ThermoNESLAB EX7, Newtown, USA) and oxygenate the perfusate by bubbling O2/CO2 (95% / 5%) gas into the solution. Monitor the pH of the solution with a pH meter (Oakton Instruments, Vernon Hills, IL) and adjust the rate of O2/CO2 bubbling to keep the pH at 7.35 ± .05. Continue monitoring pH and temperature during the experiment.
The dual optical mapping apparatus consists of two MiCAM Ultima-L CMOS cameras (SciMedia, Costa Mesa, CA) that have high spatial (100x100 pixels, 230±20 μm per pixel) and temporal (1,000-3,000 frames/sec) resolution. Fix a band-pass filter (590±15 nm, Thorlabs, Newton, NJ) in front of the designated calcium imaging camera; while, a long-pass filter (>700 nm, Thorlabs, Newton, NJ) needs to be positioned in front of the designated voltage imaging camera. The cameras are arranged perpendicular to one another by a holder, which contains a dichroic mirror (635 nm cutoff, Omega Optical, Brattleboro, VT). Immediately below the dual camera holder there is a lens (Nikon NIKKOR 55 mm 1:1.4 235052), which focuses the emission light coming from the heart onto the dichroic mirror. The working distance is approximately 3cm. The excitation light is generated by a halogen lamp (Newport Oriel Instruments, Stratford, CT; SciMedia, Costa Mesa, CA) and is passed through a heat filter, shutter, and band-pass filter (520±45 nm). A flexible light guide directs the band-pass filtered light onto the preparation, and a shutter is used to ensure that the preparation is exposed to light only during image acquisition to avoid photobleaching of the dyes.
Prepare Ag/AgCl2 electrodes for pacing and sensing in advance and install them in the chamber prior to placing the heart. Make sure that amplifiers and filters are adjusted to appropriate levels.
3. Harvest the mouse heart, cannulate, and set up Langendorff perfusion
Anesthetize the mouse with ketamine/xylazine (ketamin, 80mg/kg bodyweight; xylazine, 10 mg/kg bodyweight) and heparin (100 units) by intraperitoneal injection. Assure appropriate level of anesthesia by the lack of pain reflex.
After a mid-sternal incision, quickly remove the heart and wash it in oxygenated (95% O2, 5% CO2), constant-temperature (37±1°C) Tyrode's solution.
Using a dissecting microscope, rapidly identify the aorta and make a clean cut across the ascending aorta below the right subclavian artery. A short section of aorta is then attached to a custom made 21-gauge cannula with a flared tip. 4-0 black-braided silk suture (Surgical Specialties Corporation, Reading, PA) is used to fix the heart onto the cannula. After cannulation, the heart is retrogradely perfused and superfused with Tyrode's solution. The retrograde perfusion rate is adjusted in the range of 2-5 mL/min to keep the aortic pressure between 60 and 80 mmHg (Pressure transducer, World precision instruments Inc (WPI), Sarasota, USA; Bridge Amplifier TBM4M, WPI, Sarasota, USA).
After the heart is cannulated, lung, thymus, and fat tissue are then dissected and removed.
The isolated heart is pinned (Fine Science Tools) at the apex to the bottom of the perfusion chamber (Sylgard coated) to prevent stream-induced movement. The right and left atrial appendages are also stretched and pinned (Fine Science Tools, Inc, Foster City, CA) to the bottom of the chamber, which provides maximal surface area for optical measurements of the atria. Very important! A small silicon tube is inserted into the left ventricle through the pulmonary veins and fixed by silk suture to nearby connective tissue. This prevents solution congestion and acidification of the perfusate trapped in the left ventricle, which is especially important after the suppression of ventricular contractions with an excitation-contraction uncoupler (see Part 4 step 19).
A custom made electrode is placed on the surface of the heart to conduct the pacing stimulations, which is generated by Master-8 (A.M.P Instruments Ltd, Jerusalem, Israel) or PowerLab 26T (AD Instruments, Sydney, Australia).
A small cover glass is fixed on the surface of the solution over the heart to reduce motion artifact from the vibrating solution.
Focus the excitation light on the heart. In addition, adjust the distance between the dual camera apparatus and the heart so that maximum resolution will be obtained.
Turn off all lights in the room and commence electrical recordings using PowerLab 26T.
4. Load voltage and calcium sensitive dyes and excitation-contraction uncoupler
Warm-up 0.6 mL of blebbistatin. Mix 0.5 mL of the blebbistatin with the perfusate in the holding reservoir. Dilute the remaining 0.1 mL of blebbistatin in 1 mL of Tyrode's solution and slowly inject it (over a 20 minute period) through a drug port located near the cannula. Observe as blebbistatin gradually reduces the motion artifact.
Dilute 30 μL of voltage sensitive dye RH237 stock solution in 1 mL Tyrode's solution and slowly inject over 5-7 minutes into the same injection port as blebbistatin.
30 μL calcium indicator Rhod-2 AM is 1:1 mixed with Pluronic F127 (Invitrogen, Carlsbad, CA, 20% solution in DMSO) and then diluted in 1 mL Tyrode's solution and slowly applied over 5-10 min through the same injection port.
Wait for 5-10 minutes for blebbistatin and dyes to reach the cell membrane and cytosol. Continue with the protocol when motion is completely suppressed.
Continuously monitor ECG recordings over the entire procedure to assure normal electrical function of the heart.
Commence fluorescent signal recordings using the SciMedia custom software (SciMedia, Costa Mesa, CA).
5. Representative results:
 Figure 1. Experimental setup for the perfusion, electrical recordings and optical mapping. EM=emission; Lp=longpass
Figure 1. Experimental setup for the perfusion, electrical recordings and optical mapping. EM=emission; Lp=longpass
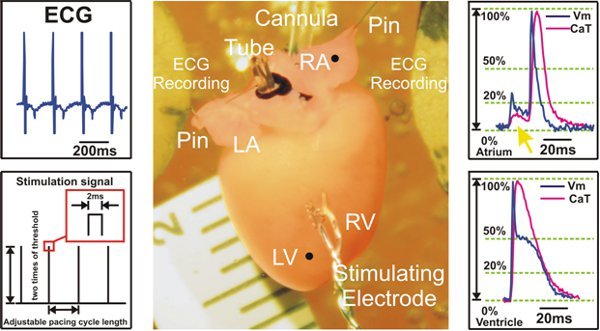 Figure 2. Experimental preparation and signal examples recorded during ventricular pacing. Left: ECG signals are collected from Ag/AgCl2 disk electrodes (Top) and an example of S1S1 stimulation protocol is shown (Bottom). Center: Langendorff mouse heart preparation. Right: Representative optical action potentials and calcium transient signals from atria (Top) and ventricles (Bottom) are shown. The yellow arrow (Top) points to the fluorescent signal scattering coming from the ventricles, which is seen in the atrial recordings. LV=left ventricle; RV=Right ventricle; LA=left atrium; RA=right atrium
Figure 2. Experimental preparation and signal examples recorded during ventricular pacing. Left: ECG signals are collected from Ag/AgCl2 disk electrodes (Top) and an example of S1S1 stimulation protocol is shown (Bottom). Center: Langendorff mouse heart preparation. Right: Representative optical action potentials and calcium transient signals from atria (Top) and ventricles (Bottom) are shown. The yellow arrow (Top) points to the fluorescent signal scattering coming from the ventricles, which is seen in the atrial recordings. LV=left ventricle; RV=Right ventricle; LA=left atrium; RA=right atrium
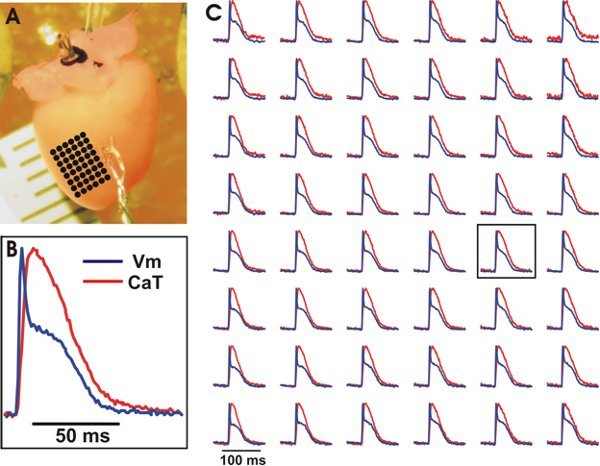 Figure 3. Representative optical recordings of Vm and CaT from the ventricles of a wild type mouse heart. A. The experimental preparation with an array of evenly spaced locations marked by black dots whose optical recordings can be seen in (C). B. Example tracing of Vm and CaT from a central location on the array (see box in (C)). C. Vm (blue) and CaT (red) from the array of evenly spaced locations. Signals were binned 3x3.
Figure 3. Representative optical recordings of Vm and CaT from the ventricles of a wild type mouse heart. A. The experimental preparation with an array of evenly spaced locations marked by black dots whose optical recordings can be seen in (C). B. Example tracing of Vm and CaT from a central location on the array (see box in (C)). C. Vm (blue) and CaT (red) from the array of evenly spaced locations. Signals were binned 3x3.
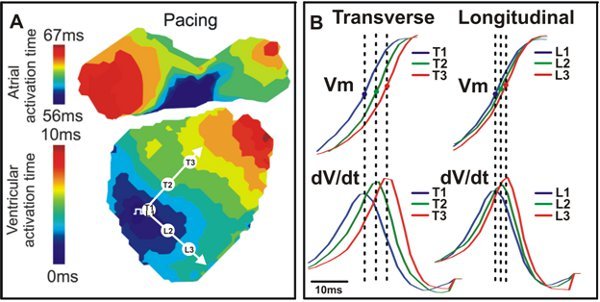 Figure 4. Activation map and conduction. A. An example activation map from a wild type mouse heart with transverse (T) and longitudinal (L) directions indicated by white arrows. B. Vm signals (Top) and dV/dt (Bottom) corresponding to the three points seen in A (T1, T2, T3; L1, L2, L3).
Figure 4. Activation map and conduction. A. An example activation map from a wild type mouse heart with transverse (T) and longitudinal (L) directions indicated by white arrows. B. Vm signals (Top) and dV/dt (Bottom) corresponding to the three points seen in A (T1, T2, T3; L1, L2, L3).
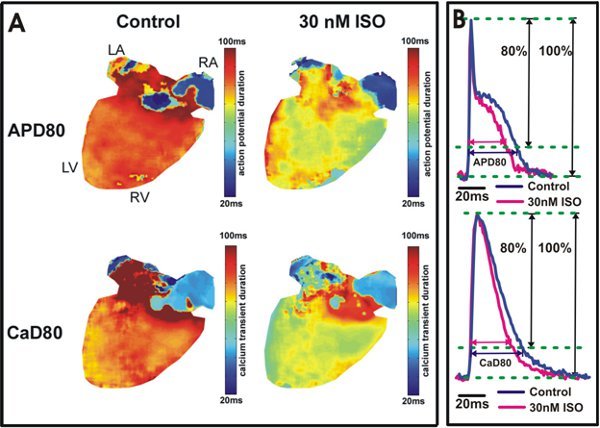 Figure 5. Action potential and calcium transient duration analysis. A. Action potential duration at 80% repolarization (APD80) and calcium transient duration at 80% relaxation (CaD80) maps are shown from a heart under control conditions (Left) and after 30 nM isoproterenol application (Right). The yellow/green color in the ventricles (Right) indicates isoproterenol shortened APD80 and CaD80. B. Example tracings of APD80 and CaD80 from the wild type mouse ventricles (Top) and atria (Bottom).
Figure 5. Action potential and calcium transient duration analysis. A. Action potential duration at 80% repolarization (APD80) and calcium transient duration at 80% relaxation (CaD80) maps are shown from a heart under control conditions (Left) and after 30 nM isoproterenol application (Right). The yellow/green color in the ventricles (Right) indicates isoproterenol shortened APD80 and CaD80. B. Example tracings of APD80 and CaD80 from the wild type mouse ventricles (Top) and atria (Bottom).
Discussion
In this experiment we modified the Langendorff perfusion method by adding a small silicon tube, which is especially crucial after the suppression of ventricular contractions with an excitation-contraction uncoupler. The silicon tube is used to prevent solution congestion, acidification of the perfusion solution, and development of ischemia in the left ventricle. The mouse heart is very sensitive to hypothermia; thus, temperature variations across the heart will cause artificial differences in action potential durations. Consequently, a heating system was implemented in the perfusion chamber in order to maintain a constant temperature of 37°C during the entirety of the experiment8. Since a Langendorff model does not retain innervation of the heart, one needs to consider adding neurotransmitters to the perfusate in order to investigate physiological sympathetic and parasympathetic tone9. Besides retrograde perfusion, addition of superfusion of the heart helps to maintain suitable environmental parameters such as pH and temperature. In this method, the Langendorff perfused heart was horizontally placed. A vertical Langendorff perfusion setup can also be used10, but may result in slightly different cardiac mechanics11. In addition to CMOS cameras, alternative detectors are also available and can be applied to map Vm and CaT simultaneously12.
Application of CMOS cameras of high spatio-temporal resolution assures the accuracy of the recordings; however, optical mapping signals are not from a single cell. Rather, each fluorescent signal comes from hundreds or thousands of cells, depending on optical magnification. The much larger ventricular fluorescence can distort atrial signals by optical scattering; therefore, careful interpretation of the optically recorded signals is required. Another limitation of the mouse preparation is the signal distortion and noise induced by the curvature of the surface due to the small size of the heart13. Conduction velocity measurements can be altered not only from the curvature of the mouse heart but also from electrode polarity and virtual electrodes. To achieve accuracy for conduction velocity, activation anisotropy, and repolarization maps, correct focusing of the camera at the surface of the heart is essential.
In this method, real time ECG recordings can supplement the optical investigation of cardiac electrophysiology. Voltage-sensitive dye (RH237) and calcium indicator (Rhod-2AM) are used in the protocol because of their fast response, similar excitation, and distinct emission spectra3,7. There are alternative combinations of dyes that can be used to measure Vm and CaT other than RH237 and Rhod-2AM3. A novel voltage-sensitive dye, PGHI, with a large Stoke's shift (>200nm) was found to allow better Vm and CaT signals because of the greater separation of the emission wavelengths between PGHI and Rhod-2AM14. Future improvements may focus on exploring novel fluorescent probes, development of new imaging detectors, and improved image processing software. Higher resolution and novel optical imaging modalities for 3D optical mapping are also important future directions of optical mapping5.
Disclosures
No conflicts of interest declared.
Acknowledgments
NIH grants R01 HL085369.
References
- Efimov IR, Rendt JM, Salama G. Optical maps of intracellular [Ca2+]i transients and action-potentials from the surface of perfused guinea-pig hearts. Circulation. 1994;90:1–1. [Google Scholar]
- Efimov IR. Optical mapping of repolarization and refractoriness from intact hearts. Circulation. 1994;90:1469–1480. doi: 10.1161/01.cir.90.3.1469. [DOI] [PubMed] [Google Scholar]
- Choi BR, Salama G. Simultaneous maps of optical action potentials and calcium transients in guinea-pig hearts: mechanisms underlying concordant alternans. J Physiol. 2000;529:171–188. doi: 10.1111/j.1469-7793.2000.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruvot EJ. Role of calcium cycling versus restitution in the mechanism of repolarization alternans. Circ Res. 2004;94:1083–1090. doi: 10.1161/01.RES.0000125629.72053.95. [DOI] [PubMed] [Google Scholar]
- Efimov IR, Nikolski VP, Salama G. Optical imaging of the heart. Circ Res. 2004;95:21–33. doi: 10.1161/01.RES.0000130529.18016.35. [DOI] [PubMed] [Google Scholar]
- Fast VG. Recording action potential using voltage sensitive dyes. In: Dhein S, Delmoar M, editors. Practical methods in cardiovascular research. New York: Springer; 2005. pp. 233–255. [Google Scholar]
- Glukhov AV. Differential K(ATP) channel pharmacology in intact mouse heart. J Mol Cell Cardiol. 2009. [DOI] [PMC free article] [PubMed]
- Baker LC. Enhanced dispersion of repolarization and refractoriness in transgenic mouse hearts promotes reentrant ventricular tachycardia. Circ Res. 2000;86:396–407. doi: 10.1161/01.res.86.4.396. [DOI] [PubMed] [Google Scholar]
- Sutherland FJ, Hearse DJ. The isolated blood and perfusion fluid perfused heart. Pharmacol Res. 2000;41:613–627. doi: 10.1006/phrs.1999.0653. [DOI] [PubMed] [Google Scholar]
- Efimov IR. Virtual electrode polarization in the far field: implications for external defibrillation. Am J Physiol Heart Circ Physiol. 2000;279:1055–1070. doi: 10.1152/ajpheart.2000.279.3.H1055. [DOI] [PubMed] [Google Scholar]
- Hammouda M, Kinosita R. The coronary circulation in the isolated heart. J Physiol. 1926;61:615–628. doi: 10.1113/jphysiol.1926.sp002322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama G, Hwang SM. Simultaneous optical mapping of intracellular free calcium and action potentials from Langendorff perfused hearts. Curr Protoc Cytom. 2009;12:17–17. doi: 10.1002/0471142956.cy1217s49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou Q. Quantitative panoramic imaging of epicardial electrical activity. Ann Biomed Eng. 2008;36:1649–1658. doi: 10.1007/s10439-008-9539-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama G. Properties of new, long-wavelength, voltage-sensitive dyes in the heart. J Membr Biol. 2005;208:125–140. doi: 10.1007/s00232-005-0826-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


