Abstract
Hsp90 is an essential and highly abundant molecular chaperone protein that has been found to regulate more than 150 eukaryotic signaling proteins, including transcription factors (e.g. nuclear receptors, p53) and protein kinases (e.g. Src, Raf, Akt kinase) involved in cell cycling, tumorigenesis, apoptosis, and multiple eukaryotic signaling pathways 1,2. Of these many 'client' proteins for hsp90, the assembly of steroid receptor•hsp90 complexes is the best defined (Figure 1). We present here an adaptable glucocorticoid receptor (GR) immunoprecipitation assay and in vitro GR•hsp90 reconstitution method that may be readily used to probe eukaryotic hsp90 functional activity, hsp90-mediated steroid receptor ligand binding, and molecular chaperone cofactor requirements. For example, this assay can be used to test hsp90 cofactor requirements and the effects of adding exogenous compounds to the reconstitution process.
The GR has been a particularly useful system for studying hsp90 because the receptor must be bound to hsp90 to have an open ligand binding cleft that is accessible to steroid 3. Endogenous, unliganded GR is present in the cytoplasm of mammalian cells noncovalently bound to hsp90. As found in the endogenous GR•hsp90 heterocomplex, the GR ligand binding cleft is open and capable of binding steroid. If hsp90 dissociates from the GR or if its function is inhibited, the receptor is unable to bind steroid and requires reconstitution of the GR•hsp90 heterocomplex before steroid binding activity is restored 4 . GR can be immunoprecipitated from cell cytosol using a monoclonal antibody, and proteins such as hsp90 complexed to the GR can be assayed by western blot. Steroid binding activity of the immunoprecipitated GR can be determined by incubating the immunopellet with [3H]steroid.
Previous experiments have shown hsp90-mediated opening of the GR ligand binding cleft requires hsp70, a second molecular chaperone also essential for eukaryotic cell viability. Biochemical activity of hsp90 and hsp70 are catalyzed by co-chaperone proteins Hop, hsp40, and p23 5. A multiprotein chaperone machinery containing hsp90, hsp70, Hop, and hsp40 are endogenously present in eukaryotic cell cytoplasm, and reticulocyte lysate provides a chaperone-rich protein source 6.
In the method presented, GR is immunoadsorbed from cell cytosol and stripped of the endogenous hsp90/hsp70 chaperone machinery using mild salt conditions. The salt-stripped GR is then incubated with reticulocyte lysate, ATP, and K+, which results in the reconstitution of the GR•hsp90 heterocomplex and reactivation of steroid binding activity 7. This method can be utilized to test the effects of various chaperone cofactors, novel proteins, and experimental hsp90 or GR inhibitors in order to determine their functional significance on hsp90-mediated steroid binding 8-11.
Protocol
1. Preparation of cell cytosol containing functional GR
Technical note: All buffers should be refrigerated and each step of this protocol, including centrifugations and incubation, should be performed on ice or at 4 °C, unless otherwise noted. The low temperature is essential to prevent degradation of the GR and protein complexes.
Using a refrigerated centrifuge, obtain a ˜1-5 ml pellet of cells that expresses a high concentration of functional GR. Examples of cell sources rich in GR include mouse fibroblast L929 cells, which express a high concentration of endogenous GR, and Sf9 cells that have been infected with recombinant GR baculovirus (e.g. the p2Bac-mGR baculovirus 12). Approximately 15-20 ml of packed cells is obtained per liter of Sf9 baculovirus cell culture. Cells should be growing exponentially prior to harvesting. Centrifuge the cell suspension at 5,000 × g for 5 min.
Wash the cell pellet three times with 15 ml of Hanks' buffered saline solution. Between washes, gently resuspend the pellet, followed by centrifuging at 5,000 × g for 5 min. Following the last wash, completely remove the supernatant.
Prepare 7.5 ml of a homogenization buffer containing HEM buffer (10 mM HEPES, 1 mM EDTA, 20 mM sodium molybdate, pH 7.4), 1 mM phenylmethylsulfonyl fluoride (PMSF), and 3 ground tablets of Complete-Mini protease inhibitor. The PMSF and Complete-Mini tablets prevent proteolysis from occurring during the subsequent steps. Complete-Mini tablets can be easily ground to a fine powder with a mortar and pestle. Add 1.5 volumes of homogenization buffer to the cell pellet.
Rupture the cells by Dounce homogenization (50 strokes) or freeze/thaw (3 cycles) technique. Dounce homogenization may maintain improved endogenous GR•hsp90 protein complexes and is faster to complete. Freeze/thaw provides researcher an opportunity to suspend the protocol at this step and continue at a later time. Dounce homogenization should be performed with the homogenizer in an ice bucket to prevent protein degradation. Freeze/thaw can be accomplished by freezing the centrifuge tube containing the cell pellet-homogenization buffer mixture with liquid nitrogen, dry ice, or -20 °C incubation, followed by thawing in a 30 °C water bath.
Separate the GR-containing cytosol from the ruptured cell particulate matter by ultracentrifugation. Transfer the homogenate to durable ultracentrifuge tubes, balance tubes by mass in pairs, and centrifuge at 100,000 × g for 30 minutes.
Remove the cytosol (supernatant) and store at -20 °C in 500 μl aliquots in 1.5 ml microcentrifuge tubes for long-term storage. To retain maximum functional GR activity, flash-freeze the aliquots in liquid nitrogen or incubate for 30 seconds in a methanol-dry ice bath prior to storage. Take caution to avoid direct skin contact with the cooling reagent.
2. Immunoadsorption of GR from cell cytosol
Technical note: Figure 2 provides a schematic overview of Steps 2-5 of this protocol.
- Prepare an immunoadsorption mixture containing a monoclonal immunoglobulin G (IgG) antibody raised against the GR to immunoadsorb the receptor prepared in Step 1. In a 1.5 ml microcentrifuge tube, combine 100 μl of thawed GR-containing cytosol, 200 μl TEGM buffer (8 mM TES, 4 mM EDTA, 50 mM NaCl, 20 mM molybdate, 10% (v/v) glycerol, pH 7.6), 100 μl of a 20% protein A-Sepharose (PAS) slurry (v/v, prepared in TEGM buffer), and 5 μg of anti-GR monoclonal IgG antibody (e.g. BuGR2). Molybdate is included in the TEGM buffer in order to help stabilize the GR-hsp90 heterocomplex, which may be useful in order to assay endogenous heterocomplex steroid binding activity 13.
- Anti-GR antibody is commercially available as purified protein. Alternatively, it may be obtained by adding 10 μl of anti-GR IgG-containing ascites to the immunoadsorption mixture. Ascites is produced by mice injected with anti-GR hybridoma cells (e.g. FiGR hybridoma cells 14), and the approximate antibody concentration obtained from ascites and used in these experiments was 0.5 μg/μl, as measured by Bradford assay.
- Prepare a negative control sample using GR-containing cytosol, TEGM buffer, and PAS (as described above), and 5 μg of an IgG that does not recognize the GR, hsp90, or other molecular chaperone proteins (referred to as a "nonimmune" antibody).
- Due to the relatively large PAS bead size, use a cut P-200 pipet tip when transferring PAS from the 20% slurry to sample tubes. Cut pipet tips are prepared by slicing 2-3 mm off of the end of commercially available P-200 tips, thus increasing the pipet tip aperture.
Place samples on a vertical rotating wheel and incubate for a minimum of 2 hours with constant rotation. Samples may be incubated for up to 8 hours without loss of activity.
- At the conclusion of the immunoadsorption incubation, remove unbound proteins from the antibody-PAS pellet ("immunopellet") by centrifugation. Separate the supernatants from immunopellets by centrifugation using a refrigerated centrifuge programmed for 1 minute at 10,000 × g, and then wash twice with 1 ml TEGM buffer and vortex. Following centrifugation and between washes, discard the supernatant being cautious to not disturb the pellet.
- Remove all the supernatant at the conclusion of the second wash. In order to ensure none of the pellet is inadvertently aspirated, use a crimped P-200 pipet tip for final supernatant aspiration. Crimped pipet tips are prepared by flattening commercially available P-200 tips using forceps.
3. Dissociation of endogenous hsp90 from GR immunopellet ("Salt stripping")
To the washed immunopellet prepared in Step 2, add 355 μl TEG buffer (TEGM buffer without sodium molybdate) and 45 μl of 5 M NaCl (final NaCl concentration = 0.5 M).
Place samples on a vertical rotating wheel and incubate for 1.5 hours with constant rotation. Samples can be incubated for up to 2 hours; however, longer incubations may lead to decreased protein recovery and lower functional activity.
Remove unbound proteins from the immunopellet by centrifugation. Spin samples for 1 minute at 10,000 × g. Wash once with 1 ml TEG buffer and once with 1 ml of 10 mM HEPES buffer, pH 7.4.
Remove all the supernatant at the conclusion of the second wash, being careful not to disturb the immunopellet, using a crimped P-200 pipet tip.
4. Reconstitution of GR•hsp90 heterocomplex using molecular chaperones, cofactors, and ATP
Technical note: A myriad of experimental conditions can be tested by including additional proteins, cofactors, activators, or inhibitors of interest into the reconstitution mixture prepared below. Total volume of reconstitution mixtures should be <120 μl.
- To each salt-stripped immunopellet prepared in Step 3, add a reconstitution mixture containing 50 μl of a molecular chaperone source and 5 μl of an ATP-generating system (10 mM HEPES, 50 mM ATP, 250 mM creatine phosphate, 20 mM magnesium acetate, 100 U/ml creatine phosphokinase, pH 7.4).
- Reconstitution reactions may be increased by supplementing the reconstitution mixture with 50 μl HKD buffer (10 mM HEPES, 100 mM KCl, 5 mM dithiothreitol (DTT), pH 7.4) and an additional 5 μl of ATP-generating system. This step may be omitted if ample reconstitution and steroid binding are observed. KCl increases hsp70 ATPase activity and DTT protects cysteine-containing proteins from oxidation 9.
- A recommended molecular chaperone source is commercially available rabbit reticulocyte lysate. Individual molecular chaperones purified from reticulocyte lysate or recombinantly expressed (e.g. 15 μg hsp90, 15 μg hsp70, 0.6 μg Hop, 6 μg p23, and 0.125 μg hsp40 in HKD buffer) may also be used.
Incubate samples in a 30 °C water bath for exactly 20 minutes. Flick tubes every 1-2 minutes in order to gently disturb the pellet and mix the reconstitution solution.
Add 1 ml TEGM buffer, vortex, and centrifuge at 10,000 × g for 1 minute. Remove supernatant and discard being careful not to disturb the pellet. Repeat for a total of three washes. Completely remove all supernatant at the conclusion of the final wash using a crimped P-200 pipet tip.
5. Analysis of reconstituted protein complexes and ligand binding activity
- Proteins in the reconstituted immunopellet can be identified by conventional denaturing polyacrylamide gel electrophoresis (SDS-PAGE) , microfluidic electrophoresis (e.g. Bio-Rad Experion), and western blotting (Fig. 3). Technical note: Excellent protocols describing SDS-PAGE 15 and western blotting 16 are currently available, courtesy of other investigators.
- Add 50 μl of SDS-PAGE sample buffer containing β-mercaptoethanol and vortex. Specific sample buffer recipes vary. Select one based on the recommendation of the manufacturer of the electrophoresis system to be employed.
- Incubate for 5 minutes in a boiling water bath or 100 °C electronic heat block.
- Centrifuge at 10,000 × g for 1 minute. The immunoadsorbing antibody, GR, and proteins complexed to GR will be dissociated from one another and released into the sample buffer supernatant.
- Transfer the supernatant to a fresh microcentrifuge tube using a crimped tip P-200 pipet tip. Discard the pellet. The sample is now prepared for analysis using commercially available SDS-PAGE or microfluidic electrophoresis systems. Complete either technique following manufacturer's instructions.
- Western blot analysis following SDS-PAGE allows for definitive identification of the immunoadsorbed GR and molecular chaperones complexed to the GR following the reconstitution incubation. Use monoclonal primary antibodies raised against GR (BuGR2), hsp90 (AC88), hsp70 (N27F3-4) and co-chaperone proteins to detect the major components of the GR•hsp90 protein complex.
- Functional activation of the salt-stripped GR following reconstitution of the GR•hsp90 protein complex can be identified by assaying steroid binding activity using a [3H]steroid (Fig. 4). For the remainder of the protocol, follow necessary precautions for safely handling tritiated samples and waste.
- To the reconstituted immunopellet, add 47.5 μl HEM buffer and 2.5 μl of 2 μM [3H]dexamethasone (final steroid concentration = 100 nM).
- Gently mix the pellet being careful to minimize the amount of sample displaced to the wall of the microcentrifuge tube.
- Incubate overnight on ice. It is not necessary to rotate or mix the sample tubes during this incubation.
- Add 1 ml TEGM buffer, gently mix, and centrifuge at 10,000 × g for 1 minute. Discard the supernatant, being mindful it contains unbound [3H]steroid. Repeat for a total of 3 washes with TEGM buffer.
- Completely remove all supernatant at the conclusion of the final wash using a crimped P-200 pipet tip. The pellet contains GR, hsp90 and other molecular chaperones complexed to the GR, and the [3H]steroid specifically bound to the receptor. Nonspecific binding can be calculated by including 1000-fold excess non-tritiated dexamethasone in the overnight incubation mixture. As an alternate negative control, the anti-GR immunoadsorbing antibody added in Step 2.2 may be replaced with a non-GR-immunoadsorbing antibody (NI).
- Suspend the washed immunopellets in 200 μl TEGM buffer and transfer to 10 ml scintillation vials using a cut P-200 pipet tip.
- Add 4.8 ml scintillation cocktail to the vials and vortex.
- The amount of [3H]steroid binding to the reconstituted GR•hsp90 protein complex can be assayed using liquid scintillation spectrometry.
6. Representative Results:
Representative SDS-PAGE and western blot data are presented in Figure 3. The endogenous GR•hsp90 heterocomplex is immunoadsorbed from cell cytosol using anti-GR antibody and individual proteins that co-immunoadsorb with the GR are visualized by Coomassie staining (Fig. 3A). Confirmation of protein identity of any component of the immunopellet at any step in the reconstitution assay can be made by SDS-PAGE calculation of molecular weight and western blotting using monoclonal antibodies raised against the protein of interest.
Reconstituted GR•hsp90 heterocomplexes are presented using Experion microfluidic electrophoresis data (Fig. 3C and 3D) and western blot analysis (Fig. 3B). The specificity of the GR immunoadsorption is confirmed by replacing the immunoadsorbing anti-GR antibody with a nonimmune antibody (Fig. 3C, NI IgG lanes). Even when excessive amounts of nonimmune antibody are used, no GR or molecular chaperones are immunoprecipitated (cf. in Fig. 3C, the GR, hsp90, and hsp70 protein bands present in the anti-GR immunoadsorption lanes with the lack of band observed in the NI IgG lanes). Likewise, it is possible to confirm the dissociation of hsp90 and other chaperone proteins from the salt-stripped GR by western blot analysis (Fig. 3B) of the stripped and reconstituted immunopellets. The heavy chain of the immunoadsorbing antibody (HC) is detectable by both Coomassie stain and western blotting, and it serves to confirm equal sized immunopellets are being visualized in each lane.
Steroid binding activity of the immunoadsorbed GR can be assayed by for all the conditions tested, and representative steroid binding data are presented in Figure 4. Endogenous GR•hsp90 heterocomplexes are immunoadsorbed, salt-stripped, and reconstituted. Using either reticulocyte lysate or purified proteins, steroid binding activity of the reconstituted GR•hsp90 heterocomplex typically returns to 75-100% of the endogenous GR•hsp90 binding activity level. Similar to the electrophoresis data, a nonimmune antibody (NI) may be used in place of the anti-GR antibody (I) in order to serve as a negative control and as a measure of nonspecific [3H]steroid binding.
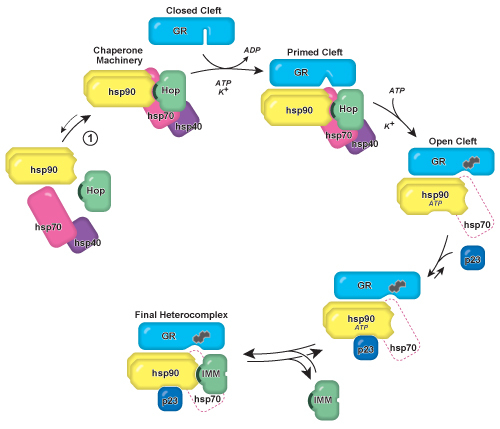 Figure 1. Model of GR•hsp90 heterocomplex assembly and GR steroid binding
cleft opening. The hsp90/hsp70-based chaperone machinery converts the GR ligand binding
domain from a folded conformation in which the steroid binding cleft is closed and not
accessible to hormone to an open cleft conformation that can be accessed (illustrated by
the heterocyclic steroid icon). The chaperone machinery is preassembled in the cell and
will spontaneously form when purified hsp90, hsp70, and Hop are mixed in solution
(Reaction 1). Hop contains a 34 amino acid tetratricopeptide repeat (TPR) domain
(illustrated as a dark crescent), and is later replaced during the heterocomplex
maturation process by a TPR domain-containing immunophilin protein. The machinery opens
the steroid binding cleft in an ATP- and K+-dependent manner, after which Hop
and most of the hsp70 and hsp40 ultimately leave the complex (illustrated as a dashed
icon). Mechanistically, hsp70 interacts with the GR prior to hsp90 and primes the receptor
for hsp90 and subsequent cleft opening. Experimentally, steroid binding and GR cleft
opening are increased if hsp90 and hsp70 are simultaneously present. The heterocomplex is
stabilized by entry of the hsp90 co-chaperone p23, which maintains hsp90 in an ATP-bound
conformation. After the exit of Hop, a high molecular weight immunophilin (IMM) can bind
hsp90, forming the final heterocomplex as it is recovered from cells. Adapted from Pratt
and Toft 1.
Figure 1. Model of GR•hsp90 heterocomplex assembly and GR steroid binding
cleft opening. The hsp90/hsp70-based chaperone machinery converts the GR ligand binding
domain from a folded conformation in which the steroid binding cleft is closed and not
accessible to hormone to an open cleft conformation that can be accessed (illustrated by
the heterocyclic steroid icon). The chaperone machinery is preassembled in the cell and
will spontaneously form when purified hsp90, hsp70, and Hop are mixed in solution
(Reaction 1). Hop contains a 34 amino acid tetratricopeptide repeat (TPR) domain
(illustrated as a dark crescent), and is later replaced during the heterocomplex
maturation process by a TPR domain-containing immunophilin protein. The machinery opens
the steroid binding cleft in an ATP- and K+-dependent manner, after which Hop
and most of the hsp70 and hsp40 ultimately leave the complex (illustrated as a dashed
icon). Mechanistically, hsp70 interacts with the GR prior to hsp90 and primes the receptor
for hsp90 and subsequent cleft opening. Experimentally, steroid binding and GR cleft
opening are increased if hsp90 and hsp70 are simultaneously present. The heterocomplex is
stabilized by entry of the hsp90 co-chaperone p23, which maintains hsp90 in an ATP-bound
conformation. After the exit of Hop, a high molecular weight immunophilin (IMM) can bind
hsp90, forming the final heterocomplex as it is recovered from cells. Adapted from Pratt
and Toft 1.
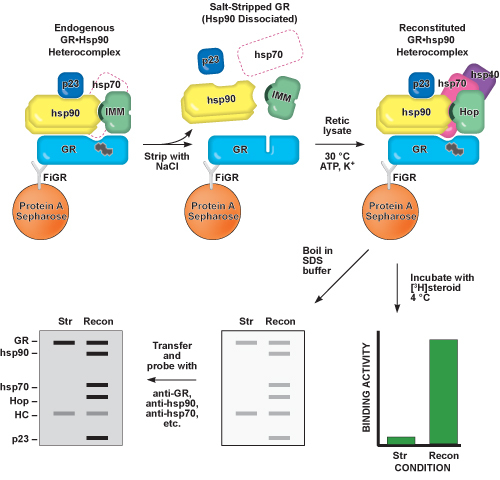 Figure 2. Schematic representation of Steps 2-5 of this protocol. The GR is
immunoadsorbed from cell cytosol using a monoclonal antibody raised against the GR (FiGR)
bound to a pellet of protein A-Sepharose. Hsp90 and other chaperone proteins present in
the endogenous GR•hsp90 heterocomplex are co-immunoadsorbed with the GR, and the GR is
able to bind to steroid. Hsp90 is dissociated from GR using a mild salt treatment,
referred to as salt-stripping (Str). The steroid binding cleft of the salt-stripped GR
closes, making it unable to bind to steroid. Intermediate GR•hsp90 heterocomplexes
containing hsp70, hsp40, and Hop, which are capable of binding steroid, can be
reconstituted (Recon) by incubating the salt-stripped GR with purified proteins or
reticulocyte lysate (Retic lysate) and cofactors. The steroid binding activity and
immunopellet protein content of each step can be identified by overnight
[3H]steroid incubation and western blotting, respectively.
Figure 2. Schematic representation of Steps 2-5 of this protocol. The GR is
immunoadsorbed from cell cytosol using a monoclonal antibody raised against the GR (FiGR)
bound to a pellet of protein A-Sepharose. Hsp90 and other chaperone proteins present in
the endogenous GR•hsp90 heterocomplex are co-immunoadsorbed with the GR, and the GR is
able to bind to steroid. Hsp90 is dissociated from GR using a mild salt treatment,
referred to as salt-stripping (Str). The steroid binding cleft of the salt-stripped GR
closes, making it unable to bind to steroid. Intermediate GR•hsp90 heterocomplexes
containing hsp70, hsp40, and Hop, which are capable of binding steroid, can be
reconstituted (Recon) by incubating the salt-stripped GR with purified proteins or
reticulocyte lysate (Retic lysate) and cofactors. The steroid binding activity and
immunopellet protein content of each step can be identified by overnight
[3H]steroid incubation and western blotting, respectively.
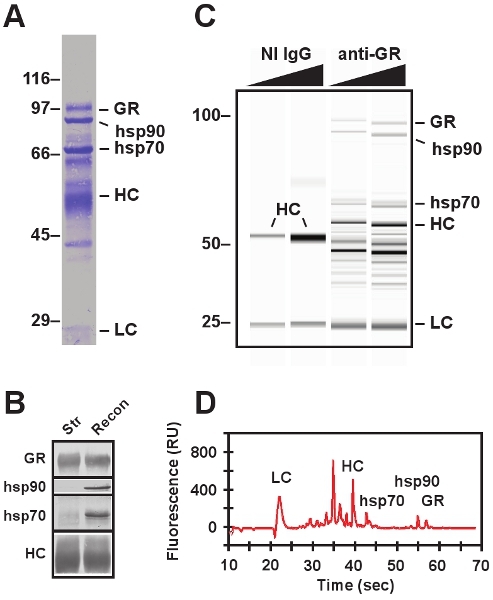 Figure 3. Electrophoretic protein analysis of immunoadsorbed complexes. A,
SDS-PAGE of reconstituted GR•hsp90 heterocomplex imaged using conventional electrophoresis
system followed by electrotransfer and Coomassie staining. In addition to GR, hsp90, and
hsp70, the heavy chain (HC) and light chain (LC) of the antibodies are visualized.
Migration of molecular weight markers (expressed in kDa) are indicated on the left. B,
Western blot of immunopellets containing salt-stripped GR (Str) and reconstituted GR•hsp90
heterocomplex (Recon). C, virtual gel of reconstituted GR•hsp90 heterocomplex generated
using an Experion microfluidic electrophoresis system. Two different concentrations of a
nonimmune (NI IgG) and immune (anti-GR) immunoadsorbing antibodies are included. NI IgGs
serve as negative controls. D, electropherogram of reconstituted GR•hsp90 heterocomplex
obtained from Experion microfluidic electrophoresis sample shown in the third lane of
panel B. Advantages of microfluidic electrophoresis include the requirement for smaller
sample volumes, decreased liquid handling and post-run cleanup, improved quantification,
and substantially shorter sample runs.
Figure 3. Electrophoretic protein analysis of immunoadsorbed complexes. A,
SDS-PAGE of reconstituted GR•hsp90 heterocomplex imaged using conventional electrophoresis
system followed by electrotransfer and Coomassie staining. In addition to GR, hsp90, and
hsp70, the heavy chain (HC) and light chain (LC) of the antibodies are visualized.
Migration of molecular weight markers (expressed in kDa) are indicated on the left. B,
Western blot of immunopellets containing salt-stripped GR (Str) and reconstituted GR•hsp90
heterocomplex (Recon). C, virtual gel of reconstituted GR•hsp90 heterocomplex generated
using an Experion microfluidic electrophoresis system. Two different concentrations of a
nonimmune (NI IgG) and immune (anti-GR) immunoadsorbing antibodies are included. NI IgGs
serve as negative controls. D, electropherogram of reconstituted GR•hsp90 heterocomplex
obtained from Experion microfluidic electrophoresis sample shown in the third lane of
panel B. Advantages of microfluidic electrophoresis include the requirement for smaller
sample volumes, decreased liquid handling and post-run cleanup, improved quantification,
and substantially shorter sample runs.
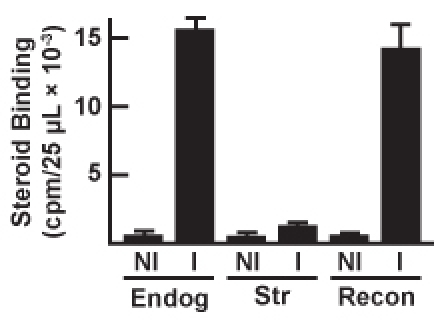 Figure 4. Steroid binding activity of immunopellets following
immunoadsorption of endogenous GR (Enodg), salt-stripped GR (Str), and reconstituted
GR•hsp90 heterocomplex (Recon). In addition to immunoadsorbing the GR with a monoclonal
anti-GR immunoadsorbing antibody (I), negative control samples for each condition were
prepared using a nonimmune immunoglobulin (NI).
Figure 4. Steroid binding activity of immunopellets following
immunoadsorption of endogenous GR (Enodg), salt-stripped GR (Str), and reconstituted
GR•hsp90 heterocomplex (Recon). In addition to immunoadsorbing the GR with a monoclonal
anti-GR immunoadsorbing antibody (I), negative control samples for each condition were
prepared using a nonimmune immunoglobulin (NI).
Discussion
The assay described above can be adapted to test a myriad of conditions affecting the chaperone actions of the hsp90/hsp70-based chaperone machinery as well as GR steroid binding. It is a modification of previously reported methods 4,8,9,17 and is designed to take advantage of recent advances in electrophoresis technology and be accessible to a broader research community. Additional cofactors or proteins of interest may be added to the GR•hsp90 heterocomplex reconstitution mixture, described in Step 4 of the protocol, in order to observe effects on steroid binding, heterocomplex protein-protein interactions, and co-factor requirements. For example, the effects of ischemia-reperfusion on hsp90 function can be modeled with the addition of increasing concentrations of a reactive oxygen species. Purified proteins with a specific feature (e.g. acetylated hsp90) or potential hsp90 inhibitors (e.g. novel geldanamycin analogs) can be added in order for their effects on steroid binding and heterocomplex formation to be determined.
Down-stream assays different than electrophoresis, western blotting, and steroid binding assays may also be employed. For example, immunoprecipitated multiprotein complexes have been analyzed by flow cytometry with substantial success 18,19. The immunoadsorbed GR•hsp90 complexes may also be assayed for hsp90/hsp70 nucleotide state or visualized using atomic force microscopy 17. Hsp90 client proteins other than the GR may be used (e.g. chaperoning checkpoint kinase I (Chk1) 20), and seminal research has been conducted using variations of this protocol with the estrogen receptor, progesterone receptor, and Src 1,13,21.
Disclosures
Select electrophoresis reagents and image analysis software were provided by Bio-Rad.
Acknowledgments
This work was funded by National Institutes of Health grant GM086822, Hope Heart Institute Basic Sciences award, and the M. J. Murdock Charitable Trust College Science Research Program. Select electrophoresis reagents and image analysis software were generously provided by Bio-Rad.
References
- Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp. Biol. Med. 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- Murphy PJM. Regulation of glucocorticoid receptor steroid binding and trafficking by the hsp90/hsp70-based chaperone machinery: implications for clinical intervention. Leukemia. 2005;19:710–712. doi: 10.1038/sj.leu.2403687. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Morishima Y, Osawa Y. The Hsp90 chaperone machinery regulates signaling by modulating ligand binding clefts. J. Biol. Chem. 2008;283:22885–22889. doi: 10.1074/jbc.R800023200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar KD, Hutchison KA, Owens-Grillo JK, Pratt WB. Reconstitution of the steroid receptor hsp90 heterocomplex assembly system of rabbit reticulocyte lysate. J. Biol. Chem. 1996;271:12833–12839. doi: 10.1074/jbc.271.22.12833. [DOI] [PubMed] [Google Scholar]
- Morishima Y. The Hsp organizer protein hop enhances the rate of but is not essential for glucocorticoid receptor folding by the multiprotein Hsp90-based chaperone system. J. Biol. Chem. 2000;275:6894–6900. doi: 10.1074/jbc.275.10.6894. [DOI] [PubMed] [Google Scholar]
- Murphy PJM, Kanelakis KC, Galigniana MD, Morishima Y, Pratt WB. Stoichiometry, abundance, and functional significance of the hsp90/hsp70-based multiprotein chaperone machinery in reticulocyte lysate. J. Biol. Chem. 2001;276:30092–30098. doi: 10.1074/jbc.M103773200. [DOI] [PubMed] [Google Scholar]
- Dittmar KD, Pratt WB. Folding of the glucocorticoid receptor by the reconstituted Hsp90-based chaperone machinery. The initial hsp90.p60.hsp70-dependent step is sufficient for creating the steroid binding conformation. J. Biol. Chem. 1997;272:13047–13054. doi: 10.1074/jbc.272.20.13047. [DOI] [PubMed] [Google Scholar]
- Kanelakis KC. Differential effects of the hsp70-binding protein BAG-1 on glucocorticoid receptor folding by the hsp90-based chaperone machinery. J. Biol. Chem. 1999;274:34134–34140. doi: 10.1074/jbc.274.48.34134. [DOI] [PubMed] [Google Scholar]
- Morishima Y, Kanelakis KC, Murphy PJM, Shewach DS, Pratt WB. Evidence for iterative ratcheting of receptor-bound hsp70 between its ATP and ADP conformations during assembly of glucocorticoid receptor.hsp90 heterocomplexes. Biochemistry. 2001;40:1109–1116. doi: 10.1021/bi002399+. [DOI] [PubMed] [Google Scholar]
- Murphy PJM. Pifithrin-alpha inhibits p53 signaling after interaction of the tumor suppressor protein with hsp90 and its nuclear translocation. J. Biol. Chem. 2004;279:30195–30201. doi: 10.1074/jbc.M403539200. [DOI] [PubMed] [Google Scholar]
- Murphy PJM, Morishima Y, Kovacs JJ, Yao TP, Pratt WB. Regulation of the dynamics of hsp90 action on the glucocorticoid receptor by acetylation/deacetylation of the chaperone. J. Biol. Chem. 2005;280:33792–33799. doi: 10.1074/jbc.M506997200. [DOI] [PubMed] [Google Scholar]
- Morishima Y, Murphy PJM, Li DP, Sanchez ER, Pratt WB. Stepwise assembly of a glucocorticoid receptor hsp90 heterocomplex resolves two sequential ATP-dependent events involving first hsp70 and then hsp90 in opening of the steroid binding pocket. J. Biol. Chem. 2000;275:18054–18060. doi: 10.1074/jbc.M000434200. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr. Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- Bodwell JE. Identification of phosphorylated sites in the mouse glucocorticoid receptor. J. Biol. Chem. 1991;266:7549–7555. [PubMed] [Google Scholar]
- Penna A, Cahalan MWestern. Blotting using the Invitrogen NuPage Novex Bis Tris minigels. J. Vis. Exp. 2007;(7):e264–e264. doi: 10.3791/264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo YS, Zhang Z. Detection of Protein Ubiquitination. J. Vis. Exp. 2009;(30):e1293–e1293. doi: 10.3791/1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PJM. Visualization and mechanism of assembly of a glucocorticoid receptor•Hsp70 complex that is primed for subsequent Hsp90-dependent opening of the steroid binding cleft. J. Biol. Chem. 2003;278:34764–34773. doi: 10.1074/jbc.M304469200. [DOI] [PubMed] [Google Scholar]
- Schrum AG. High-sensitivity detection and quantitative analysis of native protein-protein interactions and multiprotein complexes by flow cytometry. Sci STKE. 2007;389:pl2–pl2. doi: 10.1126/stke.3892007pl2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TR, Schrum AG. IP-FCM: Immunoprecipitation Detected by Flow Cytometry. J. Vis. Exp. 2010;(46):e2066–e2066. doi: 10.3791/2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felts SJ, Karnitz LM, Toft DO. Functioning of the Hsp90 machine in chaperoning checkpoint kinase I (Chk1) and the progesterone receptor (PR) Cell Stress Chaperones. 2007;12:353–363. doi: 10.1379/CSC-299.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cintron NS, Toft D. Defining the requirements for Hsp40 and Hsp70 in the Hsp90 chaperone pathway. J. Biol. Chem. 2006;281:26235–26244. doi: 10.1074/jbc.M605417200. [DOI] [PubMed] [Google Scholar]


