Abstract
Background
Major advances during the past 50 years highlight the immense potential for restoration of function after neural injury, even in the damaged adult human brain. Yet, the translation of these advances into clinically useful treatments is painstakingly slow.
Objective
Here, we consider why the traditional model of a “translational research pipeline” that transforms basic science into novel clinical practice has failed to improve rehabilitation practice for people after stroke.
Results
We find that (1) most treatments trialed in vitro and in animal models have not yet resulted in obviously useful functional gains in patients; (2) most clinical trials of restorative treatments after stroke have been limited to small-scale studies; (3) patient recruitment for larger clinical trials is difficult; (4) the determinants of patient outcomes and what patients want remain complex and ill-defined, so that basic scientists have no clear view of the clinical importance of the problems that they are addressing; (5) research in academic neuroscience centers is poorly integrated with practice in front-line hospitals and the community, where the majority of patients are treated; and (6) partnership with both industry stakeholders and patient pressure groups is poorly developed, at least in the United Kingdom where research in the translational restorative neurosciences in stroke depends on public sector research funds and private charities.
Conclusions
We argue that interaction between patients, front-line clinicians, and clinical and basic scientists is essential so that they can explore their different priorities, skills, and concerns. These interactions can be facilitated by funding research consortia that include basic and clinical scientists, clinicians and patient/carer representatives with funds targeted at those impairments that are major determinants of patient and carer outcomes. Consortia would be instrumental in developing a lexicon of common methods, standardized outcome measures, data sharing and long-term goals. Interactions of this sort would create a research-friendly, rather than only target-led, culture in front-line stroke rehabilitation services.
Keywords: Rehabilitation, Research pipeline, Translation
There are many opportunities to prevent or reverse disease, but health care systems worldwide are dominated by the treatment of people with chronic disability,1 and their care poses a daily demand on finite resources. Stroke is the leading cause of acquired disability worldwide.2 It results in a prevalence of disability of 5 to 8 per 1000 population,3 and is the commonest single cause of severe physical disablement in people living at home in the United Kingdom,4 where the annual costs of direct and informal care and lost productivity have recently been estimated at £7.0 billion.5 At 6 to 12 months poststroke, only 60% of patients presenting with hemiplegic stroke have achieved independence in personal care; 30% to 40% of survivors are depressed, 10% to 15% severely so; 50% need help with either housework, meal preparation, or shopping; and a similar number lack a meaningful social, recreational, or occupational activity during the day.6,7 Function, health-related quality of life (HRQoL) and subjective well-being remain significantly reduced 4 to 5 years poststroke.8, 9
There have been major advances in hyperacute treatment after stroke in a minority of patients using thrombolysis, but, in the majority, efforts must focus on the management of residual disability by means of rehabilitation. This process is multidimensional, and uses a 2-pronged approach to enhance an individual's functional activity and societal participation, so that life quality is subjectively improved and life is “worth living,”10 by means of (1) behavioral adaptation to loss of function involving contextual changes and psychological adjustment, and (2) restoration of physiological or psychological function by replacing or retraining parts of the central nervous system (CNS) that have been damaged or remain, to engage functions that have been lost. The latter approach was explored at a Wellcome Trust Masterclass convened in September of 2007 at Cumberland Lodge, Windsor Great Park, United Kingdom, to address key areas of the basic and clinical restorative neurosciences and how they could best translate into clinical practice during rehabilitation after stroke, and is discussed in this document.
The Problem
Remarkable advances (Appendix) in the basic and clinical neurosciences highlight the immense potential for restoration of function even in the damaged adult human brain. Fifty years ago, regrowth of connections after acute damage in the mature CNS of mammals was viewed as impossible. In the intervening years, experiments in basic science have overturned that dogma and replaced it with a model of the CNS as a dynamically changing environment where “plasticity” of neural connections is the norm. The result has been the emergence of restorative neuroscience, a discipline that aspires to take the insights about neural plasticity derived from animal models and normal man and apply them therapeutically in human patients after acute or chronic injury to the CNS. The long-term goal is to restore where possible the functions previously performed by permanently damaged tissue.
However, the translation of these advances into clinically useful treatments after brain injury of all sorts, including stroke, is painstakingly slow. Of course, more knowledge is needed to understand the limits of neural plasticity, but could the application of these new insights be accelerated by modifying the systems within which they are developed—could organizational change contribute to the development of the restorative neurosciences? The generic problems involved in successfully translating research have been increasingly discussed over the past 10 years in the United Kingdom,11–13 United States,14–16 Canada, and elsewhere. The aim of this document is to explore these questions in the specific context of stroke recovery, and to suggest some solutions.
Biological Issues: What Can Be Expected From Restorative Neurology in Stroke?
It is tempting to state that restorative neurology has swept aside Cajal's statement in 1928 that “ . . . the nerve paths are something fixed, and immutable; . . . ,” replacing it with models of plasticity for recovery that were never thought possible, and treatment strategies in rehabilitation other than training the patient to use alternative, but less effective, behavioral strategies. At present however, clinical experience still emphasizes something rather more prosaic: that (a) neural injury generally results in an irreversible loss at least of anatomical and physiological if not functional status; and that (b) after injury, very obvious functional gains can occur but these largely result from the prevention of complications secondary to the initial damage, and from adaptive interventions involving environmental modification. For example, if complications due to spasticity, immobility and detraining, and cognitive and emotional dysfunction can be prevented, and adaptative interventions provided, significant functional recovery will follow. Compared with the consequences of no treatment, still readily visible in patients from the third world, these interventions result in very significant functional benefit in many patients, and probably underpin the considerable evidence base demonstrating the effectiveness of organized care and rehabilitation poststroke.17,18 Can new treatments derived from restorative neuroscience provide additional gains beyond outcomes achievable by widespread implementation of current best practice in rehabilitation by means of organized systems of care?
At the present time, the answer is not an unequivocal “Yes.” The conclusion for the restorative neuroscientist is that introduction of novel neural conditioning techniques, shown to be of functional benefit in proof-of-principle studies, will be difficult unless they provide a step-change in functional outcome that surprises and excites patients and carers. Like thrombolysis in hyperacute stroke, achieving this end even in small groups of patients could have a talismanic effect on the more general provision of restorative treatments after acquired brain injury of all sorts.
The present status of constraint-induced movement therapy (CIMT) is perhaps a good example of how novel approaches can be viewed. The groundbreaking Phase III EXCITE trial19 in the United States has proven the effectiveness of this approach, yet widespread clinical implementation of conventional CIMT is limited. Some of the barriers to widespread clinical implementation may be overcome by changing the timing, delivery, intensity and duration of therapy. However, even modified CIMT may fail to be taken up widely within a health service and by eligible patients if outcomes merely reflect modest gains in function. A large effect size is needed to generate the clinician, patient, and carer demand required to drive organizational change in a (public) health care system. Perhaps only a combined approach to enhance plasticity—using multiple strategies to replace cells and encourage regrowth, directed by drivers of neural reorganization including drugs, cortical stimulation, and structured training programs like CIMT—is needed before there is a sufficiently significant benefit to influence clinical practice.
Process Issues: Why the “Translational Pipeline” Has Failed in Stroke
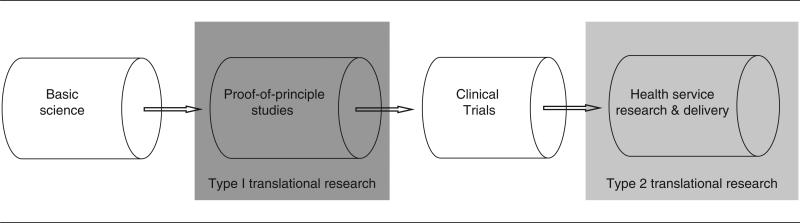
The standard model describing how scientific knowledge is converted into clinical application is the classic translational pathway or pipeline,11 adapted from the well-trodden development pathway for pharmaceuticals. In this model, discoveries are made at a preclinical level (in animals or humans), commonly called “basic science.” With this “basic science” knowledge, a strategy of intervention is derived and applied in a small-scale study, which is often termed a “proof-of-concept” study. The next stage progresses this “proof of concept” study to large-scale clinical trials; the gold hold standard for this in medicine is the multicenter randomized controlled trial. The final stage in this process is the implementation of this evidence-backed intervention by means of guidelines and protocols to restructure services and educate frontline practitioners. This classic model of translation is exemplified in Figure 1; at present, drugs take approximately 15 years to complete the journey.20
Figure 1.
The Classic Unidirectional Translational Research Pipeline and its 2 “Steps” or “Blocks,” Type 1 and Type 2
The effectiveness of the classic translational model is self-evident when the treatment target and the outcome are circumscribed and well defined. Thus, the success of treating a molecular target in cancer medicine is readily quantified by its effects on mortality but less readily by its effects on life quality; the effects of an antibiotic are easily assessed by patient cure but less easily by a reduction in hospital length of stay. These variables determine whether or not the basic scientist at one end of the pipeline has a clear view of the narrative of patient need and key outcomes, whilst at the other end of the pipeline such variables are key to whether patients can appreciate clearly the goals of basic science and have a realistic sense of what research can achieve. Research will progress if both ends of the pipeline share similar goals and the same robust scales to measure outcome.
However, this model is obscured in recovery after single incident neural injury including stroke, when drivers of need involving disability and life quality are multiple and complex, and thus treatment targets, and what patients want, are often ill-defined and poorly measured. The result is that scientists do not know what patients want, and clinicians and patients do not understand the potential that new science might have for their own individual treatment. This disconnection encourages neuroscientists to focus on “curiosity-led” targets within their individual scientific paradigms, rather than pushing interventions toward translation. Similarly, lack of “pull” or demand from clinicians, patients, and carers for better implementation of novel treatments leads to a state in which clinicians encourage coping strategies and the prevention of deterioration at best, rather than the potential benefits derived from trial entry. By contrast, in chronic relapsing or deteriorating diseases, trial entry is the norm and is demanded actively by pressure groups. When risks and benefits of potential new therapies are unclear to the scientist, clinician, and patient, and also industrial stake-holders, flow through the pipeline stalls. The resulting stagnation leaves a scientific community driven by curiosity rather than the need to translate research findings, and a clinical sector that is risk averse and resistant to change, and often charged with meeting short-term service targets.
The need for restorative neuroscience to drive the discoveries of basic science into clinical trials after neural injury, and “move research from bench to bedside,” was identified, although largely as a unidirectional process, by a UK Academy of Medical Sciences report.21 That document largely addressed Type 1 Translational Research or the T1 translational step or gap, and advocated the formation of “Regional Neurorehabilitation Research Centres” to perform clinical trials of promising new science. The document stimulated interest in the area, but has been followed by a large number of small and medium scale proof-of-principle trials that have almost universally failed to progress to larger clinical trials or to changes in front line medical treatment at a hospital or community level.
Currently, a myriad of researchers and clinicians, with ever narrowing specialization, pursue individual approaches to improve outcomes after stroke. A molecular biologist might aim to maximize dendritic regrowth, a bioengineer may aim to optimize kinematics of a movement, while a clinician might be interested in how many patients live in nursing homes after a stroke. A variety of professionals under the broad church of stroke rehabilitation research share little in terms of a common purpose. That molecular, structural, and physiological markers of outcome used in vitro or in animal models have proved unreliable predictors of human clinical outcomes, at least in trials of neuroprotection, remains relatively unacknowledged. This state of affairs is compounded by the “Principal Investigator” funding model, which has encouraged niche research rather than research crossing multiple translational steps. Each intervention proceeds at an ad hoc pace, stalling for no obvious reason, particularly at the “proof-of-principle” stage where there is a proliferation of techniques and methodologies for even a single intervention but no clear sense of how to proceed. This behavior can be traced to a lack of a research consensus and thus a financial driving force to trial an intervention at the next stage.
Solutions
Given these challenges, patients, clinicians, and researchers together must agree upon the biological and organizational catalysts that can drive change in practice, integrate the pipeline, and translate science into stroke recovery.
Restorative Translational Pipeline in Stroke
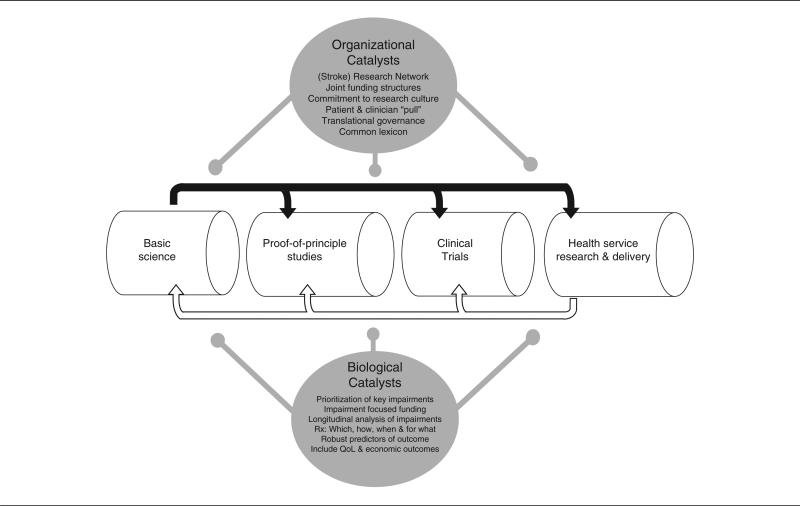
The initial idea of the Type 1 Translational Research pipeline focused on a unidirectional push from bench to bedside. Its failure to bring widespread change in practice has been recognized by adding 2 more members to the T-family. Type 2 Translational Research, the “translation of results from clinical studies into everyday clinical practice and health decision making”14; and Type 3, which aims to optimize the implementation of recommended new treatments into day-to-day clinical practice by means of practice-based research.15 This division highlights the need for a bidirectional process to transfer research-derived knowledge between components of the biomedical research pipeline,13 by means of proof-of-principle studies designed to look at “efficacy” (how well the intervention performs under controlled circumstances), and clinical studies of “effectiveness” (how well an intervention performs under more normal/“typical” circumstances) (Figure 2).
Figure 2.
The Reiterative Bidirectional Biomedical Research Pipeline with its Biological and Organizational Catalysts
The model provides a framework for a “restorative translational pipeline” in stroke, in which it should be possible to explore which treatments work, how and when to use them after neural injury, and for what type and level of impairment. These aims would be achieved by means of reiteratively agreed common goals and action plans that bring together the view from each end of the pipeline. A framework of this sort can enable a Stroke Research Network, for example the 10 local stroke research networks in the United Kingdom, to recruit patients to trials of restorative techniques, as well as to trials of preventative or neuroprotective drug treatments.
Improving Research Culture
Research at each stage of the pipeline needs to be an iterative process in which information about the successes and failures of clinical practice and the development of new research strategies feedback or feedforward into earlier or later stages of translation. At present, many frontline clinicians and therapists are poorly integrated into the restorative translational pipeline, and basic scientists have limited access to clinical practice; as a result their knowledge base and skill sets are not easily available to each other. Key to these partnerships and to the continuity of communication that encourages cross-disciplinary collaboration are knowledge about evidence-based practice, and basic and applied science amongst clinicians; the nature and constraints of daily clinical practice amongst basic scientists; and the use of a common lexicon or language between different members of the pipeline, so that the meaning, for example, of “recovery,” “plasticity,” “adaptation,” or “compensatory behavior” in the laboratory and the clinic are congruent.
Training Clinicians in Health Service Research
Observational studies show a positive association between participation of clinical teams in research and the quality of clinical care provided by a service to all patients, whether or not a particular patient is participating in research. The reasons for this improvement are unclear but the observation suggests that the development of skills that lead to participation in research may lead to better care.
The model used to train doctors in the United Kingdom hitherto has been very effective in ensuring that clinical researchers also have a good understanding of patient need and clinical practice, by enabling them to deliver clinical care in a senior clinical, often consultative role. This model of working for both a university and an NHS hospital is considered the usual expected employment arrangement for most clinical academics in the United Kingdom. In addition, a large proportion of full-time medical clinicians have undertaken a period of often high-quality research during their training. However, recent “advances” in modernizing medical careers and encouraging less active participation in science for frontline clinicians threaten to undermine this approach. Sweden, which is one of the countries with very high-quality health services and an excellent clinical research output in Europe, has a very high proportion of medical practitioners who hold a research degree.12
The training of many therapists in the United Kingdom substantially differs from this model. A low level of clinical research activity, with few trainees undertaking higher research degrees, means that many therapists have had no exposure to clinical research. There are a small number of academic positions occupied by allied health care professionals, who rarely have structures or opportunities to continue clinical practice in the local hospital or community services. As a result, the research culture may be weak in many rehabilitation units, and there may be difficulties in clinicians accepting equipoise in relation to trial entry and the role and value of established or new therapies.
The second and third translation gaps remain a major challenge for rehabilitation. Widespread collaborative understanding of the research culture amongst clinicians, and also users, is needed to negotiate the translation of new restorative and rehabilitative therapies after stroke to patients (T2) and to practice (T3).
Encourage “Patient Pull”
The public perception of stroke outcomes continues to assume inevitable disability; this reality reduces public pressure for improved outcomes after stroke rehabilitation. This problem is multifactorial and merits further investigation. Contributing factors are likely to include lack of knowledge about stroke rehabilitation and what can be done; the complexity of achievable outcomes in stroke rehabilitation compared with simple mortality; neurological and psychological deficits (eg, anosognosia and depression); carer burden; and the absence until recently of an effective acute treatment for stroke. The recent introduction of thrombolysis as an effective emergency treatment for stroke, and the concept of stroke being a “brain attack,” is likely to alter public, and also professional, assumptions that residual disability after stroke is inevitably immutable. This perception would contribute to “patient pull” and a more proactive and goal-oriented translational research framework.
Choice of Outcome Measure
Understanding that the tools needed to answer questions of effectiveness differ according to the stage of translation is critical to flow in the pipeline. For example, basic science studies often use dendritic growth as a surrogate marker for recovery, while the Barthel Index is commonly used in clinical trials. In the absence of evidence about what to measure how should one go about designing a study? The key message is simply to define the goal of the study. Thus, during early development of an intervention emphasis will likely be placed on molecular and subsequently anatomical, physiological or psychological (impairment-based) outcomes, but in clinical trials measures of functional independence and health related or subjective quality of life as well as economic impact are likely to be more important to decisions about applying the treatment to larger populations of patients.
Biological Catalysts
Techniques that facilitate neural replacement and regrowth are likely to be most effective when combined with intensive training protocols that drive plasticity toward useful functional goals. Definition and trial of combined treatments of this sort requires explicit linkage between ends of the restorative pipeline.
Learning is key to the rehabilitation process and allows the acquisition of old and new skills after neural injury. Elucidation of the structural, physiological, and psychological drivers of learning and memory should engage each part of the restorative translational pipeline.
Potentially mutable impairments that are known to be important in determining longer-term outcomes should be used to prioritize restorative treatment targets. These include neglect22; upper and lower limb weakness23; aphasia24; and anxiety, cognitive impairment, and incontinence.8,25
How each of these impairment-based drivers of long-term objective and subjective outcome achieve their effects post-stroke is likely to influence treatment targets and requires further investigation. For example, both lateralized and nonlateralized attentional deficits mediate the functional effects of neglect26; and weakness, muscle co-contraction, spasticity, and soft tissue shortening those of motor dysfunction, but to what extent in different patients at different times poststroke remains unclear. Which cognitive problems result in an inability to goal plan and thus independently manage life tasks, so that cognitive impairment correlates with a reduction in long-term life satisfaction,27 is entirely unexplored.
If clinical trials of impairment-based restorative treatments are to generate health care strategies that implement new restorative treatments, they need to demonstrate an impact on measures of health-related and subjective quality of life, as well as economic outcomes, rather than targeting outcomes at the level of disability alone. Multiple rather than single interventions may be needed to achieve this.
Robust measures must be developed that will allow us to predict, preferably early poststroke, which patients have the lowest probability of responding to existing therapies. This group is most likely to benefit from new, invasive, and potentially risky new therapies.
Organizational Catalysts
Change is more likely if funding models recognize the importance of partnerships between researchers, clinicians, and health care organizations. Specific and systematic funding should be provided to consortia of clinicians and researchers from the basic and clinical neurosciences that focus on means of reversing specific key impairments, for example aphasia, selected and prioritized by studies of patients and carers, and incorporate a governance framework that facilitates synergy across translational gaps.
There is an urgent need to improve practical support for both proof-of-principle and clinical trials of restorative interventions, to implement trials and finance the resources needed for patient recruitment and retention.28 In the United Kingdom, the formation of its Stroke Research Network (SRN) has been a big stride in this direction and makes possible recruitment of patients to controlled single case experimental designs and case series, using existing statistical methods to address effects within small groups,29 as well as larger clinical trials.
Barriers to the translation of proof-of-principle studies from bench to bedside and from bedside to the community should be identified at the earliest stages of translation and seen as legitimate targets for intervention. Funding streams should encourage grant applications that explore potential problems in translating restorative interventions. For example, proof-of-principle studies related to new interventions should focus on a prioritized impairment. Collaborative multicenter groups working in parallel could then apply common methodologies and quickly identify which intervention is most effective and should progress to clinical trial.
The translation of best evidence into improved clinical practice and patient outcomes is a complex process and often requires substantial and expensive change to health care delivery systems. Strategies to achieve this in stroke can be developed from those that have successfully led to the introduction of new therapies in other fields. Systematic reviews of trials of organizational interventions designed to improve health care are produced by the Cochrane Effective Practice and Organization of Care group (EPOC).
Studies should be encouraged to undertake collaborative research in front-line hospitals and the community, and not just academic centers.15
Step changes in the translation of treatments into rehabilitation after stroke may need international collaboration to confront funding bodies and drive the restorative pipeline.
Next Steps
The solutions suggested for the restorative pipeline after stroke are components of a complex intervention, intended to improve the pipeline's effectiveness and, ultimately, recovery of patients after stroke. The components of the proposed intervention are the product of 1 meeting of 1 “focus group” and inevitably incomplete.
Further development of the intervention's components, to prevent its derailment by competing interests and increase the chance that its components are practical and their implementation successful, could be guided by the first 2 phases, “Theory” and “Modeling,” of the Medical Research Council's guidelines30 to develop complex interventions. This step could be implemented by a steering group, supported by a “research translator,” to facilitate iterative literature review of current knowledge, initiated by the Appendix; and include perspectives from other experts and stakeholders, including funders, patient and carer groups, and health care providers not so far represented.
Conclusions
The translational pipeline is a useful model for research in the restorative neurosciences but requires refinement.
When both determinants of outcome, and thus treatment targets, and treatments are complex, the pipeline stalls and stagnates.
In these circumstances, drivers of the pipeline need to be made more explicit, to funders, researchers, clinicians, and also to patients and their advocates.
Patient- and carer-led prioritization of outcome determinants and treatment targets should be used to focus interventions and research funding streams on a few impairment-based translational pipelines.
A common language or lexicon is key to better communication and flow of ideas between clinicians and scientists and to the transmission of information in any pipeline.
These recommendations are derived from 1 meeting of 1 focus group. We hope they stimulate further discussion that leads to implementation of the translational restorative pipeline after stroke.
Acknowledgments
Conference convenors were as follows: Binith Cheeran, Richard Greenwood, John Rothwell, and James Teo. The Cumberland Consensus Group was supported by the Wellcome Trust, the Stroke Association, The Guarantors of Brain, and the British Neuroscience Association. The Cumberland Consensus Group 2007 consisted of the following persons: Ann Ashburn and Jane Burridge, School of Health Professions and Rehabilitation Sciences, University of Southampton, UK; John Bamford, University of Leeds, UK; Jean-Claude Baron, Addenbrooke's Hospital, Cambridge, UK; Philip Bath, Avril Drummond, and Alan Sunderland, University of Nottingham, UK; Jon Driver, Institute of Cognitive Neuroscience, University College, London, UK; Steve Dunnett, University of Cardiff, UK; Martin Eccles, University of Newcastle, UK; Anne Forster, Academic Unit of Elderly Care and Rehabilitation, Bradford Royal Infirmary, UK; Shaheen Hamdy, University of Manchester, UK; Peter U. Heuschmann, King's College London, London; Neville Hogan, Massachusetts Institute of Technology, USA; Roger Lemon and Jon Marsden, Sobell Dept of Motor Neuroscience, Institute of Neurology, UK; Averil Mansfield, Stroke Association, UK; Robert McCrum, patient; Isaebel Mhairi Macrae, University of Glasgow, UK; Valerie Pomeroy, University of East Anglia, UK; Cathy Price and Alex Leff, Wellcome Trust Centre for Neuroimaging, Institute of Neurology, UK; Yves Rosetti, INSERM, France; Duncan Turner, University of East London, UK; Paulette van Vliet and Alan Wing, University of Birmingham, UK; Derick Wade, Nuffield Orthopaedic Centre, Oxford, UK; Cornelius Weiller, University of Freiburg, Germany; Richard Wise, MRC Clinical Sciences Centre and Imperial College London, UK; Ulf Ziemann, J.W. Goethe-University, Germany.
Appendix Current Knowledge
Basic Science
Evidence from animal experiments, including primates, indicates that acute brain injury leads to local and contralesional increases in proteins associated with neural repair, growth promoting factors, and cellular and synaptic changes including growth of new connections.31,32 There is also evidence that larger changes occur if animals are encouraged to use the paretic limb,33,34 early after injury,35 suggesting that they may be an important mechanism underlying motor recovery after stroke.
Several strategies have been developed to maximize these changes in animal stroke models: (1) pharmacological intervention, using either (a) neuromodulators or (b) factors promoting neural regrowth; (2) neural (cortical) stimulation; and (3) cell replacement therapies.
Pharmacological intervention in animals with neuromodulators has largely focused on noradrenergic stimulation with amphetamine and benefit has been observed in primates,36 cats,37 and rats.38,39 Cholinergic stimulation with nicotine has shown benefit in rodents.40 Intervention with agents promoting neural regrowth has involved neurotrophic factors like BDNF41; other growth promoting factors such as inosine42,43 or recombinant erythropoietin44; inhibitors of myelin and glial scar associated CNS growth-inhibitory factors particularly the protein Nogo-A,45 and the chondroitin-sulphate proteoglycans (although not after stroke). Direct cortical stimulation has been shown to be beneficial in rodents46–48 and primates,49 but was not successful in 1 human trial.50 Finally, cell replacement therapy may in the future become a potential additional treatment option. To date, research has focused largely on pathological rather than functional outcomes.51 Proposed sources include fetal neural stem cells, embryonic stem cells, neuroteratocarcinoma cells, umbilical cord blood-derived nonhematopoietic stem cells, and bone marrow-derived stem cells. It is as yet unclear if the effectiveness of this approach is through direct tissue replacement or secretion of neurotrophic factors by the transplanted stem cells.
Which of these interventions has the greatest potential to revolutionize the treatment of stroke (and other forms of brain injury) is unclear. In animals these techniques appear to be best used in combination with intensive training protocols to synergistically enhance efficacy and drive plasticity toward useful goals43,52; this may also be the case in human patients. There are several caveats relevant to the majority of these preclinical studies of restorative treatments, similar to those emphasized by the CAMARADES collaboration in relation to animal studies of candidate drugs for neural protection after stroke, which include:
Most are performed in rodent models. The organization of the cortical control of movement in nonprimates is very different to that in higher primates, which makes extrapolation to recovery in human studies difficult. In addition, it is often unclear whether the functional outcome measures in animals (especially rodents that recover very quickly in stroke models) are reliable predictors of the therapeutic response in man and whether they have any relevance to the needs of a typical patient after stroke.
Most animal studies have examined small lesions in young animals, rather than the large and complex lesions in elderly humans that occur after a typical stroke. Additionally, highly inbred strains are often used that fail to reflect the diversity of the population affected by stroke.
Proof-of-Principle Studies
Movement Rehabilitation
Novel interventions focus on “add-on” treatments that could maximize the effects of conventional physical therapies. Currently these include transcranial magnetic and direct current stimulation, somatosensory stimulation, drug therapies, and robot assisted therapy.
Transcranial stimulation techniques involve repetitive transcranial magnetic stimulation (rTMS), transcranial direct current stimulation (TDCS), and paired associative stimulation (PAS). Numerous small-scale studies measuring the effects on hand and arm function have been conducted with all 3 forms but there is a high degree of heterogeneity between the protocols used in different centers. The rTMS technique alone can be subdivided by frequency of stimulation, by intensity of stimulation,53 by pattern of stimulation,54 and by site of stimulation55 and the optimum parameters are still unclear. A more direct approach has been recently trialed for safety: epidural cortical stimulation in a small group of chronic stroke patients.50 The results have been generally positive, but highly variable. More information is needed to understand which patients might benefit most from which intervention before any larger scale trials are appropriate.
These techniques have also been tried in the context of dysphagia: both transcranial direct current stimulation56,57 and rTMS58,59 can modulate excitability of cortical circuits involved in pharyngeal control and improve swallowing in dysphagic patients.57,58 However, as in other proof-of-principle studies, the patient numbers are very small and trials have only involved 1 center.
Drug-based interventions have focused on amphetamine due to the early positive effects with amphetamine in animal models. Larger human trials have not shown benefit,60,61 and noradrenergic stimulation may prove not to be the right approach in an patient population at risk of worsening control of hypertension. A dopaminergic approach may be a promising alternative.62
Robotics adapts a motor learning model toward movement rehabilitation and has been shown in small studies to be beneficial.63,64 It also allows for standardization of therapy and quantification of rehabilitation, which has been a problem dogging conventional therapy trials.65 However, while robots are useful in a research setting with small patient numbers, current building costs make robots expensive for larger studies.
There are 2 main problems in these small-scale studies. (1) There are as many versions of each technique as centers that have used them, creating a “Babel” of intervention protocols and making it impossible for results to be collated across centers. (2) The majority of studies have selected very compliant patients with relatively mild deficits. Even here, there has been only 10% to 20% improvement, often of physiological or nonfunctional behavioral outcomes. In addition, most studies have been in single sessions, rather than examining long-term application in therapeutic settings, dosage effects, or wider patient populations with more severe deficits.
Aphasia and Communication Problems
There are 2 major caveats in regard to treatments of aphasia. First, there is no animal model of an equivalent deficit that can be used to inform work in patients. Second, those who actively treat patients believe that, because of the complexity of the distributed system controlling speech and language, the deficit is highly individualized from one patient to the next. In their view this precludes large treatment trials of a standardized therapy. Although meta-analysis of RCTs of aphasia therapy suggests that treatment effects are greater when treatment is more intensive or more prolonged,66 the studies entered into this meta-analysis are old and the nature of therapy poorly specified; their relevance to current practice remains unclear.
Despite these problems, single-case experimental designs and case series with proper controls allow the conclusion that therapy can be effective for the participant(s).67,68 There is also evidence that participants’ response to specific therapies depends on the nature of their language-processing deficits.69 This evidence, however, is drawn mostly from subjects with chronic aphasia who are willing to participate in such studies. They tend to be younger, more enthusiastic, and have less comorbidity than the population of people with aphasia. Whether findings from these participants can be generalized to a wider range of people with aphasia, and especially those treated soon after onset (the typical pattern in current delivery of services), requires investigation. Some70 have raised the possibility that different treatment strategies are likely to be appropriate in acute and chronic aphasia. In the acute stage, treatments that optimize natural recovery and psychosocial adjustment may be most appropriate. In chronic aphasia treatment can concentrate on re-learning and development of compensatory strategies. There is an urgent need for further trials of well-specified therapies, with designs that allow a proper comparison of the way in which the outcome relates to the nature of the language processing impairment. Equally, we need trials comparing different treatments for the same underlying disorder to establish which is more effective and for whom.
Imaging evidence suggests that during recovery there are increases in rCBF during language tasks in both perilesional regions in the affected hemisphere and homologous regions in the unaffected right hemisphere. However, changes in perilesional regions in the affected hemisphere appear to be the most important component for functional recovery.71 Preliminary reports suggest that rTMS to the unimpaired right hemisphere in people with aphasia due to left hemisphere lesions may enhance the benefit from behavioral treatment.72,73 There are also some studies suggesting small benefits from drug treatments in combination with therapy.74
Neglect and Attention
These often combine a disorder of spatial perception with a more general disorder of attention or vigilance.26 Because patients are unaware of the nature of their deficits, “top-down” retraining strategies self-initiated by the patient are of limited use because they may operate only in specific contexts, but not at an intrinsic “automated” level. Two novel “bottom-up” therapies are available: prism adaptation and drug therapy. Prism adaptation provides a biasing visual input to rotate visual perception toward the side of the lesion in an attempt to induce a contralesional visuomotor adaptation. In contrast, some types of drug therapy (eg, guanfacine, an alpha-adrenergic receptor agonist) may enhance vigilance by an action on preserved frontal areas of cortex.75 Other drug studies have used dopaminergic compounds with varying success.76 The latter approach has proved successful in small-scale “proof-of-principle” trials, but there are no large-scale trials on a general population of neglect patients. Prism adaptation has been explored in several different centers with repeated sessions leading to lasting improvement on several spatial tests. Nevertheless, there are no studies of the most effective “dosage” (ie, prism angle, time worn, days of treatment) or how usefully they improve “real-world” functional activities. Future studies might usefully combine behavioral and drug interventions to investigate potential synergies between these approaches.76
Clinical Trials of Restorative Treatments
The EXCITE trial, for constraint-induced movement therapy (CIMT) is one of the few multicenter randomized trials for any physiotherapeutic intervention.19 This trial on over 200 patients concluded that the therapy could improve arm and hand function in patients 3 to 9 months poststroke. However, despite this clear beneficial effect, CIMT has yet to become a part of routine care. One significant problem is cost: the treatment dosage is high, requiring several hours of treatment daily for 2 weeks. Applied to an estimated 30% of patients who might benefit, this would place an enormous burden on therapy services. Further trials are being conducted to examine dosage effects and extension to other groups of patients.
Health Service Delivery
A very considerable evidence base has shown that patients who receive organized acute and early unit-based inpatient rehabilitation after stroke rather than an alternative service, usually in a general medical or geriatric ward with or without a visiting stroke team, do not stay longer in hospital and are more likely to be alive, independent, and living at home 1 year after the stroke, regardless of gender, age, and stroke severity.18 Unit-based care is more cost-effective and delivers many aspects of care not delivered by a peripatetic specialist stroke team or conventional care.77,78 Remarkably, the differences between unit-based and alternative care persist for 5 and even 10 years.79
Early supported discharge of medically stable patients with support by carers and a multidisciplinary domiciliary therapy team after mild and moderate stroke, with an admission Barthel Index (BI) of >9/20, adds to initial SU gains, and compared with conventional care showed a reduced risk of death or dependency, a hospital stay shortened by 8 days (P < .0001), a significant improvement in domestic and extended activities of daily living, and better life quality,80 which may still be evident after 5 years.81
Once in the community, patients are known to be at risk of deteriorating after stroke as a result of multiple health problems, and health-related quality of life has been shown to decline significantly in the 6 months after discharge.82 Deterioration and readmission are prevented and independence in personal, extended, and leisure-based activities of daily living improved by outpatient or domiciliary rehabilitation, delivered by either a multidisciplinary team or by a physiotherapist or, particularly, by an occupational therapist.83,84
Informal carers should be recognized as an important resource: they enable patients to remain in the community,85 and their support is likely to facilitate patient outcomes and reduce depression.86 Formal support for carers, is difficult to obtain in many countries, but Kalra and colleagues87 have shown that training informal carers in basic nursing skills and facilitation of personal care techniques reduced costs and caregiver burden, and improved psychosocial outcomes in the carer and patient, although there was still no change in patients’ mortality, institutionalization, and disability.
Prognostic Markers and Measures of Progress
There are 3 different categories of measure: (1) Neuroscientific measures; (2) Behavioral measures; (3) Measures of function and life quality.
Neuroscientific (surrogate) measures are correlational rather than causal, and thus do not substitute for other types of measures, even though they may be more objective and quantifiable. They include functional magnetic resonance imaging (fMRI), magnetoencephalography (MEG), and transcranial magnetic stimulation (TMS). They have helped develop the concept of a “residual functional architecture” particularly in relation to hand motor function,88–91 but also after aphasic and dysphagic stroke.92,93 The premise is that the brain uses existing undamaged modules in neighboring cortical regions as well as functionally related regions in the contralateral hemisphere for compensation and adaptation after injury, and that the modules used change over time after a stroke.93–95 The hypothesis is that although each stroke may be highly specific to each individual, certain patterns of functional residual architecture may be useful predictors of outcome and therapy needs. However, this has yet to be tested.
Behavioral measures try to determine how a specific behavior or performance of a task changes after an intervention or over time. They often adopt a reductionist approach to break down a task to measurable chunks. In relation to hand function this may consist of measuring force production, force control, reaction speed, muscle stiffness, or kinematics of a movement. Outcomes in studies of dysphagia can be separated into lingual and pharyngeal phases, and then further subdivided into sensory and motor components of each aspect of the task.
This can identify the nature of the recovery process, how change in different impairments contributes to recovery of a behavioral task, and whether recovery is the result of reduced impairment or a compensatory strategy. For example, recovery of leg function due to increased muscle tone that allows a joint to be stabilized, is a compensatory strategy that improves function without enhancing voluntary motor output or reducing impairment. Robotics offers an attractive method for measuring many behavioral outcomes simultaneously and objectively.
Measures of function and life quality reflect the objective and subjective impact of behavioral changes on care needs, independence, and involvement in social roles after stroke. Measures of focal function, such as the Jebsen-Taylor hand function test, the Wolf Motor Function Test, and the Fugl-Meyer scale are often useful in translational research. Generic measures of general function and life quality, for example the Barthel Index, modified Rankin Scale (mRS), and Short Form 36 (SF-36), are commonly used after stroke96,97 but may poorly represent the impact of stroke, for example as a result of dysphasia; exhibit ceiling or floor effects in different study populations; and are relatively insensitive to change over time. These difficulties are less evident with specific measures of outcome, which after stroke include the Stroke Impact Scales and Stroke Specific Quality of Life (SS-QOL) scale,98,99 although concerns remain that the impact of neuropsychological and linguistic impairments are poorly represented.
References
- 1.Hoffman C, Rice D, Sung HY. Persons with chronic conditions. Their prevalence and costs. JAMA. 1996;276:1473–1479. [PubMed] [Google Scholar]
- 2.World Health Organization . Neurological Disorders: Public Health Challenges. World Health Organization; Geneva: 2006. [Google Scholar]
- 3.Feigin VL, Lawes CMM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th Century. Lancet Neurol. 2003;2:43–53. doi: 10.1016/s1474-4422(03)00266-7. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe CDA. The impact of stroke. Br Med Bull. 2000;56:275–286. doi: 10.1258/0007142001903120. [DOI] [PubMed] [Google Scholar]
- 5.Department of Health (UK) Reducing Brain Damage: Faster Access to Better Stroke Care. National Audit Office; London: 2005. [Google Scholar]
- 6.Mayo NE, Wood-Dauphinee S, Côté R, Durcan L, Carlton J. Activity, participation and quality of life 6 months post stroke. Arch Phys Med Rehabil. 2002;83:1035–1042. doi: 10.1053/apmr.2002.33984. [DOI] [PubMed] [Google Scholar]
- 7.Patel A, Duncan P, Lai S, Studenski S. The relation between impairments and functional outcomes poststroke. Arch Phys Med Rehabil. 2000;81:1357–1363. doi: 10.1053/apmr.2000.9397. [DOI] [PubMed] [Google Scholar]
- 8.Haacke C, Althaus A, Spottke A, Siebert U, Back T, Dodel R. Long-term outcome after stroke: evaluating health-related quality of life using utility measurements. Stroke. 2006;37:193–198. doi: 10.1161/01.STR.0000196990.69412.fb. [DOI] [PubMed] [Google Scholar]
- 9.Paul SL, Sturm JW, Dewey HM, Donnan GA, Macdonell RA, Thrift AG. Long-term outcome in the North East Melbourne Stroke Incidence Study: predictors of quality of life at 5 years after stroke. Stroke. 2005;36:2082–2086. doi: 10.1161/01.STR.0000183621.32045.31. [DOI] [PubMed] [Google Scholar]
- 10.Fuhrer MJ. Subjective well-being: implications for medical rehabilitation outcomes and models of disablement. Am J Phys Med Rehabil. 1994;73:358–364. doi: 10.1097/00002060-199409000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Academy of Medical Sciences . Strengthening Clinical Research. Academy of Medical Sciences; London: 2003. [Google Scholar]
- 12.Treasury HM. Cooksey Report: A Review of UK Health Research Funding. HMSO; London: 2006. [Google Scholar]
- 13.Medical Research Council Workshop . Accelerating the Translation of Medical Research. Medical Research Council; London: 2007. [Google Scholar]
- 14.Sung NS, Crowley WF, Jr, Genel M, et al. Central challenges facing the national clinical research enterprise. JAMA. 2003;289:1278–1287. doi: 10.1001/jama.289.10.1278. [DOI] [PubMed] [Google Scholar]
- 15.Westfall JM, Mold J, Fagnan L. Practice-based research - “blue highways” on the NIH roadmap. JAMA. 2007;297:403–406. doi: 10.1001/jama.297.4.403. [DOI] [PubMed] [Google Scholar]
- 16.Woolf SH. The meaning of translational research and why it matters. JAMA. 2008;299:211–213. doi: 10.1001/jama.2007.26. [DOI] [PubMed] [Google Scholar]
- 17.Greenwood R, Ward N. Rehabilitation of stroke. In: Schapira AHV, editor. Neurology and Clinical Neuroscience. Mosby Elsevier; Philadelphia: 2007. pp. 645–660. [Google Scholar]
- 18.Stroke Unit Trialists’ Collaboration Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev. 2007;(4):CD000197. doi: 10.1002/14651858.CD000197.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Wolf SL, Winstein CJ, Miller JP, et al. EXCITE Investigators Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 20.Frantz S. Therapeutic area influences development costs. Nat Rev Drug Discov. 2004;3:4. doi: 10.1038/nrd1436. [DOI] [PubMed] [Google Scholar]
- 21.Academy of Medical Sciences . Restoring Neurological Function: Putting the Neurosciences to Work in Neurorehabilitation. Academy of Medical Sciences; London: 2004. [Google Scholar]
- 22.Sturm JW, Donnan GA, Dewey HM, et al. Determinants of handicap after stroke: the North East Melbourne Stroke Incidence Study (NEMESIS). Stroke. 2004;35:2340–2345. doi: 10.1161/01.STR.0000117573.19022.66. [DOI] [PubMed] [Google Scholar]
- 23.Ada L, O'Dwyer N, O'Neill E. Relation between spasticity, weakness and contracture of the elbow flexors and upper limb activity after stroke: an observational study. Disabil Rehabil. 2006;28:891–897. doi: 10.1080/09638280500535165. [DOI] [PubMed] [Google Scholar]
- 24.Bakas T, Kroenke K, Plue LD, Perkins SM, Williams LS. Outcomes among family caregivers of aphasic versus nonaphasic stroke survivors. Rehabil Nurs. 2006;3:33–42. doi: 10.1002/j.2048-7940.2006.tb00008.x. [DOI] [PubMed] [Google Scholar]
- 25.Patel MD, McKevitt C, Lawrence E, Rudd AG, Wolfe CD. Clinical determinants of long-term quality of life after stroke. Age Aging. 2007;36:316–322. doi: 10.1093/ageing/afm014. [DOI] [PubMed] [Google Scholar]
- 26.Parton A, Malhotra P, Husain M. Hemispatial neglect. J Neurol Neurosurg Psychiatry. 2004;75:13–21. [PMC free article] [PubMed] [Google Scholar]
- 27.Clarke P, Marshall V, Black SE, Colantonio A. Well-being after stroke in Canadian seniors: findings from the Canadian study of health and aging. Stroke. 2002;33:1016–1021. doi: 10.1161/01.str.0000013066.24300.f9. [DOI] [PubMed] [Google Scholar]
- 28.Blanton S, Morris DM, Prettyman MG, et al. Lessons learned in participant recruitment and retention: the EXCITE trial. Phys Ther. 2006;86:1520–1533. doi: 10.2522/ptj.20060091. [DOI] [PubMed] [Google Scholar]
- 29.Todman J, Dugard P. Single Case and Small-n Experimental Designs: A Practical Guide to Randomization Tests. Lawrence Erlbaum; Mahwah, NJ: 2001. [Google Scholar]
- 30.Medical Research Council (UK) A Framework for Development and Evaluation of RCTs for Complex Interventions to Improve Health. Medical Research Council; London: 2000. [Google Scholar]
- 31.Dancause N, Barbay S, Frost SB, et al. Extensive cortical rewiring after brain injury. J Neurosci. 2005;25:10167–10179. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kleim JA, Barbay S, Cooper NR, et al. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol Learn Mem. 2002;77:63–77. doi: 10.1006/nlme.2000.4004. [DOI] [PubMed] [Google Scholar]
- 33.Friel KM, Heddings AA, Nudo RJ. Effects of postlesion experience on behavioral recovery and neurophysiologic reorganization after cortical injury in primates. Neurorehabil Neural Repair. 2000;14:187–198. doi: 10.1177/154596830001400304. [DOI] [PubMed] [Google Scholar]
- 34.Maldonado MA, Allred RP, Felthauser EL, Jones TA. Motor skill training, but not voluntary exercise, improves skilled reaching after unilateral ischemic lesions of the sensorimotor cortex in rats. Neurorehabil Neural Repair. 2008;22:250–261. doi: 10.1177/1545968307308551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biernaskie J, Chernenko G, Corbett D. Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. J Neurosci. 2004;24:1245–1254. doi: 10.1523/JNEUROSCI.3834-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barbay S, Zoubina EV, Dancause N, et al. A single injection of D-amphetamine facilitates improvements in motor training following a focal cortical infarct in squirrel monkeys. Neurorehabil Neural Repair. 2006;20:455–458. doi: 10.1177/1545968306290773. [DOI] [PubMed] [Google Scholar]
- 37.Hovda DA, Feeney DM. Amphetamine and experience promote recovery of locomotor function after unilateral frontal cortex injury in the cat. Brain Res. 1984;298:358–361. doi: 10.1016/0006-8993(84)91437-9. [DOI] [PubMed] [Google Scholar]
- 38.Goldstein LB, Davis JN. Post-lesion practice and amphetamine-facilitated recovery of beam-walking in the rat. Restor Neurol Neurosci. 1990;2:311–314. doi: 10.3233/RNN-1990-1501. [DOI] [PubMed] [Google Scholar]
- 39.Hurwitz BE, Dietrich WD, McCabe PM, Alonson O, Watson BD. Amphetamine promotes recovery from sensory-motor integration deficit after thrombotic infarction of the primary somatosensory rat cortex. Stroke. 1991;22:648–654. doi: 10.1161/01.str.22.5.648. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez CL, Gharbawie OA, Kolb B. Chronic low-dose administration of nicotine facilitates recovery and synaptic change after focal ischemia in rats. Neuropharmacology. 2006;50:777–787. doi: 10.1016/j.neuropharm.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 41.Schäbitz WR, Berger C, Kollmar R, et al. Effect of brain-derived neurotrophic factor treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke. 2004;35:992–997. doi: 10.1161/01.STR.0000119754.85848.0D. [DOI] [PubMed] [Google Scholar]
- 42.Chen P, Goldberg DE, Kolb B, Lanser M, Benowitz LI. Inosine induces axonal rewiring and improves behavioral outcome after stroke. Proc Natl Acad Sci U S A. 2002;99:9031–9036. doi: 10.1073/pnas.132076299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith JM, Lunga P, Story D, et al. Inosine promotes recovery of skilled motor function in a model of focal brain injury. Brain. 2007;130:915–925. doi: 10.1093/brain/awl393. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Zhang ZG, Rhodes K, et al. Post-ischemic treatment with erythropoietin or carbamylated erythropoietin reduces infarction and improves neurological outcome in a rat model of focal cerebral ischemia. Br J Pharmacol. 2007;151:1377–1384. doi: 10.1038/sj.bjp.0707285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Papadopoulos CM, Tsai S-Y, Cheatwood JL, et al. Dendritic plasticity in the adult rat following middle cerebral artery occlusion and Nogo-a neutralization. Cereb Cortex. 2006;16:529–536. doi: 10.1093/cercor/bhi132. [DOI] [PubMed] [Google Scholar]
- 46.Adkins DL, Campos P, Quach D, Borromeo M, Schallert K, Jones TA. Epidural cortical stimulation enhances motor function after sensorimotor cortical infarcts in rats. Exp Neurol. 2006;200:356–370. doi: 10.1016/j.expneurol.2006.02.131. [DOI] [PubMed] [Google Scholar]
- 47.Kleim JA, Bruneau R, VandenBerg P, MacDonald E, Mulrooney R, Pocock D. Motor cortex stimulation enhances motor recovery and reduces peri-infarct dysfunction following ischemic insult. Neurol Res. 2003;25:789–793. doi: 10.1179/016164103771953862. [DOI] [PubMed] [Google Scholar]
- 48.Teskey GC, Flynn C, Goertzen CD, Monfils MH, Young NA. Cortical stimulation improves skilled forelimb use following a focal ischemic infarct in the rat. Neurol Res. 2003;25:794–800. doi: 10.1179/016164103771953871. [DOI] [PubMed] [Google Scholar]
- 49.Plautz EJ, Barbay S, Frost SB, et al. Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: a feasibility study in primates. Neurol Res. 2003;25:801–810. doi: 10.1179/016164103771953880. [DOI] [PubMed] [Google Scholar]
- 50.Brown JA, Lutsep HL, Weinand M, Cramer SC. Motor cortex stimulation for the enhancement of recovery from stroke: a prospective, multicenter safety study. Neurosurgery. 2006;58:464–473. doi: 10.1227/01.NEU.0000197100.63931.04. [DOI] [PubMed] [Google Scholar]
- 51.Rosser AE, Zietlow R, Dunnett SB. Stem cell transplantation for neurode-generative diseases. Curr Opin Neurol. 2007;20:688–692. doi: 10.1097/WCO.0b013e3282f132fc. [DOI] [PubMed] [Google Scholar]
- 52.Hicks AU, Hewlett K, Windle V, et al. Enriched environment enhances transplanted subventricular zone stem cell migration and functional recovery after stroke. Neuroscience. 2007;146:31–40. doi: 10.1016/j.neuroscience.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 53.Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117:2584–2596. doi: 10.1016/j.clinph.2006.06.712. [DOI] [PubMed] [Google Scholar]
- 54.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 55.Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Arch Neurol. 2004;61:1844–1848. doi: 10.1001/archneur.61.12.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hamdy S, Rothwell JC, Aziz Q, Singh KD, Thompson DG. Long-term reorganization of human motor cortex driven by short-term sensory stimulation. Nat Neurosci. 1998;1:64–8. doi: 10.1038/264. [DOI] [PubMed] [Google Scholar]
- 57.Power ML, Fraser CH, Hobson A, et al. Evaluating oral stimulation as a treatment for dysphagia after stroke. Dysphagia. 2006;21:49–55. doi: 10.1007/s00455-005-9009-0. [DOI] [PubMed] [Google Scholar]
- 58.Fraser C, Power M, Hamdy S, et al. Driving plasticity in human adult motor cortex is associated with improved motor function after brain injury. Neuron. 2002;34:831–840. doi: 10.1016/s0896-6273(02)00705-5. [DOI] [PubMed] [Google Scholar]
- 59.Gow D, Rothwell J, Hobson A, Thompson D, Hamdy S. Induction of long-term plasticity in human swallowing motor cortex following repetitive cortical stimulation. Clin Neurophysiol. 2004;115:1044–1051. doi: 10.1016/j.clinph.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 60.Gladstone DJ, Danells CJ, Armesto A, et al. Subacute therapy with amphetamine and rehabilitation for stroke study investigators. Physiotherapy coupled with dextroamphetamine for rehabilitation after hemiparetic stroke: a randomized, double-blind, placebo-controlled trial. Stroke. 2006;37:179–185. doi: 10.1161/01.STR.0000195169.42447.78. [DOI] [PubMed] [Google Scholar]
- 61.Platz T, Kim IH, Engel U, Pinkowski C, Eickhof C, Kutzner M. Amphetamine fails to facilitate motor performance and to enhance motor recovery among stroke patients with mild arm paresis: interim analysis and termination of a double blind, randomised, placebo-controlled trial. Restor Neurol Neurosci. 2005;23:271–280. [PubMed] [Google Scholar]
- 62.Floel A, Hummel F, Breitenstein C, Knecht S, Cohen LG. Dopaminergic effects on encoding of a motor memory in chronic stroke. Neurology. 2005;65:472–474. doi: 10.1212/01.wnl.0000172340.56307.5e. [DOI] [PubMed] [Google Scholar]
- 63.Aisen ML, Krebs HI, Hogan N, McDowell F, Volpe BT. The effect of robot-assisted therapy and rehabilitative training on motor recovery following stroke. Arch Neurol. 1997;54:443–446. doi: 10.1001/archneur.1997.00550160075019. [DOI] [PubMed] [Google Scholar]
- 64.Ferraro M, Palazzolo JJ, Krol J, Krebs HI, Hogan N, Volpe BT. Robot-aided sensorimotor arm training improves outcome in patients with chronic stroke. Neurology. 2003;61:1604–1607. doi: 10.1212/01.wnl.0000095963.00970.68. [DOI] [PubMed] [Google Scholar]
- 65.Ballinger C, Ashburn A, Low J, Roderick P. Unpacking the black box of therapy: a pilot study to describe occupational therapy and physiotherapy interventions for people with stroke. Clin Rehabil. 1999;13:301–309. doi: 10.1191/026921599673198490. [DOI] [PubMed] [Google Scholar]
- 66.Bhogal S, Teasell R, Speechley M. Intensity of aphasia therapy, impact on recovery. Stroke. 2003;34:987–993. doi: 10.1161/01.STR.0000062343.64383.D0. [DOI] [PubMed] [Google Scholar]
- 67.Nickels LA. Improving word finding: practice makes (closer to) perfect? Aphasiology. 2002;16:1047–1060. [Google Scholar]
- 68.Thompson CK. Single subject controlled experiments in aphasia: the science and the state of the science. J Commun Disord. 2006;39:266–291. doi: 10.1016/j.jcomdis.2006.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hickin J, Best W, Herbert R, Howard D, Osborne F. Phonological therapy for word-finding difficulties: a re-evaluation. Aphasiology. 2002;16:981–999. [Google Scholar]
- 70.Holland A, Fridriksson J. Aphasia management during the early phases of recovery following stroke. Am J Speech Lang Pathol. 2001;10:19–28. [Google Scholar]
- 71.Saur D, Lange R, Baumgaertner A, et al. Dynamics of language reorganization after stroke. Brain. 2006;129:1371–1384. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- 72.Martin PI, Naeser MA, Theoret H, et al. Transcranial magnetic stimulation as a complementary treatment for aphasia. Semin Speech Lang. 2004;25:181–91. doi: 10.1055/s-2004-825654. [DOI] [PubMed] [Google Scholar]
- 73.Naeser MA, Martin PI, Nicholas M, et al. Improved picture naming in chronic aphasia after TMS to part of right Broca's area: an open-protocol study. Brain Lang. 2005;93:95–105. doi: 10.1016/j.bandl.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 74.de Boissezon X, Peran P, de Boysson C, Démonet JF. Pharmacotherapy of aphasia: Myth or reality? Brain Lang. 2007;102:114–125. doi: 10.1016/j.bandl.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 75.Malhotra PA, Parton AD, Greenwood R, Husain M. Noradrenergic modulation of space exploration in visual neglect. Ann Neurol. 2006;59:186–190. doi: 10.1002/ana.20701. [DOI] [PubMed] [Google Scholar]
- 76.Singh-Curry V, Husain M. Rehabilitation of neglect. In: Stuss DT, Winocur G, Robertson IH, editors. Cognitive Neurorehabilitation. Cambridge University Press; Cambridge: 2008. pp. 449–463. [Google Scholar]
- 77.Evans A, Perez I, Harraf F, et al. Can differences in management processes explain different outcomes between stroke unit and stroke-team care? Lancet. 2001;358:1586–1592. doi: 10.1016/S0140-6736(01)06652-1. [DOI] [PubMed] [Google Scholar]
- 78.Moodie M, Cadilhac D, Pearce D, et al. SCOPES Study Group Economic evaluation of Australian stroke services: a prospective, multicenter study comparing dedicated stroke units with other care modalities. Stroke. 2006;37:2790–2795. doi: 10.1161/01.STR.0000245083.97460.e1. [DOI] [PubMed] [Google Scholar]
- 79.Indredavik B, Bakke F, Slordahl SA, Rokseth R, Hâheim LL. Stroke unit treatment. 10-year follow-up. Stroke. 1999;30:1524–1527. doi: 10.1161/01.str.30.8.1524. [DOI] [PubMed] [Google Scholar]
- 80.Langhorne P, Taylor G, Murray G, et al. Early supported discharge services for stroke patients: a meta-analysis of individual patients’ data. Lancet. 2005;365:501–506. doi: 10.1016/S0140-6736(05)17868-4. [DOI] [PubMed] [Google Scholar]
- 81.Thorsén AM, Holmqvist LW, de Pedro-Cuesta J, von Koch L. A randomized controlled trial of early supported discharge and continued rehabilitation at home after stroke: five-year follow-up of patient outcome. Stroke. 2005;36:297–303. doi: 10.1161/01.STR.0000152288.42701.a6. [DOI] [PubMed] [Google Scholar]
- 82.Hopman WM, Verner J. Quality of life during and after inpatient stroke rehabilitation. Stroke. 2003;34:801805. doi: 10.1161/01.STR.0000057978.15397.6F. [DOI] [PubMed] [Google Scholar]
- 83.Outpatient Therapy Trialists Rehabilitation therapy services for stroke patients living at home: systematic review of randomised trials. Lancet. 2004;363:352–356. doi: 10.1016/S0140-6736(04)15434-2. [DOI] [PubMed] [Google Scholar]
- 84.Steultjens EM, Dekker J, Bouter LM, van de Nes JCM, Cup EH, van den Ende CH. Occupational therapy for stroke patients. A systematic review. Stroke. 2003;34:676–687. doi: 10.1161/01.STR.0000057576.77308.30. [DOI] [PubMed] [Google Scholar]
- 85.Hancock R, Jarvis C. The Long Term effects of Being a Carer. HMSO; London: 1994. [Google Scholar]
- 86.Morris PL, Robinson RG, Raphael B, Bishop D. The relationship between the perception of social support in post-stroke depression in hospitalised patients. Psychiatry. 1991;54:306–316. doi: 10.1080/00332747.1991.11024559. [DOI] [PubMed] [Google Scholar]
- 87.Kalra L, Evans A, Perez I, et al. Training carers of stroke patients: randomised controlled trial. Br Med J. 2004;328:1099–1101. doi: 10.1136/bmj.328.7448.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gerloff C, Bushara K, Sailer A, et al. Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain. 2006;129:791–808. doi: 10.1093/brain/awh713. [DOI] [PubMed] [Google Scholar]
- 89.Luft AR, Waller S, Forrester L, et al. Lesion location alters brain activation in chronically impaired stroke survivors. Neuroimage. 2004;21(3):924–35. doi: 10.1016/j.neuroimage.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 90.Ward NS. Neural plasticity and recovery of function. Prog Brain Res. 2005;150:527–535. doi: 10.1016/S0079-6123(05)50036-0. [DOI] [PubMed] [Google Scholar]
- 91.Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130:170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- 92.Price CJ, Crinion J. The latest on functional imaging studies of aphasic stroke. Curr Opin Neurol. 2005;18:429–434. doi: 10.1097/01.wco.0000168081.76859.c1. [DOI] [PubMed] [Google Scholar]
- 93.Thulborn KR, Carpenter PA, Just MA. Plasticity of language-related brain function during recovery from stroke. Stroke. 1999;30:749–754. doi: 10.1161/01.str.30.4.749. [DOI] [PubMed] [Google Scholar]
- 94.Feydy A, Carlier R, Roby-Brami A, et al. Longitudinal study of motor recovery after stroke: recruitment and focusing of brain activation. Stroke. 2002;33:1610–1617. doi: 10.1161/01.str.0000017100.68294.52. [DOI] [PubMed] [Google Scholar]
- 95.Ward NS, Brown MM, Thompson AJ, Frackowiak RS. The influence of time after stroke on brain activations during a motor task. Ann Neurol. 2004;55:829–834. doi: 10.1002/ana.20099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Banks JL, Marotta CA. Outcomes validity and reliability of the Modified Rankin Scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38:1091–1096. doi: 10.1161/01.STR.0000258355.23810.c6. [DOI] [PubMed] [Google Scholar]
- 97.Patel MD, Tilling K, Lawrence E, Rudd AG, Wolfe CD, McKevitt C. Relationships between long-term stroke disability, handicap and health-related quality of life. Age Ageing. 2006;35:273–279. doi: 10.1093/ageing/afj074. [DOI] [PubMed] [Google Scholar]
- 98.Duncan PW, Lai SM, Bode RK, Perera S, DeRosa J. Stroke Impact Scale-16: a brief assessment of physical function. Neurology. 2003;60:291–296. doi: 10.1212/01.wnl.0000041493.65665.d6. [DOI] [PubMed] [Google Scholar]
- 99.Williams LS, Weinberger M, Harris LE, Clark DO, Biller J. Development of a stroke-specific quality of life (SS-QOL) scale. Stroke. 1999;30:1362–1369. doi: 10.1161/01.str.30.7.1362. [DOI] [PubMed] [Google Scholar]