SUMMARY
We estimated the effectiveness and cost-effectiveness of changes in concurrent sexual partnerships in reducing the spread of HIV in sub-Saharan Africa. Using data from Swaziland, Tanzania, Uganda, and Zambia, we estimated country-specific concurrency behavior from sexual behavior survey data on the number of partners in the past 12 months, and we developed a network model to compare the impact of three behavior changes on the HIV epidemic: (1) changes in concurrent partnership patterns to strict monogamy; (2) partnership reduction among those with the greatest number of partners; and (3) partnership reduction among all individuals. We estimated the number of new HIV infections over ten years and the cost per infection averted. Given our assumptions and model structure, we find that reducing concurrency among high-risk individuals averts the most infections and increasing monogamy the least (11.7% versus 8.7% reduction in new infections, on average, for a 10% reduction in concurrent partnerships). A campaign that costs $1 per person annually is likely cost-saving if it reduces concurrency by 9% on average, given our baseline estimates of concurrency. In sensitivity analysis, the rank ordering of behavior change scenarios was unaffected by potential over-estimation of concurrency, though the number of infections averted decreased and the cost per HIV infection averted increased. Concurrency reduction programs may be effective and cost-effective in reducing HIV incidence in sub-Saharan Africa if they can achieve even modest impacts at similar costs to past mass media campaigns in the region. Reduced concurrency among high-risk individuals appears to be most effective in reducing HIV incidence, but concurrency reduction in other risk groups may yield nearly as much benefit.
Keywords: HIV/AIDS, sub-Saharan Africa, concurrent partnerships, sexual partnership network, cost effectiveness
INTRODUCTION
HIV prevalence in parts of sub-Saharan Africa is orders of magnitude higher than in Europe or the US [1]. This disparity may be due in part to the existence of concurrent sexual partnerships, which have been documented at high rates in parts of sub-Saharan Africa [1–5]. Concurrent partnerships allow for more efficient transmission of HIV by increasing the number of individuals connected together through a network of sexual partnerships. In a monogamous relationship, the virus can be transmitted between partners, but cannot escape the partnership, whereas in a concurrent sexual network the virus can rapidly spread [6]. Previous work has suggested that for the same number of lifetime partners, having concurrent partners is associated with a more rapid spread of sexually transmitted diseases than having one partner at a time [6–8]. This is of particular concern with HIV, since transmission of the virus is most efficient during the early phase of the infection, when viral load is high and the ability to diagnose individuals is limited [9].
Individuals engage in multiple concurrent partnerships for a variety of reasons (e.g., sexual variety, increased social status, and material gain [10–14]) and to different degrees (some individuals may only have two partners simultaneously, while others may have many concurrent partners). Strategies to reduce concurrent partnerships may achieve different levels of success depending on the target population and behavior change: for example, a campaign that promotes faithfulness in married or cohabitating partnerships may have a different effect on the spread of HIV than a campaign that reduces partnerships among highly promiscuous individuals.
Reducing concurrent sexual partnerships is increasingly an important goal for HIV prevention in highly endemic areas [15–17]. A recent review of HIV prevention activities argued that partner reduction programs have had a powerful impact on containing the spread of HIV, and should be more assertively promoted [17]. Several countries in Africa have targeted concurrent sexual partnerships for HIV prevention. In the late 1980s, Uganda launched a “Zero Grazing” campaign which cost an estimated $0.23 per person in the population annually and promoted faithfulness as a means of reducing concurrent sexual partnerships [18–21]. The introduction of this campaign in Uganda was associated with a significant decrease in HIV prevalence in the early 1990s, while other countries in the region were still seeing HIV prevalence rise [20, 21]. While the “Zero Grazing” campaign may not have been solely responsible to the reduction in HIV prevalence, several analyses have concluded that other factors such as condom use or HIV mortality cannot explain the large reductions in prevalence seen in Uganda compared to its neighbors [20–24]. More recently, observational studies in Zimbabwe found a reduction in the number of individuals reporting multiple sex partners, including concurrent partners, from the late 1990s to the 2000s [25–27]. Mathematical models suggest that this change in sexual behavior contributed to the observed coincident decline in HIV prevalence, which cannot be explained by the natural history of the epidemic alone [23, 26, 27]. These examples suggest that behavior change can have a major impact on the course of the HIV epidemic.
Many African countries now focus on concurrent partnerships in their HIV prevention programs. For example, Zambia encourages faithfulness to a single partner as part of its sexual risk reduction programs [12, 28]. Botswana recently embarked on a comprehensive nationwide campaign to reduce concurrency [29], targeting the underlying motivations for engaging in multiple concurrent partnerships, such as a desire for sexual variety or material gain [30, 31]. The extent of concurrent partnership behavior change caused by such programs is uncertain.
Previous behavior change campaigns in the region have seen reductions in number of sexual partners or increases in monogamous relationships in direct response to mass media campaigns in a variety of settings [32]. Studies of various mass media campaigns in sub-Saharan Africa found changes in sexual risk behavior among individuals within the broadcast area as compared to those outside of the broadcast area: in Zimbabwe, monogamous behavior among young adults increased by 18% in response to a reproductive health multi-media campaign targeting adolescents [33]; in Tanzania, there was a 17% reduction in the number of sexual partners reported by men in the previous year in response to an educational radio drama highlighting the consequences of risky sexual behavior [34].
We sought to evaluate the relative effectiveness and cost-effectiveness of concurrency reduction campaigns based on the level and type of behavior change achieved. We used country-specific data on demographics, HIV prevalence, and sexual behavior from sub-Saharan Africa. Because the true level of concurrency in these populations is uncertain, we varied the level of concurrency in sensitivity analysis.
METHODS
Overview
We developed a stochastic network model to simulate the course of the HIV epidemic in one-month intervals over a 10-year time horizon in a population of approximately 9,000 adults aged 15–49 (details in Appendix). The model tracks sexual partnerships, entry into the population, HIV transmission and disease progression, HIV treatment, deaths from HIV and other causes, life years experienced in the population, and HIV incidence and prevalence. We used data on sexual partnership patterns and the current state of the HIV epidemic from four sub-Saharan African countries: Swaziland, Tanzania, Uganda, and Zambia (Table 1). We examined four countries in order to determine the impact of concurrency reduction in different demographic and epidemiologic settings. We chose these countries because sufficient nationally representative data was available that was generated using a consistent collection methodology across countries, on their demographic structure, HIV epidemic, and sexual behavior (see Appendix). We used the model to evaluate several types of behavior change that might be achieved by a concurrency reduction campaign and the effects that such behavior change might have on the spread of HIV. We also determined the cost-effectiveness of concurrency reduction programs as a function of program cost and the level and type of behavior change achieved.
Table 1.
Model Inputs and Sources
| Variable | Value | 95% CI | Source |
|---|---|---|---|
| Population Characteristics | |||
| Population size | ~9000* | ||
| % Married | MEASURE DHS [36–39] | ||
| Swaziland | 33% | ± 0.36% | |
| Tanzania | 52% | ± 0.42% | |
| Uganda | 58% | ± 0.33% | |
| Zambia | 58% | ± 0.68% | |
| Sexual Partnerships Parameters | |||
| Degree distribution – Swaziland (M, F) | MEASURE DHS [36] | ||
| 0 partners | 39%, 30% | ± 0.72%, ± 0.59% | |
| 1 partner | 47%, 68% | ± 0.76%, ± 0.60% | |
| 2 partners | 13%, 2% | ± 0.33%, ± 0.04% | |
| 3 partners | 0.5%, 0% | ± 0.02%, ± 0.00% | |
| 4 partners | 0.5%, 0% | ± 0.02%, ± 0.00% | |
| Degree distribution – Tanzania (M, F) | MEASURE DHS [37] | ||
| 0 partners | 29%, 24% | ± 0.53%, ± 0.44% | |
| 1 partner | 51%, 64% | ± 0.65%, ± 0.54% | |
| 2 partners | 16%, 9% | ± 0.35%, ± 0.20% | |
| 3 partners | 2%, 2% | ± 0.06%, ± 0.03% | |
| 4 partners | 2%, 1% | ± 0.05%, ± 0.03% | |
| Degree distribution – Uganda (M, F) | MEASURE DHS [38] | ||
| 0 partners | 28%, 27% | ± 0.42%, ± 0.37% | |
| 1 partner | 52%, 71% | ± 0.52%, ± 0.38% | |
| 2 partners | 17%, 2% | ± 0.30%, ± 0.03% | |
| 3 partners | 2%, 0.2% | ± 0.05%, ± 0.00% | |
| 4 partners | 1%, 0% | ± 0.02%, ± 0.00% | |
| Degree distribution – Zambia (M, F) | MEASURE DHS [39] | ||
| 0 partners | 19%, 25% | ± 0.66%, ± 0.42% | |
| 1 partner | 61%, 73% | ± 1.01%, ± 0.44% | |
| 2 partners | 15%, 2% | ± 0.55%, ± 0.04% | |
| 3 partners | 3%, 0% | ± 0.10%, ± 0.00% | |
| 4 partners | 2%, 0% | ± 0.09%, ± 0.00% | |
| Duration of partnerships (years) | Morris [8], NACA [29] | ||
| Spousal | 10 | 4.0–19.9 i | |
| Non-spousal | 1.5 | 0.8–2.4 | |
| HIV Disease Parameters | |||
| HIV transmission probabilities (per act) | Wawer [45], Quinn [73] | ||
| Acute phase | 0.0082 | 0.0039–0.0150 | |
| Chronic phase | 0.0007 | 0.0006–0.0011 | |
| With treatment | 0.0001 | 0.00008–0.00014 | |
| Initial HIV prevalence | MEASURE DHS [36–39] | ||
| Swaziland | 26.2% | ± 0.40% | |
| Tanzania | 6.7% | ± 0.11% | |
| Uganda | 5.8% | ± 0.08% | |
| Zambia | 15.3% | ± 0.26% | |
| CD4 count at HIV treatment initiation | 200 cell/µl | N/A | WHO [54] |
| HIV treatment coverage (of eligible patients) | UNAIDS [1] | ||
| Swaziland | 42% | 36%–50% ii | |
| Tanzania | 31% | 26%–38% | |
| Uganda | 33% | 27%–40% | |
| Zambia | 46% | 40%–56% | |
| HIV additional mortality | Badri [49] | ||
| < 200 cells/µL | 0.0222 | 0.0190–0.0271 | |
| 200 – 350 cells/µL | 0.0066 | 0.0042–0.0100 | |
| > 350 cells/µL | 0.0042 | 0.0025–0.0058 |
We initialized the network model with a population of 4000 men. We formed partnerships according to the degree distribution for each country.34–37 The number of women in the network was then determined by these partnerships. For the degree distributions we used, this led to approximately 4900 women in the population (the number varied slightly by country), and thus a population size of approximately 9000 individuals.
Range found in literature.
Low and high estimates.
Population Characteristics
We stratified the model population by age and gender to match the population demographics of the four study countries [35]. At baseline, our infected population was age- and gender-matched to the HIV prevalence in each country, determined from demographic and health survey data [36–39].
Individuals enter the population at age 15. We used population pyramids at baseline to determine the number of males and females aging into the population each month [35]. Individuals exit the population at age 50 (unless involved in a sexual partnership) or due to death. We used age- and gender-specific death rates, adjusted for HIV mortality, to estimate the risk of death among uninfected individuals [40, 41]. HIV-infected individuals experience an additional mortality risk, determined by their disease state.
Sexual Partnership Network
We constructed a network model with gender-specific concurrent sexual partnership degree distributions estimated from population surveillance data for the four study countries (Table 1) [36–39]. These surveys collected data on the number of sexual partners reported by men and women in the past 12 months, but did not differentiate between concurrent and serial partnerships. We used the number of sexual partners reported in the past 12 months as a surrogate measure for the number of concurrent partnerships. In sensitivity analysis, we considered lower values for the number of concurrent partnerships.
The degree distribution describes the fraction of individuals in the population who have a given number of partnerships. Less than 0.1% of the population in each country reported more than 4 concurrent sexual partnerships, so we assumed that individuals have at most 4 sexual partnerships at any time. On average, men report having more sexual partners than women [36–39]. We balanced the reported partnership distribution at baseline by adjusting the female-to-male ratio appropriately. Over the 10-year time horizon, we assume a fixed degree distribution; that is, the fraction of individuals who are involved in 1, 2, or more concurrent partnerships is stable from month to month.
We modeled two types of partnerships: spousal and non-spousal. The number of spousal partnerships was determined by the proportion of married adults in the general population. On average, spousal partnerships last longer than non-spousal relationships (Table 1). The model captures the formation and dissolution of partnerships each month. Each partnership has a constant monthly probability of dissolution. Spousal partnerships have a lower monthly probability of dissolution than non-spousal partnerships. Partnership dissolution also occurs in the event of the death of a partner. We chose partnership formation probabilities to balance partnership dissolution rates while maintaining the reported gender-specific partnership distributions (see Appendix and [42])
HIV Transmission and Progression
The model includes both acute and chronic HIV infection. Acute infection is characterized by high infectivity for a period of 3 months [43, 44]. The subsequent chronic infection phase is characterized by a slow decline in CD4 counts and a lower infectivity [45–47].
Changes in CD4 counts influence an infected individual’s risk of HIV-related mortality and of developing an AIDS-defining illness (ADI). We modified a previous model of HIV disease progression to estimate HIV-related mortality as a function of CD4 counts [48]. As CD4 counts drop, an individual’s risk of developing an ADI and HIV-related mortality increases [49, 50]. Individuals become eligible for treatment when their CD4 counts fall below 200 cells/µl or when they experience an ADI. Country-specific coverage of antiretroviral therapy (ART) determines the fraction of eligible individuals who ultimately receive treatment [1]. When an infected individual receives effective ART, CD4 counts rise as a function of the CD4 count nadir and time on treatment [51–53]. However if treatment fails, CD4 counts again decline. We modeled treatment options based on World Health Organization (WHO) guidelines, and treatment failure rates based on clinical experience in southern Africa [54, 55].
The probability of acquiring HIV infection depends on the HIV disease state of the infected partner and the level of sexual activity. The risk of infection per sexual act is substantially higher during acute infection than during chronic infection with and without effective treatment (Table 1) [47]. We estimated the level of sexual activity by using the number of sex acts per month described in a cohort of discordant couples, and then adjusted these values to calibrate the model [47].
Model Calibration
We calibrated our model to projected HIV prevalence trends from the UNAIDS Spectrum Package [1, 56]. We chose HIV prevalence as a calibration measure because of the availability of longitudinal HIV prevalence data that has been obtained with a consistent methodology over time. In the four study countries, HIV prevalence is estimated to be stable or in slight decline [1]. To match projected country-specific HIV prevalence trends, we adjusted the monthly risk of HIV infection per partnership (Table 2). These monthly infection risks differ by country and implicitly reflect potential differences in sexual behavior across countries, such as differences in coital frequency, condom use, and prevalence of male circumcision.
Table 2.
Model calibration parameters and measures.
| Swaziland | Tanzania | Uganda | Zambia | |
|---|---|---|---|---|
| Calibration Parameter | ||||
| HIV monthly transmission probabilities (per partnership) | ||||
| Acute phase | 0.34 | 0.18 | 0.25 | 0.25 |
| Chronic phase | 0.029 | 0.015 | 0.021 | 0.022 |
| With treatment | 0.0041 | 0.0022 | 0.0030 | 0.0031 |
| Calibration Measures | ||||
| Change in HIV Prevalence (%) | ||||
| Data, 2004–2007 [1] | −0.4% | −0.3% | −0.9% | 0.1% |
| (low and high estimates indicated) | (−2.5%, 1.7%) | (−1.0%, 0.5%) | (−2.1%, −0.1%) | (−2.1%, 2.2%) |
| Model prediction, 2004–2007 | −0.9% | −0.1% | −0.2% | −1.0% |
| (95% confidence intervals) | (−1.8%, −0.2%) | (−0.7%, 0.4%) | (−0.7%, 0.2%) | (−1.6%, −0.3%) |
| Number of partners | ||||
| Data [36–39] | ||||
| Married (%) | 33% | 52% | 58% | 58% |
| (95% confidence intervals) | (32%, 34%) | (51%, 53%) | (57%, 59%) | (56%, 60%) |
| More than 1 concurrent partner | 14% | 20% | 20% | 20% |
| (95% confidence intervals) | (13%, 15%) | (19%, 21%) | (19%, 21%) | (18%, 22%) |
| Model prediction | ||||
| Married (%) | 31% | 52% | 58% | 57% |
| (95% confidence intervals) | (30%, 32%) | (51%, 53%) | (58%, 59%) | (57%, 58%) |
| More than 1 concurrent partner | 13% | 20% | 21% | 19% |
| (95% confidence intervals) | (12%, 13%) | (19%, 20%) | (20%, 21%) | (19%, 20%) |
The monthly probabilities of HIV transmission were adjusted to match country-specific HIV prevalence trends. Other measures, such as number of married and unmarried partners, are also well-matched.
Behavior Change Scenarios
A campaign to reduce concurrent partnerships may achieve different changes in sexual behavior, depending on the campaign message and target populations. We considered the following types of behavior change that might be achieved:
Increased Monogamy: individuals with more than one partnership change to having at most one partnership at any one time. This may be achieved by promoting faithfulness to a single partner.
High-Risk Partnership Reduction: individuals with the highest number of concurrent partnerships reduce their number of partners, but do not necessarily become monogamous. This may be achieved by a campaign with a partnership reduction message targeting individuals with (or behaviors leading to) a high number of concurrent partnerships.
Untargeted Partnership Reduction: individuals with more than one partnership reduce their number of sexual partners. This may be achieved by a broad partnership reduction campaign targeting all individuals with concurrent partnerships.
We compared the relative effectiveness of concurrency reduction under these behavior change scenarios to answer two important questions: 1) Which type of behavior change achieves the greatest benefits? 2) How much better is the most effective behavior change compared to other (and perhaps more realistic) behavior changes? For example, it may be more difficult to change the behavior of high-risk individuals. We sought to determine how much benefit is gained if high-risk individuals are targeted and, therefore, how important it is to find effective behavior change messages for such individuals.
All behavior change scenarios were compared against a baseline projection of the HIV epidemic in the absence of any changes in concurrent sexual partnerships (Status Quo). Because the effectiveness of concurrent partnership reduction campaigns is unknown, we varied the program effectiveness for each of the scenarios from 0% to 50%. We measured effectiveness as the percentage of non-monogamous individuals who become monogamous under the Increased Monogamy scenario. We compared the results of the other scenarios by removing the same number of partnerships. For example, 10% effectiveness for each of the scenarios implies that the number of concurrent partnerships removed is equal to 10% of the number of partnerships removed under Increased Monogamy. If an Increased Monogamy program that is 10% effective involves a reduction of 200 partnerships, then 10% effectiveness in each of the other scenarios involves 200 fewer partnerships.
Analyses
For each of the three behavior change scenarios, we estimated the number of HIV infections averted over a 10-year time horizon as a function of program effectiveness. We simulated each behavior change scenario 500 times to obtain stable estimates. We express a reduction in the number of new HIV infections as a percentage reduction compared to the Status Quo.
We also examined the cost-effectiveness of concurrency reduction using cost estimates of behavior change campaigns implemented in Uganda and Botswana. Uganda’s “Zero Grazing” campaign was estimated to cost approximately $0.23 per person annually [18], while Botswana’s campaign targeting multiple concurrent partnerships has a budget of over $1 per person annually (T. Kasper, personal communication, August 8, 2009). We estimated the cost per HIV infection averted for the Increased Monogamy scenario as a function of behavior change and annual campaign costs per person.
RESULTS
Status Quo
The Status Quo was associated with an estimated average drop in HIV prevalence of 1.0% (0.3% – 1.9%) in the four study countries over the next 10 years, consistent with UNAIDS estimates of the prevalence trends [56]. We estimated an average HIV incidence of 2.3% (1.1% – 4.3%) in the first year and 1.5% (0.7% – 2.9%) after 10 years, consistent with recent estimates [57, 58].
New HIV Infections
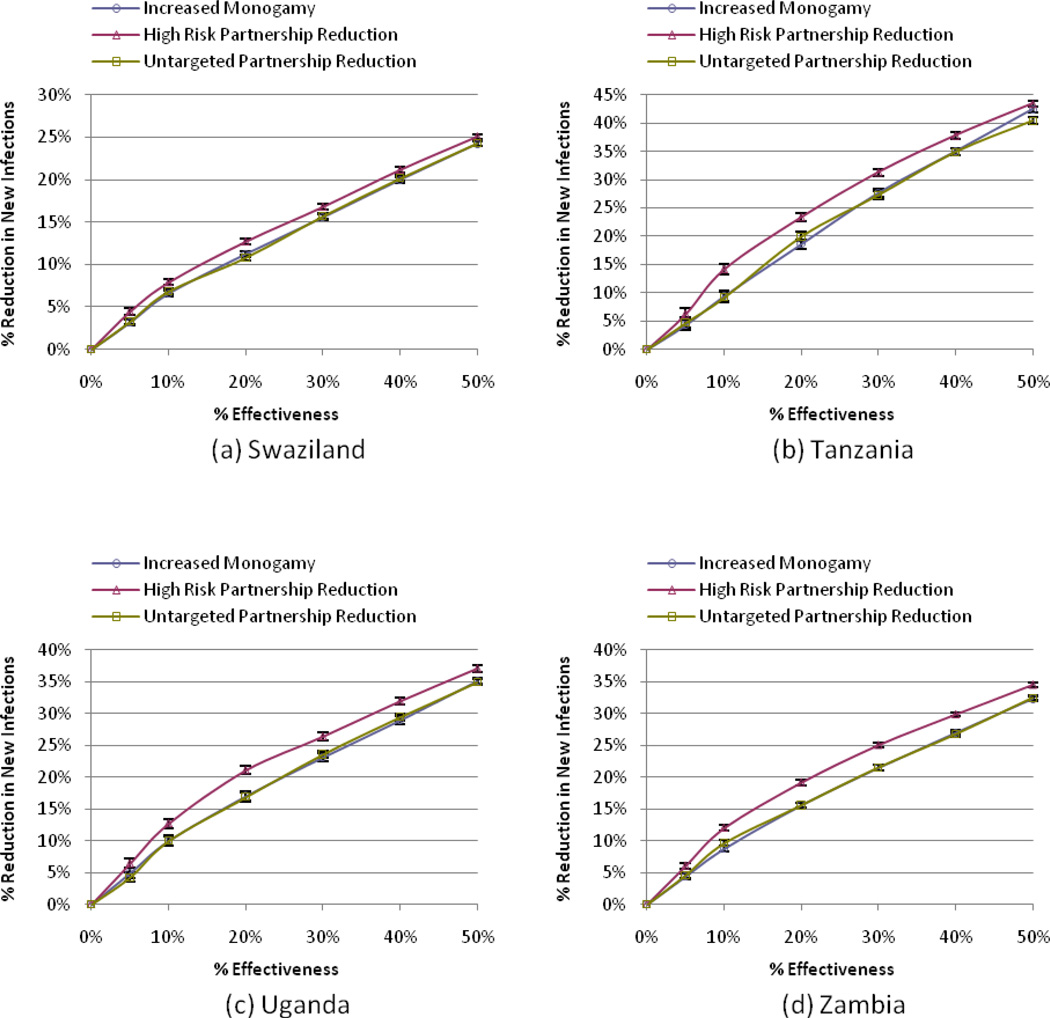
Each modeled behavior change scenario resulted in fewer HIV infections compared to the Status Quo (Figure 1). Across all four study countries and for any level of behavior change, the High-Risk Partnership Reduction scenario resulted in the greatest reduction in new infections. For example, a program targeting high-risk individuals with 10% effectiveness led to an average decrease in new infections of 11.7% (7.9% – 14.2%) over 10 years in the four study countries, as compared to average decreases of 8.7% (6.6% – 10.0%) and 8.9% (6.8% – 10.0%) in new infections under the Increased Monogamy and Untargeted Partnership Reduction scenarios, respectively. The reduction in new infections for each country is shown in Figure 1 for a range of program effectiveness from 0% to 50%.
Figure 1. The effect of behavior change (percentage reduction in concurrent partnerships) on reducing new HIV infections.
The figure shows the estimated percentage reduction in the number of new HIV infections (compared to the Status Quo) for a range of campaign effectiveness from 0% to 50% under the different behavior change scenarios (Increased Monogamy, High-Risk Partnership Reduction, Untargeted Partnership Reduction) for the four study countries, Swaziland (a), Tanzania (b), Uganda (c), and Zambia (d). 95% confidence intervals are indicated for each sample point.
Cost-Effectiveness
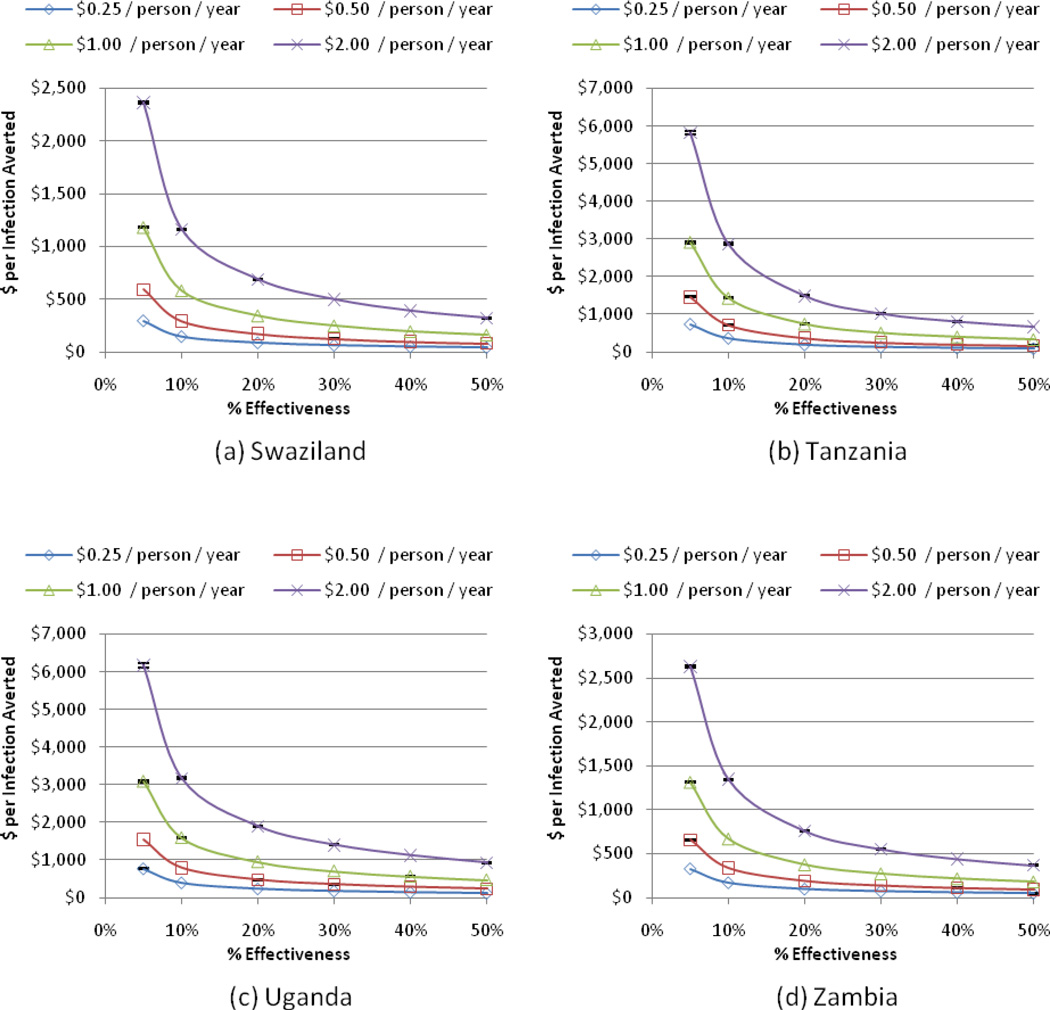
We estimated the cost per HIV infection averted for a single scenario (Increased Monogamy) in each study country as a function of behavior change achieved and annual program cost (Figure 1). Using an estimate of $1,200 – $3,800 for the lifetime medical cost of caring for an HIV-infected individual in Africa [59–62], our analysis suggests that concurrency reduction is cost-saving under a broad range of cost and effectiveness assumptions. For example, according to model predictions, a program that costs $0.25 per person annually (similar to Uganda’s “Zero Grazing” campaign) [18], would need a program effectiveness of less than 5% in any of the study countries to be cost-saving, while a program that costs $1 per person annually (similar to Botswana’s campaign (T. Kasper, personal communication, August 8, 2009)) would need to be 9% (<5% – 15%) effective on average. A program that costs $2 per person annually would need to be 20% (10% – 35%) effective in order to be cost-saving, and less than 5% (<5% – 12%) effective to cost less than $5,000 per infection averted.
Sensitivity Analysis
Our primary analysis includes sensitivity analysis on a range of concurrency, demographic, and epidemic patterns in sub-Saharan Africa, as well as the impacts of different levels of behavior change up to a program effectiveness of 50%. In sensitivity analysis, we considered the case where concurrency is completely eliminated (100% behavior change). In this case the model predicts an average decrease in new infections of 54.8%.
We relied upon sexual behavior surveys to estimate concurrency behavior in the study countries. However, different survey methodologies can lead to different estimates of numbers of sexual partners [63]. The sexual behavior survey data used in this analysis provided information on the number of sexual partners in the past 12 months reported by men and women but did not differentiate between concurrent and serial partnerships. These surveys may therefore overestimate concurrency behavior. Overestimating the number of concurrent partnerships in the study countries may overstate the benefits of concurrency reduction. We therefore conducted a sensitivity analysis in which we assumed that these surveys overestimated concurrency by 50%. With levels of concurrency that are half as high as we estimated in the base case, we found that fewer infections are averted under the three behavior change scenarios as compared to the Status Quo (e.g., 30% versus 45% reduction in new infections in Tanzania for a 50% reduction in concurrency) with a greater cost per HIV infection averted (e.g., $2,400 versus $1,400 per HIV infection averted in Tanzania for a program that is 10% effective and costs $1 per person annually). However, the rank ordering of the different types of behavior change was unaffected (Appendix Figure 4).
In sexual behavior survey data, men and women typically report different numbers of partners. In a heterosexual population, the total number of partnerships engaged in by men should equal the total number of partnerships engaged in by women. To resolve this discrepancy, in our base case analyses we adjusted the male-to-female ratio appropriately and left the reported gender-specific partnership distributions unchanged. For two of the study countries, this ratio was quite different than that estimated from demographic data (1:1.3 versus 1:1 in Uganda and 1:1.4 versus 1:1 in Zambia; Appendix Table 1). In sensitivity analysis, we explored the effects of fixing the male-to-female ratio to 1:1 and then averaging the male- and female-specific partnership distributions and applying this average distribution to both men and women. With an average partnership distribution, slightly fewer infections are averted for each of the three behavior change scenarios as compared to the Status Quo (e.g., 30% versus 35% reduction in new infections in Zambia for a 50% reduction in concurrency). However, the rank ordering of the different types of behavior change was again unchanged (Appendix Figure 5).
We also varied a number of other parameters (e.g., rate of condom use in non-spousal partnerships, duration of non-spousal partnerships, level of access to HIV treatment, and the CD4 count threshold for HIV treatment eligibility). Changes in the duration of non-spousal partnerships, level of access to HIV treatment, and HIV treatment eligibility criteria had little effect on the number of infections averted through concurrency reduction. Reducing the risk of HIV transmission in non-spousal partnerships (e.g., increasing condom use) resulted in fewer infections averted for a given level of concurrency reduction; however, over all parameter ranges considered in sensitivity analysis, the rank ordering of the different types of behavior change in averting HIV infections was unchanged (Appendix Table 2).
DISCUSSION
Using a stochastic network model based on data from four representative sub-Saharan African countries with a large HIV burden, we provide the first estimate of the epidemiologic benefits and the cost-effectiveness of changes in sexual behavior that may arise from concurrent partnership reduction campaigns. Our analysis suggests that reducing concurrent partnerships among high-risk individuals would result in the greatest reduction in new HIV infections compared to the Status Quo. However, all three of the modeled behavior change scenarios achieved relatively similar reductions in new HIV infections. In contrast to previous work which argues that concurrency reduction without targeting the most connected individuals will fail [64], our results suggest that the message and target population matter less than the extent of partnership reduction.
Concurrent sexual partnerships increase the rate of HIV transmission by creating a connected network, and high-risk individuals are the most important links in such a network. This explains our finding that reducing the number of partnerships among the highest risk individuals has the greatest impact on HIV transmission. However, reaching the most promiscuous individuals and changing their behaviors is often difficult. Our analyses suggest that other forms of concurrency reduction provide relatively similar benefits, without the need to specifically target groups that may be hard to reach. These findings lend support to previous suggestions that reaching all members of a sexual network, not just the most promiscuous, is of great importance in preventing HIV [65].
Campaigns explicitly targeting concurrent partnership reduction are relatively new and their effectiveness has yet to be measured. However, our analyses suggest that if campaigns can achieve at least modest reductions in concurrency at similar costs as other mass media campaigns, they are likely to be cost-effective and even cost saving. For example, given our baseline estimates of concurrency, our model predicts that a campaign that costs $1 per person annually need only reduce concurrent partnerships by less 5% to 15% in the study countries considered to be cost-saving, when comparing the cost per HIV infection averted to the lifetime medical costs of an HIV infection. For a campaign that is 10% effective in reducing concurrent partnerships and that costs $1 per person annually, we estimate that the cost per HIV infection averted ranges from $582 to $1,583 in the study countries considered. This places concurrency reduction on par with other established HIV prevention interventions in Africa: the costs per HIV infection averted for male circumcision in South Africa and prevention of mother-to-child transmission are $181 and $20-$2,198, respectively [61, 66–68].
We estimate that the maximal potential reduction in new infections due to concurrency reduction (i.e., if there are no concurrent partnerships) is about 55%, corresponding to 0.2, 2.0, 1.5, and 1.4 million infections averted in Swaziland, Tanzania, Uganda, and Zambia, respectively, over 10 years. While this is a substantial reduction in new infections, it highlights the importance of implementing complementary HIV prevention approaches. Even complete elimination of multiple concurrent sexual partnerships cannot stop HIV transmission.
Our analysis focused on the benefits of concurrency reduction for HIV prevention. However, programs that reduce concurrency may also generate additional benefits, such as reduction in the incidence of other sexually transmitted diseases. Targeting concurrent sexual partnerships may also have social benefits such as reducing intergenerational sex, a phenomenon that has likely contributed to the feminization of the HIV epidemic in Africa, and reducing domestic violence, which is commonly associated with having multiple sexual partners [11, 69, 70].
The results presented here are contingent on the prevalence of concurrency in each of the study countries. Our estimates of concurrency behavior relied on limited data. We assumed that sexual behavior survey data on the number of sexual partners reported in the past 12 months could be used as a surrogate for the concurrency behavior in the population. This assumption may overestimate the true concurrency behavior. At the same time, sexual behavior surveys may underestimate true behavior patterns. While discussions are ongoing about how best to measure concurrency using survey methods [71], sexual behavior surveys have yet to systematically incorporate these recommendations. In an effort to quantify the importance of this limitation on our results, we varied the prevalence of concurrency in sensitivity analysis to account for potential overestimation. We found that the rank ordering of the behavior change scenarios was unchanged, though the expected number of infections averted decreased as the prevalence of concurrency was reduced and the cost per infection averted increased. This highlights the need for better estimates of baseline sexual behavior, including the prevalence of concurrent partnerships, when considering investments in campaigns to change sexual behaviors.
Our analysis has several additional limitations. First, we assessed the relative effectiveness of different types of behavior change, but do not comment on the feasibility of such behavior changes. These considerations may vary by country. Second, our prevalence estimates depend on HIV mortality, which may change as treatment coverage changes in sub-Saharan Africa. Finally, our analysis was limited by the data informing the network structure in our model. We based the sexual partnership network structure for each of the four study countries on country-specific survey data. This data only provided us with partnership distributions, and we assumed non-preferential mixing lacking data to informing us otherwise, though populations typically do exhibit some kind of assortative mixing [72]. Mixing patterns induced by cross-generational partnerships, in particular, could prove extremely important in the context of concurrent partnership reduction campaigns. Future work characterizing these relationships would greatly help in evaluating the impact of concurrency reduction on the course of the epidemic and could inform an extension of the current model incorporating preferential mixing patterns.
The message(s) that a concurrency reduction campaign chooses to convey have important implications. The target population may change, the cultural background may affect the message’s effectiveness, and the cost of reaching the target population may vary. Our analysis suggests that the beneficial effect of concurrency reduction is only minimally dependent on the target population and message, and greatly dependent on the achieved reduction in concurrency. Moreover, our analysis suggests that concurrency reduction campaigns can be cost-effective, or even cost-saving, under a broad range of plausible cost and effectiveness assumptions, and even if the level of concurrency is half as high as we estimated in our base case. This lends further support to the potential importance of these campaigns as a means of controlling HIV in sub-Saharan Africa.
Supplementary Material
Figure 2. Cost per HIV infection averted for the Increased Monogamy Scenario.
This figure shows the cost per HIV infection averted for the Increased Monogamy scenario, as a function of annual program costs (measured as $/person/year) and program effectiveness (measured as the percentage reduction in concurrency). Costs and infections averted are discounted to the present at 3% per year. Results are shown for each of the four study countries, Swaziland (a), Tanzania (b), Uganda (c), and Zambia (d). 95% confidence intervals are indicated for each sample point.
ACKNOWLEDGEMENTS
This work was funded by the National Institute on Drug Abuse.
This research was funded by the National Institute on Drug Abuse, Grant Number R01-DA15612. Eva Enns is supported by a National Defense Science and Engineering Graduate Fellowship, a National Science Foundation Graduate Fellowship, and a Rambus Stanford Graduate Fellowship. Thomas Igeme was supported by a Stanford Undergraduate Research Fellowship. Eran Bendavid is supported by the National Institute of Allergy and Infectious Diseases (K01-AI084582). We appreciate the help of Tobias Kasper in providing important input on Botswana’s campaign to reduce concurrent sexual partnerships. The funding agencies had no part in the study design, the collection of data, the analysis, or the conclusions. No medical writers or editors were used for this manuscript.
REFERENCES
- 1.UNAIDS. Report on the Global AIDS Epidemic. Geneva: Joint United Nations Programme on HIV AIDS; 2008. [Google Scholar]
- 2.Morris M, Epstein H, Wawer M. Timing Is Everything: International Variations in Historical Sexual Partnership Concurrency and HIV Prevalence. PLos One. 2010;5(11):e14092. doi: 10.1371/journal.pone.0014092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lagarde E, Auvert B, Caraël M, et al. Concurrent sexual partnerships and HIV prevalence in five urban communities of sub-Saharan Africa. AIDS. 2001;15(7):877. doi: 10.1097/00002030-200105040-00008. [DOI] [PubMed] [Google Scholar]
- 4.Carter MW, Kraft JM, Koppenhaver T, et al. “A bull cannot be contained in a single kraal”: concurrent sexual partnerships in Botswana. AIDS Behav. 2007;11(6):822–830. doi: 10.1007/s10461-006-9203-6. [DOI] [PubMed] [Google Scholar]
- 5.Morris M, Wawer MI, Podhisita C. The Thailand and Ugandan sexual network studies. In: Morris M, editor. Network Epidemiology: A Handbook for Survey Design and Data Collection. Oxford: Oxford University Press; 2004. [Google Scholar]
- 6.Morris M, Kretzschmar M. Concurrent partnerships and the spread of HIV. AIDS. 1997;11(5):641–648. doi: 10.1097/00002030-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Kretzschmar M, Morris M. Measures of concurrency in networks and the spread of infectious disease. Math Biosci. 1996 Apr 15;133(2):165–195. doi: 10.1016/0025-5564(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 8.Morris M, Kretzschmar M. A microsimulation study of the effect of concurrent partnerships on the spread of HIV in Uganda. Math Popul Stud. 2000;8(2):109–133. [Google Scholar]
- 9.Pilcher CD, Tien HC, Eron JJ, Jr, et al. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J Infect Dis. 2004;189(10):1785–1792. doi: 10.1086/386333. [DOI] [PubMed] [Google Scholar]
- 10.Gourvenac D, Taruberekera N, Mochaka O, Kasper T. Multiple Concurrent Partnerships Among Men and Women Aged 15–34 in Botswana. Gaborone, Botswana: PSI-Botswana; 2007. [Google Scholar]
- 11.Jana M, Nkambule M, Tumbo D. One Love: Multiple and Concurrent Sexual Partnerships in Southern Africa. Johannesburg, South Africa: Soul City Institute for Health & Development Communications; 2008. [Google Scholar]
- 12.Serlemitsos E. Mass media campaigns on multiple concurrent sexual partnerships (MCP) in Zambia. Conference on Addressing Multiple and Concurrent Partnerships in Southern Africa: Developing Guidance for Bold Action; Gaborone, Botswana. 2009. [Google Scholar]
- 13.Mah T, Halperin DT. Concurrent sexual partnerships and the HIV epidemic in Africa: evidence to move forward. AIDS Behav. 2008 doi: 10.1007/s10461-008-9433-x. [DOI] [PubMed] [Google Scholar]
- 14.Shelton J. Why multiple sex partners? Lancet. 2009;374(9687):367–369. doi: 10.1016/S0140-6736(09)61399-4. [DOI] [PubMed] [Google Scholar]
- 15.Wilson D, Halperin DT. “Know your epidemic, know your response”: a useful approach, if we get it right. Lancet. 2008;372(9637):423–426. doi: 10.1016/S0140-6736(08)60883-1. [DOI] [PubMed] [Google Scholar]
- 16.Halperin DT, Epstein H. Concurrent sexual partnerships help to explain Africa's high HIV prevalence: implications for prevention. Lancet. 2004 Jul 3–9;364(9428):4–6. doi: 10.1016/S0140-6736(04)16606-3. [DOI] [PubMed] [Google Scholar]
- 17.Potts M, Halperin DT, Kirby D, et al. Public health. Reassessing HIV prevention. Science. 2008 May 9;320(5877):749–750. doi: 10.1126/science.1153843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Low-Beer D. This is a routinely avoidable disease. Financial Times. 2003 November 28; [Google Scholar]
- 19.Ciantia F, Orach S, Pariyo GW, et al. HIV prevention conundrum: did the Pope have a case? J Med Pers. 2009;7(2):63–69. [Google Scholar]
- 20.Green E, Nantulya V, Stoneburner R, J S. What Happened in Uganda? Declining HIV Prevalence, Behavior Change, and the National Response. USAID. 2002 [Google Scholar]
- 21.Green EC, Halperin DT, Nantulya V, Hogle JA. Uganda's HIV prevention success: the role of sexual behavior change and the national response. AIDS Behav. 2006;10(4):335–346. doi: 10.1007/s10461-006-9073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shelton JD, Halperin DT, Nantulya V, Potts M, Gayle HD, Holmes KK. Partner reduction is crucial for balanced "ABC" approach to HIV prevention. BMJ. 2004 Apr 10;328(7444):891–893. doi: 10.1136/bmj.328.7444.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hallett TB, Aberle-Grasse J, Bello G, et al. Declines in HIV prevalence can be associated with changing sexual behaviour in Uganda, urban Kenya, Zimbabwe, and urban Haiti. Sex Transm Infect. 2006;82 Suppl 1:1–8. doi: 10.1136/sti.2005.016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoneburner RL, Low-Beer D. Population-level HIV declines and behavioral risk avoidance in Uganda. Science. 2004;304(5671):714–718. doi: 10.1126/science.1093166. [DOI] [PubMed] [Google Scholar]
- 25.Halperin DT, Mugurungi O, Hallett TB, et al. A Surprising Prevention Success: Why Did the HIV Epidemic Decline in Zimbabwe? PLoS Med. 2011;8(2):e1000414. doi: 10.1371/journal.pmed.1000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregson S, Garnett GP, Nyamukapa CA, et al. HIV decline associated with behavior change in eastern Zimbabwe. Science. 2006;311(5761):664–666. doi: 10.1126/science.1121054. [DOI] [PubMed] [Google Scholar]
- 27.Gregson S, Gonese E, Hallett TB, et al. HIV decline in Zimbabwe due to reductions in risky sex? Evidence from a comprehensive epidemiological review. Int J Epidemiol. 2010;39(5):1–13. doi: 10.1093/ije/dyq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.HIV/AIDS/STI/TB Council of Zambia. National HIV AIDS Communication Strategy. [cited April 9, 2009];2005 Available from: http://zambia.jhuccp.org/resources/nac_pubs/NationalHIVAIDSCommunicationStrategy.pdf.
- 29.National AIDS Coordinating Agency. National Campaign Plan: Multiple Concurrent Partnerships. [cited March 2009];2009 Available from: http://www.psiwash.org/resources/pubs/National%20MCP%20strategy.pdf.
- 30.National AIDS Coordinating Agency. Botswana Campaign on MCP. Johannesburg, South Africa: UNAIDS/Soul City Regional Meeting on Multiple Concurrent Partnerships; 2008. [Google Scholar]
- 31.Matlhare RK. An overview of National MCP Campaign Botswana. Conference on Addressing Multiple and Concurrent Partnerships in Southern Africa: Developing Guidance for Bold Action; Gaborone, Botswana. 2009. [Google Scholar]
- 32.Bertrand J, O'Reilly K, Denison J, Anhang R, Sweat M. Systematic review of the effectiveness of mass communication programs to change HIV/AIDS-related behaviors in developing countries. Health Educ Res. 2006;21(4):567–597. doi: 10.1093/her/cyl036. [DOI] [PubMed] [Google Scholar]
- 33.Kim YM, Kols A, Nyakauru R, Marangwanda C, Chibatamoto P. Promoting Sexual Responsibility Among Young People in Zimbabwe. Int Fam Plan Perspect. 2001;27(1):11–19. [Google Scholar]
- 34.Vaughan PW, Rogers EM, Singhal A, Swalehe RM. Entertainment-Education and HIV/AIDS Prevention: A field experiment in Tanzania. J Health Comm. 2000;5 Suppl:81–100. doi: 10.1080/10810730050019573. [DOI] [PubMed] [Google Scholar]
- 35.US Census Bureau. International Data Base. [cited January 29, 2009];2008 Available from: http://www.census.gov/ipc/www/idb/tables.html.
- 36.MEASURE DHS. Swaziland Demographic and Health Survey. [cited January 2009];2006 Available from: http://www.measuredhs.com/countries/country_main.cfm?ctry_id=220&c=Swaziland.
- 37.MEASURE DHS. Tanzania Demographic and Health Survey. [cited January 2009];2003 Available from: http://www.measuredhs.com/countries/country_main.cfm?ctry_id=39&c=Tanzania.
- 38.MEASURE DHS. Uganda Demographic and Health Survey. [cited January 2009];2004 Available from: http://www.measuredhs.com/countries/country_main.cfm?ctry_id=44&c=Uganda.
- 39.MEASURE DHS. Zambia Demographic and Health Survey. [cited January 2009];2001 Available from: http://www.measuredhs.com/countries/country_main.cfm?ctry_id=47&c=Zambia.
- 40.World Health Organization. Life Tables for WHO Member States. [cited November 2008];2006 Available from: http://www.who.int/whosis/database/life_tables/life_tables.cfm.
- 41.Marston M, Todd J, Glynn JR, et al. Estimating 'net' HIV-related mortality and the importance of background mortality rates. AIDS. 2007 Nov;21 Suppl 6:S65–S71. doi: 10.1097/01.aids.0000299412.82893.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Enns EA, Brandeau ML. Inferring Network Structure and Dynamics in Network-Based Disease Simulation. Health Care Manage Sci. 2011 doi: 10.1007/s10729-011-9150-2. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vergis EN, Mellors JW. Natural history of HIV-1 infection. Infect Dis Clin North Am. 2000;14(4):809–825. doi: 10.1016/s0891-5520(05)70135-5. [DOI] [PubMed] [Google Scholar]
- 44.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis. 2008 Sep 1;198(5):687–693. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- 45.Wawer MJ, Gray RH, Sewankambo NK, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191(9):1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 46.McCormick AW, Walensky RP, Lipsitch M, et al. The effect of antiretroviral therapy on secondary transmission of HIV among men who have sex with men. Clin Infect Dis. 2007 Apr 15;44(8):1115–1122. doi: 10.1086/512816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001 Apr 14;357(9263):1149–1153. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 48.Bendavid E, Young SD, Katzenstein DA, Bayoumi AM, Sanders GM, Owens DK. Cost-effectiveness of HIV monitoring strategies in resource-limited settings – a southern African analysis. Arch Intern Med. 2008 September 22;168(17):1910–1918. doi: 10.1001/archinternmed.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Badri M, Lawn SD, Wood R. Short-term risk of AIDS or death in people infected with HIV-1 before antiretroviral therapy in South Africa: a longitudinal study. Lancet. 2006;368(9543):1254–1259. doi: 10.1016/S0140-6736(06)69117-4. [DOI] [PubMed] [Google Scholar]
- 50.Holmes CB, Wood R, Badri M, et al. CD4 decline and incidence of opportunistic infections in Cape Town, South Africa: implications for prophylaxis and treatment. J Acquir Immune Defic Syndr. 2006;42(4):464–469. doi: 10.1097/01.qai.0000225729.79610.b7. [DOI] [PubMed] [Google Scholar]
- 51.Lawn SD, Myer L, Bekker LG, Wood R. CD4 cell count recovery among HIV-infected patients with very advanced immunodeficiency commencing antiretroviral treatment in sub-Saharan Africa. BMC Infect Dis. 2006;6:59. doi: 10.1186/1471-2334-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaufmann GR, Perrin L, Pantaleo G, et al. CD4 T-lymphocyte recovery in individuals with advanced HIV-1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study. Arch Intern Med. 2003 Oct 13;163(18):2187–2195. doi: 10.1001/archinte.163.18.2187. [DOI] [PubMed] [Google Scholar]
- 53.Kaufmann GR, Furrer H, Ledergerber B, et al. Characteristics, determinants, and clinical relevance of CD4 T cell recovery to <500 cells/microL in HIV type 1-infected individuals receiving potent antiretroviral therapy. Clin Infect Dis. 2005;41(3):361–372. doi: 10.1086/431484. [DOI] [PubMed] [Google Scholar]
- 54.World Health Organization. Geneva: 2006. Antiretroviral Therapy For HIV Infection in Adults And Adolescents: Recommendations for a Public Health Approach. Revision. [PubMed] [Google Scholar]
- 55.Orrell C, Harling G, Lawn SD, et al. Conservation of first-line antiretroviral treatment regimen where therapeutic options are limited. Antivir Ther. 2007;12(1):83–88. [PubMed] [Google Scholar]
- 56.Stover J, Walker N, Grassly NC, Marston M. Projecting the demographic impact of AIDS and the number of people in need of treatment: updates to the Spectrum projection package. Sex Transm Infect. 2006;82 Suppl 3:iii45–iii50. doi: 10.1136/sti.2006.020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matovu JKB, Gray RH, Kiwanuka N, et al. Repeat voluntary HIV counseling and testing (VCT), sexual risk behavior and HIV incidence in Rakai, Uganda. AIDS Behav. 2007;11(1):71–78. doi: 10.1007/s10461-006-9170-y. [DOI] [PubMed] [Google Scholar]
- 58.Shelton JD, Halperin DT, Wilson D. Has global HIV incidence peaked? Lancet. 2006;367(9517):1120–1122. doi: 10.1016/S0140-6736(06)68436-5. [DOI] [PubMed] [Google Scholar]
- 59.Stover J, Bertozzi S, Gutierrez J-P, et al. The global impact of scaling up HIV/AIDS prevention programs in low- and middle-income countries. Science. 2006;311(5766):1474–1476. doi: 10.1126/science.1121176. [DOI] [PubMed] [Google Scholar]
- 60.Cleary S, Boulle A, McIntyre D, Coetzee D. Cost-Effectiveness of Antiretroviral Treatment for HIV-Positive Adults in a South African Township. Cape Town: Health Systems Trust; 2004. [Google Scholar]
- 61.Kahn JG, Marseille E, Auvert B. Cost-effectiveness of male circumcision for HIV prevention in a South African setting. PLoS Med. 2006 December;3(12):2349–2358. doi: 10.1371/journal.pmed.0030517. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yazdanpanah Y, Losina E, Anglaret X, et al. Clinical impact and cost-effectiveness of co-trimoxazole prophylaxis in patients with HIV/AIDS in Cote d'Ivoire: a trial-based analysis. AIDS. 2005 Aug 12;19(12):1299–1308. doi: 10.1097/01.aids.0000180101.80888.c6. [DOI] [PubMed] [Google Scholar]
- 63.LePont F, Pech N, Boelle P, et al. A new scale for measuring dynamic patterns of sexual partnership and concurrency: application to three French Caribbean regions. Sex Transm Dis. 2003;30(1):6–9. doi: 10.1097/00007435-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 64.Liljeros F, Edling CR, Amaral LAN, Stanley HE, Åberg Y. The web of human sexual contacts. Nature. 2001;411(6840):907–908. doi: 10.1038/35082140. [DOI] [PubMed] [Google Scholar]
- 65.Jones JH, Handcock MS. Social networks: Sexual contacts and epidemic thresholds. Nature. 2003 Jun 5;423(6940):605–606. doi: 10.1038/423605a. discussion 6. [DOI] [PubMed] [Google Scholar]
- 66.Sweat M, Gregorich S, Sangiwa G, et al. Cost-effectiveness of voluntary HIV-1 counselling and testing in reducing sexual transmission of HIV-1 in Kenya and Tanzania. Lancet. 2000 Jul 8;356(9224):113–121. doi: 10.1016/S0140-6736(00)02447-8. [DOI] [PubMed] [Google Scholar]
- 67.Soderlund N, Zwi K, Kinghorn A, Gray G. Prevention of vertical transmission of HIV: analysis of cost effectiveness of options available in South Africa. BMJ. 1999;318(7199):1650–1656. doi: 10.1136/bmj.318.7199.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilkinson D, Floyd K, Gilks C. Antiretroviral drugs as a public health intervention for pregnant HIV-infected women in rural South Africa: an issue of cost-effectiveness and capacity. AIDS. 1998;12(13):1675–1682. doi: 10.1097/00002030-199813000-00016. [DOI] [PubMed] [Google Scholar]
- 69.Hallett TB, Gregson S, Lewis JJC, Lopman BA, Garnett GP. Behaviour change in generalised HIV epidemics: impact of reducing cross-generational sex and delaying age at sexual debut. BMJ. 2007;83 Suppl 1:50–54. doi: 10.1136/sti.2006.023606. [DOI] [PubMed] [Google Scholar]
- 70.Andersson N, Ho-Foster A, Mitchell S, Scheepers E, Goldstein S. Risk factors for domestic physical violence: national cross-sectional household surveys in eight southern African countries. BMC Womens Health. 2007;7:11. doi: 10.1186/1472-6874-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.UNAIDS Reference Group on Estimates, Modelling and Projections. Consultation on Concurrent Sexual Partnerships. Nairobi: UNAIDS; 2009. [Google Scholar]
- 72.Garnett G, Hughes J, Anderson R, et al. Sexual Mixing Patterns of Patients Attending Sexually Transmitted Diseases Clinics. Sex Transm Dis. 1996;23(3):248–257. doi: 10.1097/00007435-199605000-00015. [DOI] [PubMed] [Google Scholar]
- 73.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342(13):921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.